Scabiosa Genus: A Rich Source of Bioactive Metabolites
Abstract
:1. Introduction
2. Scabiosa Genus: Traditional and Pharmacological Applications
3. Structural Pattern of the Secondary Metabolites Isolated from Scabiosa Species
4. In Vivo Assessments of Nominated Metabolites
4.1. Flavonoid-Type Metabolites
4.2. Iridoid Type Metabolites
4.3. Pentacyclic Triterpenoid Type Metabolites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1847, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Lehbili, M.; Magid, A.A.; Hubert, J.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Renault, J.-H.; Nuzillard, J.-M.; Morjani, H.; Abedini, A.; Gangloff, S.C.; et al. Two new bis-iridoids isolated from Scabiosa stellata and their antibacterial, antioxidant, anti-tyrosinase and cytotoxic activities. Fitoterapia 2018, 125, 41–48. [Google Scholar] [CrossRef] [PubMed]
- The Plant List Database. Available online: http://www.theplantlist.org/tpl1.1/search?q=Scabiosa (accessed on 10 September 2018).
- George, E.B.; Ronald, J.T. Toxic Plants of North America; John Wiley and Sons: Oxford, UK, 2013; pp. 319–322. [Google Scholar]
- Carlson, S.E.; Linder, P.H.; Donoghue, M.J. The historical biogeography of Scabiosa (dipsacaceae): Implications for Old World plant disjunctions. J. Biogeogr. 2012, 39, 1086–1100. [Google Scholar] [CrossRef]
- Mostafa, E.-N.; Sedigheh, N.-S. Palynological study of some Iranian species of Scabiosa L. (Caprifoliaceae). Bangladesh J. Plant Taxon. 2016, 23, 215–222. [Google Scholar] [CrossRef]
- Quezel, P.; Santa, S. Nouvelle Flore de l’Algérie et des Régions Désertiques Méridionales; du CNRS: Paris, France, 1963; pp. 890–893. [Google Scholar]
- Erarslan, Z.B.; Yeşil, Y. The anatomical properties of Scabiosa atropurpurea L. (Caprifoliaceae). Istanbul J. Pharm. 2018, 48, 1–5. [Google Scholar]
- Girre, L. Connaître et Reconnaître Les Plantes Médicinales in Bulletin des Bibliothèques de France (BBF). Available online: http://bbf.enssib.fr/consulter/bbf-1980-07-0373-023 (accessed on 6 October 2018).
- Ferrer-Gallego, P.P. Lectotypification of Linnaean names in the genus Scabiosa (Dipsacaceae). Taxon 2014, 63, 1353–1357. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Committee. Drug Standards of Ministry of Public Health of China (Mongolian medicine Fascicule); Chemical Industry Press: Beijing, China, 1998. [Google Scholar]
- Bonet, M.À.; Parada, M.; Selga, A.; Vallès, J. Studies on pharmaceutical ethnobotany in the regions of L’Alt Empordà and Les Guilleries (Catalonia, Iberian Peninsula). J. Ethnopharmacol. 1999, 68, 145–168. [Google Scholar] [CrossRef]
- Gras, A.; Garnatje, T.; Ibáñez, N.; López-Pujol, J.; Nualart, N.; Vallès, J. Medicinal plant uses and names from the herbarium of Francesc Bolòs (1773–1844). J. Ethnopharmacol. 2017, 204, 142–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigat, M.; Bonet, M.À.; Garcia, S.; Garnatje, T.; Vallès, J. Studies on pharmaceutical ethnobotany in the high river Ter valley (Pyrenees, Catalonia, Iberian Peninsula). J. Ethnopharmacol. 2007, 113, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Kose, L.S.; Moteetee, A.; Vuuren, S.V. Ethnobotanical survey of medicinal plants used in the Maseru district of Lesotho. J. Ethnopharmacol. 2015, 170, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Moteetee, A.; Kose, L.S. Medicinal plants used in Lesotho for treatment of reproductive and post reproductive problems. J. Ethnopharmacol. 2016, 194, 827–849. [Google Scholar] [CrossRef] [PubMed]
- Bammi, J.; Douira, A. Les plantes médicinales dans la forêt de L’Achach (Plateau Central, Maroc). Acta Bot. Malacit. 2002, 27, 131–145. [Google Scholar]
- Xu, H.; Ma, Q.; Ma, J.; Wu, Z.; Wang, Y.; Ma, C. Hepato-protective effects and chemical constituents of a bioactive fraction of the traditional compound medicine-Gurigumu-7. BMC Complement. Altern. Med. 2016, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.N.; Bolraa, S.; Ji, M.; He, Q.Q.; Ma, C.M. Quantification and antioxidant and anti-HCV activities of the constituents from the inflorescences of Scabiosa comosa and S. tschilliensis. Nat. Prod. Res. 2016, 30, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Hlila, M.B.; Mosbah, H.; Mssada, K.; Jannet, H.B.; Aouni, M.; Selmi, B. Acetylcholinesterase inhibitory and antioxidante properties of roots extracts from the Tunisian Scabiosa arenaria Forssk. Ind. Crop. Prod. 2015, 67, 62–69. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Malca-García, G.; Glenn, A.; Sharon, D.; Chait, G.; Díaz, D.; Pourmand, K.; Jonat, B.; Somogy, S.; Guardado, G.; et al. Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. J. Ethnopharmacol. 2010, 132, 101–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elhawary, S.S.; Eltantawy, M.E.; Sleem, A.A.; Abdallah, H.M.; Mohamed, N.M. Investigation of phenolic content and biological activities of Scabiosa atropurpurea L. World Appl. Sci. J. 2011, 15, 311–317. [Google Scholar]
- Christopoulou, C.; Graikou, K.; Chinou, I. Chemosystematic value of chemical constituents from Scabiosa hymettia (Dipsacaceae). Chem. Biodivers. 2008, 5, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Vuuren, S.F.v.; Naidoo, D. An antimicrobial investigation of plants used traditionally in southern Africa to treat sexually transmitted infections. J. Ethnopharmacol. 2010, 130, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Li, X.; Bi, K.; Zhang, Y.; Huang, J.; Zhang, R. Variation of active constituents and antioxidant activity in Scabiosa tschiliensis Grüning from different stages. J. Food Sci. Technol. 2017, 54, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.A.; Otoom, N.K.; Al-Jaber, H.; Saleh, A.M.; Zarga, M.H.A.; Afifi, F.U.; Orabi, S.T.A. New flavonol glycoside from Scabiosa prolifera L. aerial parts with in vitro antioxidant and cytotoxic activities. Nat. Prod. Res. 2017, 31, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Hlila, B.M.; Mosbah, H.; Majouli, K.; Nejma, A.B.; Jannet, H.B.; Mastouri, M.; Aouni, M.; Selmi, B. Antimicrobial activity of Scabiosa arenaria Forssk. extracts and pure compounds using bioguided fractionation. Chem. Biodivers. 2016, 13, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Perdetzoglou, D.; Skaltsa, H.; Tzakou, O.; Harval, C. Comparative phytochemical and morphological study of two species of the Scabiosa L. genus. Feddes Repert. 1994, 105, 157–165. [Google Scholar] [CrossRef]
- Rahmouni, N.; Pinto, D.C.G.A.; Beghidja, N.; Benayache, S.; Silva, A.M.S. Scabiosa stellata L. phenolic content clarifies its antioxidant activity. Molecules 2018, 23, 1285. [Google Scholar] [CrossRef] [PubMed]
- Zemtsova, G.N.; Bandyukova, V.A.; Dzhumyrko, S.F. Flavones and phenolic acids of Scabiosa olgae. Chem. Nat. Compd. 1972, 8, 662. [Google Scholar] [CrossRef]
- Lehbili, M.; Magid, A.A.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Morjani, H.; Harakat, D.; Kabouche, Z. Triterpenoid saponins from Scabiosa stellata collected in North-eastern Algeria. Phytochemistry 2018, 150, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Polat, E.; Alankus-Caliskan, O.; Karayildirim, T.; Bedir, E. Iridoids from Scabiosa atropurpurea L. subsp. maritima Arc. (L.). Biochem. Syst. Ecol. 2010, 38, 253–255. [Google Scholar] [CrossRef]
- Papalexandrou, A.; Magiatis, P.; Perdetzoglou, D.; Skaltsounis, A.L.; Chinou, I.B.; Harvala, C. Iridoids from Scabiosa variifolia (Dipsacaceae) growing in Greece. Biochem. Syst. Ecol. 2003, 31, 91–93. [Google Scholar] [CrossRef]
- Zheng, Q.; Koike, K.; Han, L.K.; Okuda, H.; Nikaido, T. New biologically active triterpenoid saponins from Scabiosa tschiliensis. J. Nat. Prod. 2004, 67, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Baykal, T.; Panayir, T.; Tasdemir, D.; Sticher, O.; Çalis, I. Triterpene saponins from Scabiosa rotata. Phytochemistry 1998, 48, 867–873. [Google Scholar] [CrossRef]
- Akimailiev, S.A.; Putieva, Z.M.; Alimbaeva, P.K.; Abubakirov, N.K. Triterpene glycosides of Scabiosa soogorica. V. β-Sitosterol β-d-glucopyranoside and songoroside A. Khim. Prir. Soedin. 1988, 1988, 885–886. [Google Scholar]
- Rahmouni, N.; Pinto, D.C.G.A.; Santos, S.A.O.; Beghidja, N.; Silva, A.M.S. Lipophilic composition of Scabiosa stellata L.: An underexploited plant from Batna (Algeria). Chem. Pap. 2018, 72, 753–762. [Google Scholar] [CrossRef]
- Cárdenas, M.; Marder, M.; Blank, V.C.; Roguin, L.P. Antitumor of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines. Bioorg. Med. Chem. 2006, 14, 2966–2971. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, V.; Hnatyszyn, O.; Acevedo, C.; Megías, J.; Alcaraz, M.J.; Ferraro, G. Flavonoids from Artemisia copa with anti-inflammatory activity. Planta Med. 2006, 72, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Beyer, G.; Melzig, M.F. Effects of selected flavonoids and caffeic acid derivatives on hypoxanthine-xanthine oxidase-induced toxicity in cultivated Human cells. Planta Med. 2003, 69, 1125–1129. [Google Scholar] [PubMed]
- Verma, A.K.; Pratap, R. The biological potential of flavones. Nat. Prod. Rep. 2010, 27, 1571–1593. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The story of beta-sitosterol: A review. Eur. J. Med. Plants 2014, 4, 590–609. [Google Scholar] [CrossRef]
- Haque, M.N.; Bhuiyan, M.M.H.; Moon, I.S. Stigmasterol activates Cdc42-Arp2 and Erk1/2-Creb pathways to enrich glutamatergic synapses in cultures of brain neurons. Nutr. Res. 2018, 56, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E.L. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine 1998, 5, 147–163. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A.; Menichini, F. Biological and Pharmacological activities of iridoids: Recent developments. Mini-Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Marques, V.; Farah, A. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chem. 2009, 113, 1370–1376. [Google Scholar] [CrossRef]
- Kuril’chenko, V.A.; Zemtsova, G.N.; Bandyukova, V.Y. A chemical study of Scabiosa bipinnata. Khim. Prir. Soedin. 1971, 534–535. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid componds? New Phytol. 2016, 2016. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Sung, B.; Kannappan, R.; Aggarwal, B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins 2010, 2, 2428–2466. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.M.; Goyal, S.N.; Patil, C.R. Multifunctional pentacyclic triterpenoids as adjuvants in cancer chemotherapy: A review. RSC Adv. 2014, 4, 33370–33382. [Google Scholar] [CrossRef]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants-rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Ata, A. Oleanolic acid and related derivatives as medicinally important compounds. J. Enzym. Inhib. Med. Chem. 2008, 23, 739–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolini, F.; Burmistrova, O.; Marrero, M.T.; Torres, F.; Hernández, C.; Quintana, J.; Estévez, F. Induction of G2/M phase arrest and apoptosis by the flavonoid tamarixetin on Human leukemia cells. Mol. Carcinog. 2014, 53, 939–950. [Google Scholar] [PubMed]
- Hayamizu, K.; Morimoto, S.; Nonaka, M.; Hoka, S.; Sasaguri, T. Cardiotonic actions of quercetin and its metabolite tamarixetin through a digitalis-like enhancement of Ca2+ transients. Arch Biochem. Biophys. 2018, 637, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Bharitkar, Y.P.; Hazra, A.; Pal, U.; Verma, S.; Jana, S.; Singh, U.P.; Maiti, N.C.; Mondal, N.B.; Swarnakar, S. Tamarixetin 3-O-β-d-glucopyranoside from Azadirachta indica leaves: Gastroprotective role through inhibition of matrix metalloproteinase-9 activity in mice. J. Nat. Prod. 2017, 80, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Plant polyphenols—XI: The structure of acylated anthocyanins. Phytochemistry 1964, 3, 151–160. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Liu, Y.; Chu, H.; Duan, H. Synthesis and biological activity of trans-tiliroside derivatives as potent anti-diabetic agents. Molecules 2010, 15, 9174–9183. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Li, C.-B.; Jin, M.-N.; Shi, L.-H.; Duan, H.-Q.; Niu, W.-Y. Synthesis and biological activity of novel tiliroside derivants. Eur. J. Med. Chem. 2011, 46, 5189–5195. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, R.; Aderogba, M.; Olajide, O.A. Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-kB/p38-mediated neuroinflammation in activated BV2 microglia. Biochim. Biophys. Acta 2014, 1840, 3311–3319. [Google Scholar] [CrossRef] [PubMed]
- Da’i, M.; Wikantyasning, E.R.; Wahyuni, A.S.; Kusumawati, I.T.D.; Saifudin, A.; Suhendi, A. Antiproliferative properties of tiliroside from Guazuma ulmifolia lamk on T47D and MCF7 cancer cell lines. Natl. J. Physiol. Pharm. Pharmacol. 2016, 6, 627–633. [Google Scholar] [CrossRef]
- Barbosa, E.; Calzada, F.; Campos, R. In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican tradicional medicine for the treatment of diarrhea. J. Ethnopharmacol. 2007, 109, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Máñez, S.; Giner, R.M.; Cerdá-Nicolás, M.; Ríos, J.-L. Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside. Eur. J. Pharmacol. 2003, 461, 53–61. [Google Scholar] [CrossRef]
- Jin, X.; Song, S.; Wang, J.; Zhang, Q.; Qiu, F.; Zhao, F. Tiliroside, the major component of Agrimonia pilosa Ledeb ethanol extract, inhibits MAPK/JNK/p38-mediated inflammation in lipopolysaccharide-activated RAW 264.7 macrophages. Exp. Ther. Med. 2016, 12, 499–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, G.C.; Pereira, A.C.; Rezende, B.A.; da Silva, J.F.P.; Cruz, J.S.; de Souza, M.F.V.; Gomes, R.A.; Teles, Y.C.F.; Cortes, S.F.; Lemos, V.S. Mechanism of the antihypertensive and vasorelaxant effects of the flavonoif tiliroside in resistance arteries. Planta Med. 2013, 79, 1003–1008. [Google Scholar] [PubMed]
- Perkin, A.G. CI.-Colouring matters of the New Zealand dyewood puriri, Vitex littoralis. Part I. J. Chem. Soc. Trans. 1898, 73, 1019–1031. [Google Scholar] [CrossRef]
- Baldim, J.L.; Alcântara, B.G.V.; Domingos, O.S.; Soares, M.G.; Caldas, I.S.; Novaes, R.D.; Oliveira, T.B.; Lago, J.H.G.; Chagas-Paula, D.A. The correlation between chemical structures and antioxidant, prooxidant, and antitrypanosomatid properties of flavonoids. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Lang, W.; Feng, X.; Das, S.; Maier, J.; Jeffries, C.; Shelat, A.; Rivas, F. Novel vitexin-inspired scaffold against leukemia. Eur. J. Med. Chem. 2018, 146, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Gaitan, E.; Cooksey, R.C.; Legan, J.; Lindsay, R.H. Antithyroid effects in vivo and in vitro of vitexin: A C-glucosylflavone in millet. J. Clin. Endocrinol. Metab. 1995, 80, 1144–1147. [Google Scholar] [PubMed]
- Das, M.C.; Sandhu, P.; Gupta, P.; Rudrapaul, P.; De, U.C.; Tribedi, P.; Akhter, Y.; Bhattacharjee, S. Attenuation of Pseudomonas aeruginosa biofilm formation by vitexin: A combinatorial study with azithromycin and gentamicin. Sci. Rep. 2016, 6, 23347. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, L.; Zhang, X. Vitexin mitigates myocardial ischemia reperfusion-induced damage by inhibiting excessive autophagy to suppress apoptosis via the PI3K/Akt/mTOR signaling cascade. RSC Adv. 2017, 7, 56406–56416. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Li, F.; Wang, W. Vitexin protects dopaminergic neurons in MPTP-induced Parkinson’s disease through PI3K/Akt signaling pathway. Drug Des. Dev. Ther. 2018, 12, 565–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, S.I.G.; Rios-Santos, F.; Balogun, S.O.; Martins, D.T.O. Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine 2016, 23, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Min, J.; Huang, W.-X.; Wang, X.; Peng, Y.; Han, S.; Yin, J.; Liu, W.-H.; He, X.-H.; Peng, B.-W. Vitexin reduces epilepsy after hypoxic ischemia in the neonatal brain via inhibition of NKCC1. J. Neuroinflamm. 2018, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- He, J.-D.; Wang, Z.; Li, S.-P.; Xu, Y.-J.; Yu, Y.; Ding, Y.-J.; Yu, W.-L.; Zhang, R.-X.; Zhang, H.-M.; Du, H.-Y. Vitexin suppresses autophagy to induce apoptosis in hepatocellular carcinoma via activation of the JNK signaling pathway. Oncotarget 2016, 7, 84520–84532. [Google Scholar] [PubMed]
- Bhardwaj, M.; Paul, S.; Jakhar, R.; Khan, I.; Kang, J.I.; Kim, H.M.; Yun, J.W.; Lee, S.-J.; Cho, H.J.; Lee, H.G.; et al. Vitexin confers HSF-1 mediated autophagic cell death by activating JNK and ApoL1 in colorectal carcinoma cells. Oncotarget 2017, 8, 112426–112441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, M.; Cho, H.J.; Paul, S.; Jakhar, R.; Khan, I.; Lee, S.-J.; Kim, B.-Y.; Krishnan, M.; Khaket, T.P.; Lee, H.G.; et al. Vitexin induces apoptosis by suppressing autophagy in multi-drug resistant colorectal cancer cells. Oncotarget 2018, 9, 3278–3291. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review. Ann. N. Y. Acad. Sci. 2017, 1401, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Yi, X.-J.; Huang, X.-J.; Li, M.; Li, J.; Yang, G.-Z.; Gao, Y.; Muhammad, A. Hepatoprotective activity of iridoids, seco-iridoids and analogs glycosides from Gentianaceae on HepG2 cells via CYP3A4 induction and mitochondrial pathway. Food Funct. 2018, 9, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, H.; Guo, F.; Wu, Y.; Li, Y. Antinociceptive and anti-inflammatory activities of a standardizedextractract of bis-iridoids from Pterocephalus hookeri. J. Ethnopharmacol. 2018, 216, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Mncwangi, N.; Vermaak, I. Anti-inflammatory iridoids of botanical origin. Curr. Med. Chem. 2012, 19, 2104–2127. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Antidiabetic potential of monoterpenes: A case of small molecules punching above their weight. Int. J. Mol. Sci. 2018, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- West, B.J.; Deng, S.; Uwaya, A.; Isami, F.; Abe, Y.; Yamagishi, S.-I.; Jensen, C.J. Iridoids are natural glycation inhibitors. Glycoconj. J. 2016, 33, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Jaishree, V.; Badami, S.; Kumar, M.R.; Tamizhmani, T. Antinociceptive activity of swertiamarin isolated from Enicostemma axillare. Phytomedicine 2009, 16, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.P.; Soni, S.; Parikh, P.; Gosai, J.; Chruvattil, R.; Gupta, S. Swertiamarin: An active lead from Enicostemma littorale regulates hepatic and adipose tissue gene expression by targeting PPAR-γ and improves insulin sensitivity in experimental NIDDM rat model. Evid.-Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, J.; Hassan, N.; Amin, S.; Mir, S.R. Swertiamarin contributes to glucose homeostasis via inhibition of carbohydrate metabolizing enzymes. J. Nat. Remed. 2016, 4, 125–130. [Google Scholar] [CrossRef]
- Saravanan, S.; Pandikumar, P.; Babu, N.P.; Islam, V.I.H.; Thirugnanasambantham, K.; Paulraj, M.G.; Balakrishna, K.; Ignacimuthu, S. In vivo and in vitro immunomodulatory potential of swertiamarin isolated from Enicostema axillare (Lam.) A. Raynal that acts as an anti-inflammatory agent. Inflammation 2014, 37, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Islam, V.I.H.; Babu, N.P.; Pandikumar, P.; Thirugnanasambantham, K.; Chellappandian, M.; Raj, C.S.D.; Paulraj, M.G.; Ignacimuthu, S. Swertiamarin attenuates inflammation mediators via modulating NF-κB/I κB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur. J. Pharm. Sci. 2014, 56, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Hairul-Islam, M.I.; Saravanan, S.; Thirugnanasambantham, K.; Chellappandian, M.; Raj, C.S.D.; Karikalan, K.; Paulraj, M.G.; Ignacimuthu, S. Swertiamarin, a natural steroid, prevent bone erosion by modulating RANKL/RANK/OPG signaling. Int. Immunopharmacol. 2017, 53, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Šiler, B.; Mišić, D.; Nestorović, J.; Banjanac, T.; Glamočlija, J.; Soković, M.; Ćirić, A. Antibacterial and antifungal screening of Centaurium pulchellum crude extracts and main secoiridoid compounds. Nat. Prod. Commun. 2010, 5, 1525–1530. [Google Scholar] [PubMed]
- Shitlani, D.; Choudhary, R.; Pandey, D.P.; Bodakhe, S.H. Ameliorative antimalarial effects of the combination of rutin and swertiamarin on malarial parasites. Asian Pac. J. Trop. Dis. 2016, 6, 453–459. [Google Scholar] [CrossRef]
- Jeong, Y.T.; Jeong, S.C.; Hwang, J.S.; Kim, J.H. Modulation effects of sweroside isolated from the Lonicera japonica on melanin synthesis. Chem. Biol. Interac. 2015, 238, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-L.; Li, J.-D.; Wang, W.-L.; Yang, C.; Li, Z.-Y. Sweroside eradicated leukemia cells and attenuated pathogenic processes in mice by inducing apoptosis. Biomed. Pharm. 2017, 95, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Sung, S.H.; Kim, S.H.; Jang, Y.P.; Oh, T.H.; Kim, Y.C. Cognitive-enhancing activity of loganin isolated from Cornus officinalis in scopolamine-induced amnesic mice. Arch Pharm. Res. 2009, 32, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Kim, H.-C.; Lee, S.-Y.; Jang, C.-G. Loganin improves learning and memory impairments induced by scopolamine in mice. Eur. J. Pharm. 2009, 619, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Babri, S.; Azami, S.H.; Mohaddes, G. Effect of acute administration of loganin on special memory in diabetic male rats. Adv. Pharm. Bull. 2013, 3, 91–95. [Google Scholar] [PubMed]
- Tseng, Y.-T.; Chen, C.-S.; Jong, Y.-J.; Chang, F.-R.; Lo, Y.-C. Loganin possesses neuroprotective properties, restores SMN protein and activates protein synthesis positive regulator Akt/mTOR in experimental models of spinal muscular atrophy. Pharm. Res. 2016, 111, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-L.; Zhang, S.-P.; Hou, J.; Zhu, H.-B. Effect of loganin on experimental diabetic nephropathy. Phytomedicine 2012, 19, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, H.; Lv, G.; Liu, B.; Lee, M.K.K.; Lu, C.; Lv, X.; Wu, Y. Loganin attenuates diabetic nephropathy in C57BL/6J mice with diabetes induced by streptozotocin and fed with diets containing high level of advanced glycation end products. Life Sci. 2015, 123, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-H.; Wu, C.-H.; Cheng, C.-H.; Chien, C.-T. Ba-Wei-Di-Huang-Wan through its active ingredient loganin counteracts substance P-enhanced NF-κB/ICAM-1 signaling in rats with bladder hyperactivity. Neurourol. Urodynam. 2016, 35, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Shi, L.; Zhao, C.; Shen, B.; Tian, Y.; Feng, H. Loganin inhibits the inflammatory response in mouse 3T3L1 adipocytes and mouse model. Int. Immunopharm. 2016, 36, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Schinella, G.; Aquila, S.; Dade, M.; Giner, R.; Recio, M.C.; Spegazzini, E.; Buschiazzo, P.; Tournier, H.; Rios, J.L. Anti-inflammatory and apoptotic activities of pomolic acid isolated from Cecropia pachystachya. Planta Med. 2008, 74, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Baek, S.; Shin, D.; Lee, J.; Roh, J.-L. Hederagenin induces apoptosis in cisplatin-resistant head and neck cancer cells by inhibiting the Nrf2-ARE antioxidant pathway. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.-Z.; Qi, Q.; Tao, L.; Zhao, Q.; Mu, R.; Gu, H.-Y.; Wang, M.; Feng, X.; Guo, Q.-L. Macranthoside B, a hederagenin saponin extracted from Lonicera macranthoides and its anti-tumor activities in vitro and in vivo. Food Chem. Toxicol. 2009, 47, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, D.; Demuner, A.J.; Barbosa, L.C.A.; Csuk, R.; Heller, L. Hederagenin as a triterpene template for the development of new antitumor compounds. Eur. J. Med. Chem. 2015, 105, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.S.; Yuan, Y.; Song, G.; Lin, S.Q. Inhibitory effect of ursolic acid and oleanolic acid from Eriobotrya fragrans on A549 cell viability in vivo. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Abdelmageed, N.; Morad, S.A.S.; Elghoneimy, A.A.; Syrovets, T.; Simmet, T.; El-zorba, H.; El-Banna, H.A.; Cabot, M.; Abdel-Aziz, M.I. Oleanolic acid methyl ester, a novel cytotoxic mitocan, induces cell cycle arrest and ROS-mediated cell death in castration-resistant prostate cancer PC-3 cells. Biomed. Pharm. 2017, 96, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Caunii, A.; Oprean, C.; Cristea, M.; Ivan, A.; Danciu, C.; Tatu, C.; Paunescu, V.; Marti, D.; Tzanakakis, G.; Spandidos, D.A.; et al. Effects of ursolic and oleanolic on SK-MEL-2 melanoma cells: In vitro and in vivo assays. Int. J. Oncol. 2017, 51, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Yin, J.; Shao, J.; Yu, Y.; Yang, L.; Wang, Y.; Xie, M.; Fussenegger, M.; Ye, H. A synthetic-biology-inspired therapeutic strategy for targeting and treating hepatogenous diabetes. Mol. Ther. 2017, 25, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Przybylska-Gornowicz, B.; Zakłos-Szyda, M.; Dąbrowski, M.; Michalczuk, L.; Koziołkiewicz, M.; Babuchowski, A.; Zielonka, Ł.; Lewczuk, B.; Gajęcki, M.T. The influence of a natural triterpene preparation on the gastrointestinal tract of gilts streptozocin-induced diabetes and on cell metabolic activity. J. Funct. Foods 2017, 33, 11–20. [Google Scholar] [CrossRef]
- Munhoz, A.C.M.; Fröde, T.S. Isolated compounds from natural products with potential antidiabetic activity —A systematic review. Curr. Diabetes Rev. 2018, 14, 36–106. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wu, G.; Cheng, X.; Fan, J.; Peng, J.; Su, H.; Xu, Z.; Cao, M.; Long, Z.; Hao, Y.; et al. Oleanolic acid attenuates PCBs-induced adiposity and insulin resistance via HNF1b-mediated regulation of redox and PPAR γ signaling. Free Radic. Biol. Med. 2018, 124, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Beaufay, C.; Hérent, M.-F.; Quetin-Leclercq, J.; Bero, J. In vivo anti-malarial activity and toxicity studies of triterpenic esters isolated from Keetia leucantha and crude extracts. Malar. J. 2017, 16, 406. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhou, F.; Song, Z.; Huang, H.; Chen, Y.; Shen, Y.; Jia, Y.; Chen, J. Oleanolic acid protects against pathogenesis of atherosclerosis, possibly via FXR-mediated angiotensin (Ang)-(1-7) upregulation. Biomed. Pharmacol. 2018, 97, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Bernabé-García, A.; Armero-Barranco, D.; Liarte, S.; Ruzafa-Martínez, M.; Ramos-morcillo, A.J.; Nicolás, F.J. Oleanolic acid induces migration in Mv1Lu and MDA-MB-231 epithelial cells involving EGF receptor and MAP kinases activation. PLoS ONE 2017, 12, e0172574. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-G.; Lee, H.J.; Kim, K.T.; Hwang, S.-C.; Lee, C.J.; Park, J.S. Effect of oleanolic acid on the activity, secretion and gene expression of matrix metalloproteinase-3 in articular chondrocytes in vitro and the production of matrix metalloproteinase-3 in vivo. Korean J. Physiol. Pharmacol. 2017, 21, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Guo, M.; Fu, Q.; He, Z. Application of hot melt extrusión to enhance the dissolution and oral bioavailability of oleanolic acid. Asian J. Pharm. Sci. 2017, 12, 66–72. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Xu, X.; Gao, N.; Liu, X. Preparation, characterization and in vivo pharmacokinetic study of PVP-modified oleanolic acid liposomes. Int. J. Pharm. 2017, 517, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liang, C.; Liu, H.; Li, Z.; Chen, R.; Zhou, M.; Li, D.; Ye, Q.; Luo, C.; Sun, J. Polymeric nanoparticles developed by vitamin E-modified aliphatic polycarbonate polymer to promote oral absorption of oleanolic acid. Asian J. Pharm. Sci. 2017, 12, 586–593. [Google Scholar] [CrossRef]
- Xia, X.; Liu, H.; Lv, H.; Zhang, J.; Zhou, J.; Zhao, Z. Preparation, characterization, and in vitro/vivo studies of oleanolic acid-loaded lactoferrin nanoparticles. Drug Des. Dev. Ther. 2017, 11, 1417–1427. [Google Scholar] [CrossRef] [PubMed]


| Nº | Name 1 | Plant Part (Solvent) | Species |
|---|---|---|---|
| Flavonoid Derivatives | |||
| 1 | Apigenin a | Whole plant (MeOH) [28] Whole plant (EtOH) [29] | S. tenuis [28] S. stellata [29] |
| 2 | Astragallin b | Flowering plants (CH2Cl2/MeOH) [23] | S. hymettia [23] |
| 3 | Cynaroside b | Whole plant (MeOH or ButOH) [28] Aerial (leaves and stems) parts (EtOH) [22] Epigeal part (MeOH) [30] | S. atropurpurea [22] S. olgae [30] S. tenuis [28] S. argentea [28] |
| 4 | Diosmetin-7-O-β-glucoside b | Whole plant (ButOH) [28] | S. argentea [28] |
| 5 | Hyperin 3,b | Whole plant (EtOH) [2] | S. stellata [2] |
| 6 | Isoorientin b | Whole plant (EtOH) [2,29] Whole plant (ButOH) [28] | S. argentea [28] S. stellata [2,29] |
| 7 | Isovitexin b | Whole plant (MeOH) [28] | S. tenuis [28] |
| 8 | Kaempferol-3-O-[3-O-acetyl-6-O-(E)-p-coumaroyl]-β-d-glucoside b | Flowering plants (CH2Cl2/MeOH) [23] Whole plant (EtOH) [31] | S. hymettia [23] S. stellata [31] |
| 9 | Lucenin 2,b | Whole plant (EtOH) [29] | S. stellata [29] |
| 10 | Luteolin a | Aerial (leaves and stems) parts (EtOH) [22] Whole plant (EtOH) [29] Whole plant (MeOH) [28] | S. atropurpurea [22] S. tenuis [28] S. stellata [29] |
| 11 | Luteolin-7-O-β-gentiobioside c | Whole plant (MeOH or ButOH) [28] | S. argentea [28] S. tenuis [28] |
| 12 | Luteolin-7-O-rutinoside c | Aerial (leaves and stems) parts (EtOH) [22] | S. atropurpurea [22] |
| 13 | Quercetin a | Whole plant (ButOH) [28] | S. argentea [28] |
| 14 | Quercetin-3-O-arabinoside b | Whole plant (ButOH) [28] | S. argentea [28] |
| 15 | Quercetin-3-O-galactoside b | Whole plant (ButOH) [28] | S. argentea [28] |
| 16 | Swertiajaponin b | Whole plant (EtOH) [2] | S. stellata [2] |
| 17 | Tamarixetin 3-β-l-rhamnosyl-(1→2)[β-l-rhamnosyl-(1→6)]β-d-glucoside] d | Whole plant (EtOH) [29] | S. stellata [29] |
| 18 | Tiliroside b | Whole plant (EtOH) [29] | S. stellata [29] |
| 19 | Vitexin b | Whole plant (MeOH) [28] | S. tenuis [28] |
| Terpenoid derivatives | |||
| 20 | 7-O-(E-Caffeoyl)sylvestroside I c | Whole plant (EtOH) [2] | S. stellata [2] |
| 21 | 7-O-(E-p-Coumaroyl)sylvestroside I c | Whole plant (EtOH) [2] | S. stellata [2] |
| 22 | Cantleyoside c | Flowers (MeOH) [32] Whole plant (MeOH) [33] | S. atropurpurea [32] S. variifolia [33] |
| 23 | Eustomoruside b | Whole plant (EtOH) [2] | S. stellata [2] |
| 24 | Eustomoside b | Whole plant (EtOH) [2] | S. stellata [2] |
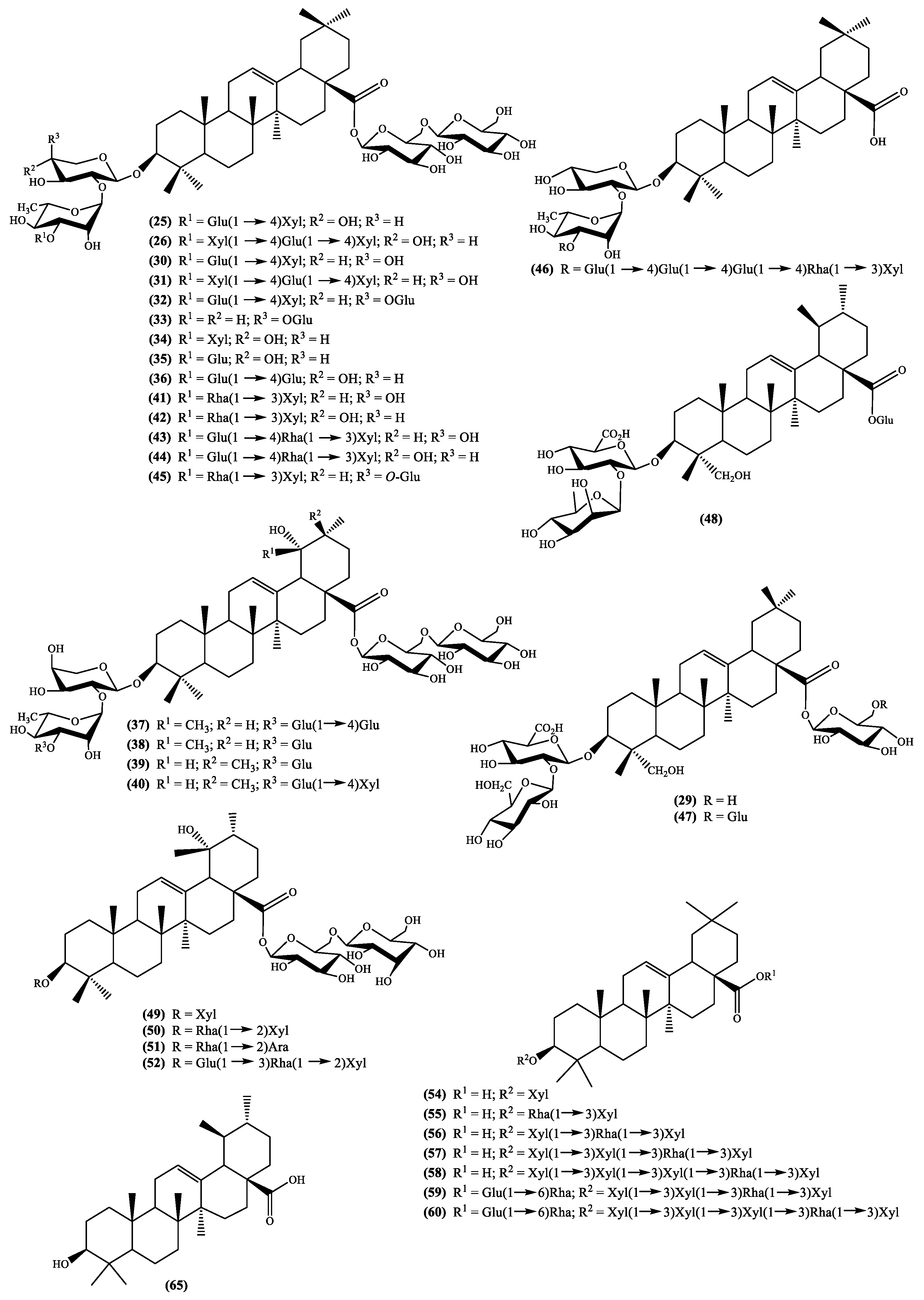
| 25,26 | Hookeroside A g and B h | Whole plant (MeOH) [34] | S. tschilliensis [34] |
| 27 | Loganic acid b | Flowering plants (CH2Cl2/MeOH) [23] Flowers (MeOH) [32] Whole plant (MeOH) [33] | S. hymettia [23] S. atropurpurea [32] S. variifolia [33] |
| 28 | Loganin b | Flowering plants (CH2Cl2/MeOH) [23] Flowers (MeOH) [32] Whole plant (MeOH) [33] | S. hymettia [23] S. atropurpurea [32] S. variifolia [33] |
| 29 | Palustroside III d | Whole plant (EtOH) [31] | S. stellata [31] |
| 30 to 40 | Scabiosaponin A g, B h, C h, D f, E f, F f, G g, H g, I f, J f and K g | Whole plant (MeOH) [34] | S. tschilliensis [34] |
| 41 to 48 | Scabiostellatosides A g, B g, C h, D h, E h, F h, G e and H d | Whole plant (EtOH) [31] | S. stellata [31] |
| 49 to 52 | Scabrioside A d, B e, C e, and D f | Roots (MeOH) [35] | S. rotata [35] |
| 53 | Septemfidoside c | Whole plant (EtOH) [2] | S. stellata [2] |
| 54 to 60 | Songoroside A b, C c, E d, G e, I f, M g and O h | Roots (EtOH) [36] | S. songarica2 [36] |
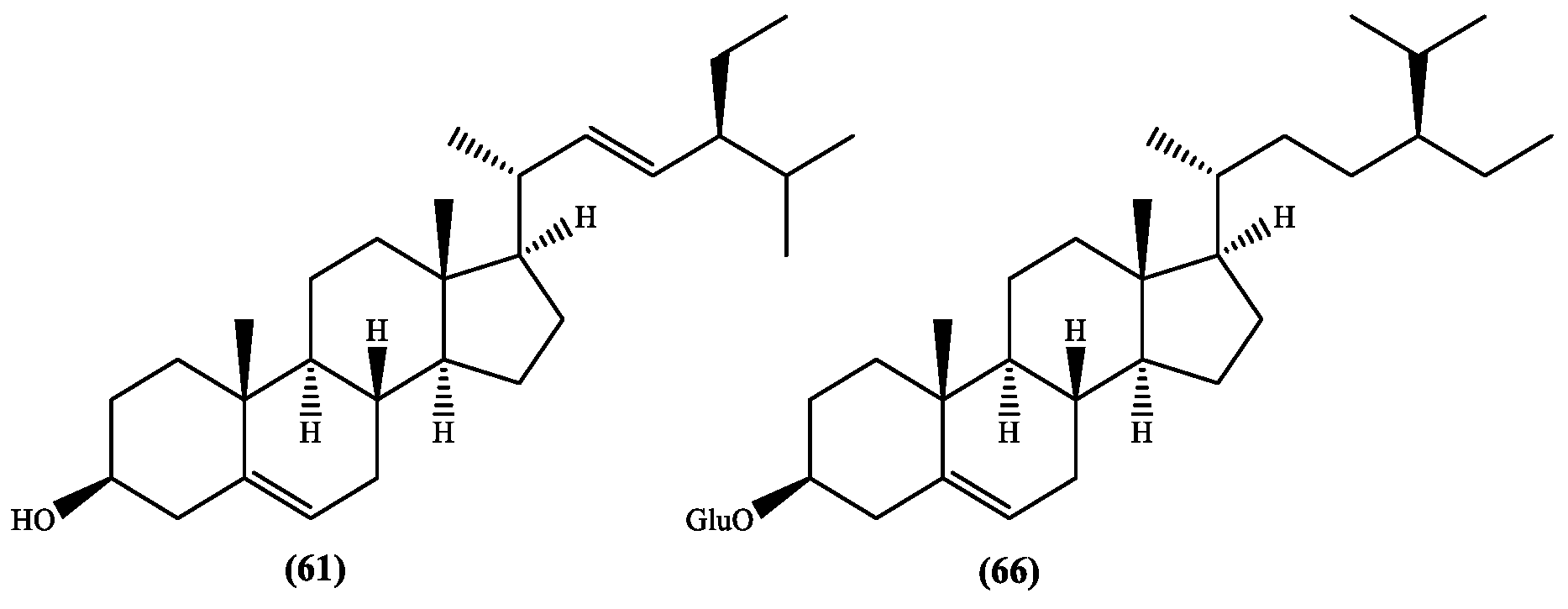
| 61 | Stigmasterol a | Whole plant (hexane) [37] | S. stellata [37] |
| 62 | Sweroside b | Whole plant (EtOH) [2] Flowers (MeOH) [32] Whole plant (MeOH) [33] | S. atropurpurea [32] S. variifolia [33] S. stellata [2] |
| 63 | Swertiamarin b | Flowering plants (CH2Cl2/MeOH) [23] Flowers (MeOH) [32] Whole plant (MeOH) [33] | S. hymettia [23] S. atropurpurea [32] S. variifolia [33] |
| 64 | Sylvestroside I c | Whole plant (EtOH) [2] | S. stellata [2] |
| 65 | Ursolic acid a | Whole plant (EtOH) [31] Whole plant (hexane) [37] | S. stellata [32,37] |
| 66 | β-Sitosterol-β-d-glucoside b | Whole plant (hexane) [37] | S. stellata [37] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, D.C.G.A.; Rahmouni, N.; Beghidja, N.; Silva, A.M.S. Scabiosa Genus: A Rich Source of Bioactive Metabolites. Medicines 2018, 5, 110. https://doi.org/10.3390/medicines5040110
Pinto DCGA, Rahmouni N, Beghidja N, Silva AMS. Scabiosa Genus: A Rich Source of Bioactive Metabolites. Medicines. 2018; 5(4):110. https://doi.org/10.3390/medicines5040110
Chicago/Turabian StylePinto, Diana C. G. A., Naima Rahmouni, Noureddine Beghidja, and Artur M. S. Silva. 2018. "Scabiosa Genus: A Rich Source of Bioactive Metabolites" Medicines 5, no. 4: 110. https://doi.org/10.3390/medicines5040110