Clastogenicity and Aneugenicity of 1,4-Benzoquinone in Different Lineages of Mouse Hematopoietic Stem/Progenitor Cells
Abstract
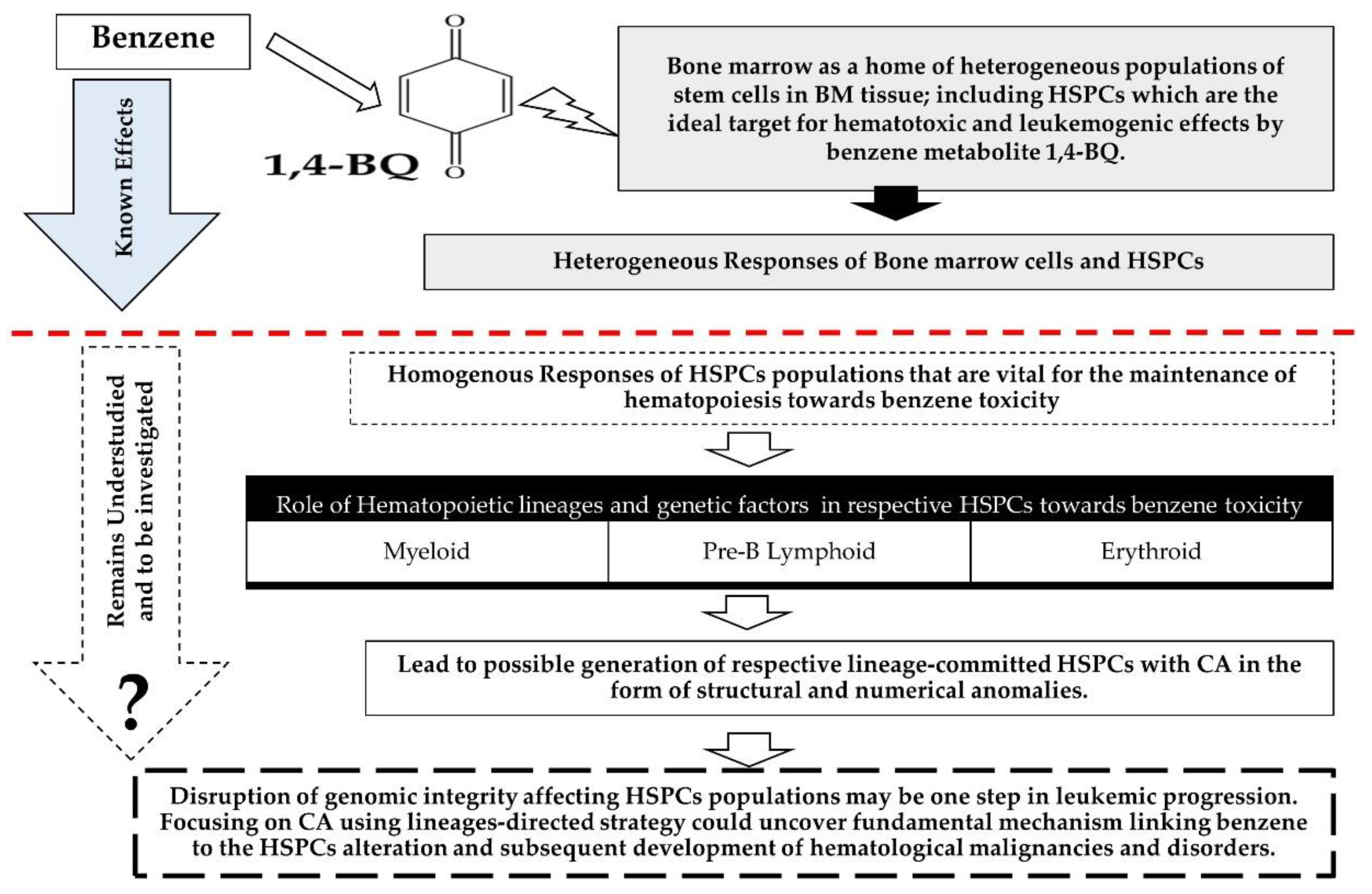
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Culturing of MBMCs
2.3. Treatment of MBMCs with 1,4-BQ
2.4. Enrichment of Erythroid, Myeloid, and Pre-B Lymphoid Progenitor Cells Using Colony-Forming Cell (CFC) Assay
2.5. Slide Preparation for Cytogenetic Analyses on 1,4-BQ-Treated MBMCs
2.6. Slide Preparation for Cytogenetic Analyses on 1,4-BQ-Treated HPCs
2.7. Cytogenetic Analyses on 1,4-BQ-Treated MBMCs and HPCs Using GenASIs Bandview Software
2.8. Statistical Analysis
3. Results
3.1. Clastogenicity and Aneugenicity of 1,4-BQ-Exposed MBMCs
3.2. Clastogenicity and Aneugenicity of 1,4-BQ-Exposed Myeloid Progenitor Cells
3.3. Clastogenicity and Aneugenicity of 1,4-BQ-Exposed Pre-B Lymphoid Progenitor Cells
3.4. Clastogenicity and Aneugenicity of 1,4-BQ-Exposed Erythroid Progenitor Cells
3.5. Differences of Cytogenetic Status between the Three Different Lineages of Hematopoietic Progenitors after 1,4-BQ Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McHale, C.M.; Zhang, L.; Smith, M.T. Current understanding of the mechanism of benzene-induced leukemia in humans: Implications for risk assessment. Carcinogenesis 2012, 33, 240–252. [Google Scholar] [CrossRef]
- Snyder, R. Benzene’s toxicity: A consolidated short review of human and animal studies by HA Khan. Hum. Exp. Toxicol. 2007, 26, 687–696. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, Q.; Ji, Z.; Li, G.; Shen, M.; Vermeulen, R.; Guo, W.; Hubbard, A.E.; McHale, C.M.; Rappaport, S.M.; et al. Leukemia-related chromosomal loss detected in hematopoietic progenitor cells of benzene-exposed workers. Leukemia 2012, 26, 2494–2498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ross, D.; Zhou, H. Relationships between metabolic and non-metabolic susceptibility factors in benzene toxicity. Chem. Biol. Interact. 2010, 184, 222–228. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, Q.; Guo, W.; Hubbard, A.E.; Li, G.; Rappaport, S.M.; McHale, C.M.; Shen, M.; Ji, Z.; Vermeulen, R.; et al. Chromosome-wide aneuploidy study (CWAS) in workers exposed to an established leukemogen, benzene. Carcinogenesis 2011, 32, 605–612. [Google Scholar] [CrossRef][Green Version]
- McHale, C.M.; Lan, Q.; Corso, C.; Li, G.; Zhang, L.; Vermeulen, R.; Curry, J.D.; Shen, M.; Turakulov, R.; Higuchi, R.; et al. Chromosome translocations in workers exposed to benzene. J. Natl. Cancer Inst. Monogr. 2008, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Major, J.; Jakab, M.; Kiss, G.; Tompa, A. Chromosome aberration, sister-chromatid exchange, proliferative rate index, and serum thiocyanate concentration in smokers exposed to low-dose benzene. Environ. Mol. Mutagenes. 1994, 23, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Fracasso, M.E.; Doria, D.; Bartolucci, G.B.; Carrieri, M.; Lovreglio, P.; Ballini, A.; Soleo, L.; Tranfo, G.; Manno, M. Low air levels of benzene: Correlation between biomarkers of exposure and genotoxic effects. Toxicol. Lett. 2010, 192, 22–28. [Google Scholar] [CrossRef] [PubMed]
- McHale, C.M.; Zhang, L.; Lan, Q.; Vermeulen, R.; Li, G.; Hubbard, A.E.; Porter, K.E.; Thomas, R.; Portier, C.J.; Shen, M.; et al. Global gene expression profiling of a population exposed to a range of benzene levels. Environ. Health Perspect. 2011, 119, 628–634. [Google Scholar] [CrossRef]
- Thomas, R.; Hubbard, A.E.; McHale, C.M.; Zhang, L.; Rappaport, S.M.; Lan, Q.; Rothman, N.; Vermeulen, R.; Guyton, K.Z.; Jinot, J.; et al. Characterization of changes in gene expression and biochemical pathways at low levels of benzene exposure. PLoS ONE 2014, 9, e91828. [Google Scholar] [CrossRef]
- Passegué, E.; Jamieson, C.H.; Ailles, L.E.; Weissman, I.L. Normal and leukemic hematopoiesis: Are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc. Natl. Acad. Sci. USA 2003, 100 (Suppl. S1), 11842–11849. [Google Scholar] [CrossRef]
- Seita, J.; Weissman, I.L. Hematopoietic stem cell: Self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cho, S.; Spangrude, G.J. Hematopoietic stem cells: Generation and self-renewal. Cell Death Differ. 2007, 14, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Rizo, A.; Vellenga, E.; de Haan, G.; Schuringa, J.J. Signaling pathways in self-renewing hematopoietic and leukemic stem cells: Do all stem cells need a niche? Hum. Mol. Genet. 2006, 15, R210–R219. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, X.; Bi, Y.; Ma, Q. Stem cell and benzene-induced malignancy and hematotoxicity. Chem. Res. Toxicol. 2012, 25, 1303–1315. [Google Scholar] [CrossRef]
- Chondrou, V.; Trochoutsou, K.; Panayides, A.; Efthimiou, M.; Stephanou, G.; Demopoulos, N.A. Combined study on clastogenic, aneugenic and apoptotic properties of doxorubicin in human cells in vitro. J. Biol. Res. 2018, 25, 17. [Google Scholar] [CrossRef]
- Moro, A.M.; Sauer, E.; Brucker, N.; Charão, M.F.; Gauer, B.; do Nascimento, S.N.; Goethel, G.; Duarte, M.; Garcia, S.C. Evaluation of immunological, inflammatory, and oxidative stress biomarkers in gasoline station attendants. BMC Pharmacol. Toxicol. 2019, 20, 75. [Google Scholar] [CrossRef]
- Tsiftsoglou, A.S.; Bonovolias, I.D.; Tsiftsoglou, S.A. Multilevel targeting of hematopoietic stem cell self-renewal, differentiation and apoptosis for leukemia therapy. Pharmacol. Ther. 2009, 122, 264–280. [Google Scholar] [CrossRef]
- Chow, P.W.; Rajab, N.F.; Chua, K.H.; Chan, K.M.; Abd Hamid, Z. Differential responses of lineages-committed hematopoietic progenitors and altered expression of self-renewal and differentiation-related genes in 1,4-benzoquinone (1,4-BQ) exposure. Toxicol. In Vitro 2018, 46, 122–128. [Google Scholar] [CrossRef]
- Idris, J.; Hamid, Z.; Ng, K.E.; Chow, P.W.; Shuib, S.; Mathialagan, R.D. Elucidating Lineage-Specific Myelotoxicity and Chromosomal Abberation Status in Hydroquinone-Exposed Hematopoietic Stem / Progenitor Cells. Life Sci. Med. Biomed. 2018, 2, 10. [Google Scholar] [CrossRef]
- Mathialagan, R.D.; Abd Hamid, Z.; Ng, Q.M.; Rajab, N.F.; Shuib, S.; Abdul Razak, S.R. Bone Marrow Oxidative Stress and Acquired Lineage-Specific Genotoxicity in Hematopoietic Stem/Progenitor Cells Exposed to 1,4-Benzoquinone. Int. J. Environ. Res. Public Health 2020, 17, 5865. [Google Scholar] [CrossRef]
- Lan, Q.; Zhang, L.; Li, G.; Vermeulen, R.; Weinberg, R.S.; Dosemeci, M.; Rappaport, S.M.; Shen, M.; Alter, B.P.; Wu, Y.; et al. Hematotoxicity in workers exposed to low levels of benzene. Science 2004, 306, 1774–1776. [Google Scholar] [CrossRef] [PubMed]
- Hiraku, Y.; Kawanishi, S. Oxidative DNA damage and apoptosis induced by benzene metabolites. Cancer Res. 1996, 56, 5172–5178. [Google Scholar]
- Faiola, B.; Fuller, E.S.; Wong, V.A.; Pluta, L.; Abernethy, D.J.; Rose, J.; Recio, L. Exposure of hematopoietic stem cells to benzene or 1,4-benzoquinone induces gender-specific gene expression. Stem Cells 2004, 22, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.F.; Peng, C.H.; Yu, X.Y.; Yang, X.J.; Yan, H.T. Expression and methylation analysis of p15 and p16 in mouse bone marrow cells exposed to 1,4-benzoquinone. Hum. Exp. Toxicol. 2012, 31, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.W.; Abdul Hamid, Z.; Chan, K.M.; Inayat-Hussain, S.H.; Rajab, N.F. Lineage-related cytotoxicity and clonogenic profile of 1,4-benzoquinone-exposed hematopoietic stem and progenitor cells. Toxicol. Appl. Pharmacol. 2015, 284, 8–15. [Google Scholar] [CrossRef]
- Hamid, Z.A.; Tan, H.Y.; Chow, P.W.; Harto, K.A.W.; Chan, C.Y.; Mohamed, J. The Role of N-Acetylcysteine Supplementation on the Oxidative Stress Levels, Genotoxicity and Lineage Commitment Potential of Ex Vivo Murine Haematopoietic Stem/Progenitor Cells. Sultan Qaboos Univ. Med. J. 2018, 18, e130–e136. [Google Scholar] [CrossRef]
- Chan, C.Y.; Hamid, Z.; Taib, I.S.; Yee, T.; Wak Harto, M.; Chow, P.W. Effects of n-acetyl-cysteine supplementation on ex-vivo clonogenicity and oxidative profile of lineage-committed hematopoietic stem/progenitor cells. J. Teknol. 2018, 80. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, J.; Xiong, M.; Wei, H.; Tan, K.; Yin, L.; Pu, Y. Altered Expression of Genes in Signaling Pathways Regulating Proliferation of Hematopoietic Stem and Progenitor Cells in Mice with Subchronic Benzene Exposure. Int. J. Environ. Res. Public Health 2015, 12, 9298–9313. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, H.; Yang, S.; Guo, L.; Li, Z.; Wang, W.; Wang, S.; Huang, W.; Wang, L.; Yang, T.; et al. Comparison of toxicity of benzene metabolite hydroquinone in hematopoietic stem cells derived from murine embryonic yolk sac and adult bone marrow. PLoS ONE 2013, 8, e71153. [Google Scholar] [CrossRef]
- Dewi, R.; Hamid, Z.A.; Rajab, N.F.; Shuib, S.; Razak, S.A. Genetic, epigenetic, and lineage-directed mechanisms in benzene-induced malignancies and hematotoxicity targeting hematopoietic stem cells niche. Hum. Exp. Toxicol. 2020, 39, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, J.; Tan, K.; Sun, R.; Yin, L.; Pu, Y. Benzene-Induced Aberrant miRNA Expression Profile in Hematopoietic Progenitor Cells in C57BL/6 Mice. Int. J. Mol. Sci. 2015, 16, 27058–27071. [Google Scholar] [CrossRef]
- Costa-Amaral, I.C.; Carvalho, L.V.B.; Santos, M.V.C.; Valente, D.; Pereira, A.C.; Figueiredo, V.O.; Souza, J.M.; Castro, V.S.; Trancoso, M.F.; Fonseca, A.S.A.; et al. Environmental Assessment and Evaluation of Oxidative Stress and Genotoxicity Biomarkers Related to Chronic Occupational Exposure to Benzene. Int. J. Environ. Res. Public Health 2019, 16, 2240. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T. Advances in understanding benzene health effects and susceptibility. Annu. Rev. Public Health 2010, 31, 133–148. [Google Scholar] [CrossRef]
- Abernethy, D.J.; Kleymenova, E.V.; Rose, J.; Recio, L.; Faiola, B. Human CD34+ hematopoietic progenitor cells are sensitive targets for toxicity induced by 1,4-benzoquinone. Toxicol. Sci. Off. J. Soc. Toxicol. 2004, 79, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Carbonari, D.; Chiarella, P.; Mansi, A.; Pigini, D.; Iavicoli, S.; Tranfo, G. Biomarkers of susceptibility following benzene exposure: Influence of genetic polymorphisms on benzene metabolism and health effects. Biomark. Med. 2016, 10, 145–163. [Google Scholar] [CrossRef]
- Tung, E.W.; Philbrook, N.A.; Macdonald, K.D.; Winn, L.M. DNA double-strand breaks and DNA recombination in benzene metabolite-induced genotoxicity. Toxicol. Sci. Off. J. Soc. Toxicol. 2012, 126, 569–577. [Google Scholar] [CrossRef]
- Yager, J.W.; Eastmond, D.A.; Robertson, M.L.; Paradisin, W.M.; Smith, M.T. Characterization of micronuclei induced in human lymphocytes by benzene metabolites. Cancer Res. 1990, 50, 393–399. [Google Scholar]
- Baker, R.K.; Kurz, E.U.; Pyatt, D.W.; Irons, R.D.; Kroll, D.J. Benzene metabolites antagonize etoposide-stabilized cleavable complexes of DNA topoisomerase IIalpha. Blood 2001, 98, 830–833. [Google Scholar] [CrossRef]
- Chen, H.; Eastmond, D.A. Topoisomerase inhibition by phenolic metabolites: A potential mechanism for benzene’s clastogenic effects. Carcinogenesis 1995, 16, 2301–2307. [Google Scholar] [CrossRef]
- Frantz, C.E.; Chen, H.; Eastmond, D.A. Inhibition of human topoisomerase II in vitro by bioactive benzene metabolites. Environ. Health Perspect. 1996, 104 (Suppl. 6), 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Hutt, A.M.; Kalf, G.F. Inhibition of human DNA topoisomerase II by hydroquinone and p-benzoquinone, reactive metabolites of benzene. Environ. Health Perspect. 1996, 104 (Suppl. 6), 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, R.H., Jr.; Bromberg, K.D.; Felix, C.A.; Osheroff, N. 1,4-Benzoquinone is a topoisomerase II poison. Biochemistry 2004, 43, 7563–7574. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Winn, L.M. The effects of 1,4-benzoquinone on c-Myb and topoisomerase II in K-562 cells. Mutat. Res. 2008, 645, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C. Cellular roles of DNA topoisomerases: A molecular perspective. Nat. Rev. Mol. Cell Biol. 2002, 3, 430–440. [Google Scholar] [CrossRef]
- Daniloski, Z.; Bisht, K.K.; McStay, B.; Smith, S. Resolution of human ribosomal DNA occurs in anaphase, dependent on tankyrase 1, condensin II, and topoisomerase IIα. Genes Dev. 2019, 33, 276–281. [Google Scholar] [CrossRef]
- Smith, N.A.; Byl, J.A.; Mercer, S.L.; Deweese, J.E.; Osheroff, N. Etoposide quinone is a covalent poison of human topoisomerase IIβ. Biochemistry 2014, 53, 3229–3236. [Google Scholar] [CrossRef]
- Choudhury, R.C.; Palo, A.K.; Sahu, P. Cytogenetic risk assessment of etoposide from mouse bone marrow. J. Appl. Toxicol. JAT 2004, 24, 115–122. [Google Scholar] [CrossRef]
- Inayat-Hussain, S.H.; Winski, S.L.; Ross, D. Differential involvement of caspases in hydroquinone-induced apoptosis in human leukemic hl-60 and jurkat cells. Toxicol. Appl. Pharmacol. 2001, 175, 95–103. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, H.M.; Shi, C.Y.; Ong, C.N. Benzene metabolites enhance reactive oxygen species generation in HL60 human leukemia cells. Hum. Exp. Toxicol. 1996, 15, 422–427. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, H.T.; Ye, Y.J.; Zhou, L.F.; Hu, W.; Zhang, G.H.; Sun, P.; Au, W.; Xia, Z.L. Association between Polymorphisms of Metabolic Enzyme Genes and Chromosomal Damage in Benzene-Exposed Workers in China. J. Occup. Environ. Med. 2017, 59, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Gowans, I.D.; Lorimore, S.A.; McIlrath, J.M.; Wright, E.G. Genotype-dependent induction of transmissible chromosomal instability by gamma-radiation and the benzene metabolite hydroquinone. Cancer Res. 2005, 65, 3527–3530. [Google Scholar] [CrossRef] [PubMed]
- Kalitsis, P.; Griffiths, B.; Choo, K. Mouse telocentric sequences reveal a high rate of homogenization and possible role in Robertsonian translocation. Proc. Natl. Acad. Sci. USA 2006, 103, 8786–8791. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, L.G.; Lupski, J.R. Molecular mechanisms for constitutional chromosomal rearrangements in humans. Annu. Rev. Genet. 2000, 34, 297–329. [Google Scholar] [CrossRef]
- Guffei, A.; Lichtensztejn, Z.; Gonçalves Dos Santos Silva, A.; Louis, S.F.; Caporali, A.; Mai, S. c-Myc-dependent formation of Robertsonian translocation chromosomes in mouse cells. Neoplasia 2007, 9, 578–588. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Guillén, R.A.; Capilla, L.; Reig-Viader, R.; Martínez-Plana, M.; Pardo-Camacho, C.; Andrés-Nieto, M.; Ventura, J.; Ruiz-Herrera, A. On the origin of Robertsonian fusions in nature: Evidence of telomere shortening in wild house mice. J. Evol. Biol. 2015, 28, 241–249. [Google Scholar] [CrossRef]
- Kawanishi, S.; Oikawa, S. Mechanism of telomere shortening by oxidative stress. Ann. N. Y. Acad. Sci. 2004, 1019, 278–284. [Google Scholar] [CrossRef]
- Yin, R.; Zhang, D.; Song, Y.; Zhu, B.-Z.; Wang, H. Potent DNA damage by polyhalogenated quinones and H2O2 via a metal-independent and Intercalation-enhanced oxidation mechanism. Sci. Rep. 2013, 3, 1269. [Google Scholar] [CrossRef]
- Eastmond, D.A.; Rupa, D.S.; Hasegawa, L.S. Detection of hyperdiploidy and chromosome breakage in interphase human lymphocytes following exposure to the benzene metabolite hydroquinone using multicolor fluorescence in situ hybridization with DNA probes. Mutat. Res. 1994, 322, 9–20. [Google Scholar] [CrossRef]
- Zhang, L.; Rothman, N.; Wang, Y.; Hayes, R.B.; Bechtold, W.; Venkatesh, P.; Yin, S.; Wang, Y.; Dosemeci, M.; Li, G.; et al. Interphase cytogenetics of workers exposed to benzene. Environ. Health Perspect. 1996, 104 (Suppl. 6), 1325–1329. [Google Scholar] [CrossRef][Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, P.W.; Abd Hamid, Z.; Mathialagan, R.D.; Rajab, N.F.; Shuib, S.; Sulong, S. Clastogenicity and Aneugenicity of 1,4-Benzoquinone in Different Lineages of Mouse Hematopoietic Stem/Progenitor Cells. Toxics 2021, 9, 107. https://doi.org/10.3390/toxics9050107
Chow PW, Abd Hamid Z, Mathialagan RD, Rajab NF, Shuib S, Sulong S. Clastogenicity and Aneugenicity of 1,4-Benzoquinone in Different Lineages of Mouse Hematopoietic Stem/Progenitor Cells. Toxics. 2021; 9(5):107. https://doi.org/10.3390/toxics9050107
Chicago/Turabian StyleChow, Paik Wah, Zariyantey Abd Hamid, Ramya Dewi Mathialagan, Nor Fadilah Rajab, Salwati Shuib, and Sarina Sulong. 2021. "Clastogenicity and Aneugenicity of 1,4-Benzoquinone in Different Lineages of Mouse Hematopoietic Stem/Progenitor Cells" Toxics 9, no. 5: 107. https://doi.org/10.3390/toxics9050107
APA StyleChow, P. W., Abd Hamid, Z., Mathialagan, R. D., Rajab, N. F., Shuib, S., & Sulong, S. (2021). Clastogenicity and Aneugenicity of 1,4-Benzoquinone in Different Lineages of Mouse Hematopoietic Stem/Progenitor Cells. Toxics, 9(5), 107. https://doi.org/10.3390/toxics9050107





