Short-Term Exposure to Ambient Air Pollution and Increased Emergency Room Visits for Skin Diseases in Beijing, China
Abstract
1. Introduction
2. Materials and methods
2.1. Study Design and Population
2.2. Air Pollutants and Meteorological Data
2.3. Statistical Analysis
3. Results
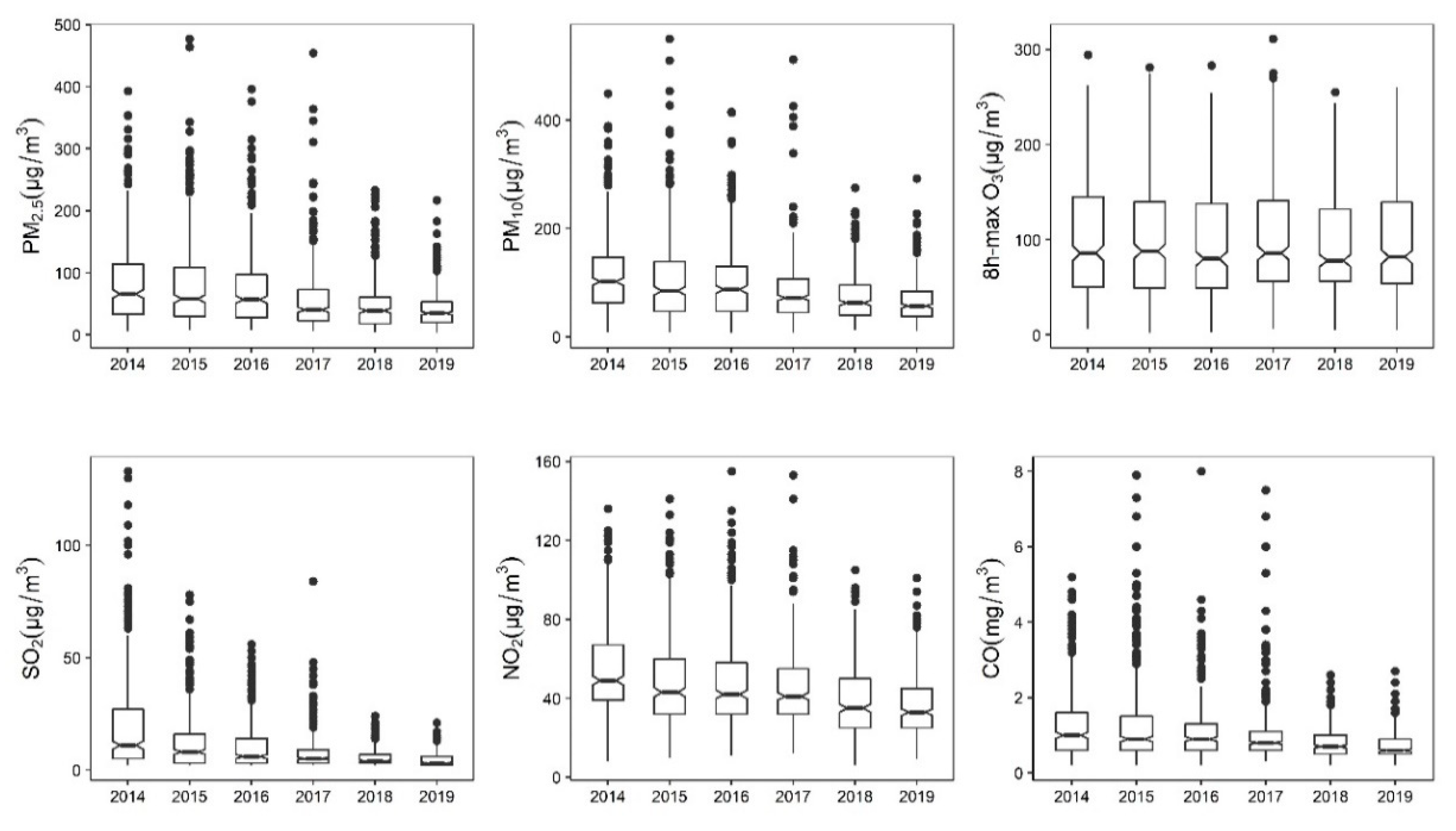
3.1. Descriptive Results
3.2. Associations between Ambient Air Pollutants and Emergency Room Visits for Skin Diseases
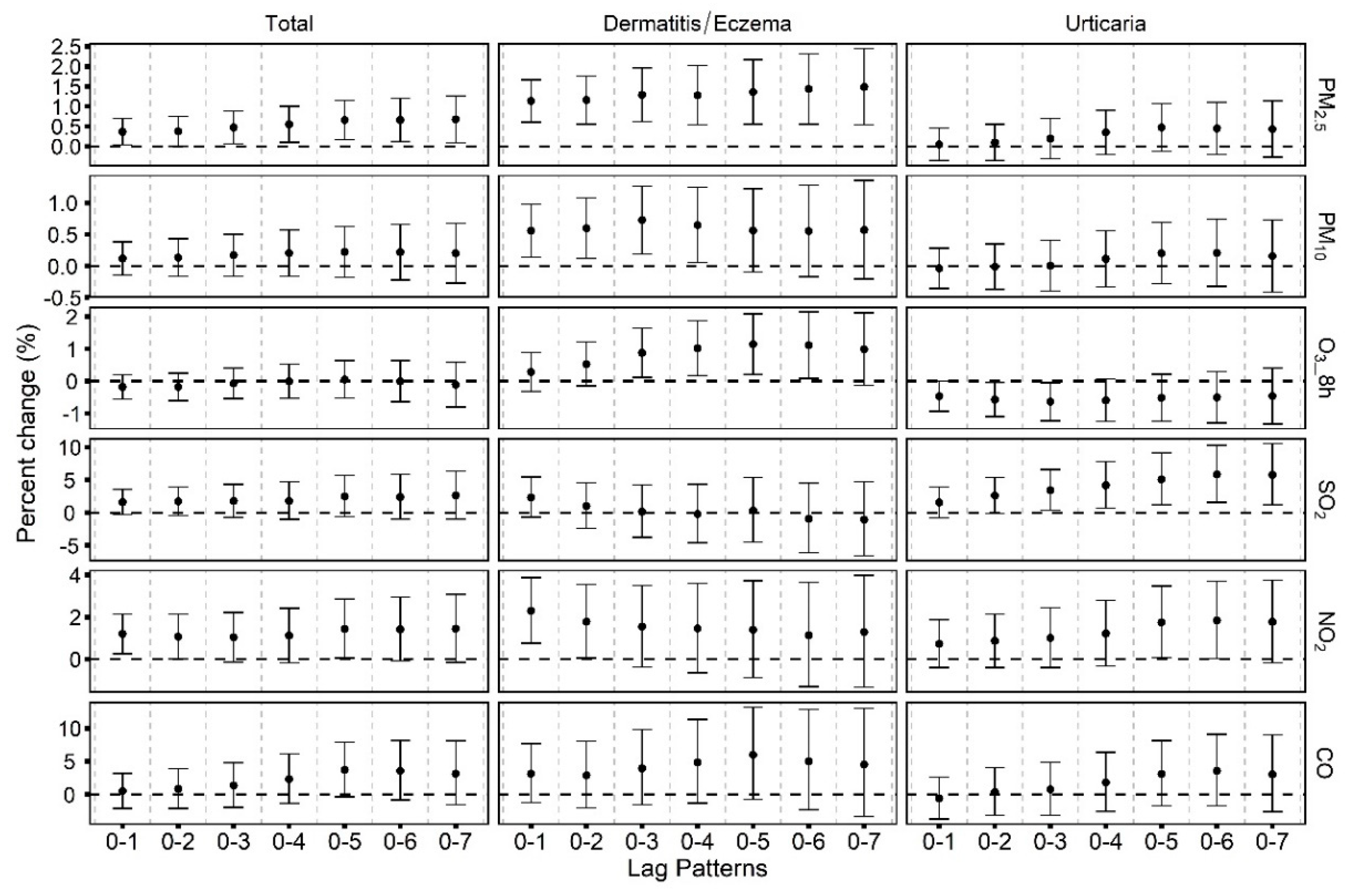
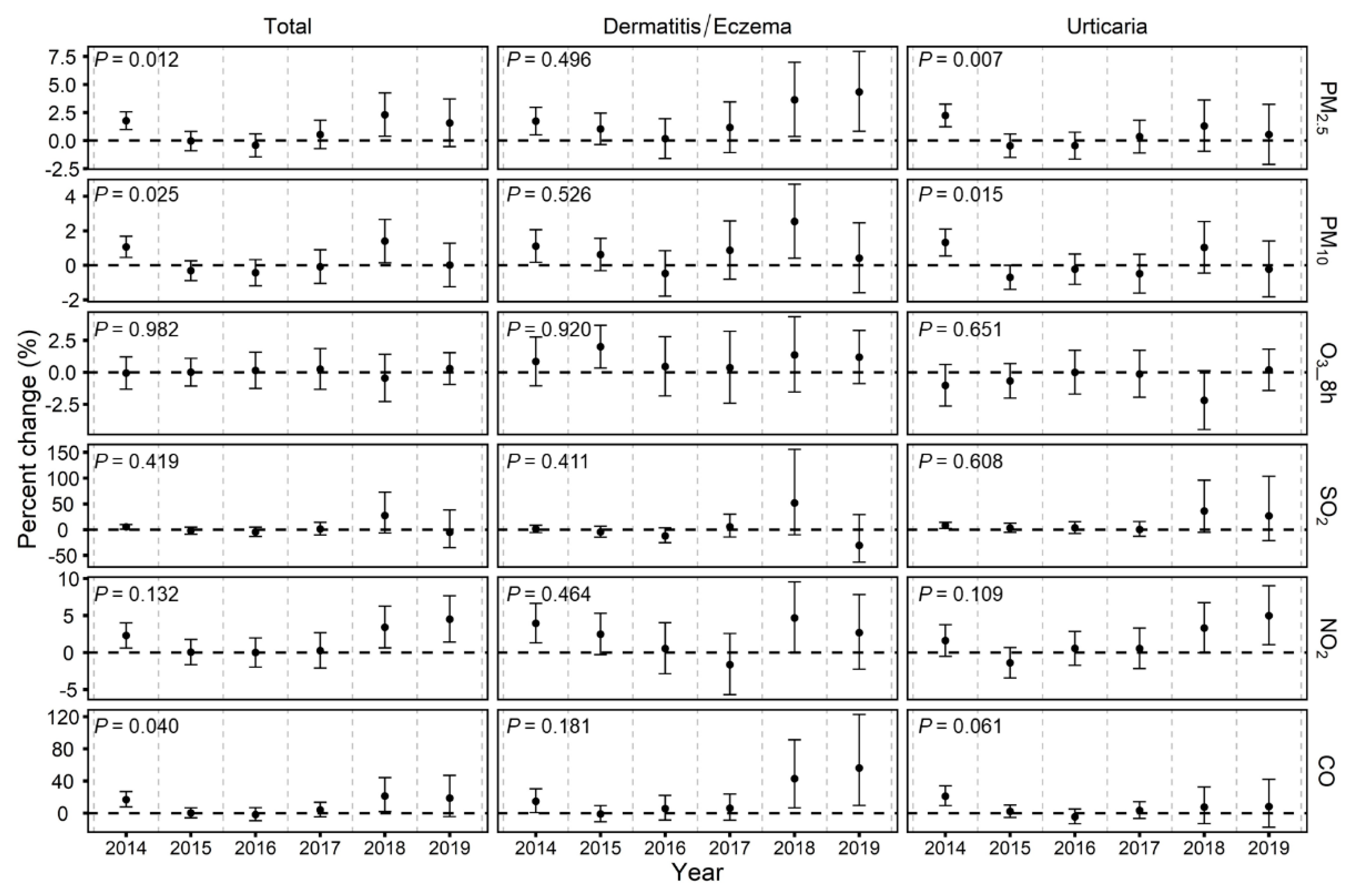
3.3. Temporal Variability of the Associations between Air Pollutants and Emergency Room Visits for Skin Diseases
3.4. Additional Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.; Saldiva, P.H.N.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Ahn, K. The role of air pollutants in atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 993–999. [Google Scholar] [CrossRef]
- Nguyen, G.H.; Andersen, L.K.; Davis, M.D.P. Climate change and atopic dermatitis: Is there a link? Int. J. Dermatol. 2019, 58, 279–282. [Google Scholar] [CrossRef]
- Guo, Q.; Liang, F.; Tian, L.; Schikowski, T.; Liu, W.; Pan, X. Ambient air pollution and the hospital outpatient visits for eczema and dermatitis in Beijing: A time-stratified case-crossover analysis. Environ. Sci. Process. Impacts 2019, 21, 163–173. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Chen, R.; Kan, H.; Song, W.; Tan, J.; Xu, F.; Xu, J. Ambient Air Pollution, Meteorological Factors and Outpatient Visits for Eczema in Shanghai, China: A Time-Series Analysis. Int. J. Environ. Res. Public Health 2016, 13, 1106. [Google Scholar] [CrossRef] [PubMed]
- Patella, V.; Florio, G.; Palmieri, M.; Bousquet, J.; Tonacci, A.; Giuliano, A.; Gangemi, S. Atopic dermatitis severity during exposure to air pollutants and weather changes with an Artificial Neural Network (ANN) analysis. Pediatr. Allergy Immunol. 2020, 31, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.T.; Ku, K.C.; Chen, D.Y.; Lin, C.H.; Tsuang, B.J.; Chen, Y.H. Adult atopic dermatitis and exposure to air pollutants—A nationwide population-based study. Ann. Allergy Asthma Immunol 2017, 118, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Baïz, N.; Banerjee, S.; Charpin, D.A.; Caillaud, D.; de Blay, F.; Raherison, C.; Lavaud, F.; Annesi-Maesano, I. The relationships between ambient air pollutants and childhood asthma and eczema are modified by emotion and conduct problems. Ann. Epidemiol. 2013, 23, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pan, X.; Vierkötter, A.; Guo, Q.; Wang, X.; Wang, Q.; Seité, S.; Moyal, D.; Schikowski, T.; Krutmann, J. A Time-Series Study of the Effect of Air Pollution on Outpatient Visits for Acne Vulgaris in Beijing. Skin Pharmacol. Physiol. 2018, 31, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Viera, M.S.; Marrot, L.; Chuberre, B.; Dreno, B. The impact of airborne pollution on skin. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Ma, F.; Qin, C.B.; Li, Y.F. Spatiotemporal trends in PM2.5 levels from 2013 to 2017 and regional demarcations for joint prevention and control of atmospheric pollution in China. Chemosphere 2018, 210, 1176–1184. [Google Scholar] [CrossRef]
- Cai, S.; Wang, Y.; Zhao, B.; Wang, S.; Chang, X.; Hao, J. The impact of the “Air Pollution Prevention and Control Action Plan” on PM2.5 concentrations in Jing-Jin-Ji region during 2012–2020. Sci. Total Environ. 2017, 580, 197–209. [Google Scholar] [CrossRef]
- Maji, K.J.; Li, V.O.; Lam, J.C. Effects of China’s current Air Pollution Prevention and Control Action Plan on air pollution patterns, health risks and mortalities in Beijing 2014–2018. Chemosphere 2020, 260, 127572. [Google Scholar] [CrossRef]
- Huang, J.; Pan, X.; Guo, X.; Li, G. Health impact of China’s Air Pollution Prevention and Control Action Plan: An analysis of national air quality monitoring and mortality data. Lancet Planet. Health 2018, 2, e313–e323. [Google Scholar] [CrossRef]
- Baghestani, S.; Zare, S.; Mahboobi, A.A. Skin disease patterns in Hormozgan, Iran. Int. J. Dermatol. 2005, 44, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qiu, H.; Wang, L.; Duan, Z.; Yu, H.; Deng, R.; Zhang, Y.; Zhou, L. Risks of hospital admissions from a spectrum of causes associated with particulate matter pollution. Sci. Total Environ. 2019, 656, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Lee, K.K.; McAllister, D.A.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; Newby, D.E.; Mills, N.L. Short term exposure to air pollution and stroke: Systematic review and meta-analysis. BMJ 2015, 350, h1295. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, H.; Zhao, Z.; Xiang, X.; Li, M.; Juan, J.; Song, J.; Cao, Y.; Wang, X.; Chen, L.; et al. Association between ambient air pollution and daily hospital admissions for ischemic stroke: A nationwide time-series analysis. PLoS Med. 2018, 15, e1002668. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklöv, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Shi, W.; Sun, Q.; Du, P.; Tang, S.; Chen, C.; Sun, Z.; Wang, J.; Li, T.; Shi, X. Modification Effects of Temperature on the Ozone-Mortality Relationship: A Nationwide Multicounty Study in China. Environ. Sci. Technol. 2020, 54, 2859–2868. [Google Scholar] [CrossRef]
- Gu, X.; Guo, T.; Si, Y.; Wang, J.; Zhang, W.; Deng, F.; Chen, L.; Wei, C.; Lin, S.; Guo, X.; et al. Association Between Ambient Air Pollution and Daily Hospital Admissions for Depression in 75 Chinese Cities. Am. J. Psychiatry 2020, 177, 735–743. [Google Scholar] [CrossRef]
- Renzi, M.; Stafoggia, M.; Faustini, A.; Cesaroni, G.; Cattani, G.; Forastiere, F. Analysis of Temporal Variability in the Short-term Effects of Ambient Air Pollutants on Nonaccidental Mortality in Rome, Italy (1998–2014). Environ. Health Perspect. 2017, 125, 067019. [Google Scholar] [CrossRef]
- Shin, H.H.; Stieb, D.M.; Jessiman, B.; Goldberg, M.S.; Brion, O.; Brook, J.; Ramsay, T.; Burnett, R.T. A temporal, multicity model to estimate the effects of short-term exposure to ambient air pollution on health. Environ. Health Perspect. 2008, 116, 1147–1153. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, K. Fine particulate matter components and mortality in Greater Houston: Did the risk reduce from 2000 to 2011? Sci. Total Environ. 2015, 538, 162–168. [Google Scholar] [CrossRef]
- Liang, L.; Cai, Y.; Barratt, B.; Lyu, B.; Chan, Q.; Hansell, A.L.; Xie, W.; Zhang, D.; Kelly, F.J.; Tong, Z. Associations between daily air quality and hospitalisations for acute exacerbation of chronic obstructive pulmonary disease in Beijing, 2013-17: An ecological analysis. Lancet Planet. Health 2019, 3, e270–e279. [Google Scholar] [CrossRef]
- Morgan, J.L.L.; Shauchuk, A.; Meyers, J.L.; Altemeier, A.; Qiao, X.; Jones, M.; Smith, E.D.; Jiang, J. Quantifying the Deposition of Airborne Particulate Matter Pollution on Skin Using Elemental Markers. Environ. Sci. Technol. 2020, 54, 15958–15967. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Liu, W.; Li, L.; Pan, X.; Crawford, M.; Sore, G.; Seite, S. Pollution and skin: From epidemiological and mechanistic studies to clinical implications. J. Dermatol. Sci. 2014, 76, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.M.; Liao, L.Y.; Li, L.; Yi, F.; Meng, H.; He, Y.F.; Guo, M.M. Skin inflammation induced by ambient particulate matter in China. Sci. Total Environ. 2019, 682, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Karagün, E.; Yıldız, P.; Cangür, Ş. Effects of climate and air pollution factors on outpatient visits for eczema: A time series analysis. Arch. Dermatol. Res. 2020, 313, 49–55. [Google Scholar] [CrossRef]
- Chi, R.; Li, H.; Wang, Q.; Zhai, Q.; Wang, D.; Wu, M.; Liu, Q.; Wu, S.; Ma, Q.; Deng, F.; et al. Association of emergency room visits for respiratory diseases with sources of ambient PM2.5. J. Environ. Sci. 2019, 86, 154–163. [Google Scholar] [CrossRef]
- Kousha, T.; Valacchi, G. The Air Quality Health Index and Emergency Department Visits for Urticaria in Windsor, Canada. J. Toxicol. Environ. Health Part A 2015, 78, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Langrish, J.P.; Nair, H.; McAllister, D.A.; Hunter, A.L.; Donaldson, K.; Newby, D.E.; Mills, N.L. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet 2013, 382, 1039–1048. [Google Scholar] [CrossRef]
- Taj, T.; Malmqvist, E.; Stroh, E.; Åström, D.O.; Jakobsson, K.; Oudin, A. Short-Term Associations between Air Pollution Concentrations and Respiratory Health-Comparing Primary Health Care Visits, Hospital Admissions, and Emergency Department Visits in a Multi-Municipality Study. Int. J. Environ. Res. Public Health 2017, 14, 587. [Google Scholar] [CrossRef]
- Perez, L.; Grize, L.; Infanger, D.; Künzli, N.; Sommer, H.; Alt, G.M.; Schindler, C. Associations of daily levels of PM10 and NO2 with emergency hospital admissions and mortality in Switzerland: Trends and missed prevention potential over the last decade. Environ. Res. 2015, 140, 554–561. [Google Scholar] [CrossRef]
- Shin, H.H.; Parajuli, R.P.; Maquiling, A.; Smith-Doiron, M. Temporal trends in associations between ozone and circulatory mortality in age and sex in Canada during 1984–2012. Sci. Total Environ. 2020, 724, 137944. [Google Scholar] [CrossRef] [PubMed]
- Breitner, S.; Stölzel, M.; Cyrys, J.; Pitz, M.; Wölke, G.; Kreyling, W.; Küchenhoff, H.; Heinrich, J.; Wichmann, H.E.; Peters, A. Short-term mortality rates during a decade of improved air quality in Erfurt, Germany. Environ. Health Perspect. 2009, 117, 448–454. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Zeger, S.L.; White, R.H.; Samet, J.M. Particulate air pollution and mortality in the United States: Did the risks change from 1987 to 2000? Am. J. Epidemiol. 2007, 166, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, H.; Lee, J.T. Temporal variability of short term effects of PM10 on mortality in Seoul, Korea. Sci. Total Environ. 2018, 644, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huang, L.; Li, J.; Ying, Q.; Zhang, H.; Liu, X.; Liao, H.; Li, N.; Liu, Z.; Mao, Y.; et al. Sources of particulate matter in China: Insights from source apportionment studies published in 1987–2017. Environ. Int. 2018, 115, 343–357. [Google Scholar] [CrossRef]
- Ewen, C.; Anagnostopoulou, M.A.; Ward, N.I. Monitoring of heavy metal levels in roadside dusts of Thessaloniki, Greece in relation to motor vehicle traffic density and flow. Environ. Monit. Assess. 2009, 157, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, M.; Eleftheriadis, K.; Smolik, J.; Colbeck, I.; Kallos, G.; Drossinos, Y.; Zdimal, V.; Vecera, Z.; Mihalopoulos, N.; Mikuska, P.; et al. Dynamics of fine particles and photo-oxidants in the Eastern Mediterranean (SUB-AERO). Atmos. Environ. 2006, 40, 6214–6228. [Google Scholar] [CrossRef]
- Lei, X.; Chen, R.; Wang, C.; Shi, J.; Zhao, Z.; Li, W.; Yan, B.; Chillrud, S.; Cai, J.; Kan, H. Personal Fine Particulate Matter Constituents, Increased Systemic Inflammation, and the Role of DNA Hypomethylation. Environ. Sci. Technol. 2019, 53, 9837–9844. [Google Scholar] [CrossRef]
- Emmanouil, C.; Drositi, E.; Vasilatou, V.; Diapouli, E.; Krikonis, K.; Eleftheriadis, K.; Kungolos, A. Study on particulate matter air pollution, source origin, and human health risk based of PM10 metal content in Volos City, Greece. Toxicol. Environ. Chem. 2017, 99, 691–709. [Google Scholar] [CrossRef]
- Nutten, S. Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab. 2015, 66 (Suppl. S1), 8–16. [Google Scholar] [CrossRef]

| Variable | Total (N = 45,094) | Dermatitis/Eczema (N = 16,891) | Urticaria (N = 23,832) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Sex a | ||||||
| Male | 18,251 | 40.5 | 6394 | 37.9 | 9649 | 40.5 |
| Female | 26,843 | 59.5 | 10,497 | 62.1 | 14,183 | 59.5 |
| Age (years) a | ||||||
| 0–18 | 9022 | 20.0 | 3618 | 21.4 | 4929 | 20.7 |
| 19–39 | 25,004 | 55.4 | 8958 | 53.0 | 13,882 | 58.2 |
| ≥40 | 11,068 | 24.5 | 4315 | 25.5 | 5021 | 21.1 |
| Season | ||||||
| Cold (November–April) | 17,615 | 39.1 | 6227 | 36.9 | 9761 | 41.0 |
| Warm (May–October) | 27,479 | 60.9 | 10,664 | 63.1 | 14,071 | 59.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, W.; Zhao, J.; Li, H.; Wu, J.; Deng, F.; Ma, Q.; Guo, X. Short-Term Exposure to Ambient Air Pollution and Increased Emergency Room Visits for Skin Diseases in Beijing, China. Toxics 2021, 9, 108. https://doi.org/10.3390/toxics9050108
Wang W, Zhang W, Zhao J, Li H, Wu J, Deng F, Ma Q, Guo X. Short-Term Exposure to Ambient Air Pollution and Increased Emergency Room Visits for Skin Diseases in Beijing, China. Toxics. 2021; 9(5):108. https://doi.org/10.3390/toxics9050108
Chicago/Turabian StyleWang, Wanzhou, Wenlou Zhang, Jingjing Zhao, Hongyu Li, Jun Wu, Furong Deng, Qingbian Ma, and Xinbiao Guo. 2021. "Short-Term Exposure to Ambient Air Pollution and Increased Emergency Room Visits for Skin Diseases in Beijing, China" Toxics 9, no. 5: 108. https://doi.org/10.3390/toxics9050108
APA StyleWang, W., Zhang, W., Zhao, J., Li, H., Wu, J., Deng, F., Ma, Q., & Guo, X. (2021). Short-Term Exposure to Ambient Air Pollution and Increased Emergency Room Visits for Skin Diseases in Beijing, China. Toxics, 9(5), 108. https://doi.org/10.3390/toxics9050108






