Ammonia Removal Using Biotrickling Filters: Part B: Determination of the Nitrogen Accumulation in the Scrubbing Liquid at a Livestock Facility Using Electrical Conductivity Measurement
Abstract
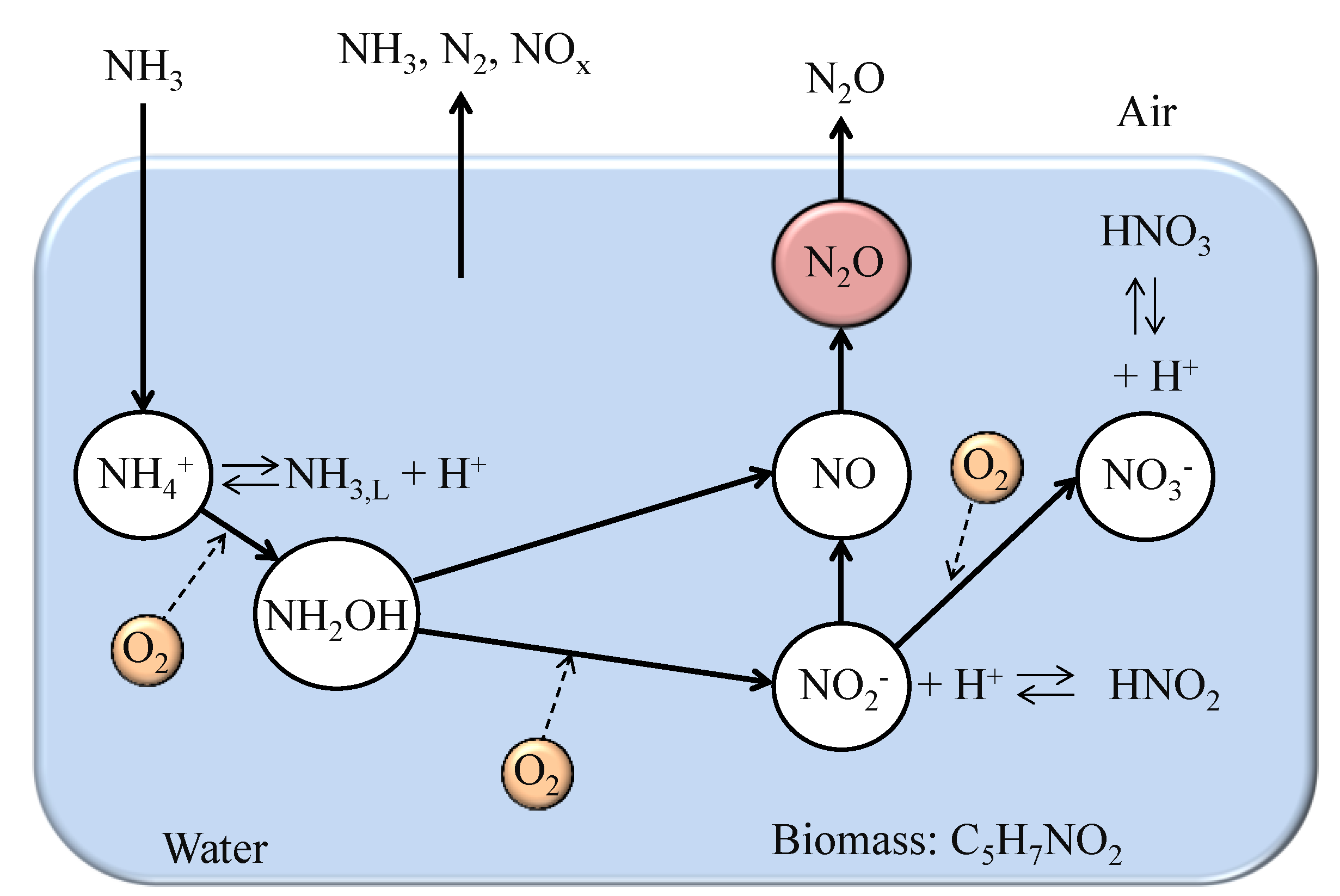
1. Introduction
2. Amount of Nitrogen Accumulated in Water
3. Description of Biotrickling Filter
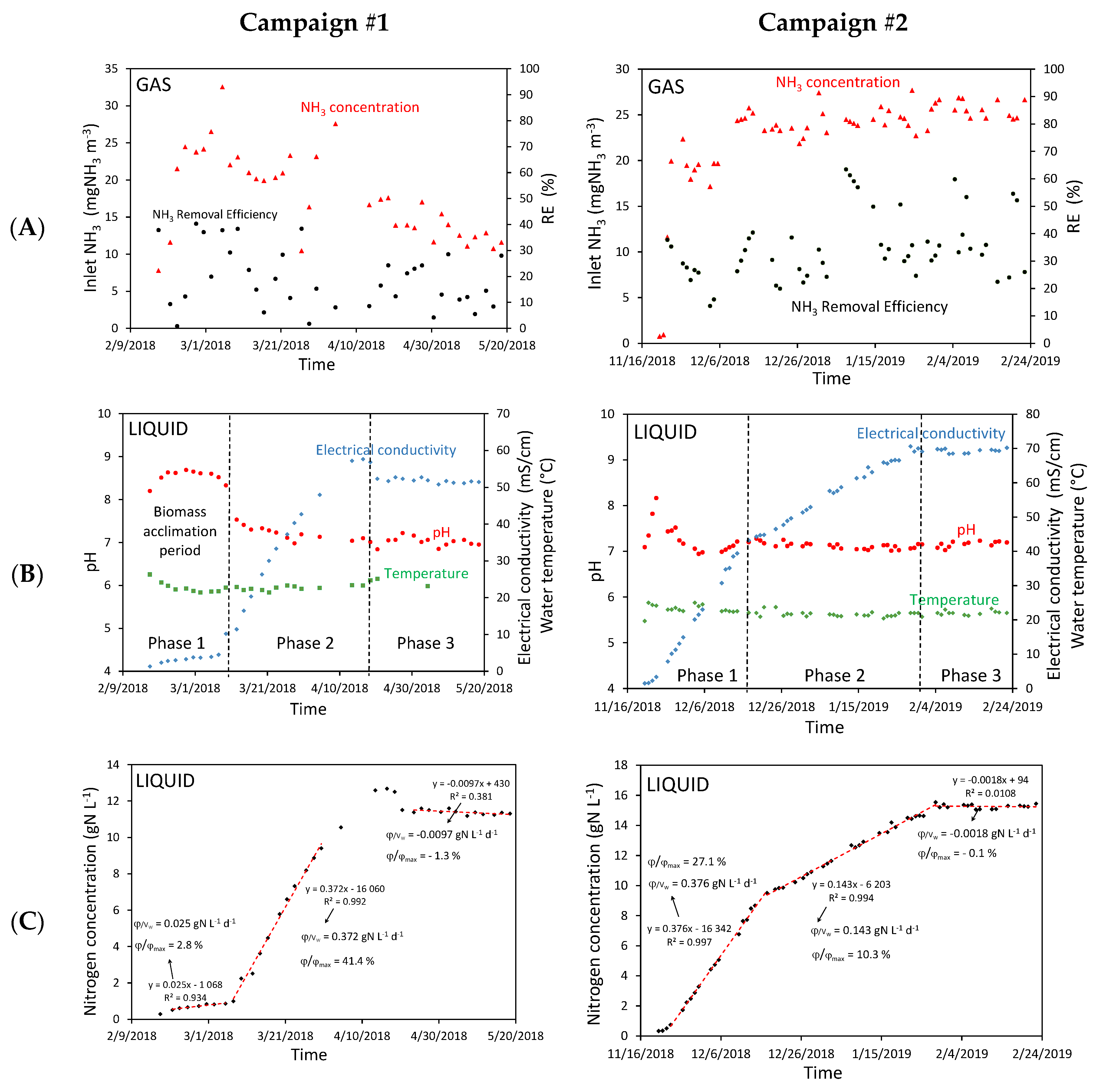
4. Results
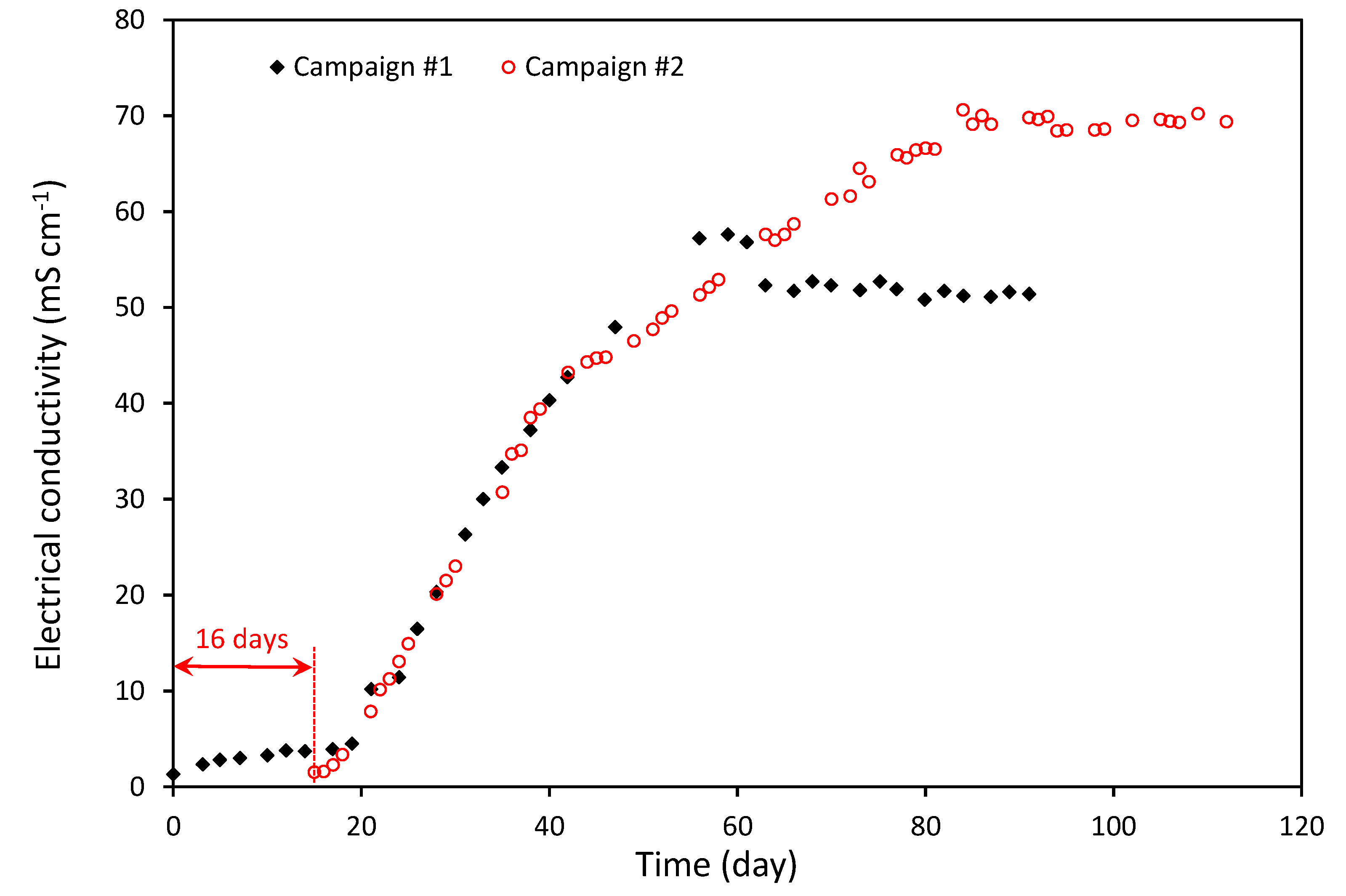
4.1. Experiment Results
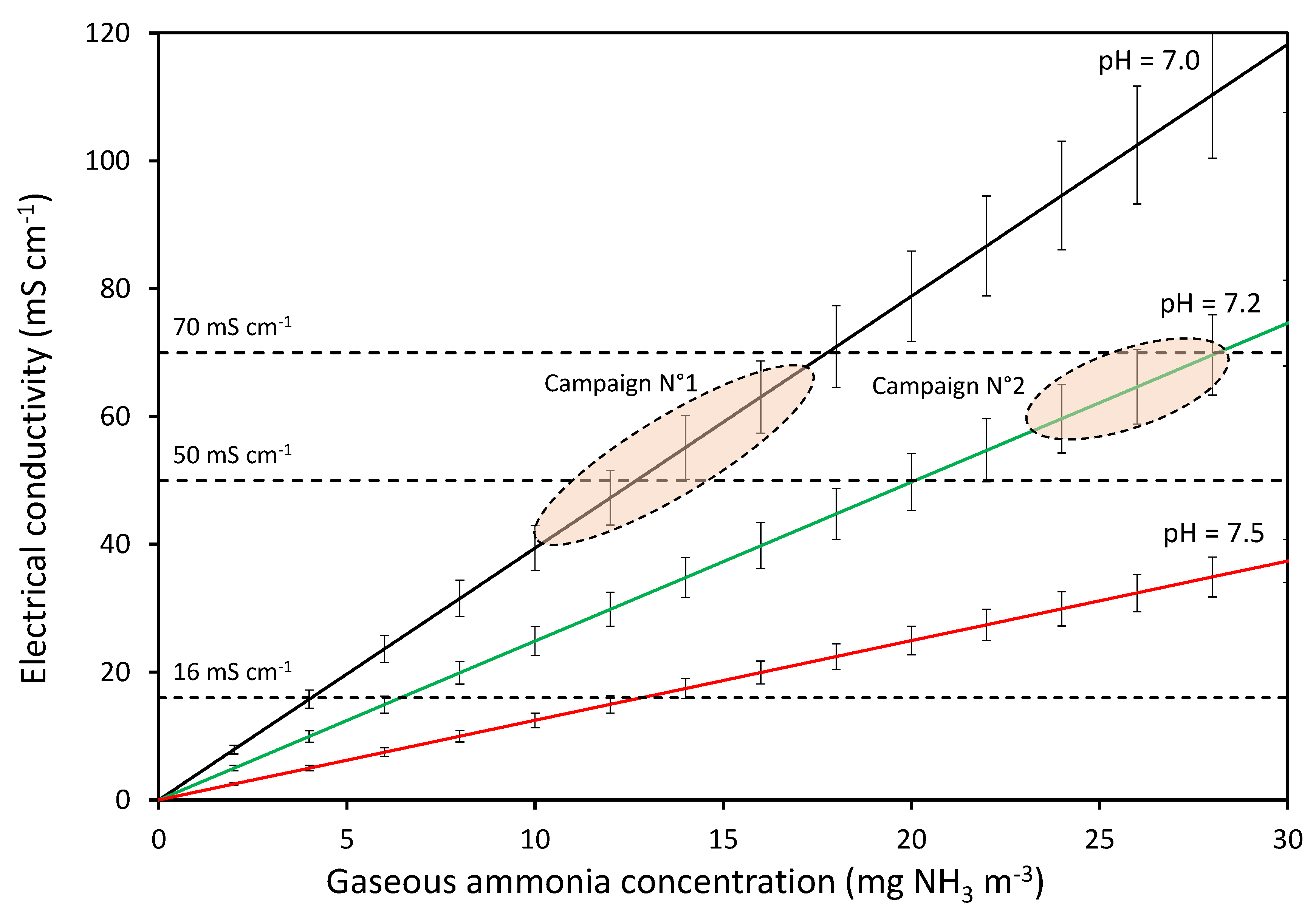
4.2. Influence of EC Value
4.3. Advantages of EC Measurement
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- REF Commission Implementing Decision (EU) 2017/302 of 15 February 2017; Establishing Best Available Techniques (BAT) Conclusions, under Directive 2010/75/EU of the European Parliament and of the Council, for the Intensive Rearing of Poultry or Pigs. 2017, Brussels, (40–42). Available online: http://eippcb.jrc.ec.europa.eu/reference/ (accessed on 9 January 2018).
- Winkel, A.; Demeyer, P.; Feilberg, A.; Jørgensen, M.; Puterflam, J.; Engel, P. Measurement of Particulate Matter: Recommendations for the VERA Test Protocol on Air Cleaning Technologies; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2014. [Google Scholar]
- Loyon, L.; Burton, C.H.; Misselbrook, T.; Webb, J.; Philippe, F.X.; Aguilar, M.; Doreau, M.; Hassouna, M.; Veldkamp, T.; Dourmad, J.Y.; et al. Best available technology for European livestock farms: Availability, effectiveness and uptake. J. Environ. Manag. 2016, 166, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Philippe, F.X.; Cabaraux, J.F.; Nicks, B. Ammonia emissions from pig houses: Influencing factors and mitigation techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- Copelli, S.; Raboni, M.; Derudi, M.; Nano, G.; Torretta, V. Comparison between absorption and biological activity on the efficiency of the biotrickling filtration of gaseous streams containing ammonia. Environ. Sci. Pollut. Res. 2017, 24, 23207–23218. [Google Scholar] [CrossRef] [PubMed]
- Raboni, M.; Torretta, V. A modified biotrickling filter for nitrification-denitrification in the treatment of an ammonia-contaminated air stream. Environ. Sci. Pollut. Res. 2016, 23, 24256–24264. [Google Scholar] [CrossRef] [PubMed]
- Kennes, C.; Veiga, M.C. Biotrickling Filters. In Air Pollution Prevention and Control: Bioreactors and Bioenergy, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 121–138. ISBN 978-1-118-52336-0. [Google Scholar]
- Van der Heyden, C.; Demeyer, P.; Volcke, E.I.P. Mitigating emissions from pig and poultry housing facilities through air scrubbers and biofilters: State-of-the-art and perspectives. Biosyst. Eng. 2015, 134, 74–93. [Google Scholar] [CrossRef]
- Desloover, J.; Vlaeminck, S.E.; Clauwaert, P.; Verstraete, W.; Boon, N. Strategies to mitigate N2O emissions from biological nitrogen removal systems. Curr. Opin. Biotechnol. 2012, 23, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Kampschreur, M.J.; Temmink, H.; Kleerebezem, R.; Jetten, M.S.M.; van Loosdrecht, M.C.M. Nitrous oxide emission during wastewater treatment. Water Res. 2009, 43, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Melse, R.W.; Ploegaert, J.P.M.; Ogink, N.W.M. Biotrickling filter for the treatment of exhaust air from a pig rearing building: Ammonia removal performance and its fluctuations. Biosyst. Eng. 2012, 113, 242–252. [Google Scholar] [CrossRef]
- Dumont, E. Impact of the treatment of NH3 emissions from pig farms on greenhouse gas emissions. Quantitative assessment from the literature data. New Biotechnol. 2018, 46, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Benkaraache, S.; Lemay, J.F.; Landry, D.; Drogui, P.; Tyagi, R.D. High-strength ammonium wastewater treatment by MBR: Steady-state nitrification kinetic parameters. J. Water Process. Eng. 2019, 32, 100945. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Hamon, L.; Andrès, Y.; Dumont, E. Aerial Pollutants in Swine Buildings: A Review of Their Characterization and Methods to Reduce Them. Environ. Sci. Technol. 2012, 46, 12287–12301. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, E.; Bezerra, T.; Lafuente, J.; Gabriel, D. Performance, limitations and microbial diversity of a biotrickling filter for the treatment of high loads of ammonia. Chem. Eng. J. 2017, 311, 91–99. [Google Scholar] [CrossRef]
- Vaddella, V.; Ndegwa, P.; Jiang, A. An Empirical Model of Ammonium Ion Dissociation in Liquid Dairy Manure. Trans. ASABE 2011, 54, 1119–1126. [Google Scholar] [CrossRef]
- Ottosen, L.D.M.; Juhler, S.; Guldberg, L.B.; Feilberg, A.; Revsbech, N.P.; Nielsen, L.P. Regulation of ammonia oxidation in biotrickling airfilters with high ammonium load. Chem. Eng. J. 2011, 167, 198–205. [Google Scholar] [CrossRef]
- Vannecke, T.P.W.; Bernet, N.; Winkler, M.K.H.; Santa-Catalina, G.; Steyer, J.P.; Volcke, E.I.P. Influence of Process Dynamics on the Microbial Diversity in a Nitrifying Biofilm Reactor: Correlation Analysis and Simulation Study. Biotechnol. Bioeng. 2016, 113, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of Nitrification by Ammonia and Nitrous Acid. J. Water Pollut. Control. Fed. 1976, 48, 835–852. [Google Scholar] [PubMed]
- Clegg, S.L.; Brimblecombe, P. Solubility of ammonia in pure aqueous and multicomponent solutions. J. Phys. Chem. 1989, 93, 7237–7248. [Google Scholar] [CrossRef]

| Substance | H = CGas/CLiquid (Dimensionless) |
|---|---|
| Ammonia (NH3) | 6.84 × 10−4 |
| Nitrogen (N2) | 63.1 |
| Nitrous oxide (N2O) | 1.68 |
| Nitric oxide (NO) | 21.2 |
| Nitrogen dioxide (NO2) | 2.88 |
| Nitrogen trioxide (NO3) | 1.06 |
| Nitrous acid (HNO2) | 8.41 × 10−4 |
| Nitric acid (HNO3) | 1.92 × 10−7 |
| Campaign #1 | Campaign #2 | ||
|---|---|---|---|
| Phase 1 | φ/VW (gN L−1 d−1) | 0.0248 | 0.376 |
| φ/(0.22 VW) (mS cm−1 d−1) | 0.113 | 1.71 | |
| φ (gN d−1) | 13.6 | 206.2 | |
| (%) | 2.8 | 27.1 | |
| Nitrogen accumulated in water (gN h−1 m−3packing material) | 1.6 | 23.6 | |
| Phase 2 | φ/VW (gN L−1 d−1) | 0.372 | 0.143 |
| φ/(0.22 VW) (mS cm−1 d−1) | 1.69 | 0.65 | |
| φ (gN d−1) | 203.9 | 78.4 | |
| (%) | 41.4 | 10.3 | |
| Nitrogen accumulated in water (gN h−1 m−3packing material) | 23.3 | 9.0 | |
| Phase 3 | φ/VW (gN L−1 d−1) | −0.0097 | −0.0018 |
| φ/(0.22 VW) (mS cm−1 d−1) | −0.044 | −0.0069 | |
| φ (gN d−1) | −5.3 | −0.8 | |
| (%) | −1.3 | −0.1 | |
| Nitrogen accumulated in water (gN h−1 m−3packing material) | −0.6 | −0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumont, É.; Lagadec, S.; Guingand, N.; Loyon, L.; Amrane, A.; Couvert, A. Ammonia Removal Using Biotrickling Filters: Part B: Determination of the Nitrogen Accumulation in the Scrubbing Liquid at a Livestock Facility Using Electrical Conductivity Measurement. ChemEngineering 2020, 4, 50. https://doi.org/10.3390/chemengineering4030050
Dumont É, Lagadec S, Guingand N, Loyon L, Amrane A, Couvert A. Ammonia Removal Using Biotrickling Filters: Part B: Determination of the Nitrogen Accumulation in the Scrubbing Liquid at a Livestock Facility Using Electrical Conductivity Measurement. ChemEngineering. 2020; 4(3):50. https://doi.org/10.3390/chemengineering4030050
Chicago/Turabian StyleDumont, Éric, Solène Lagadec, Nadine Guingand, Laurence Loyon, Abdeltif Amrane, and Annabelle Couvert. 2020. "Ammonia Removal Using Biotrickling Filters: Part B: Determination of the Nitrogen Accumulation in the Scrubbing Liquid at a Livestock Facility Using Electrical Conductivity Measurement" ChemEngineering 4, no. 3: 50. https://doi.org/10.3390/chemengineering4030050
APA StyleDumont, É., Lagadec, S., Guingand, N., Loyon, L., Amrane, A., & Couvert, A. (2020). Ammonia Removal Using Biotrickling Filters: Part B: Determination of the Nitrogen Accumulation in the Scrubbing Liquid at a Livestock Facility Using Electrical Conductivity Measurement. ChemEngineering, 4(3), 50. https://doi.org/10.3390/chemengineering4030050






