Review of NMR Studies for Oilwell Cements and Their Importance
Abstract
1. Introduction
2. Materials and Methods
3. Results
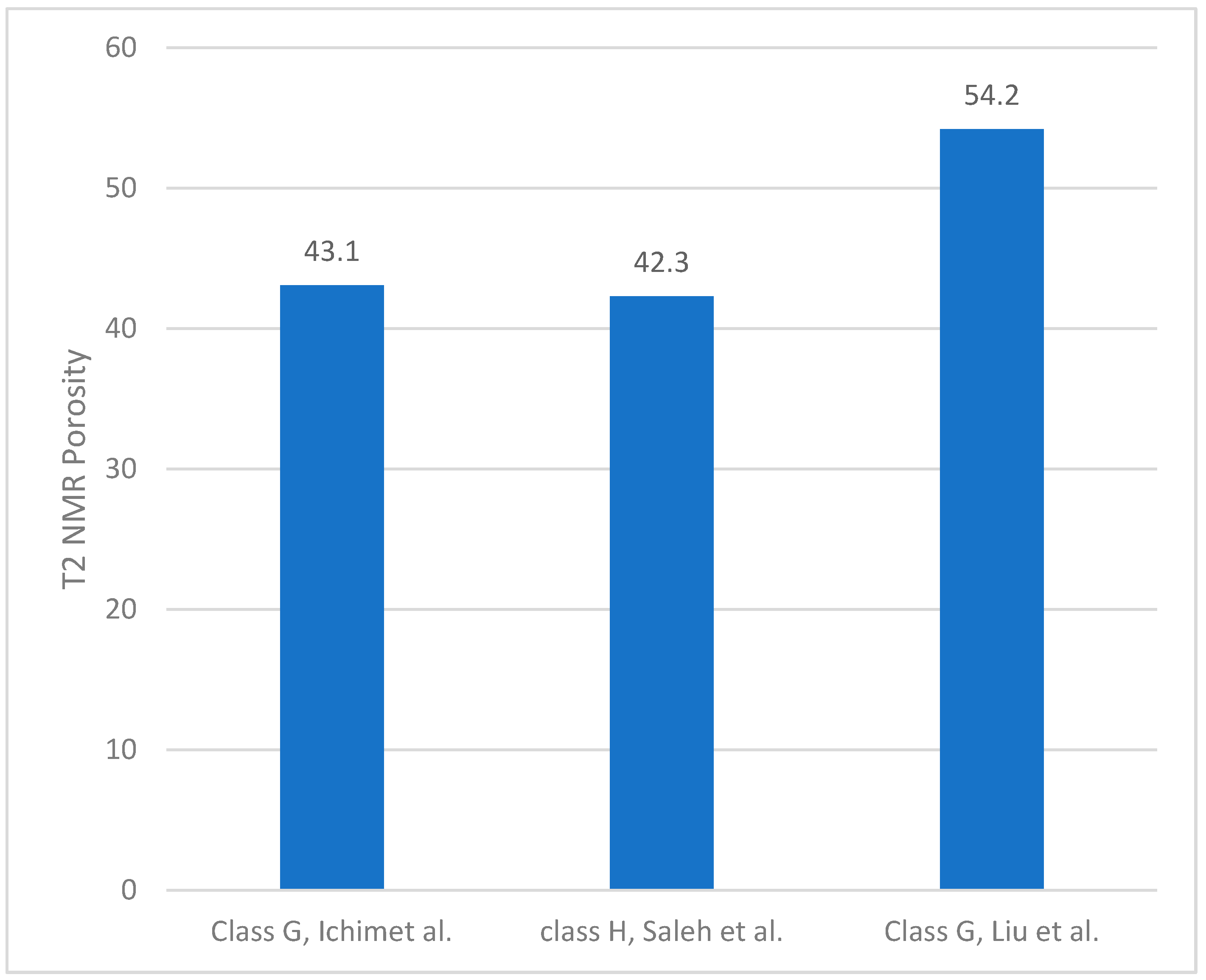
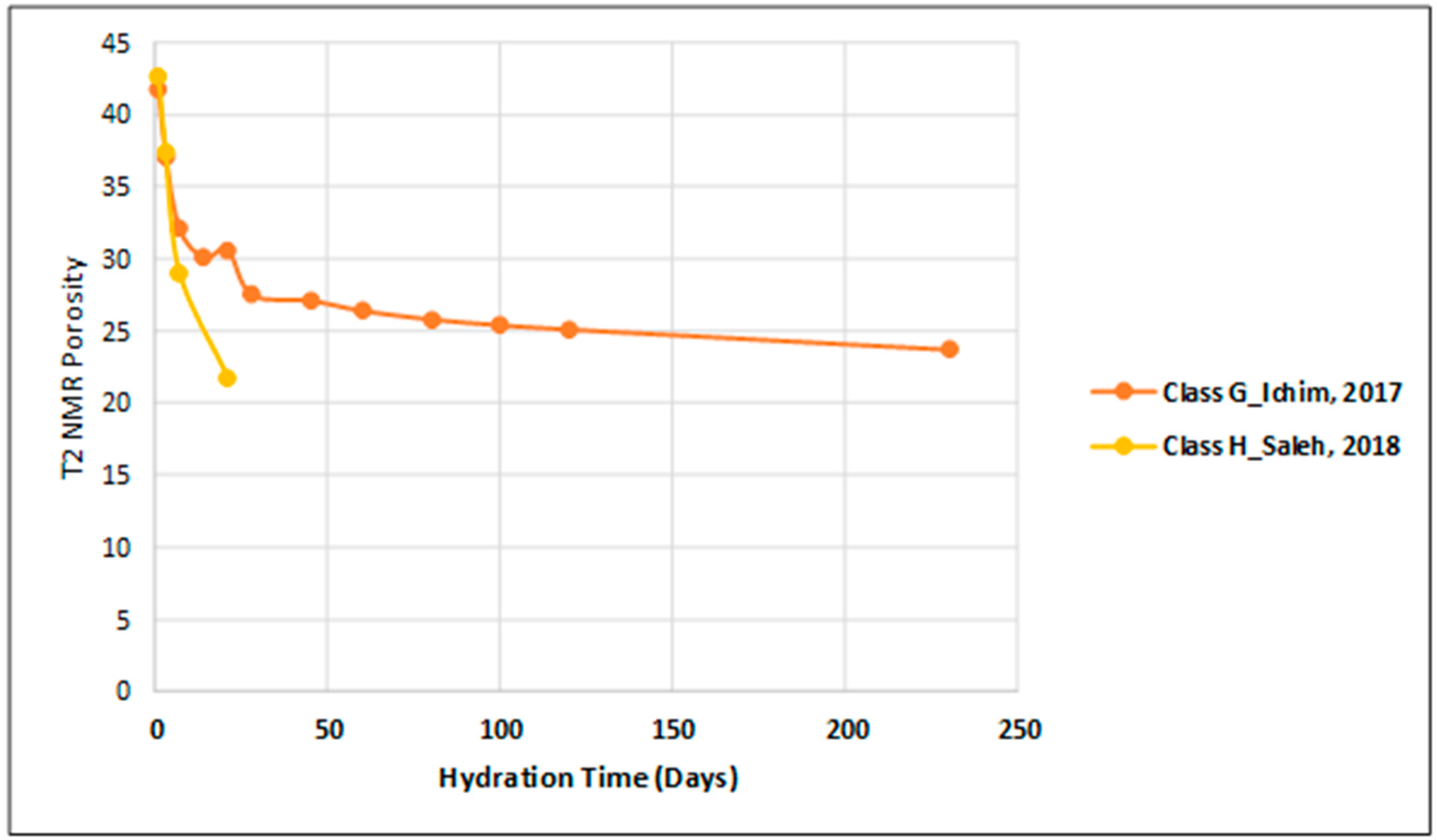
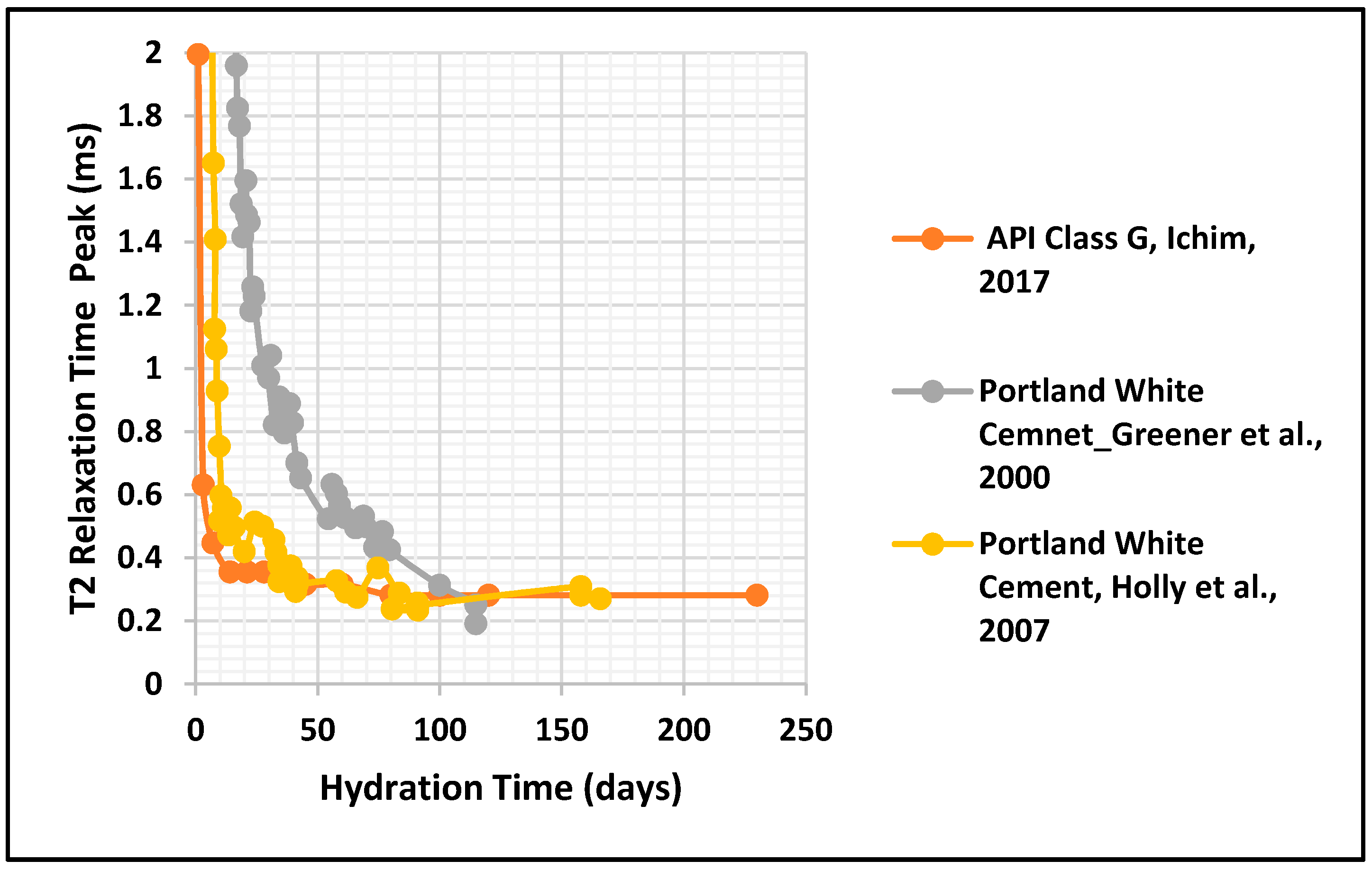
3.1. Porosity and T2 Relaxation Time Comparison
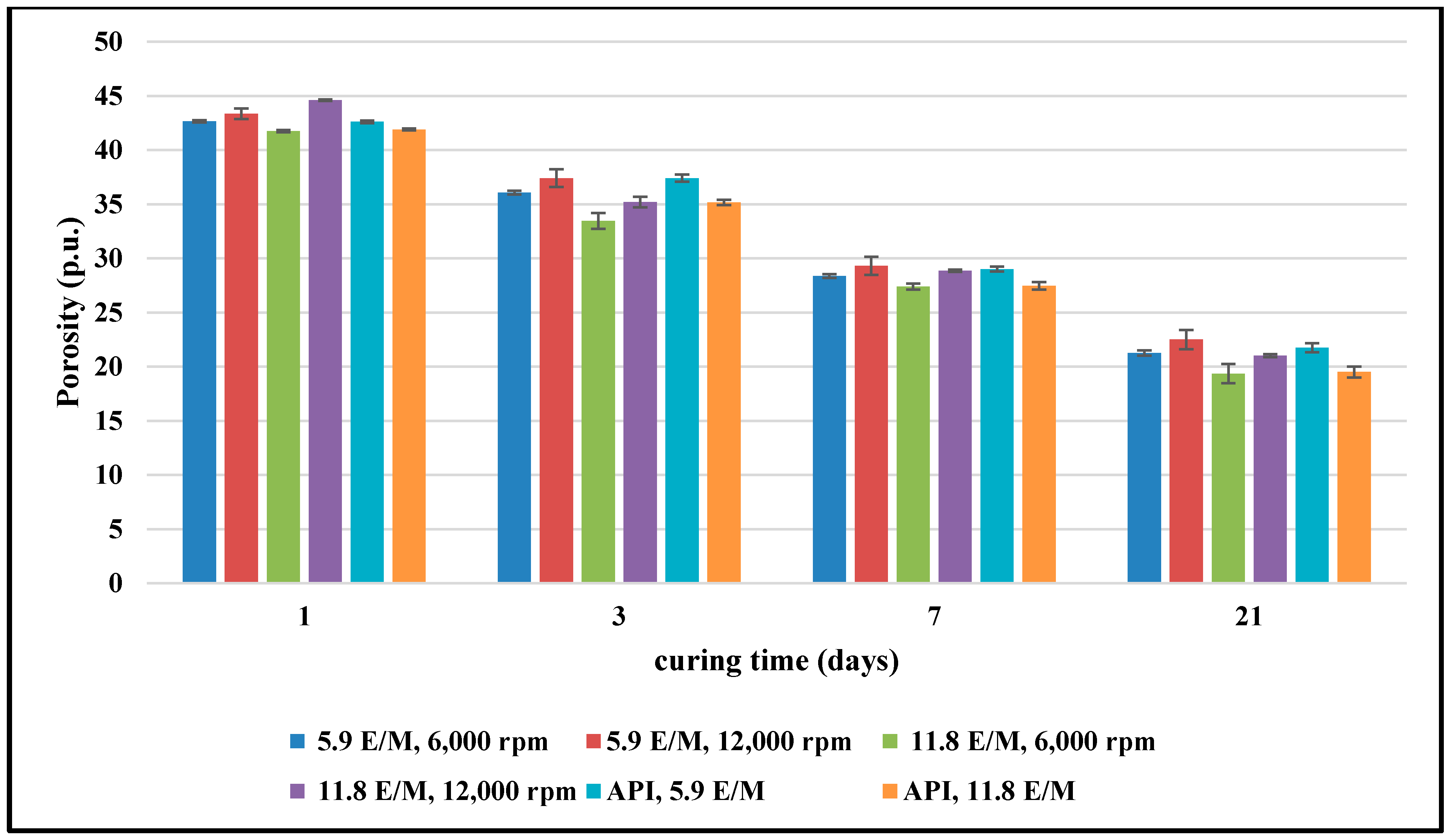
3.2. The Effect of the Cement Mixing Process on the NMR Porosity Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CSHCSH | calcium silicate hydrate |
| NMR | nuclear magnetic resonance |
| LF NMR | Low-frequency nuclear magnetic resonance |
| T1 | longitudinal relaxation time |
| T2 | transversal relaxation time |
| PSD | pore size distribution |
| UCS | unconfined compressive strength |
| CT | computer tomograph |
| SEM | scanning electron microscope |
| API | American Petroleum Institute |
References
- Kleinberg, R.L.; Farooqui, S.A.; Horsfield, M.A. Tl/T2 Ratio and Frequency Dependence of NMR Relaxation in Porous Sedimentary Rocks. J. Colloid Interface Sci. 1993, 158, 195–198. [Google Scholar] [CrossRef]
- Dunn, K.J.; Bergman, D.J.; LaTorraca, G.A. (Eds.) Nuclear Magnetic Resonance: Petrophysical and Logging Applications; Elsevier: Amsterdam, The Netherlands, 2002; Volume 32. [Google Scholar]
- Coates, G.R.; Xiao, L.; Prammer, M.G. NMR Logging Principles and Applications; Elsevier Science: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Bloembergen, N.; Purcell, E.M.; Pound, R.V.; Levenson, M.D.; Mazur, E.; Pershan, P.; Shen, Y.R. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Resonances 1990, 73, 411–444. [Google Scholar] [CrossRef]
- Kenyon, W.E. Nuclear magnetic resonance as a petrophysical measurement. Nucl. Geophys. 1992, 6, 153–171. [Google Scholar]
- Kleinberg, R.; Kenyon, W.; Mitra, P. Mechanism of NMR Relaxation of Fluids in Rock. J. Magn. Reson. Ser. A 1994, 108, 206–214. [Google Scholar] [CrossRef]
- Tinni, A.; Odusina, E.O.; Sulucarnain, I.D.; Sondergeld, C.H.; Rai, C.S. Nuclear-Magnetic-Resonance Response of Brine, Oil, and Methane in Organic-Rich Shales. SPE Reserv. Eval. Eng. 2015, 18, 400–406. [Google Scholar] [CrossRef]
- Cowan, B.P. Nuclear Magnetic Resonance and Relaxation; Cambridge University Press (CUP): Cambridge, UK, 1997. [Google Scholar]
- Sondergeld, C.; Tinni, A.; Rai, C.; Besov, A. NMR Considerations in Shale Evaluation; Society of Petrophysicists and Well-Log Analysts: Reykjawik, Iceland, 2016. [Google Scholar]
- Timur, A. Pulsed Nuclear Magnetic Resonance Studies of Porosity, Movable Fluid, and Permeability of Sandstones. J. Pet. Technol. 1969, 21, 775–786. [Google Scholar] [CrossRef]
- Kenyon, W.; Howard, J.; Sezginer, A.; Straley, C.; Matteson, A.; Horkowitz, K.; Ehrlich, R. Pore-Size Distribution and NMR in Microporous Cherty Sandstones Paper LL Trans. In Proceedings of the SPWLA, 30th Annual Logging Symposium, Denver, CO, USA, 11–14 June 1989. [Google Scholar]
- Sulucarnain, I.D.; Sondergeld, C.H.; Rai, C.S. An NMR Study of Shale Wettability and Effective Surface Relaxivity. In Proceedings of the All Days; Society of Petroleum Engineers (SPE), Calgary, AB, Canada, 20 October 2012. [Google Scholar]
- Le Saoût, G.; Lécolier, E.; Rivereau, A.; Zanni, H. Study of oilwell cements by solid-state NMR. Comptes Rendus Chim. 2004, 7, 383–388. [Google Scholar] [CrossRef]
- Griffin, A. Significance of Nanosilica Incorporation in Type G Oil Well Cement Pastes. 2013. Available online: https://digitalrepository.unm.edu/ce_etds/84 (accessed on 31 March 2021).
- Li, Q.; Hurt, A.P.; Coleman, N.J. The Application of 29Si NMR Spectroscopy to the Analysis of Calcium Silicate-Based Cement using Biodentine™ as an Example. J. Funct. Biomater. 2019, 10, 25. [Google Scholar] [CrossRef]
- Gajewicz, A.; Gartner, E.; Kang, K.; McDonald, P.; Yermakou, V. A 1H NMR relaxometry investigation of gel-pore drying shrinkage in cement pastes. Cem. Concr. Res. 2016, 86, 12–19. [Google Scholar] [CrossRef]
- Fourmentin, M.; Faure, P.; Rodts, S.; Peter, U.; Lesueur, D.; Daviller, D.; Coussot, P. NMR Observation of Water Transfer between a Cement Paste and a Porous Medium, Cement and Concrete Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 95. [Google Scholar]
- Korb, J.-P. NMR and nuclear spin relaxation of cement and concrete materials. Curr. Opin. Colloid Interface Sci. 2009, 14, 192–202. [Google Scholar] [CrossRef]
- Blinc, R.; Burgar, M.; Lahajnar, G.; Rožmarin, M.; Rutar, V.; Kocuvan, I.; Ursic, J. NMR Relaxation Study of Adsorbed Water in Cement and C3S Pastes. J. Am. Ceram. Soc. 1978, 61, 35–37. [Google Scholar] [CrossRef]
- Schreiner, L.J.; MacTavish, J.C.; Miljković, L.; Pintar, M.; Blinc, R.; Lahajnar, G.; Lasic, D.; Reeves, L.W. NMR Line Shape-Spin-Lattice Relaxation Correlation Study of Portland Cement Hydration. J. Am. Ceram. Soc. 1985, 68, 10–16. [Google Scholar] [CrossRef]
- Greener, J.; Peemoeller, H.; Choi, C.; Holly, R.; Reardon, E.J.; Hansson, C.M.; Pintak, M.M. Monitoring of Hydra-tion of White Cement Paste with Proton NMR. J. Am. Ceram. Soc. 2000, 83, 623–627. [Google Scholar] [CrossRef]
- Valori, A.; McDonald, P.; Scrivener, K. The morphology of C–S–H: Lessons from 1H nuclear magnetic resonance relaxometry. Cem. Concr. Res. 2013, 49, 65–81. [Google Scholar] [CrossRef]
- Muller, A.C.A.; Scrivener, K.L.; Gajewicz, A.M.; McDonald, P.J. Densification of C–S–H Measured by 1H NMR Relaxometry. J. Phys. Chem. C 2013, 117, 403–412. [Google Scholar] [CrossRef]
- Ichim, A.C. Experimental Determination of Oilfield Cement Properties and Their Influence on Well Integrity. Master’s Thesis, University of Oklahoma, Norman, Oklahoma, 2017. [Google Scholar]
- Saleh, F.K. Investigation of Oilwell Cement Mixing: Experimental Studies and Implications or Mixing Energy Theory. Ph.D. Dissertation, University of Oklahoma, Norman, Oklahoma, 2018. [Google Scholar]
- Liu, K.; Cheng, X.; Zhang, C.; Gao, X.; Zhuang, J.; Guo, X. Evolution of pore structure of oil well cement slurry in suspension–solid transition stage. Constr. Build. Mater. 2019, 214, 382–398. [Google Scholar] [CrossRef]
- Holly, R.; Reardon, E.J.; Hansson, C.M.; Peemoeller, H. Proton spin–spin relaxation study of the effect of tempera-ture on white cement hydration. J. Am. Ceram. Soc. 2007, 90, 570–577. [Google Scholar] [CrossRef]
- Anand, V.; Ali, M.R.; Al-Adani, N.; Willis, D.; Freedman, R.; Hamichi, F.; Abubakar, A.; Grover, R.; Neto, O.; Aboud, M.; et al. New Generation NMR Tool for Robust, Continuous T1 and T2 Measurements. In Proceedings of the SPWLA 56th Annual Logging Symposium, Society of Petrophysicists and Well-Log Analysts, Long Beach, CA, USA, 18–22 August 2015. [Google Scholar]
- Bourgoyne, A.T., Jr.; Millheim, K.K.; Chenevert, M.E.; Young, F.S., Jr. Applied Drilling Engineering; SPE: Houston, TX, USA, 1991. [Google Scholar]
- Straley, C.; Rossini, D.; Vinegar, H.; Morris, C. Core Analysis by Low Field NMR, SCA-9404. Log Anal. 1994, 38, SPWLA-1997-v38n2a3. [Google Scholar]
- Walkley, B.; Provis, J. Solid-state nuclear magnetic resonance spectroscopy of cements. Mater. Today Adv. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Ai, K.; Zhu, L. An NMR-Based Experimental Study on the Pore Structure of the Hydration Process of Mine Filling Slurry. Adv. Civ. Eng. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Maharidge, R.; Bottiglieri, A.; Dighe, S.S.; Holley, A.; Zhang, H.; Koch, A. Development of Permeability and Mechanical Properties of Class G Cement from Slurry to Set. SPE Annu. Tech. Conf. Exhib. 2016. [Google Scholar] [CrossRef]
| Authors | Temp/Press | Measured Porosity/Curing Time | Cement Type | Other Measurements | NMR Machine/Type | Observations |
|---|---|---|---|---|---|---|
| Blinc et al., 1978 [19] | room temperature | Porosity not reported. T1 and T2 versus hydration time | Portland cement (PS 550) | T1 and T2 values decrease (inverse proportionally) with hydration as a result of the larger active surface and the number of adsorptive sites, demonstrating that NMR can be an effective tool to study the hydration process | 1H nuclear magnetic resonance. Frequency of 60 MHz | T1 and T2 relaxation times were measured on cement samples mixed using distilled and D2O water. The hydration process was observed in a time interval of 10 min to 28 days. |
| Schreiner et al., 1985 [20] | room temperature | Porosity not reported. T1 and T2 versus hydration time | Portland cement, Oxide concentration was given. W/C ratio of 0.42 | The proton relaxation times, T1, and T2, were measured using a pulse NMR spectrometer | Not specified | On the basis of the time-evolution data of TI and T2 and of the proton magnetization fractions, the hydration process was divided into four dissimilar stages, associated with specific NMR attributes, which allowed a better description of the liquid and solid phases at the specific hydration time stamp |
| Greener et al. (2000) [21] | room temperature (21 °C) | No porosity reported | white cement (Portland cement with very low concentration of Fe2O3) | spin–spin relaxation on white cement as a function of time | 26 MHz system, Proton NMR | Good correlation between the NMR results and measurable chemical and stoichiometric variations inside the cement structure during the hydration process. These findings allowed a better description of the CSH gel structure |
| Valori et al., 2013 [22] | cement pastes with w/c ratios from 0.26 to 0.5 at T = 23.5 °C | Studies of morphology of CSHCSH | white cement pastes | T1 evolution as a function of hydration time. Mass and volume composition of cement pastes. The T1–T2 correlation spectrum | 1H nuclear magnetic resonance freq.not published | The CSH density and composition of wet material were measured. The above process ignores the gel pore water. The CSH desnity was also inferred using the degree of hydration and ettringite fraction as measured by other conventional methods. |
| Muller et al., 2013 [23] | temperature-controlled room at 20 °C | No porosity reported. CSH morphology using NMR. Gel and inter-hydrate pores | Aalborg Portland cement was used for these experiments. The water-to-cement ratios were varied from w/c = 0.32, 0.40, to 0.48. | CSH Morphology study. T2 relaxation time versus 10 days of hydration reported. | A Bruker Minispec NMR spectrometer operating at 7.5 MHz was used for these experiments. | The NMR experiments allowed a detailed description of the CSH morphology in particular to visualize the hydration process as a function of the cement–water ratio. |
| Ichim, 2017 [24] | 25 °C, 50 °C and 75 °C, atmospheric pressure | T2 NMR porosity measurement up to 230 days (five different recipes) | Five different cement slurry mixes: Class G-Neat, 4% Bentonite cement, 10% Bentonite cement, 4% Salt cement, 12% Salt cement | T2 Relaxation Time Peak, distribution of the saturation profile, T1–T2 NMR Measurements and their evolution with time and temperature (helped to understand hydration process) | 2 MHz Oxford Geo Spec 2TM instrument | At elevated curing temperatures, the initial porosity decreased by up to 25%. Variations in NMR porosity values with time were observed at higher curing temperatures of 50 °C and 75 °C. Correlations with UCS development, T1–T2 spectra, and DHK-Sprite measurements helped to understand hydration |
| Fourmentin et al. 2017 [17] | Non-oilwell cement Atmospheric conditions | Non-oilwell cement w/c ranging from 0.3 to 0.63 | Relaxation time T1 and their distribution | 20 MHz Bruker Minispec MQ20 ND series | Interaction between cement paste and porous medium which has a strong impact on water exchange during hydration phase | |
| Saleh, 2018 [25] | Atmospheric | 1–21 days T2 NMR porosity measurements for six types of different mixing methods | Class H-Neat | T2 spectra versus curing time (1–21 days). Porosity–UCS correlation | 2 MHz Oxford Geo Spec 2TM instrument | The NMR cement porosity data were proportional to the UCS results. Cement slurries with longer mixing times led to higher UCS and reduced porosity. |
| Liu et al., 2019 [26] | Not reported | porosity data versus hydration time reported (720 h) | Class G cement was used. Main additive was CaCl2 with a concentration that ranged from 0 to 1. | T2 was reported | LF-NMR T2 spectra, frequency not reported | The experimental LF-NMR T2 spectra, nitrogen adsorption data, and SEM images were used, showing that that the porosity of the fresh cement was approximately 58%. |
| Gajewicz et al. 2019 [16] | Atmospheric Samples where cycled between dry and wet state | T2 reported for crystalline phases, gell pores, capillary pores, hydrate interlayers and Inter-hydrates pores | Non-oilfield cement W/C of 0.4 | T2 and gel pores | NMR T2, 20 and 23 MHz | NMR was able to prove that a redistribution of porosity between fine and coarse pores exists when samples are cycled between wet and dry states. |
| Oxide. | Percentage (White Cement) | Percentage (API Class G) |
|---|---|---|
| CaO | 69.34 | 64.45 |
| SiO2 | 25.01 | 14.07 |
| SO3 | 2.1 | 2.98 |
| Al2O3 | 1.91 | 4.11 |
| MGO | 0.56 | 1.55 |
| FE2O3 | 0.32 | 5.53 |
| NA2O | 0.17 | NA |
| K2O | 0.12 | 0.05 |
| TIO3 | 0.09 | NA |
| CL | 0.008 | NA |
| Others | NA | 5.31 |
| Loss on ignition | 0.41 | 1.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, F.K.; Teodoriu, C.; Sondergeld, C. Review of NMR Studies for Oilwell Cements and Their Importance. ChemEngineering 2021, 5, 18. https://doi.org/10.3390/chemengineering5020018
Saleh FK, Teodoriu C, Sondergeld C. Review of NMR Studies for Oilwell Cements and Their Importance. ChemEngineering. 2021; 5(2):18. https://doi.org/10.3390/chemengineering5020018
Chicago/Turabian StyleSaleh, Fatemeh K., Catalin Teodoriu, and Carl Sondergeld. 2021. "Review of NMR Studies for Oilwell Cements and Their Importance" ChemEngineering 5, no. 2: 18. https://doi.org/10.3390/chemengineering5020018
APA StyleSaleh, F. K., Teodoriu, C., & Sondergeld, C. (2021). Review of NMR Studies for Oilwell Cements and Their Importance. ChemEngineering, 5(2), 18. https://doi.org/10.3390/chemengineering5020018







