Review on Toxic Effects of Di(2-ethylhexyl) Phthalate on Zebrafish Embryos
Abstract
:1. Introduction
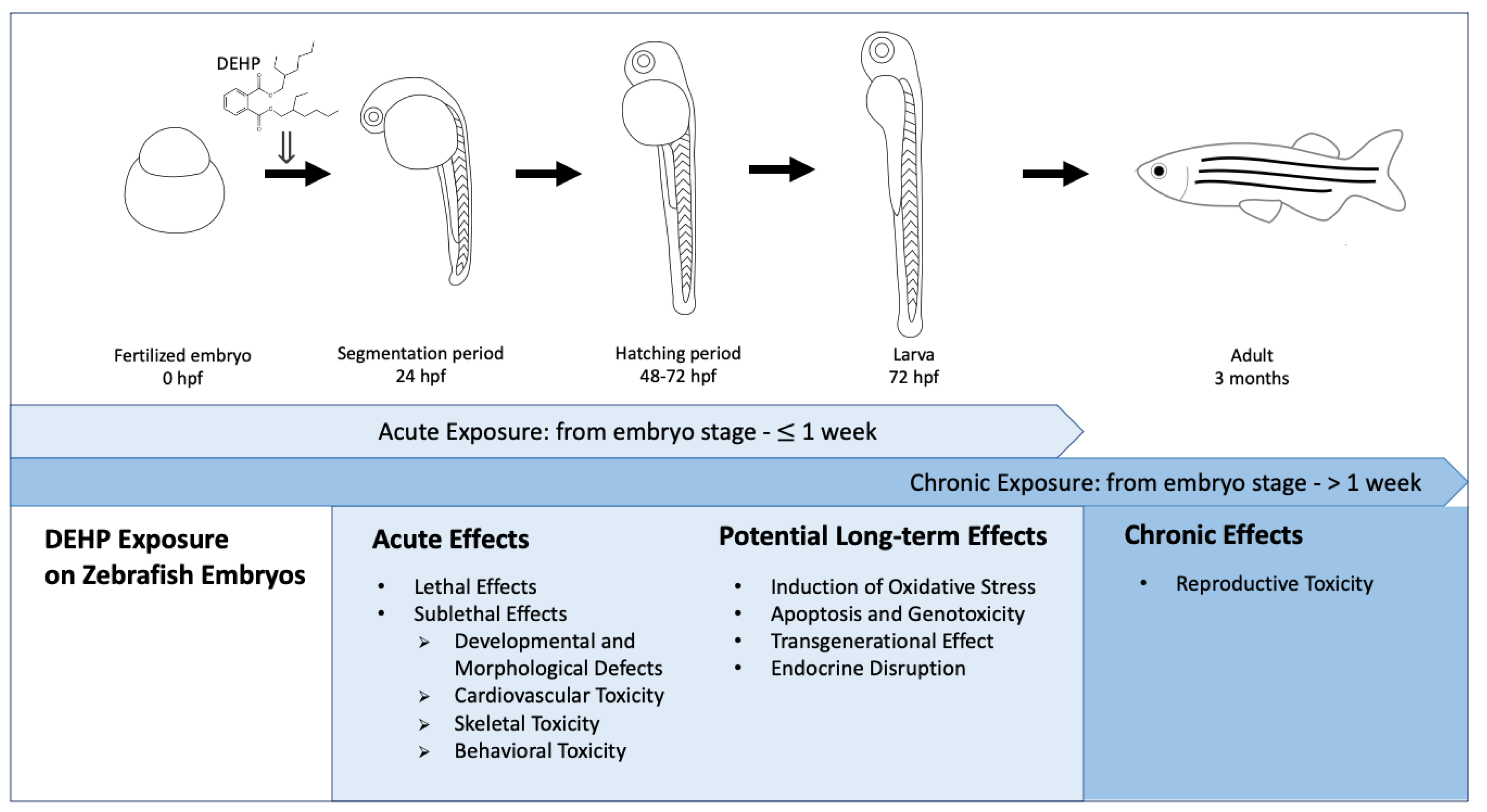
2. Acute Effects Induced by Acute Exposure
2.1. Lethal Effects
2.2. Sublethal Effects
2.2.1. Developmental and Morphological Defects
2.2.2. Cardiovascular Toxicity
2.2.3. Skeletal Toxicity
2.2.4. Behavioral Toxicity
2.3. Regulation of Genes and Possible Mechanisms
3. Potential Long-Term Effects Induced by Acute Exposure
3.1. Induction of Oxidative Stress
3.2. Apoptosis and Genotoxicity
3.3. Transgenerational Effect
3.4. Endocrine Disruption
4. Effects from Chronic Exposure
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Brazel, C.S. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z. Observing Phthalate Leaching from Plasticized Polymer Films at the Molecular Level. Langmuir 2014, 30, 4933–4944. [Google Scholar] [CrossRef] [PubMed]
- Alp, A.C.; Yerlikaya, P. Phthalate ester migration into food: Effect of packaging material and time. Eur. Food Res. Technol. 2020, 246, 425–435. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Marie, C.; Vendittelli, F.; Sauvant-Rochat, M.-P. Obstetrical Outcomes and Biomarkers to Assess Exposure to Phthalates: A Review. Environ. Int. 2015, 83, 116–136. [Google Scholar] [CrossRef]
- Zarean, M.; Keikha, M.; Poursafa, P.; Khalighinejad, P.; Amin, M.; Kelishadi, R. A Systematic Review on the Adverse Health Effects of Di-2-Ethylhexyl Phthalate. Environ. Sci. Pollut. Res. 2016, 23, 24642–24693. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Identification of Carcinogenic Hazards to Humans; International Agency for Research on Cancer: Lyon, France, 2013; Volume 101. [Google Scholar]
- United States Environmental Protection Agency (US EPA). Priority Pollutant List; United States Environmental Protection Agency: Washington, DC, USA, 2014. [Google Scholar]
- European Union (EU). Commission Regulation (EU) 2018/2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R2005&from=EN (accessed on 15 March 2021).
- United States Consumer Product Safety Commission (US CPSC). Consumer Product Safety Improvement Act of 2008; United States Consumer Product Safety Commission: Bethesda, MD, USA, 2008. [Google Scholar]
- Canada Consumer Product Safety Act (CCPSA). Phthalate Regulations (SOR/2016–188). Available online: https://laws-lois.justice.gc.ca/eng/regulations/SOR-2016-188/index.html (accessed on 15 March 2021).
- Högberg, J.; Hanberg, A.; Berglund, M.; Skerfving, S.; Remberger, M.; Calafat, A.M.; Filipsson, A.F.; Jansson, B.; Johansson, N.; Appelgren, M. Phthalate Diesters and Their Metabolites in Human Breast Milk, Blood or Serum, and Urine as Biomarkers of Exposure in Vulnerable Populations. Environ. Health Perspect. 2008, 116, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.; Samandar, E.; Preaujr, J.; Reidy, J.; Needham, L.; Calafat, A. Quantification of 22 Phthalate Metabolites in Human Urine☆. J. Chromatogr. B 2007, 860, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Alomirah, H.; Cho, H.-S.; Minh, T.B.; Mohd, M.A.; Nakata, H.; Kannan, K. Occurrence of Phthalate Metabolites in Human Urine from Several Asian Countries. Environ. Sci. Technol. 2011, 45, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.; Rozati, R.; Reddy, B.; Raman, N. General Gynaecology: Association of Phthalate Esters with Endometriosis in Indian Women. BJOG 2006, 113, 515–520. [Google Scholar] [CrossRef]
- Joensen, U.N.; Frederiksen, H.; Jensen, M.B.; Lauritsen, M.P.; Olesen, I.A.; Lassen, T.H.; Andersson, A.-M.; Jørgensen, N. Phthalate Excretion Pattern and Testicular Function: A Study of 881 Healthy Danish Men. Environ. Health Perspect. 2012, 120, 1397–1403. [Google Scholar] [CrossRef] [Green Version]
- James-Todd, T.; Stahlhut, R.; Meeker, J.D.; Powell, S.-G.; Hauser, R.; Huang, T.; Rich-Edwards, J. Urinary Phthalate Metabolite Concentrations and Diabetes among Women in the National Health and Nutrition Examination Survey (NHANES) 2001–2008. Environ. Health Perspect. 2012, 120, 1307–1313. [Google Scholar] [CrossRef] [Green Version]
- Yaghjyan, L.; Sites, S.; Ruan, Y.; Chang, S.-H. Associations of Urinary Phthalates with Body Mass Index, Waist Circumference and Serum Lipids among Females: National Health and Nutrition Examination Survey 1999–2004. Int. J. Obes. 2015, 39, 994–1000. [Google Scholar] [CrossRef] [Green Version]
- López-Carrillo, L.; Hernández-Ramírez, R.U.; Calafat, A.M.; Torres-Sánchez, L.; Galván-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrián, M.E. Exposure to Phthalates and Breast Cancer Risk in Northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef]
- Hoppin, J.A.; Jaramillo, R.; London, S.J.; Bertelsen, R.J.; Salo, P.M.; Sandler, D.P.; Zeldin, D.C. Phthalate Exposure and Allergy in the U.S. Population: Results from NHANES 2005–2006. Environ. Health Perspect. 2013, 121, 1129–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Yang, Y.; Wang, R.; Wang, Y.; Ruan, Q.; Lu, Y. Perinatal Exposure to Di-(2-Ethylhexyl) Phthalate Affects Anxiety- and Depression-like Behaviors in Mice. Chemosphere 2015, 124, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Lin, P.-C.; Park, C.J.; Best-Popescu, C.; Bakry, H.H.; Abosalem, M.E.; Abdelaleem, N.M.; Flaws, J.A.; Ko, C. Prenatal Exposure to DEHP Induces Neuronal Degeneration and Neurobehavioral Abnormalities in Adult Male Mice. Toxicol. Sci. 2018, 164, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Hao, C. Perinatal Exposure to Diethyl-Hexyl-Phthalate Induces Obesity in Mice. Front. Biosci. 2013, E5, 725–733. [Google Scholar] [CrossRef] [Green Version]
- Niermann, S.; Rattan, S.; Brehm, E.; Flaws, J.A. Prenatal Exposure to Di-(2-Ethylhexyl) Phthalate (DEHP) Affects Reproductive Outcomes in Female Mice. Reprod. Toxicol. 2015, 53, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-Q.; Chen, J.-N.; Cai, X.-H.; Chen, G.-R.; Gao, Y.; Ge, R.-S.; Wu, H.-S.; Lin, Z.-L.; Lin, J. Perinatal Exposure to Di-(2-Ethylhexyl) Phthalate Leads to Restricted Growth and Delayed Lung Maturation in Newborn Rats. J. Perinat. Med. 2010, 38. [Google Scholar] [CrossRef] [PubMed]
- Love, D.R.; Pichler, F.B.; Dodd, A.; Copp, B.R.; Greenwood, D.R. Technology for high-throughput screens: The present and future using zebrafish. Curr. Opin. Biotechnol. 2004, 15, 564–571. [Google Scholar] [CrossRef]
- Sarvaiya, V.N.; Sadariya, K.A.; Rana, M.P.; Thaker, A.M. Zebrafish as model organism for drug discovery and toxicity testing: A review. Vet. Med. Sci. 2014, 2, 31–38. [Google Scholar]
- Dooley, K. Zebrafish: A Model System for the Study of Human Disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Segner, H. Zebrafish (Danio Rerio) as a Model Organism for Investigating Endocrine Disruption. Comp. Biochem. Physiol. C Toxicol. 2009, 149, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Li, G.; Feng, W.; Liu, L.; Zhang, J.; Wu, W.; Xu, L.; Yan, Y. Chlorpyrifos is Estrogenic and Alters Embryonic Hatching, Cell Proliferation and Apoptosis in Zebrafish. Chem. Biol. Interact. 2015, 239, 26–33. [Google Scholar] [CrossRef]
- Mu, X.; Huang, Y.; Li, X.; Lei, Y.; Teng, M.; Li, X.; Wang, C.; Li, Y. Developmental Effects and Estrogenicity of Bisphenol A Alternatives in a Zebrafish Embryo Model. Environ. Sci. Technol. 2018, 52, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Moreman, J.; Lee, O.; Trznadel, M.; David, A.; Kudoh, T.; Tyler, C.R. Acute Toxicity, Teratogenic, and Estrogenic Effects of Bisphenol A and Its Alternative Replacements Bisphenol S, Bisphenol F, and Bisphenol AF in Zebrafish Embryo-Larvae. Environ. Sci. Technol. 2017, 51, 12796–12805. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Nagel, R. DarT: The Embryo Test with the Zebrafish Danio Rerio—A General Model in Ecotoxicology and Toxicology. Altex 2002, 19 (Suppl 1/02), 38–48. [Google Scholar]
- Pu, S.-Y.; Hamid, N.; Ren, Y.-W.; Pei, D.-S. Effects of Phthalate Acid Esters on Zebrafish Larvae: Development and Skeletal Morphogenesis. Chemosphere 2020, 246, 125808. [Google Scholar] [CrossRef] [PubMed]
- Hamid, N.; Junaid, M.; Manzoor, R.; Jia, P.-P.; Pei, D.-S. Prioritizing Phthalate Esters (PAEs) Using Experimental in Vitro/Vivo Toxicity Assays and Computational in Silico Approaches. J. Hazard. Mater. 2020, 398, 122851. [Google Scholar] [CrossRef]
- Muhammad, S.; Zhang, Z.; Pavase, T.R.; Guo, H. Long-Term Exposure of Two Plasticizers Di (2-Ethylhexyl) Phthalate (DEHP) and Acetyl Tributyl Citrate (ATBC): Toxic Effects on Gonadal Development and Reproduction of Zebrafish (“Danio Rerio”). Indian J. Mar. Sci. 2018, 47, 9. [Google Scholar]
- Ustundag, U.V.; Unal, I.; Ates, P.S.; Alturfan, A.A.; Yigitbasi, T.; Emekli Alturfan, E. Oxidant-Antioxidant Status and c-Myc Expression in BPA and DEHP-Exposed Zebrafish Embryos. Eur. J. Biol. 2017, 76, 26–30. [Google Scholar] [CrossRef]
- Boran, H.; Terzi, S. Bis(2-Ethylhexyl) Phthalate Induces DNA Strand Breaks and Gene Expression Alterations in Larval Zebrafish Danio Rerio. Toxicol. Ind. Health 2019, 35, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Luo, J.; Liu, Y.; Yang, X. The Oxidative Stress Responses Caused by Phthalate Acid Esters Increases MRNA Abundance of Base Excision Repair (BER) Genes in Vivo and in Vitro. Ecotoxicol. Environ. Saf. 2021, 208, 111525. [Google Scholar] [CrossRef]
- McCollum, C.W.; Ducharme, N.A.; Bondesson, M.; Gustafsson, J.-A. Developmental Toxicity Screening in Zebrafish. Birth Defects Res. C Embryo Today 2011, 93, 67–114. [Google Scholar] [CrossRef]
- Üstündağ, Ü.V.; Ünal, İ.; Ateş, P.S.; Alturfan, A.A.; Yiğitbaşı, T. Bisphenol A and Di(2-Ethylhexyl) Phthalate Exert Divergent Effects on Apoptosis and the Wnt/β-Catenin Pathway in Zebrafish Embryos: A Possible Mechanism of Endocrine Disrupting Chemical Action. Toxicol. Ind. Health 2017, 33, 901–910. [Google Scholar] [CrossRef]
- Kinch, C.D.; Kurrasch, D.M.; Habibi, H.R. Adverse Morphological Development in Embryonic Zebrafish Exposed to Environmental Concentrations of Contaminants Individually and in Mixture. Aquat. Toxicol. 2016, 175, 286–298. [Google Scholar] [CrossRef]
- Tran, C.M.; Do, T.N.; Kim, K.-T. Comparative Analysis of Neurotoxicity of Six Phthalates in Zebrafish Embryos. Toxics 2021, 9, 5. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Choi, K.; Kim, K.-T. Comparative Analysis of Endocrine Disrupting Effects of Major Phthalates in Employed Two Cell Lines (MVLN and H295R) and Embryonic Zebrafish Assay. Environ. Res. 2019, 172, 319–325. [Google Scholar] [CrossRef]
- Mu, X.; Huang, Y.; Li, J.; Yang, K.; Yang, W.; Shen, G.; Li, X.; Lei, Y.; Pang, S.; Wang, C. New Insights into the Mechanism of Phthalate-Induced Developmental Effects. Environ. Pollut. 2018, 241, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-B.; Kim, G.-E.; Kim, Y.J.; On, J.; Park, C.-G.; Kwon, Y.-S.; Pyo, H.; Yeom, D.-H.; Cho, S.-H. Reproductive Dysfunction Linked to Alteration of Endocrine Activities in Zebrafish Exposed to Mono-(2-Ethylhexyl) Phthalate (MEHP). Environ. Pollut. 2020, 265, 114362. [Google Scholar] [CrossRef]
- Kamstra, J.H.; Sales, L.B.; Aleström, P.; Legler, J. Differential DNA Methylation at Conserved Non-Genic Elements and Evidence for Transgenerational Inheritance Following Developmental Exposure to Mono(2-Ethylhexyl) Phthalate and 5-Azacytidine in Zebrafish. Epigenetics Chromatin 2017, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Sant, K.E.; Moreau, H.M.; Williams, L.M.; Jocobs, H.M.; Bowsher, A.M.; Boisvert, J.D.; Smolowitz, R.M.; Pantazis, J.; Annunziato, K.; Nguyen, M.; et al. Embryonic Exposures to Mono-2-Ethylhexyl Phthalate Induce Larval Steatosis in Zebrafish Independent of Nrf2a Signaling.Pdf. J. Dev. Orig. Health Dis. 2020, 12, 132–140. [Google Scholar] [CrossRef]
- Mu, X.; Chen, X.; Liu, J.; Yuan, L.; Wang, D.; Qian, L.; Qian, Y.; Shen, G.; Huang, Y.; Li, X. A Multi-Omics Approach Reveals Molecular Mechanisms by Which Phthalates Induce Cardiac Defects in Zebrafish (Danio Rerio). Environ. Pollut. 2020, 265, 113876. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Liu, J.; Lin, Z.; Chen, X.; Yuan, L.; Shen, G.; Yang, W.; Wang, D.; Huang, Y.; Pang, S. Evaluation of the Spinal Effects of Phthalates in a Zebrafish Embryo Assay. Chemosphere 2020, 249, 126144. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Snyder, M.P. Integrative Omics for Health and Disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Junaid, M.; Jia, P.-P.; Tang, Y.-M.; Xiong, W.-X.; Huang, H.-Y.; Strauss, P.R.; Li, W.-G.; Pei, D.-S. Mechanistic Toxicity of DEHP at Environmentally Relevant Concentrations (ERCs) and Ecological Risk Assessment in the Three Gorges Reservoir Area, China. Environ. Pollut. 2018, 242, 1939–1949. [Google Scholar] [CrossRef]
- Scholz, S.; Fischer, S.; Gündel, U.; Küster, E.; Luckenbach, T.; Voelker, D. The Zebrafish Embryo Model in Environmental Risk Assessment—Applications beyond Acute Toxicity Testing. Environ. Sci. Pollut. Res. 2008, 15, 394–404. [Google Scholar] [CrossRef]
- Caldwell, J.C. DEHP: Genotoxicity and potential carcinogenic mechanisms—A review. Mutat. Res. 2012, 751, 82–157. [Google Scholar] [CrossRef]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of Reactive Oxygen Species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.-P.; Ma, Y.-B.; Lu, C.-J.; Mirza, Z.; Zhang, W.; Jia, Y.-F.; Li, W.-G.; Pei, D.-S. The Effects of Disturbance on Hypothalamus-Pituitary-Thyroid (HPT) Axis in Zebrafish Larvae after Exposure to DEHP. PLoS ONE 2016, 11, e0155762. [Google Scholar] [CrossRef]
- An, Y.; Lee, H.; Kim, K. Developmental Toxicity and Endocrine Disruption Effect of 6 Phthalates in Zebrafish Embryos. In Proceedings of the Annual Conference of Japan Society of Material Cycles and Waste Management The 29th Annual Conference of Japan Society of Material Cycles and Waste Management (p. 621), Nagoya, Japan, 12–14 September 2018. [Google Scholar]
- He, J.-H.; Gao, J.-M.; Huang, C.-J.; Li, C.-Q. Zebrafish Models for Assessing Developmental and Reproductive Toxicity. Neurotoxicol. Teratol. 2014, 42, 35–42. [Google Scholar] [CrossRef]
- Zhai, W.; Huang, Z.; Chen, L.; Feng, C.; Li, B.; Li, T. Thyroid Endocrine Disruption in Zebrafish Larvae after Exposure to Mono-(2-Ethylhexyl) Phthalate (MEHP). PLoS ONE 2014, 9, e92465. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Clark, C.W.; Conlin, S.M.; Brown, S.E.; Timme-Laragy, A.R. Mapping Glutathione Utilization in the Developing Zebrafish (Danio Rerio) Embryo. Redox Biol. 2019, 26, 101235. [Google Scholar] [CrossRef]
- Jacobs, H.M.; Sant, K.E.; Basnet, A.; Williams, L.M.; Moss, J.B.; Timme-Laragy, A.R. Embryonic Exposure to Mono(2-Ethylhexyl) Phthalate (MEHP) Disrupts Pancreatic Organogenesis in Zebrafish (Danio Rerio). Chemosphere 2018, 195, 498–507. [Google Scholar] [CrossRef]
- Sant, K.; Jacobs, H.; Xu, J.; Borofski, K.; Moss, L.; Moss, J.; Timme-Laragy, A. Assessment of Toxicological Perturbations and Variants of Pancreatic Islet Development in the Zebrafish Model. Toxics 2016, 4, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, D.; Xin, G.-Y.; Junaid, M.; Wang, Y.; Ma, Y.-B. DEHP Chronic Exposure Disturbs the Gut Microbial Community and Metabolic Homeostasis: Gender-Based Differences in Zebrafish. Res. Sq. 2020, Preprint. [Google Scholar] [CrossRef] [Green Version]
- Padilla, S.; Corum, D.; Padnos, B.; Hunter, D.L.; Beam, A.; Houck, K.A.; Sipes, N.; Kleinstreuer, N.; Knudsen, T.; Dix, D.J. Zebrafish developmental screening of the ToxCastTM Phase I chemical library. Reprod. Toxicol. 2012, 33, 174–187. [Google Scholar] [CrossRef]
- Dodd, A.; Curtis, P.M.; Williams, L.C.; Love, D.R. Zebrafish: Bridging the gap between development and disease. Hum. Mol. Genet. 2000, 9, 2443–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Zon, L.I. The zebrafish as a model for human disease. In Fish Physiology; Perry, S.F., Ekker, M., Farrel, A.P., Brauner, C.J., Eds.; Academic Press: Burlington, MA, USA, 2010. [Google Scholar]
- Tao, T.; Peng, J. Liver development in zebrafish. J. Genet. Genom. 2009, 36, 325–334. [Google Scholar] [CrossRef]
- Blanton, M.L.; Specker, J.L. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction. Crit. Rev. Toxicol. 2007, 37, 97–115. [Google Scholar] [CrossRef] [PubMed]
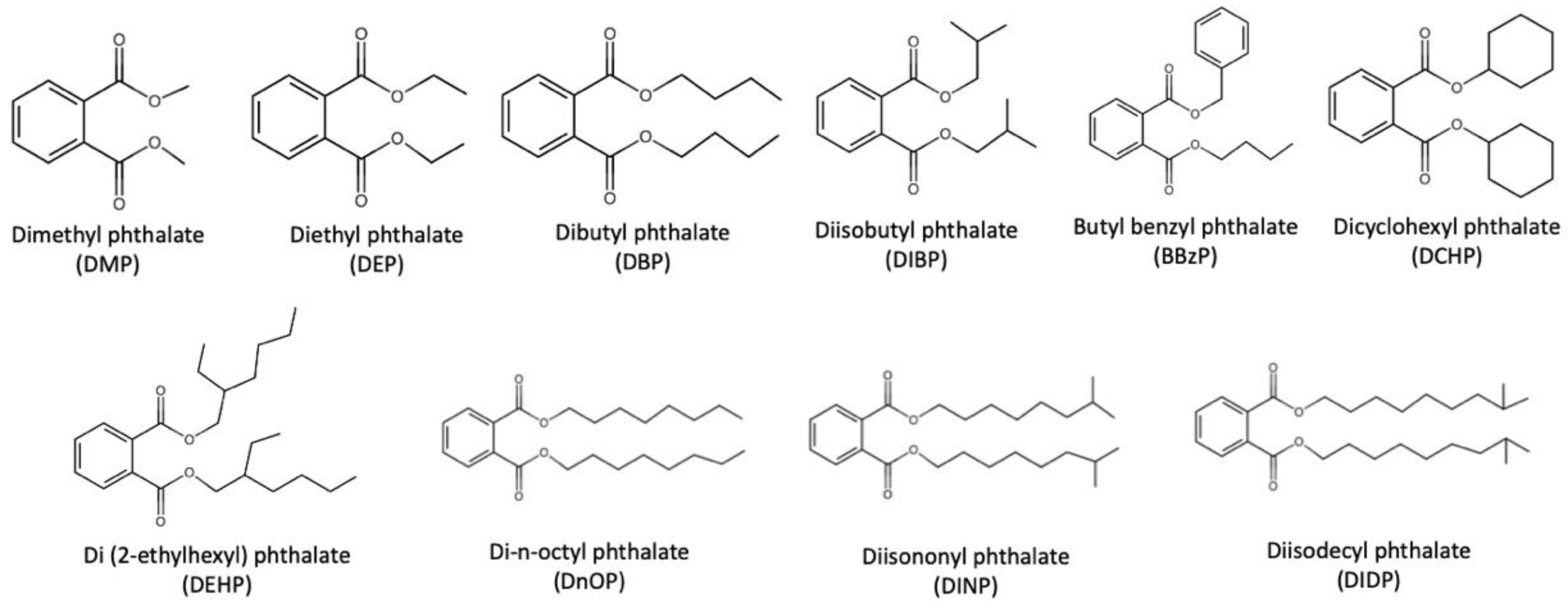

| (a) DEHP | ||||
|---|---|---|---|---|
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| mortality rate increased | 0.5 µg/L | 27–72 hpf | 72 hpf | [39] |
| 25 µg/L | 3–168 hpf | 168 hpf | [38] | |
| 200 mg/L | 1–168 hpf | 168 hpf | [37] | |
| survival (LC50) | 2.5 µg/L | start at embryo stage—72 hpf | 72 hpf | [40] |
| 54.02 mg/L | 72–168 hpf | 72–168 hpf | [41] | |
| (b) MEHP | ||||
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| mortality rate increased | ** 2.8 mg/L | start at embryo stage—36/48/60/72/96 hpf | ** 2.8 mg/L: 60, 72, 96 hpf; ** 7.0 mg/L & ** 14 mg/L: 36, 48, 60, 72, 96 hpf | [42] |
| (a) DEHP | ||||
|---|---|---|---|---|
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| hatching delayed | 0.5 µg/L | 27–72 hpf | 72 hpf | [39] |
| 2.5 µg/L | 4–48/72 hpf | 48, 72 hpf | [44] | |
| deformity rate increased | 25 µg/L | 3–168 hpf | 168 hpf | [38] |
| 200 mg/L | 1–168 hpf | 168 hpf | [37] | |
| body length decreased | * 2.0 µg/L | 3–24 hpf | 24 hpf | [45] |
| 100 mg/L | 2–120 hpf | 120 hpf | [46] | |
| 100 mg/L | 6–168 hpf | 168 hpf | [47] | |
| tail length decreased | * 2.0 µg/L | 3–24 hpf | 24 hpf | [45] |
| swim bladder inflation inhibited | 500 µg/L | 72–168 hpf | 168 hpf | [41] |
| yolk sac edema | 2.5 µg/L | 4–72 hpf | 72 hpf | [44] |
| 50 µg/L | 2–48 hpf | 48 hpf | [48] | |
| yolk extension length decreased | * 2.0 µg/L | 3–24 hpf | 24 hpf | [45] |
| hyperemia | 10 mg/L | 72–168 hpf | 168 hpf | [41] |
| dark pigmentation | 10 mg/L | 72–168 hpf | 168 hpf | [41] |
| forebrain length decreased | * 2.0 µg/L | 3–48 hpf | 48 hpf | [45] |
| head width decreased | * 2.0 µg/L | 3–24 hpf | 24 hpf | [45] |
| (b) MEHP | ||||
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| hatching delayed | ** 2.8 mg/L | 4–76 hpf | 76 hpf | [42] |
| deformity rate increased | ** 14 mg/L | 4–76 hpf | 76 hpf | [42] |
| body length decreased | ** 7.0 mg/L | 4–76 hpf | 76 hpf | [42] |
| body length decreased | ** 8.4 mg/L | start at embryo stage—3/6 dpf | 3/6 dpf | [50] |
| swim bladder abnormality | 200 µg/L | 6–96 hpf | 96 hpf | [51] |
| no observed effect concentration (NOEC) | <6.09 mg/L | 6–144 hpf | 144 hpf | [49] |
| effective concentration, 10% (EC10) | 9.77 mg/L | 6–144 hpf | 144 hpf | [49] |
| effective concentration, 50% (EC50) | 29.98 mg/L | 6–144 hpf | 144 hpf | [49] |
| effective concentration, 100% (EC100) | 50.0 mg/L | 6–144 hpf | 144 hpf | [49] |
| (a) DEHP | ||||
|---|---|---|---|---|
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| heart rate decreased | * 20 mg/L | 4–76 hpf | 76 hpf | [42] |
| 120 mg/L | 1–72 hpf | 72 hpf | [37] | |
| heart rate increased | 25, 50 µg/L | 3–168 hpf | 168 hpf | [38] |
| pericardial edema | 2.5 µg/L | 4–72 hpf | 72 hpf | [44] |
| 250 µg/L | 1.5–72/96 hpf | 72, 96 hpf | [52] | |
| 10 mg/L | 72–168 hpf | 168 hpf | [42] | |
| apoptosis signal increased in heart region | 250 µg/L | 1.5–72 hpf | 72 hpf | [52] |
| (b) MEHP | ||||
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| heart rate decreased | ** 14 mg/L | 4–76 hpf | 76 hpf | [42] |
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
|---|---|---|---|---|
| bent spinal curvature | 2.5 µg/L | 4–72 hpf | 72 hpf | [44] |
| 200 mg/L | 1–168 hpf | 168 hpf | [37] | |
| spinal dysplasia (cartilage effect) | 200 mg/L | 1–168 hpf | 168 hpf | [37] |
| (a) DEHP | ||||
|---|---|---|---|---|
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| spontaneous movement decreased (no. of spontaneous movements in 20 s) | 50 µg/L | 2–24 hpf | 24 hpf | [53] |
| movement inhibition | 90 mg/L | 1–24 hpf | 24 hpf | [37] |
| locomotor activity inhibited | * 20 mg/L | 4–100 hpf | 100 hpf | [42] |
| locomotor activity (dark) (bursting) decreased | 5 µg/L | 2–120 hpf | 120 hpf | [46] |
| locomotor activity (dark) (cruising) decreased | 5 µg/L | 2–120 hpf | 120 hpf | [46] |
| locomotor activity (light) (swimming) increased | 10 mg/L | 2–120 hpf | 120 hpf | [46] |
| locomotor activity (light) (cruising) increased | 10 mg/L | 2–120 hpf | 120 hpf | [46] |
| locomotor activity (light) (freezeing) increased | 1 mg/L | 2–120 hpf | 120 hpf | [46] |
| (b) MEHP | ||||
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| locomotor activity inhibited | ** 14 mg/L | 4–100 hpf | 100 hpf | [42] |
| (a) DEHP | ||||
|---|---|---|---|---|
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| intense PCNA staining | 2.5 µg/L | 4–48 hpf | 48 hpf | [44] |
| intense wnt3a staining | 2.5 µg/L | 4–48 hpf | 48 hpf | [45] |
| intense β-catenin staining | 2.5 µg/L | 4–48 hpf | 48 hpf | [46] |
| gsk3β mRNA expression increased | 2.5 µg/L | 4–72 hpf | 72 hpf | [44] |
| bmp2 transcript level decreased | 50 µg/L | 2–96 hpf | 96 hpf | [53] |
| ngs transcript level increased | 50 µg/L | 2–96 hpf | 96 hpf | [53] |
| co18ala transcript level increased | 50 µg/L | 2–96 hpf | 96 hpf | [53] |
| co18ala transcript level decreased | 250 µg/L | 2–96 hpf | 96 hpf | [53] |
| klh14a transcript level increased | 50 µg/L | 2–96 hpf | 96 hpf | [53] |
| smyd2b transcript level increased | 50 µg/L | 2–96 hpf | 96 hpf | [53] |
| spp1 transcript level increased | 50 µg/L | 2–96 hpf | 96 hpf | [53] |
| stac3 transcript level increased | 50 µg/L | 2–96 hpf | 96 hpf | [53] |
| steroid binding pathway induction (paqr5b, nr1h4, fabp10a) | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| cyclase activator activity pathway induction (guca1a, guca1d) | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| actomyosin structure organization pathway induction (klhl41a, csrp3, cnn1b, ctnt) | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| chemokine receptor binding pathway induction(ccl27a, ccl39.3) | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| myofibril assembly pathway induction (csrp3, klhl41a, ctnt) | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| notochord development pathway induction (col8a1a, ngs, fbn2b) | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| skeletal system development pathway induction (spp1) | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| heart contraction and heart process pathway induction (smyd2b,csrp3) | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| nppa transcript level altered | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| My17 transcript level altered | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| Tbx5b transcript level altered | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| smyd2b transcript level altered | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| ctnt transcript level altered | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| cmlc1 transcript level altered | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| hypomethylation (nppa, ctnt) | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| hypermethylation (tbx5b) | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| immune system-related genes (cci27a, tnfaip2b, casp3b, rnasel3, traf3ip2b, diexf, trpm2, park2, smyd2b, creb312, il-1b, nfkb1, tp63, caspa) altered | 50 µg/L | 2–96 hpf | 96 hpf | [48] |
| lipid metabolism and skeletal development related genes (paqr5b, nr1h4, gc, fabp10a, esyt3, zfos-411a11.2, alox5a, apoba, atp8b1, osbp19, ch25hl1.2, gfpt1, klhl41a, klhl40b, stac3, ucmab, spp1, caspa, hapln1b) altered | 50 µg/L | 2–96 hpf | 96 hpf | [48] |
| sp7 transcript level increased | 200 mg/L | 1–168 hpf | 168 hpf | [37] |
| runx2b transcript level increased | 200 mg/L | 1–168 hpf | 168 hpf | [38] |
| gpc4a transcript level increased | 200 mg/L | 1–168 hpf | 168 hpf | [39] |
| shha transcript level increased | 200 mg/L | 1–168 hpf | 168 hpf | [40] |
| elav13 expression decreased, Fluorescence intensity reduction inTg (HuC:eGFP) | 500 µg/L | 2–72 hpf | 72 hpf | [46] |
| ache transcript level increased | 50 µg/L | 2–120 hpf | 120 hpf | [46] |
| th transcript level decreased | 500 µg/L | 2–120 hpf | 121 hpf | [46] |
| pik3r1 mRNA expression increased | 400 µg/L | 2–168 hpf | 168 hpf | [55] |
| akt1 mRNA expression increased | 400 µg/L | 2–168 hpf | 168 hpf | [55] |
| mtor mRNA expression increased | 400 µg/L | 2–168 hpf | 168 hpf | [55] |
| ps6kb mRNA expression increased | 200 µg/L | 2–168 hpf | 168 hpf | [55] |
| AhR activity induction, Fluorescence intensity increased in Tg(cyp1a:gfp) | 33 µg/L | 2–120 hpf | 120 hpf | [55] |
| Cerg1 level decreased | 50 µg/L | 2–96 hpf | 96 hpf | [48] |
| Che level decreased | 50 µg/L | 2–96 hpf | 96 hpf | [48] |
| DG level decreased | 50 µg/L | 2–96 hpf | 96 hpf | [48] |
| TG level decreased | 50 µg/L | 2–96 hpf | 96 hpf | [48] |
| FA level decreased | 50 µg/L | 2–96 hpf | 96 hpf | [48] |
| lipase activity regulation, lipid absorption, lipid catabolism, lipid digestion, lipid metabolism and lipid transport increased | 50 µg/L | 2–96 hpf | 96 hpf | [48] |
| (b) MEHP | ||||
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| increased vacuolization and type of vacuolization in liver | 200 µg/L | 6–96 hpf | 96 hpf | [51] |
| lipid accumulation in liver & brain | 200 µg/L | 6–120 hpf | 15 dpf | [51] |
| fabp1a1 expression increased | 200 µg/L | 6–120 hpf | 15 dpf | [51] |
| (a) DEHP | ||||
|---|---|---|---|---|
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| lipid peroxidation (LPO) increased | 2.5 µg/L | start at embryo stage—72 hpf | 72 hpf | [40] |
| glutathione S-transferase (GST) decreased | 2.5 µg/L | start at embryo stage—72 hpf | 72 hpf | [40] |
| CAT expression increased | 50 µg/L | 3–96 hpf | 96 hpf | [38] |
| CuSOD expression increased | 50 µg/L | 3–96 hpf | 96 hpf | [39] |
| MnSOD expression increased | 50 µg/L | 3–96 hpf | 96 hpf | [40] |
| ROS generation increased | * 20 mg/L | 4–28 hpf | 28 hpf | [42] |
| SOD activity decreased | * 20 mg/L | 4–28 hpf | 28 hpf | [42] |
| (b) MEHP | ||||
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| ROS generation increased | ** 14 mg/L | 4–28 hpf | 28 hpf | [42] |
| SOD activity decreased | ** 14 mg/L | 4–28 hpf | 28 hpf | [42] |
| (a) DEHP | ||||
|---|---|---|---|---|
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| apoptosis signal increased | 50 µg/L | 3–96 hpf | 96 hpf | [38] |
| apoptosis signal increased (heart region) | 250µg/L | 1.5–72 hpf | 72 hpf | [52] |
| bax mRNA expression decreased | 50 µg/L | 3–96 hpf | 96 hpf | [38] |
| bax mRNA expression increased | * 20 mg/L | 4–28 hpf | 28 hpf | [42] |
| bcl2 mRNA expression decreased | * 9.8 mg/L | 4–28 hpf | 28 hpf | [42] |
| ctnt transcription level increased | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| ctnt protein level increased | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| nppa transcription level increased | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| Nppa protein level increased | 50 µg/L | 1.5–96 hpf | 96 hpf | [52] |
| cas8 mRNA expression increased | 50 µg/L | 3–96 hpf | 96 hpf | [38] |
| cas9 mRNA expression increased | 50 µg/L | 3–96 hpf | 96 hpf | [38] |
| pf3 mRNA expression increased | 50 µg/L | 3–96 hpf | 96 hpf | [38] |
| ogg1 mRNA expression increased | * 20 mg/L | 4–28 hpf | 28 hpf | [42] |
| parp1 mRNA expression increased | * 3.9 mg/L | 4–28 hpf | 28 hpf | [42] |
| pcna mRNA expression increased | * 3.9 mg/L | 4–28 hpf | 28 hpf | [42] |
| polb mRNA expression increased | * 3.9 mg/L | 4–28 hpf | 28 hpf | [42] |
| pold mRNA expression decreased | * 3.9 mg/L | 4–28 hpf | 28 hpf | [42] |
| fen1 mRNA expression increased | * 20 mg/L | 4–28 hpf | 28 hpf | [42] |
| lig1 mRNA expression increased | * 3.9 mg/L | 4–28 hpf | 28 hpf | [42] |
| c-myc transcript level increased | 2.5 µg/L | start at embryo stage—72 hpf | 72 hpf | [40] |
| DNA breaks increased | 5 mg/L | 72–168 hpf | 168 hpf | [41] |
| p53 mRNA expression increased | 500 µg/L | 72–168 hpf | 168 hpf | [41] |
| rad51 expression increased | 1 mg/L | 72–168 hpf | 168 hpf | [41] |
| xrcc5 expression increased | 1 mg/L | 72–168 hpf | 168 hpf | [41] |
| (b) MEHP | ||||
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| bax mRNA level increased | ** 2.8 mg/L | 4–28 hpf | 28 hpf | [42] |
| bcl2 mRNA level decreased | ** 14 mg/L | 4–28 hpf | 28 hpf | [42] |
| ogg1 mRNA expression increased | ** 2.8 mg/L | 4–28 hpf | 28 hpf | [42] |
| nthl1 mRNA expression increased | ** 2.8 mg/L | 4–28 hpf | 28 hpf | [42] |
| apex1 mRNA expression increased | ** 7.0 mg/L | 4–28 hpf | 28 hpf | [42] |
| parp1 mRNA expression increased | ** 2.8 mg/L | 4–28 hpf | 28 hpf | [42] |
| xrcc1 mRNA expression increased | ** 7.0 mg/L | 4–28 hpf | 28 hpf | [42] |
| lig3 mRNA expression increased | ** 2.8 mg/L | 4–28 hpf | 28 hpf | [42] |
| ung mRNA expression increased | ** 2.8 mg/L | 4–28 hpf | 28 hpf | [42] |
| pcna mRNA expression increased | ** 2.8 mg/L | 4–28 hpf | 28 hpf | [42] |
| polb mRNA expression decreased | ** 7.0 mg/L | 4–28 hpf | 28 hpf | [42] |
| pold mRNA expression decreased | ** 2.8 mg/L | 4–28 hpf | 28 hpf | [42] |
| fen1 mRNA expression increased | ** 7.0 mg/L | 4–28 hpf | 28 hpf | [42] |
| lig1 mRNA expression increased | ** 14 mg/L | 4–28 hpf | 28 hpf | [42] |
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
|---|---|---|---|---|
| dnmt gene expressions altered (dnmt1, dnmt3aa, dnmt3ab, dnmt3bb.1, dnmt3bb.2) | F0: ** 8.4 mg/L | F0: start at embryo stage—6 dpf | F0 6 dpf | [50] |
| global demethylation in liver | F0: ** 8.4 mg/L | F0: start at embryo stage—6 dpf | F0 adult female | [50] |
| global hmC level decreased | F0: ** 8.4 mg/L | F0: start at embryo stage—6 dpf | F0 6 dpf | [50] |
| global hmC level decreased in brain tissue | F0: ** 8.4 mg/L | F0: start at embryo stage—6 dpf | F0 adult male | [50] |
| average methylation increased at zfCNEs | F0: ** 8.4 mg/L | F0: start at embryo stage—6 dpf | F0 6 dpf | [50] |
| adipogenesis pathway increased | F0: ** 8.4 mg/L | F0: start at embryo stage—6 dpf | F0 6 dpf | [50] |
| transgenerational effect: DNA methylation at cbfa2t2 locus and specific CpG sites among F0, F1 and F2 generations | F0: ** 8.4 mg/L | F0: start at embryo stage—6 dpf | F0 6 dpf, F1 6 dpf, F2 6 dpf, F0 15 dpf and F0 adult sperm | [50] |
| (a) DEHP | ||||
|---|---|---|---|---|
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| triiodothyronine (T3) level increased | 400 µg/L | 2–168 hpf | 168 hpf | [59] |
| triiodothyronine (T3) level increased | 250 µg/L | 2–96 hpf | 96 hpf | [48] |
| thyroxinem (T4) level increased | 400 µg/L | 2–168 hpf | 168 hpf | [59] |
| thyroxinem (T4) level increased | 250 µg/L | 2–96 hpf | 96 hpf | [48] |
| estrogen receptor alpha (erα) mRNA increased | 50 µg/L | 3–96 hpf | 96 hpf | [38] |
| estrogen receptor alpha (erα) mRNA increased | 250 µg/L | 2–96 hpf | 96 hpf | [48] |
| estrogen receptor alpha (ERα) gene expression increased | 100 µg/L | start at embryo stage—168 hpf | 168 hpf | [60] |
| estrogen receptor alpha (ERα) protein level increased | 250 µg/L | 2–96 hpf | 96 hpf | [48] |
| estrogen receptor alpha (erα) transcript level decreased | 10 µg/L | 6–168 hpf | 168 hpf | [47] |
| estrogen receptor beta (erβ) mRNA increased | 50 µg/L | 3–96 hpf | 96 hpf | [38] |
| cytochrome P450 aromatase (cyp19a) expression increased | 25 µg/L | 3–96 hpf | 96 hpf | [38] |
| cytochrome P450 aromatase (Cyp19b) gene expression increased | 100 µg/L | start at embryo stage—168 hpf | 168 hpf | [60] |
| cytochrome P450 aromatase (cyp19a1b) transcript level increased | 10 µg/L | 6–168 hpf | 168 hpf | [47] |
| vitellogenin (vtg) mRNA expression increased | 25 µg/L | 3–96 hpf | 96 hpf | [38] |
| vitellogenin (Vtg) gene expression increased | 100 µg/L | start at embryo stage—168 hpf | 168 hpf | [60] |
| vitellogenin (vtg1) transcript level increased | 100 µg/L | 6–168 hpf | 168 hpf | [47] |
| thyroid stimulating hormone (tshβ) mRNA expression increased | 100 µg/L | 2–168 hpf | 168 hpf | [59] |
| corticotrophin releasing hormone (crh) mRNA expression increased | 200 µg/L | 2–168 hpf | 168 hpf | [59] |
| NK2 homeobox 1 (nkx2.1) mRNA expression increased | 200 µg/L | 2–168 hpf | 168 hpf | [59] |
| thyroglobulin (tg) mRNA expression increased | 400 µg/L | 2–168 hpf | 168 hpf | [59] |
| uridinediphosphate-glucuronosyl-transferase (ugtlab) mRNA expression decreased | 200 µg/L | 2–168 hpf | 168 hpf | [59] |
| iodothyronine deiodinase (dio2) mRNA expression increased | 400 µg/L | 2–168 hpf | 168 hpf | [59] |
| transthyretin (ttr) mRNA expression increased | 200 µg/L | 2–168 hpf | 168 hpf | [59] |
| androgen receptor (ar) mRNA expression increased | 50 µg/L | 3–96 hpf | 96 hpf | [38] |
| 17βestradiol level increased | 250 µg/L | 2–96 hpf | 96 hpf | [48] |
| cancer cell migration (from yolk to gut and tail) | 400 µg/L | 72–78 hpf | 78 hpf | [55] |
| (b) MEHP | ||||
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
| triiodothyronine (T3) level increased | 200 µg/L | 2–168 hpf | 168 hpf | [62] |
| thyroxinem (T4) level decreased | 200 µg/L | 2–168 hpf | 168 hpf | [62] |
| thyroid stimulating hormone (TSHβ) transcription increased | 40 µg/L | 2–168 hpf | 168 hpf | [62] |
| NK2 Homeobox 1 (Nkx2.1) transcription increased | 8 µg/L | 2–168 hpf | 168 hpf | [62] |
| thyroglobulin (TG) transcription increased | 200 µg/L | 2–168 hpf | 168 hpf | [62] |
| UDP-glucuronosyltransferases (UGT1ab) transcription increased | 40 µg/L | 2–168 hpf | 168 hpf | [62] |
| iodothyronine deiodinase (Dio1) transcription increased | 8 µg/L | 2–168 hpf | 168 hpf | [62] |
| iodothyronine deiodinase (Dio2) transcription increased | 40 µg/L | 2–168 hpf | 168 hpf | [62] |
| transthyretin (TTR) transcription decreased | 200 µg/L | 2–168 hpf | 168 hpf | [62] |
| paired box 8 (Pax8) transcription increased | 8 µg/L | 2–168 hpf | 168 hpf | [62] |
| sodium/iodide symporter (NIS) transcription increased | 40 µg/L | 2–168 hpf | 168 hpf | [62] |
| GSH disruption in body, heart, brain ventricle and somite 12 (MCB fluorescence decreased) | 200 µg/L | 3–48/72 hpf | 48/72 hpf | [63] |
| hypomorphic islets (β-cell cluster area decreased)(Tg (ins:GFP)) | ** 195 µg/L | 3–48 hpf | 48 hpf | [65] |
| pancreatic islet variants and defects decreased | ** 195 µg/L | 3–48 hpf | 48 hpf | [65] |
| hypomorphic islets (β-cell cluster area decreased)(Tg (ins:GFP)) | 200 µg/L | 3–48/72/96/168 hpf | 48/72/96/168 hpf | [64] |
| hypomorphic islets (α-cell cluster area decreased) (Tg (gcga:GFP)) | 200 µg/L | 3–48/72/96/168 hpf | 48/72/96/168 hpf | [64] |
| frequency of pancreatic islet morphology variants increased | 200 µg/L | 3–48/72/96/168 hpf | 48/72/96/168 hpf | [64] |
| pancreatic structure altered in wt zebrafish embryo | 200 µg/L | 3–48/72/96 hpf | 48/72/96 hpf | [64] |
| pancreatic structure altered in m zebrafish embryo | 200 µg/L | 3–48/72/96 hpf | 48/72/96 hpf | [64] |
| preproinsulin a (insa) expression decreased | 200 µg/L | 3–96 hpf | 96 hpf | [64] |
| somatostatin 2 (sst2) expression decreased | 200 µg/L | 3–96 hpf | 96 hpf | [64] |
| pancreasspecifictranscription factor 1a (ptf1a) expression decreased | 200 µg/L | 3–96 hpf | 96 hpf | [64] |
| glutathione-disulfide reductase (gsr) expression increased | 200 µg/L | 3–96 hpf | 96 hpf | [64] |
| glutathione S-transferase pi 1 (gstp) expression decreased | 200 µg/L | 3–96 hpf | 96 hpf | [64] |
| Endpoint | Lowest Effective Dose | Exposure | Endpoint Time | References |
|---|---|---|---|---|
| mortality rate increased | 0.5 µg/L | 27 hpf—1 month | 1 month | [39] |
| body length increased (male) | 33 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| body length decreased (male) | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
| body length increased (female) | 10 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| body length decreased (female) | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
| body weight increased (male) | 10 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| body weight decreased (male) | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
| body weight increased (female) | 10 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| body weight decreased (female) | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
| intestinal microbiota alteration (female) | 100 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| intestinal microbiota alteration (male) | 100 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| intestinal bacteria alteration (female) | 100 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| intestinal bacteria alteration (male) | 100 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| villus width decreased (male) | 100 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| tunica muscularis thickness decreased (female) | 100 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| goblet cells per villus decreased (female) | 100 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| tlr-5 mRNA expression decreased (female) | 100 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| il-1β mRNA expression decreased (female) | 10 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| il-8 mRNA expression increased (male) | 10 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| il-8 mRNA expression increased (female) | 10 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| TG content increased (male) | 33 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| TG content increased (female) | 10 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| PY content increased (male) | 10 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| PY content increased (female) | 33 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| FA content increased (male) | 10 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| Glu content increased (female) | 10 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| conditional factor increased (male) | 33 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| conditional factor increased (female) | 10 µg/L | start at embryo stage—3.5 month | 3.5 month | [66] |
| Gonad somatic index (GSI) decreased (male) | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
| Gonad somatic index (GSI) decreased (female) | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
| egg production decreased | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
| non-fertilization rate increased | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
| spermatogonia and spermatocyte decreased (male) | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
| tubules distruption (male) | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
| ovary development inhibition (female) | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
| number of vitellogenic oocytes decreased (female) | 0.5 µg/L | 27 hpf—6 month | 6 month | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwan, W.S.; Roy, V.A.L.; Yu, K.N. Review on Toxic Effects of Di(2-ethylhexyl) Phthalate on Zebrafish Embryos. Toxics 2021, 9, 193. https://doi.org/10.3390/toxics9080193
Kwan WS, Roy VAL, Yu KN. Review on Toxic Effects of Di(2-ethylhexyl) Phthalate on Zebrafish Embryos. Toxics. 2021; 9(8):193. https://doi.org/10.3390/toxics9080193
Chicago/Turabian StyleKwan, Wing Sum, Vellaisamy A. L. Roy, and Kwan Ngok Yu. 2021. "Review on Toxic Effects of Di(2-ethylhexyl) Phthalate on Zebrafish Embryos" Toxics 9, no. 8: 193. https://doi.org/10.3390/toxics9080193
APA StyleKwan, W. S., Roy, V. A. L., & Yu, K. N. (2021). Review on Toxic Effects of Di(2-ethylhexyl) Phthalate on Zebrafish Embryos. Toxics, 9(8), 193. https://doi.org/10.3390/toxics9080193




_Kwan_Ngok_Yu.png)

