Synthesis of Non-Cubic Nitride Phases of Va-Group Metals (V, Nb, and Ta) from Metal Powders in Stream of NH3 Gas under Concentrated Solar Radiation
Abstract
1. Introduction
- ε-MN: hexagonal MN;
- δ-MN: fcc MN;
- γ-M4N3: tetragonal MN with deficient N at around N/M = 0.75;
- β-M2N: hexagonal sub-nitride phase;
- α-M(N): primary solid solution of N in metallic M.
2. Experimental
3. Results and Discussion
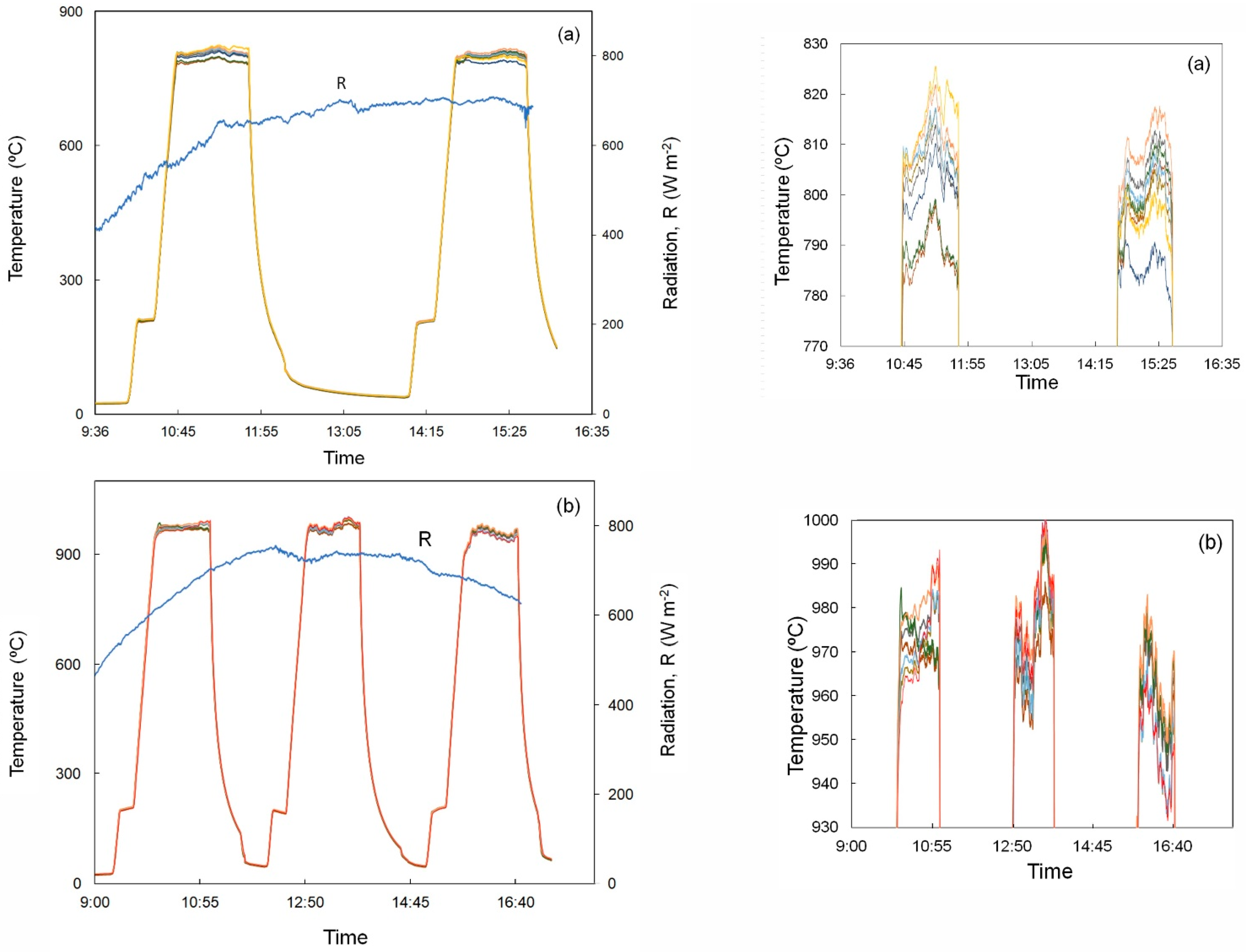
3.1. Nitriding under Flowing NH3
3.2. X-ray Diffraction Analysis
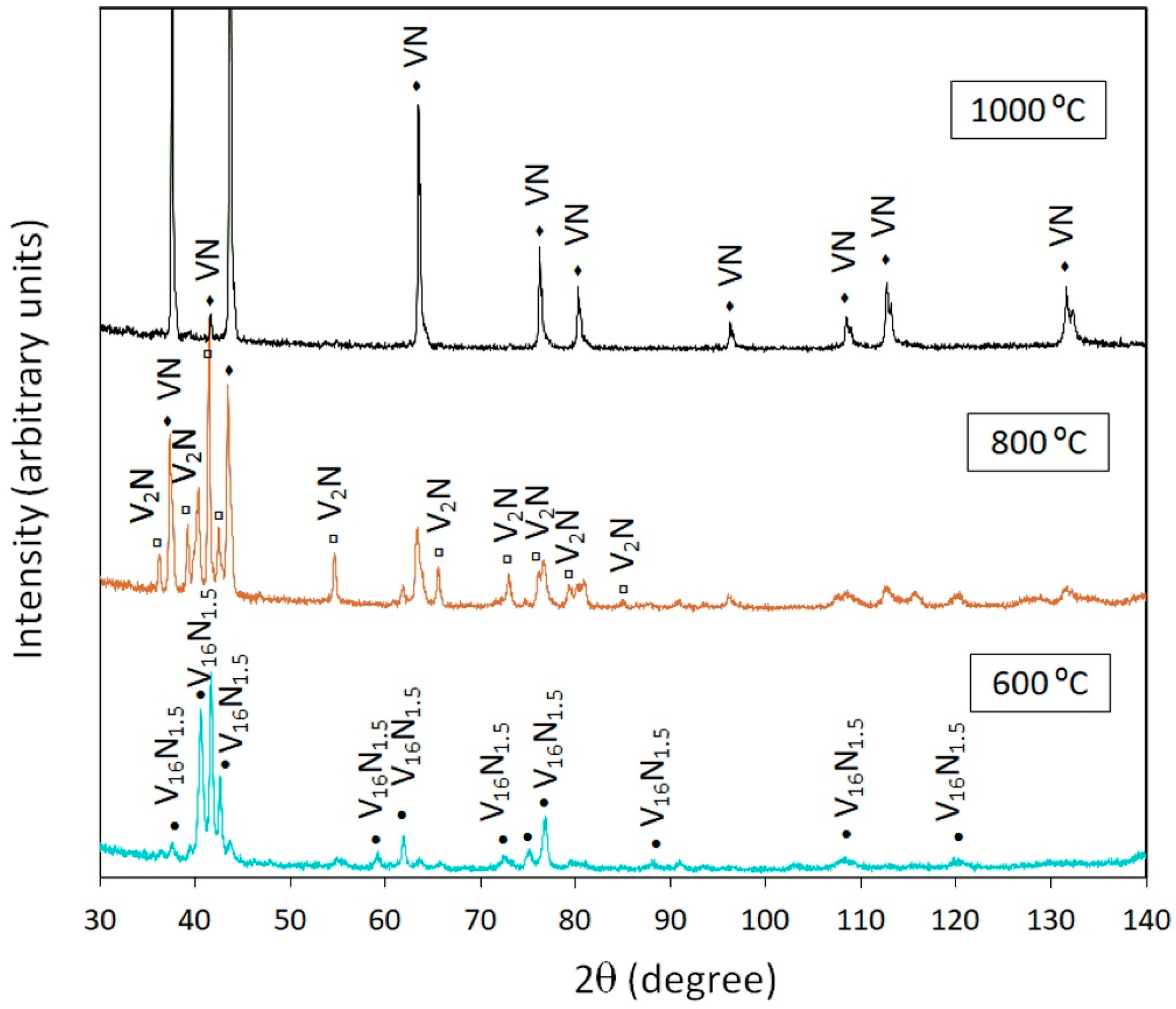
3.2.1. Vanadium
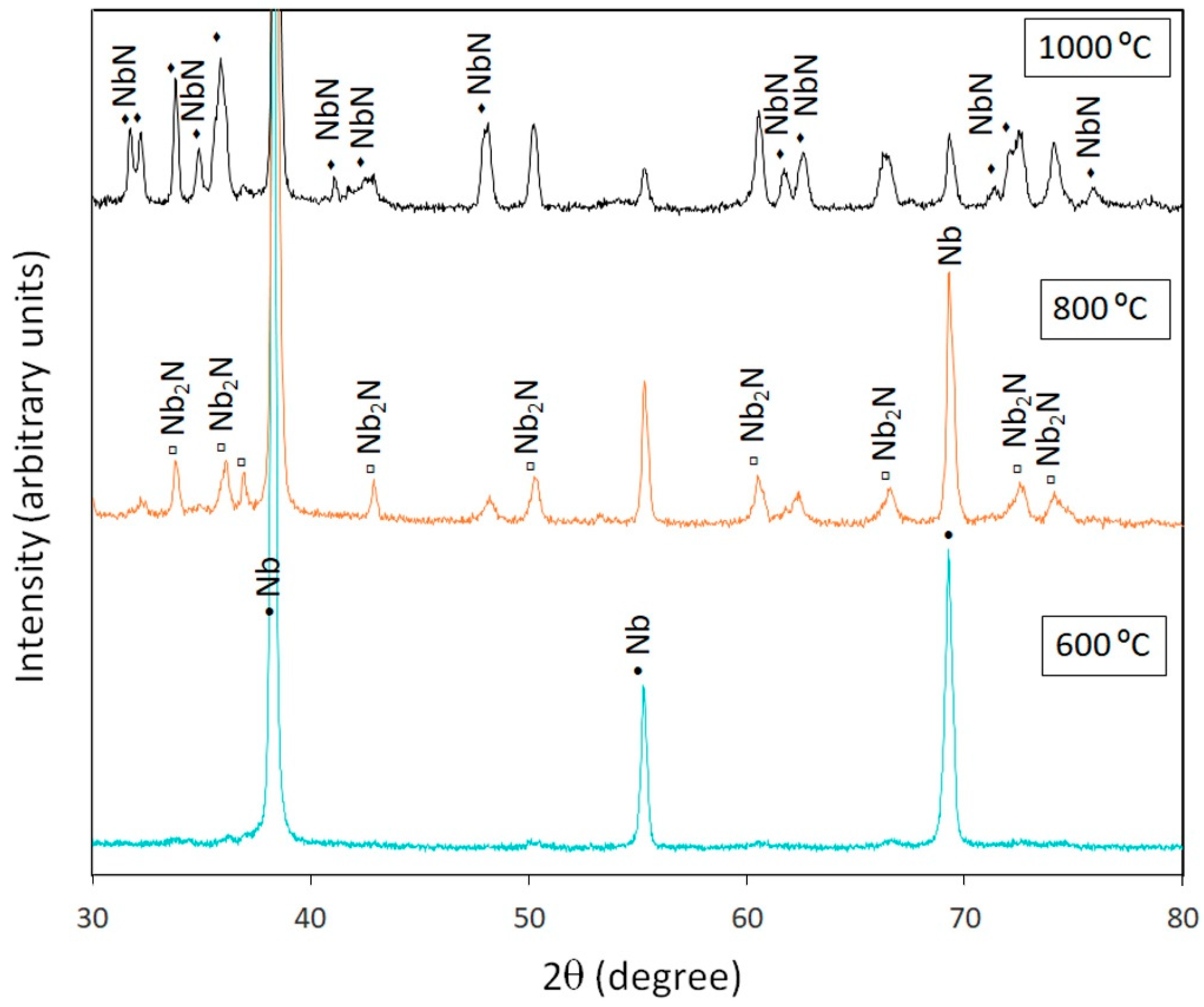
3.2.2. Niobium
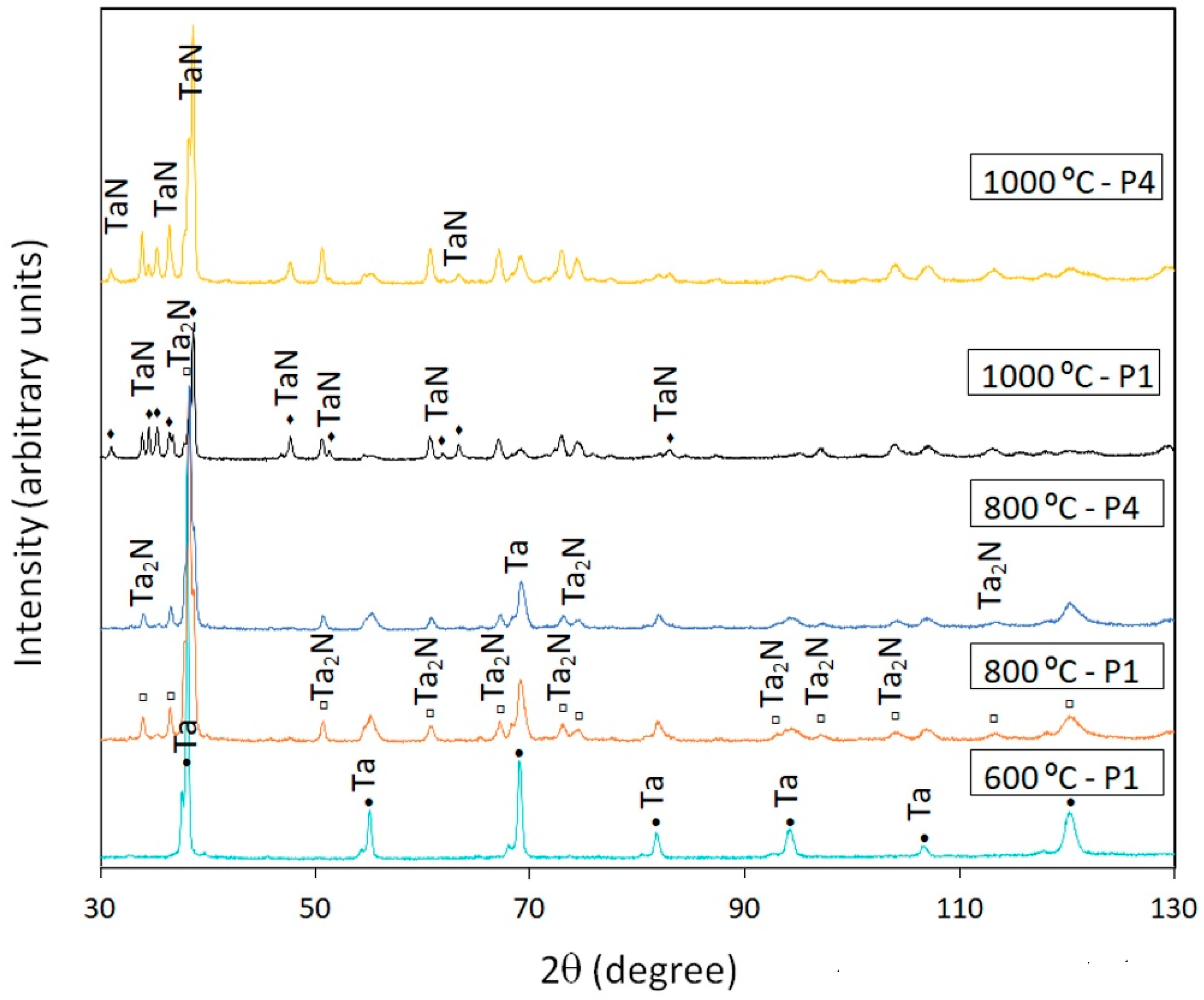
3.2.3. Tantalum
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hägg, G. X-radiation experiments on molybdenum nitrides and tungsten nitrides. Z. für Phys. Chem. -Abt. B 1930, 7, 339–362. [Google Scholar]
- Lehrer, E. Iron-hydrogen-ammoniac balance. Z. für Elektrochem. und Angew. Phys. Chem. 1930, 36, 383–392. [Google Scholar]
- Yoshizawa, H.; Shohoji, N.; Katsura, M.; Sano, T.; Yato, T. Nitrierung des metalls bzw. metallcarbides unter ammoniakstrom. Technol. Rep. Osaka Univ. 1977, 27, 363–370. [Google Scholar]
- Shohoji, N.; Marcelo, T.; Katsura, M. Influence of metastable species (non-graphitic carbon and ammonia gas) in the reactants on the composition of the reaction product (carbide, carbo-nitride and nitride). Solid State Ion. 1990, 38, 187–194. [Google Scholar] [CrossRef]
- Katsura, M. Thermodynamics of nitride and hydride formation by the reaction of metals with flowing NH3. J. Alloys Compd. 1992, 182, 91–102. [Google Scholar] [CrossRef]
- Shohoji, N.; Oliveira, F.A.C.; Fernandes, J.C.; Rosa, L.G.; Rodríguez, J.; Cañadas, I.; Ramos, C.; Magalhães, T.; Cestari, F. Synthesizing higher nitride of molybdenum (Mo) and iron (Fe) in ammonia (NH3) gas stream under irradiation of concentrated solar beam in a solar furnace. Mater. und Werkst. 2013, 44, 959–971. [Google Scholar] [CrossRef]
- Oliveira, F.A.C.; Rosa, L.G.; Fernandes, J.C.; Rodríguez, J.; Cañadas, I.; Magalhães, T.; Shohoji, N. Nitriding VIa-group metals (Cr, Mo and W) in stream of NH3 gas under concentrated solar irradiation in a solar furnace at PSA (Plataforma Solar de Almería). Sol. Energy 2015, 114, 51–60. [Google Scholar] [CrossRef][Green Version]
- Oliveira, F.A.C.; Vasques, I.F.; Fernandes, J.C.; Cañadas, I.; Rodríguez, J.; Rosa, L.G.; Shohoji, N. Reactions of IVa-group metals, Ti and Zr, with uncracked NH3 gas at a temperature in the range between 600 and 800 °C under heating with concentrated solar beam at PSA. Sol. Energy 2016, 138, 119–127. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Oliveira, F.A.C.; Rosa, L.G.; Rodríguez, J.; Cañadas, I.; Magalhães, T.; Shohoji, N. Low-temperature short-time nitriding of Va-group metals, V, Nb and Ta, in uncracked NH3 gas under heating with concentrated solar power (CSP). Ciência Tecnol. dos Mater. 2016, 28, 112–116. [Google Scholar] [CrossRef]
- Rosa, L.G. Solar heat for materials processing: A review on recent achievements and a prospect on future trends. ChemEngineering 2019, 3, 83. [Google Scholar] [CrossRef]
- Shohoji, N.; Oliveira, F.A.C.; Galindo, J.; Fernandes, J.C.; Rodríguez, J.; Cañadas, I.; Rosa, L.G. Influence of linear flow velocity of uncracked ammonia (NH3) gas on formation of higher nitrides, δ-MoN and ε-Fe2N, under concentrated solar irradiation in the SF40 solar furnace at PSA. Int. J. Mater. Chem. 2019, 9, 1–12. [Google Scholar] [CrossRef][Green Version]
- Rodríguez, J.; Cañadas, I.; Zarza, E. New PSA high concentration solar furnace SF40. AIP Conf. Proc. 2016, 1734, 070028. [Google Scholar] [CrossRef]
- Brauer, G.; Esselborn, R. Nitridphasen des niobs. Z. für Anorg. und Allg. Chem. 1961, 309, 151–170. [Google Scholar] [CrossRef]
- Terao, N. Structure des nitrures de niobium. Jpn. J. Appl. Phys. 1965, 4, 353–367. [Google Scholar] [CrossRef]
- Shohoji, N.; Marcelo, T. Tetragonality in crystal lattice of zirconium dihydride. J. Mater. Sci. Lett. 1987, 6, 1251–1253. [Google Scholar] [CrossRef]
- Pereira, J.C.G.; Rahmani, K.; Rosa, L.G. Computer modelling of the optical behaviour of homogenisers in high-flux solar furnaces. Energies 2021, 14, 1828. [Google Scholar] [CrossRef]
- Brauer, G.; Jander, J. Die nitride des niobs. Z. für Anorg. und Allg. Chem. 1952, 270, 160–178. [Google Scholar] [CrossRef]
- Lengauer, W.; Ettmayer, P. Preparation and properties of compact cubic δ-NbN1–x. Mon. für Chem. Chem. Mon. 1986, 117, 275–286. [Google Scholar] [CrossRef]
- Gatterer, J.; Dufek, G.; Ettmayer, P.; Kieffer, R. The cubic tantalum mononitride (B1) and its miscibility with the isotypic mononitrides and monocarbides of the 4a and 5a group metals. Mon. für Chem. Chem. Mon. 1975, 106, 1137–1147. [Google Scholar] [CrossRef]
- Conroy, L.E.; Christensen, A.N. Preparation and crystal structure of β-Ta2N. J. Solid State Chem. 1977, 20, 205–207. [Google Scholar] [CrossRef]
- Nishimaki, K.; Nakagawa, T.; Yamamoto, T.A.; Katsura, M. Equilibrium between flowing NH3 and synthesized FeNx at various positions along flow of reaction gas. Technol. Rep. Osaka Univ. 1998, 48, 153–156. [Google Scholar]
- Nishimaki, K.; Ohmae, S.; Yamamoto, T.A.; Katsura, M. Formation of iron–nitrides by the reaction of iron nanoparticles with a stream of ammonia. Nanostruct. Mater. 1999, 12, 527–530. [Google Scholar] [CrossRef]
- Brauer, G.; Zapp, K.H. Die nitride des tantals. Z. für Anorg. und Allg. Chem. 1954, 277, 129–139. [Google Scholar] [CrossRef]
- Schönberg, N. An X-ray study of the tantalum-nitrogen system. Acta Chem. Scand. 1954, 8, 199–203. [Google Scholar] [CrossRef]
- Christensen, A.N.; Lebech, B. A reinvestigation of the structure of ε-tantalum nitride. Acta Crystallogr. 1978, B34, 261–263. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, W.; Huang, F. Facile and economical synthesis of nitrogen-rich tantalum nitrides via an ammonia looping process under confined space. New J. Chem. 2020, 44, 9158–9162. [Google Scholar] [CrossRef]
- Peng, X.; Pi, C.; Zhang, X.; Li, S.; Huo, K.; Chu, P.K. Recent progress of transition metal nitrides for efficient electrocatalytic water splitting. Sustain. Energy Fuels 2019, 3, 366–381. [Google Scholar] [CrossRef]


| Reagent | Purity [%] | Particle Size [μm] | Supplier |
|---|---|---|---|
| V (powder) | 99.0 | <45 | Goodfellow Cambridge Ltd., Huntingdon, UK |
| Nb (powder) | 99.5 | <125 | L. Light & Co., Ltd., Colnbrook, UK |
| Ta (powder) | 99.9 | <75 | Goodfellow Cambridge Ltd., Huntingdon, UK |
| NH3 (gas) | 99.8 | - | Carburos Metálicos, Barcelona, Spain |
| N2 (gas) | 99.99 | - | Carburos Metálicos, Barcelona, Spain |
| Test Run | Sample | Temperature [°C] | Phases Identified |
|---|---|---|---|
| T1–1 | Nb | 800 | Nb, NbO, Nb2N |
| T1–4 | Nb | 800 | Nb, NbO, Nb2N |
| T2–1 | V | 800 | V2N, VN |
| T2–4 | V | 800 | V2N, VN |
| T3–1 | Ta | 800 | Ta2N, Ta |
| T3–4 | Ta | 800 | Ta2N, Ta |
| T7–1 | Nb | 600 | Nb |
| T7–4 | Nb | 600 | Nb |
| T8–1 | V | 600 | V16N1.5, V2N |
| T8–4 | V | 600 | V16N1.5 |
| T9–1 | Ta | 600 | Ta, TaN0.1 |
| T9–4 | Ta | 600 | Ta |
| T13–1 | Nb | 1000 | Nb2N, NbN |
| T13–4 | Nb | 1000 | Nb2N, NbN |
| T14–1 | V | 1000 | VN |
| T14–4 | V | 1000 | VN (traces V, V2N) |
| T15–1 | Ta | 1000 | TaN, Ta2N, Ta |
| T15–4 | Ta | 1000 | TaN, Ta2N, Ta |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shohoji, N.; Oliveira, F.A.C.; Galindo, J.; Rodríguez, J.; Cañadas, I.; Fernandes, J.C.; Rosa, L.G. Synthesis of Non-Cubic Nitride Phases of Va-Group Metals (V, Nb, and Ta) from Metal Powders in Stream of NH3 Gas under Concentrated Solar Radiation. ChemEngineering 2021, 5, 19. https://doi.org/10.3390/chemengineering5020019
Shohoji N, Oliveira FAC, Galindo J, Rodríguez J, Cañadas I, Fernandes JC, Rosa LG. Synthesis of Non-Cubic Nitride Phases of Va-Group Metals (V, Nb, and Ta) from Metal Powders in Stream of NH3 Gas under Concentrated Solar Radiation. ChemEngineering. 2021; 5(2):19. https://doi.org/10.3390/chemengineering5020019
Chicago/Turabian StyleShohoji, Nobumitsu, Fernando Almeida Costa Oliveira, José Galindo, José Rodríguez, Inmaculada Cañadas, Jorge Cruz Fernandes, and Luís Guerra Rosa. 2021. "Synthesis of Non-Cubic Nitride Phases of Va-Group Metals (V, Nb, and Ta) from Metal Powders in Stream of NH3 Gas under Concentrated Solar Radiation" ChemEngineering 5, no. 2: 19. https://doi.org/10.3390/chemengineering5020019
APA StyleShohoji, N., Oliveira, F. A. C., Galindo, J., Rodríguez, J., Cañadas, I., Fernandes, J. C., & Rosa, L. G. (2021). Synthesis of Non-Cubic Nitride Phases of Va-Group Metals (V, Nb, and Ta) from Metal Powders in Stream of NH3 Gas under Concentrated Solar Radiation. ChemEngineering, 5(2), 19. https://doi.org/10.3390/chemengineering5020019











