In Vivo Effects of Neonicotinoid-Sulfoximine Insecticide Sulfoxaflor on Acetylcholinesterase Activity in the Tissues of Zebrafish (Danio rerio)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Test Conditions
2.3. Determination of the 50% Lethal Concentration Value of Sulfoxaflor in Zebrafish
2.4. Acute Toxicity Tests
2.5. Preparation of Tissue Homogenates
2.6. Determination of Acetylcholinesterase Enzyme Activity
2.7. Determination of Protein Levels
2.8. Statistical Analysis
3. Results
3.1. Determination of the 96 h LC50 Value of Sulfoxaflor for Zebrafish
3.2. Changes in AChE Enzyme Activity in the Brain
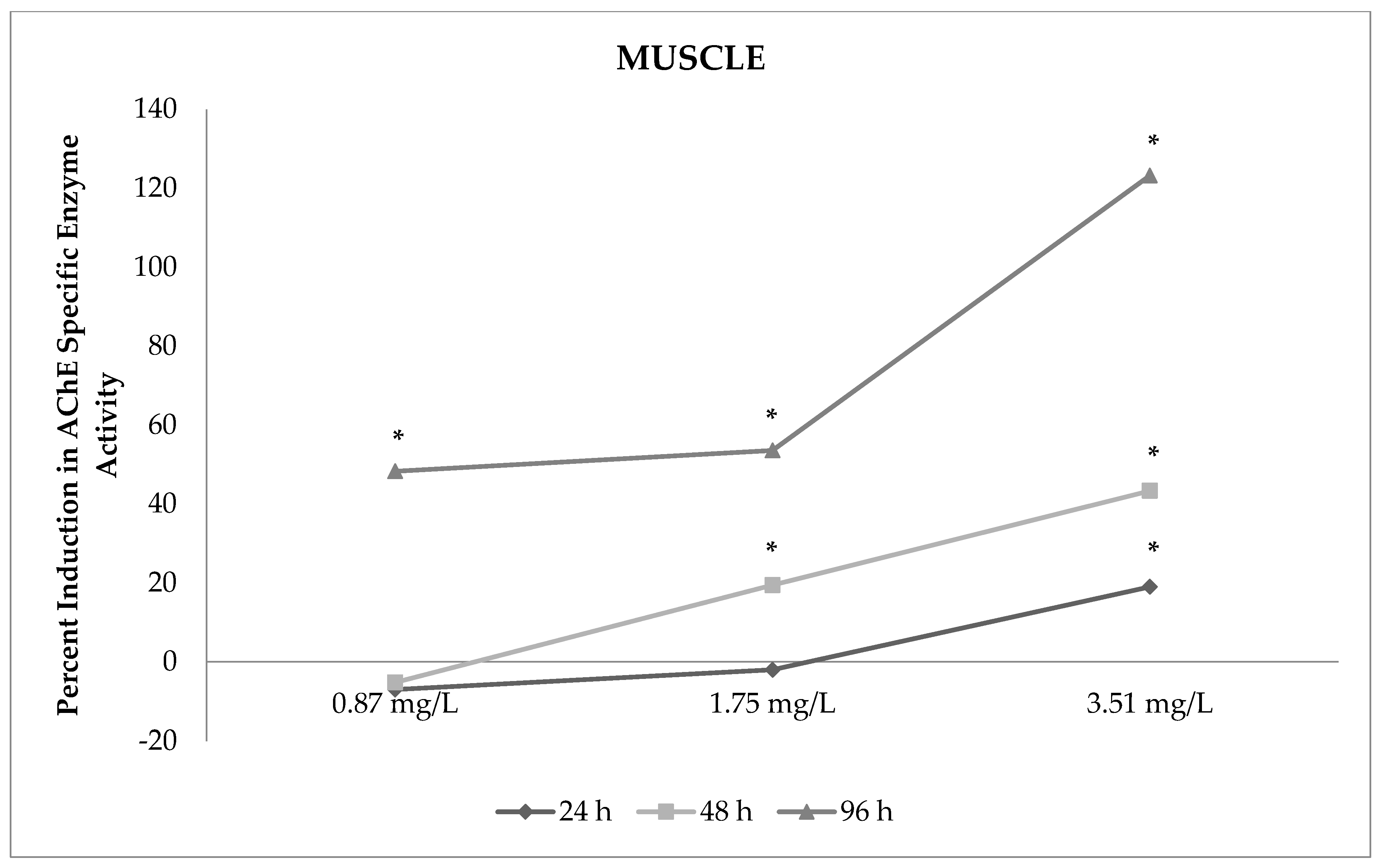
3.3. Changes in AChE Enzyme Activity in Muscles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Loso, M.R.; Watson, G.B.; Sparks, T.C.; Rogers, R.B.; Huang, J.X.; Gerwick, B.C.; Babcock, J.M.; Kelley, D.; Hegde, V.B.; et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 2011, 59, 2950–2957. [Google Scholar] [CrossRef]
- Ellis-Hutchings, R.G.; Rasoulpour, R.J.; Terry, C.; Carney, E.W.; Billington, R. Human relevance framework evaluation of a novel rat developmental toxicity mode of action induced by sulfoxaflor. Critic. Rev. Toxicol. 2014, 44, 45–62. [Google Scholar] [CrossRef]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sánchex-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Response to Public Comments on EPA’s Proposed Registration of the New Active Ingredient Sulfoxaflor for Use on Multiple Commodities, Turf Grass, and Ornamen-Tals, EPA-HQ-OPP-2010-0889; USEPA: Washington, DC, USA, 2010.
- USEPA. Registration Decision for the New Active Ingredient Sulfoxaflor. Registration of the New Active Ingredient Sulfoxaflor for Use on Multiple Commodities, Turf Grass and Ornamentals, EPA-HQ-OPP-2010-0889-0396; USEPA: Washington, DC, USA, 2013.
- Siviter, H.; Brown, M.J.F.; Leadbeater, E. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 2018, 561, 109–112. [Google Scholar] [CrossRef]
- LeBaron, M.J.; Geter, D.R.; Rasoulpour, R.J.; Gollapudi, B.B.; Thomas, J.; Murray, J.; Kan, H.L.; Wood, A.J.; Elcombe, C.; Vardy, A.; et al. An integrated approach for prospectively investigating a mode-of-action for rodent liver effects. Toxicol. Appl. Pharmacol. 2013, 270, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Rasoulpour, R.J.; Terry, C.; LeBaron, M.J.; Stebbins, K.; Ellis-Hutchings, R.J.; Billington, R. Mode-of-action and human relevance framework analysis for rat Leydig cell tumors associated with sulfoxaflor. Crit. Rev. Toxicol. 2014, 44, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Rasoulpour, R.J.; Ellis-Hutchings, R.G.; Terry, C.; Millar, N.S.; Zablotny, C.L.; Gibb, A.; Marshall, V.; Collins, T.; Carney, E.W.; Billington, R. A novel mode-of-action mediated by the fetal muscle nicotinic acetylcholine receptor resulting in developmental toxicity in rats. Toxicol. Sci. 2012, 127, 522–534. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R. Neonicotinoids-from zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticides: Highlights of a symposium on strategic molecular designs. J. Agric. Food Chem. 2011, 59, 2883–2886. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef]
- Gibbons, D.; Morrissey, C.; Mineau, P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ann, J.; Akk, G. Activation and modulation of human α4β2 nicotinic acetylcholine receptors by the neonicotinoids clothianidin and imidacloprid. J. Neurosci. Res. 2011, 89, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Song, J.H.; Shono, T.; Narahashi, T. Modulation of the neuronal nicotinic acetylcholine receptor-channel by the nitromethylene heterocycle imidacloprid. J. Pharmacol. Exp. Ther. 1998, 285, 731–738. [Google Scholar] [PubMed]
- Matsuda, K.; Buckingham, S.D.; Freeman, J.C.; Squire, M.D.; Baylis, H.A.; Sattelle, D.B. Effects of the α subunit on imidacloprid sensitivity of recombinant nicotinic acetylcholine receptors. Br. J. Pharmacol. 1998, 123, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Matsuda, K.; Otake, M.; Kuwamura, M.; Shimomura, M.; Komai, K.; Akamatsu, M.; Raymond, V.; Sattelle, D.B. Diverse actions of neonicotinoids on chicken α7, α4β2 and Drosophila-chicken SADβ2 and ALSβ2 hybrid nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Neuropharmacology 2003, 45, 33–144. [Google Scholar] [CrossRef]
- Toshima, K.; Ihara, M.; Kanaoka, S.; Tarumoto, K.; Yamada, A.; Sattelle, D.B.; Matsuda, K. Potentiating and blocking actions of neonicotinoids on the response to acetylcholine of the neuronal α4β2 nicotinic acetylcholine receptor. J. Pestic. Sci. 2008, 33, 146–151. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Minor structural changes in nicotinoid insecticides confer differential subtype selectivity for mammalian nicotinic acetylcholine receptors. Br. J. Pharmacol. 1999, 127, 115–122. [Google Scholar] [CrossRef]
- Ensley, S.M. Neonicotinoids. In Veterinary Toxicology: Basic and Clinical Principles; Gupta, R.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 596–598. [Google Scholar]
- Abou-Donia, M.B.; Goldstein, L.B.; Bullman, S.; Tu, T.; Khan, W.A.; Dechkovskaia, A.M.; Abdel-Rahman, A.A. Imidacloprid induces neuro behavioural deficits and increases expression of glial fibrillary acidic protein in the motor cortex and hippocampus in offspring rats following in utero exposure. J. Toxicol. Environ. Health Sci. Part A 2008, 71, 119–130. [Google Scholar] [CrossRef]
- Rodrigues, K.J.A.; Santana, M.B.; Do Nascimento, J.L.M.; Picanço-Diniz, D.L.W.; Maues, L.A.L.; Santos, S.N.; Ferreira, V.M.M.; Alfonso, M.; Duran, R.; Faro, L.R.F. Behavioural and biochemical effects of neonicotinoid thiamethoxam on the cholinergic system in rats. Ecotox. Environ. Saf. 2010, 73, 101–107. [Google Scholar] [CrossRef]
- Ozdemir, H.H.; Kara, M.; Yumrutas, O.; Uckardes, F.; Eraslan, E.; Demir, C.F.; Bal, R. Determination of the effects on learning and memory performance and related gene expressions of clothianidin in rat models. Cogn. Neurodynamics 2014, 8, 411–441. [Google Scholar] [CrossRef]
- Lonare, M.; Kumar, M.; Raut, S.; Badgujar, P.; Doltade, S.; Telang, A. Evaluation of imidacloprid-induced neurotoxicity in male rats: A protective effect of curcumin. Neurochem. Int. 2014, 78, 122–129. [Google Scholar] [CrossRef]
- Ford, K.A.; Casida, J.E. Chloropyridinyl neonicotinoid insecticides: Diverse molecular substituents contribute to facile metabolism in Mice. Chem. Res. Toxicol. 2006, 19, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Terayama, H.; Endo, H.; Tsukamoto, H.; Matsumoto, K.; Umezu, M.; Kanazawa, T.; Ito, M.; Sato, T.; Naito, M.; Kawakami, S.; et al. Acetamiprid accumulates in different amounts in murine brain regions. Int. J. Environ. Res. Public. Health 2016, 13, 937. [Google Scholar] [CrossRef]
- Jackson, C.J.; Oakeshott, J.G.; Sanchez-Hernandez, J.C.; Wheelock, C.E. Carboxylesterases in the metabolism and toxicity of pesticides. In Anticholinesterase Pesticides: Metabolism, Neurotoxicity, and Epidemiology; Satoh, T., Gupta, R.C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 57–75. [Google Scholar]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Schettino, T. Acetylcholinesterase as a biomarker in environmental and occupational medicine: New insights and future perspectives. Biomed. Res. Int. 2013, 2013, 321213. [Google Scholar] [CrossRef] [PubMed]
- Massoulie, J.; Sussman, J.; Bon, S.; Silman, I. Structure and functions of acetylcholinesterase and butyrylcholinesterase. In Progress in Brain Research; Claudio Cuello, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 139–198. [Google Scholar]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Topal, A.; Alak, G.; Ozkaraca, M.; Yeltekin, A.C.; Comaklı, S.; Acıl, G.; Kokturk, M.; Atamanalp, M. Neurotoxic responses in brain tissues of rainbow trout exposed to imidacloprid pesticide: Assessment of 8-hydroxy-2-deoxyguanosineactivity, oxidative stress and acetylcholinesterase activity. Chemosphere 2017, 175, 186–191. [Google Scholar] [CrossRef]
- Cheghib, Y.; Chouahda, S.; Soltani, N. Side-effects of a neonicotinoid insecticide (actaraÒ) on a non-target larvivorous fish Gambusia affinis: Growth and biomarker responses. Egypt J. Aquat. Res. 2020, in press. [Google Scholar] [CrossRef]
- Vohra, P.; Singh, K.; Gurinder, K.; Sangha, K. Physiological, biochemical and histological alterations induced by administration of imidacloprid in female albino rats. Pestic. Biochem. Phys. 2014, 110, 50–56. [Google Scholar] [CrossRef]
- Boily, M.; Sarrasin, B.; Deblois, C.; Aras, P.; Chagnon, M. Acetylcholinesterase in honey bees (Apis mellifera) exposed to neonicotinoids, atrazine and glyphosate: Laboratory and field experiments. Environ. Sci. Pollut. Res. 2013, 20, 5603–5614. [Google Scholar] [CrossRef]
- Samson-Robert, O.; Labrie, G.; Mercier, P.; Chagnon, M.; Derome, N.; Fournier, V. Increased acetylcholinesterase expression in bumble bees during neonicotinoid-coated corn sowing. Sci. Rep. 2015, 5, 12636. [Google Scholar] [CrossRef]
- Morakchi, S.; Maiza, A.; Farine, P.; Soltani, N. Effects of a neonicotinoid insecticide (acetamiprid) on acetylcholinesterase activity and cuticular hydrocarbons profil in German cockroaches. Commun. Agric. Appl. Biol. Sci. 2005, 70, 843–848. [Google Scholar] [PubMed]
- Tian, X.; Yang, W.; Wang, D.; Zhao, Y.; Yao, R.; Ma, L.; Ge, C.; Li, X.; Huang, Z.; He, L.; et al. Chronic brain toxicity response of juvenile Chinese rare minnows (Gobiocypris rarus) to the neonicotinoid insecticides imidacloprid and nitenpyram. Chemosphere 2018, 210, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Parveen, M.; Kumar, S. Recent Trends in the Acetylcholinesterase System; IOS Press: Amsterdam, The Netherlands, 2005; pp. 1–240. [Google Scholar]
- Bertrand, C.; Chatonnet, A.; Takke, C.; Yan, Y.L.; Postlethwait, J.; Toutant, J.P.; Cousin, X. Zebrafish acetylcholinesterase is encoded by a single gene localized on linkage group 7. Gene structure and polymorphism; molecular forms and expression pattern during development. J. Biol. Chem. 2001, 276, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Fraysse, B.; Mons, R.; Garric, J. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals. Ecotoxicol. Environ. Saf. 2006, 63, 253–267. [Google Scholar] [CrossRef]
- Linney, E.; Upchurch, L.; Donerly, S. Zebrafish as a neurotoxicological model. Neurotoxicol. Teratol. 2004, 26, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.T.; Nass, R.; Boyd, W.A.; Freedman, J.H.; Dong, K.; Narahashi, T. Use of non-mammalian alternative models for neurotoxicological study. Neurotoxicology 2008, 29, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Selderslaghs, I.W.T.; Hooyberghs, J.; Coen, W.D.; Witters, H.E. Locomotor activity in zebrafish embryos: A new method to assess developmental neurotoxicity. Neurotox. Teratol. 2010, 32, 60–471. [Google Scholar] [CrossRef]
- Dow AgroScience. 2017. Available online: https://www.dowagro.com/tr-tr/turkiye/products/insektisitler/transform-500-wg.html (accessed on 19 August 2020).
- APHA; AWWA; WEF. Standard Methods. American Public Health Association, 20th ed.; American Public Health Association: Washington, DC, USA; New York, NY, USA, 1998; pp. 1–784. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Anders, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Habig, C.; Di Giulio, R.T. Biochemical characteristics of cholinesterases in aquatic organisms. In Cholinesterase Inhibiting Insecticides; Their Impact on Wildlife and Environment; Mineau, P., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1991; pp. 19–33. [Google Scholar]
- Zhang, T.; Yang, M.; Pan, H.; Li, S.; Ren, B.; Ren, Z.; Xing, N.; Qi, L.; Ren, Q.; Xu, S.; et al. Does time difference of the acetylcholinesterase (AChE) inhibition in different tissues exist? A case study of zebra fish (Danio rerio) exposed to cadmium chloride and deltamethrin. Chemosphere 2016, 168, 908–916. [Google Scholar] [CrossRef]
- Marinho, C.S.; Matias, M.V.F.; Toledo, E.K.M.; Smaniotto, S.; Ximenes-da-Silva, A.; Tonholo, J.; Santos, E.L.; Machado, S.S.; Zanta, C.L.P.S. Toxicity of silver nanoparticles on different tissues in adult Danio rerio. Fish. Physiol. Biochem. 2021. [Google Scholar] [CrossRef]
- Schmidel, A.J.; Assmann, K.L.; Werlang, C.C.; Bertoncello, K.T.; Francescon, F.; Rambo, C.L.; Beltrame, G.M.; Calegari, D.; Batista, C.B.; Blaser, R.E.; et al. Subchronic atrazine exposure changes defensive behaviour profile and disrupts brain acetylcholinesterase activity of zebrafish. Neurotoxicol. Teratol. 2014, 44, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Piner, P.; Üner, N. In vivo acetylcholinesterase inhibition in the tissues of spinosad exposed Oreochromis niloticus. Environ. Toxicol. Pharmacol. 2014, 34, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Gyori, J.; Farkas, A.; Stolyar, O.; Szekacs, A.; Mörtl, M.; Vehovszky, A. Inhibitory effects of four neonicotinoid active ingredients on acetylcholine esterase activity. Acta Biol. Hung. 2017, 68, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Teralı, K. An evaluation of neonicotinoids’ potential to inhibit human cholinesterases: Protein-ligand docking and interaction profiling studies. J. Mol. Graph. Model. 2018, 84, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Yang, L.; Zhao, Q.; Caen, J.P.; He, H.Y.; Jin, Q.H.; Guo, L.H.; Alemany, M.; Zhang, L.Y.; Shi, Y.F. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ. 2002, 9, 790–800. [Google Scholar] [CrossRef]
- Jin, Q.H.; He, H.Y.; Shi, Y.F.; Lu, H.; Zhang, X.J. Overexpression of acetylcholinesterase inhibited cell proliferation and promoted apoptosis in NRK cells. Acta Pharmacol. Sin. 2004, 25, 1013–1021. [Google Scholar]
- Legradi, J.B.; Di Paolo, C.; Kraak, M.H.S.; van der Geest, H.G.; Schymanski, E.L.; Williams, A.J.; Dingemans, M.M.L.; Massei, R.; Brack, W.; Cousin, X.; et al. An ecotoxicological view on neurotoxicity assessment. Environ. Sci. Eur. 2018, 30, 46. [Google Scholar] [CrossRef]
- Costa, B.P.D.; Moura, L.A.; Pinto, S.A.G.; Lima-Maximino, M.; Maximino, C. Review zebrafish models in neural and behavioral Toxicology across the life stages. Fishes 2020, 5, 23. [Google Scholar] [CrossRef]
- Levin, E.D.; Swain, H.A.; Donerly, S.; Linney, E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol. Teratol. 2004, 26, 719–723. [Google Scholar] [CrossRef]
- Sandahl, J.F.; Baldwin, D.H.; Jenkins, J.J.; Scholz, N.L. Comparative thresholds for acetylcholinesterase inhibition and behavioral impairment in coho salmon exposed to chlorpyrifos. Environ. Toxicol. Chem. 2005, 24, 136–145. [Google Scholar] [CrossRef]
- Almeida, J.R.; Oliveira, C.; Gravato, C.; Guilhermino, L. Linking behavioural alterations with biomarkers responses in the European seabass Dicentrarchus labrax L. exposed to the organophosphate pesticide fenitrothion. Ecotoxicology 2010, 19, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Lauridsen, H.; Buels, K.; Chi, L.-H.; LaDu, J.; Bruun, D.A.; Olson, J.R.; Tanguay, R.L.; Lein, P.J. Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol. Sci. 2011, 121, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Khalil, F.; Kang, I.J.; Undap, S.; Tasmin, R.; Qiu, X.; Shimasaki, Y.; Oshima, Y. Alterations in social behavior of Japanese medaka (Oryzias latipes) in response to sublethal chlorpyrifos exposure. Chemosphere 2013, 92, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Dubetz, C.; Palace, V.P. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total. Environ. 2015, 505, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]

| Lethal Concentrations | Sulfoxaflor (mg/L) | 95% Confidence Limits | |
|---|---|---|---|
| Lower | Upper | ||
| LC1 | 17.347 | 12.27 | 20.782 |
| LC5 | 21.331 | 16.621 | 24.403 |
| LC10 | 23.816 | 19.501 | 26.64 |
| LC15 | 25.654 | 21.685 | 28.308 |
| LC50 | 35.13 | 32.469 | 38.298 |
| LC85 | 48.106 | 43.11 | 58.428 |
| LC90 | 51.819 | 45.757 | 65.051 |
| LC95 | 57.855 | 49.898 | 76.398 |
| LC99 | 71.140 | 58.538 | 103.587 |
| Slope ± SEM | 7.592 ± 1.281 | ||
| Intercept ± SE | −11.734 ± 1.967 | ||
| χ2 value | 3.873 | ||
| p | <0.05 | ||
| Exposure Periods | AChE Enzyme Activity/Brain | |||
|---|---|---|---|---|
| Group I (Control) | Group II (0.87 mg/L Sulfoxaflor) | Group III (1.75 mg/L Sulfoxaflor) | Group IV (3.51 mg/L Sulfoxaflor) | |
| 24 h | 0.588 ± 0.015 bx | 0.485 ± 0.034 cy | 0.649 ± 0.041 abxy | 0.737 ± 0.029 ay |
| 48 h | 0.569 ± 0.019 ax | 0.548 ± 0.048 ay | 0.571 ± 0.064 ay | 0.490 ± 0.014 az |
| 96 h | 0.585 ± 0.021 cx | 0.746 ± 0.019 bx | 0.757 ± 0.020 bx | 1.073 ± 0.062 ax |
| Exposure Periods | AChE Enzyme Activity/Muscle | |||
|---|---|---|---|---|
| Group I (Control) | Group II (0.87 mg/L Sulfoxaflor) | Group III (1.75 mg/L Sulfoxaflor) | Group IV (3.51 mg/L Sulfoxaflor) | |
| 24 h | 0.699 ± 0.018 bx | 0.650 ± 0.029 bx | 0.685 ± 0.024 bz | 0.832 ± 0.030 ay |
| 48 h | 0.662 ± 0.010 bx | 0.674 ± 0.021 bx | 0.804 ± 0.019 ay | 0.823 ± 0.019 ay |
| 96 h | 0.671 ± 0.026 cx | 1.03 ± 0.083 by | 0.90 ± 0.028 bx | 1.207 ± 0.041 ax |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piner Benli, P.; Çelik, M. In Vivo Effects of Neonicotinoid-Sulfoximine Insecticide Sulfoxaflor on Acetylcholinesterase Activity in the Tissues of Zebrafish (Danio rerio). Toxics 2021, 9, 73. https://doi.org/10.3390/toxics9040073
Piner Benli P, Çelik M. In Vivo Effects of Neonicotinoid-Sulfoximine Insecticide Sulfoxaflor on Acetylcholinesterase Activity in the Tissues of Zebrafish (Danio rerio). Toxics. 2021; 9(4):73. https://doi.org/10.3390/toxics9040073
Chicago/Turabian StylePiner Benli, Petek, and Mehmet Çelik. 2021. "In Vivo Effects of Neonicotinoid-Sulfoximine Insecticide Sulfoxaflor on Acetylcholinesterase Activity in the Tissues of Zebrafish (Danio rerio)" Toxics 9, no. 4: 73. https://doi.org/10.3390/toxics9040073
APA StylePiner Benli, P., & Çelik, M. (2021). In Vivo Effects of Neonicotinoid-Sulfoximine Insecticide Sulfoxaflor on Acetylcholinesterase Activity in the Tissues of Zebrafish (Danio rerio). Toxics, 9(4), 73. https://doi.org/10.3390/toxics9040073






