Optimization of Exopolysaccharide (EPS) Production by Rhodotorula mucilaginosa sp. GUMS16
Abstract
:1. Introduction
2. Materials and Methods
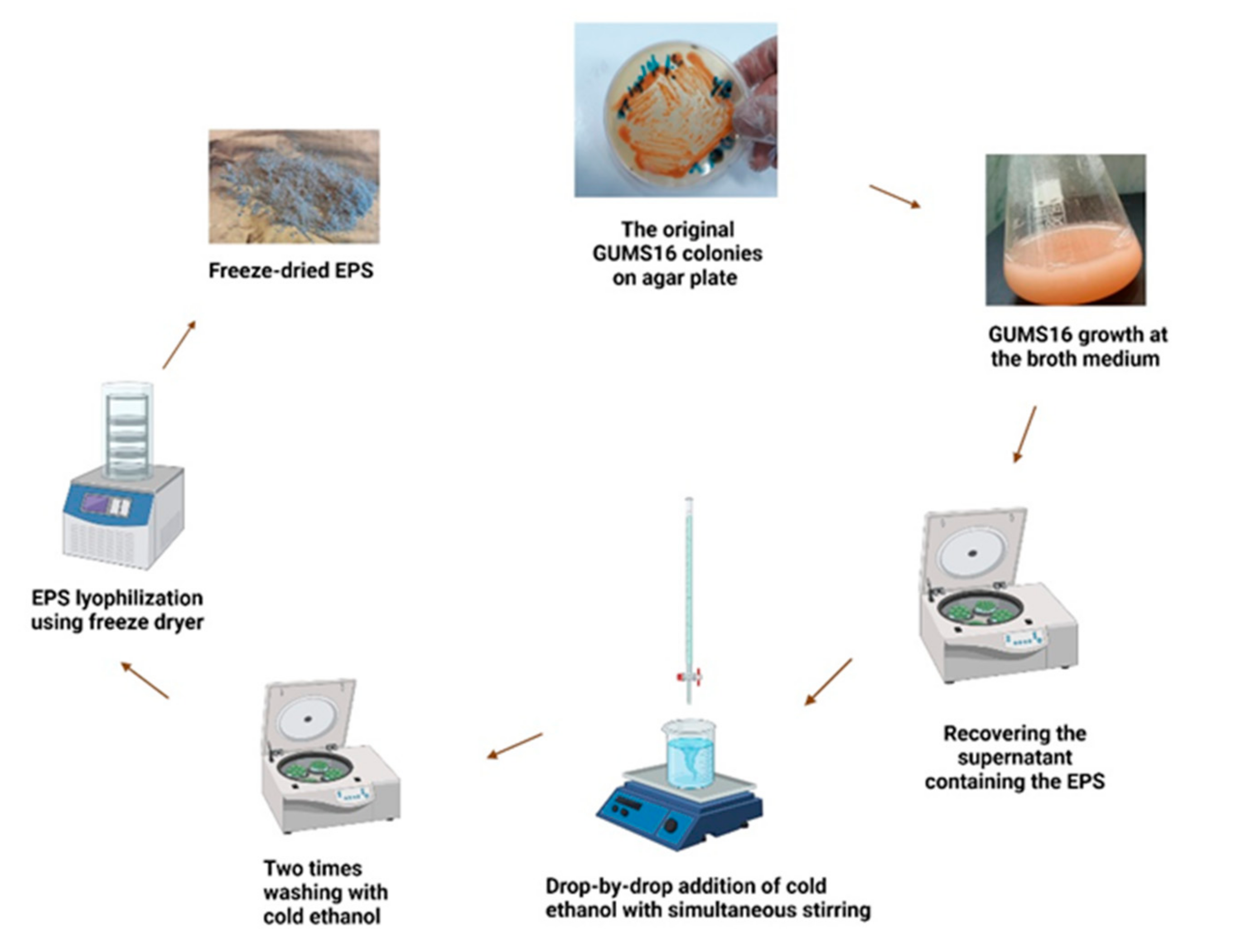
2.1. Microorganism
2.2. Preparation of Inoculum
2.3. Experimental Design, Statistical Analysis, and Optimization
2.4. Culture Conditions
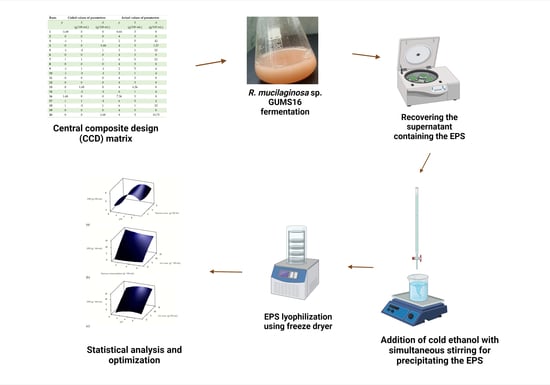
2.5. Recovery of the EPS
3. Results and Discussions
3.1. Model Fitting
3.2. Effects of the Process Variables
3.2.1. Effect of pH
3.2.2. Effect of Ammonium Sulfate Concentration
3.2.3. Effect of Sucrose Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shanmugam, M.; Abirami, R.G. Microbial Polysaccharides—Chemistry and Applications. J. Biol. Act. Prod. Nat. 2019, 9, 73–78. [Google Scholar] [CrossRef]
- Luft, L.; Confortin, T.C.; Todero, I.; Zabot, G.L.; Mazutti, M.A. An overview of fungal biopolymers: Bioemulsifiers and biosurfactants compounds production. Crit. Rev. Biotechnol. 2020, 40, 1059–1080. [Google Scholar] [CrossRef]
- Castillo, N.A.; Valdez, A.L.; Farina, J.I. Microbial production of Scleroglucan and downstream processing. Front. Microbiol. 2015, 6, 1106. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, K.; Ponnusami, V. Review on production, downstream processing and characterization of microbial pullulan. Carbohydr. Polym. 2017, 173, 573–591. [Google Scholar]
- Hamidi, M.; Mirzaei, R.; Delattre, C.; Khanaki, K.; Pierre, G.; Gardarin, C.; Petit, E.; Karimitabar, F.; Faezi, S. Characterization of a new exopolysaccharide produced by Halorubrum sp. TBZ112 and evaluation of its anti-proliferative effect on gastric cancer cells. 3 Biotech 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Smelcerovic, A.; Knezevic-Jugovic, Z.; Petronijevic, Z. Microbial polysaccharides and their derivatives as current and prospective pharmaceuticals. Curr. Pharm. Des. 2008, 14, 3168–3195. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Rathour, R.; Medhi, K.; Tyagi, B.; Thakur, I.S. 3-Microbial-derived natural bioproducts for a sustainable environment: A bioprospective for waste to wealth. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 51–85. [Google Scholar]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [Green Version]
- Ansorena, D.; Astiasarán, I. Fermented Foods: Composition and Health effects. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 649–655. [Google Scholar]
- Bomfeti, C.A.; Florentino, L.A.; Guimarães, A.P.; Cardoso, P.G.; Guerreiro, M.C.; Moreira, F.M.d.S. Exopolysaccharides produced by the symbiotic nitrogen-fixing bacteria of leguminosae. Revista Brasileira Ciência Solo 2011, 35, 657–671. [Google Scholar] [CrossRef] [Green Version]
- Ziadi, M.; Bouzaiene, T.; M’Hir, S.; Zaafouri, K.; Mokhtar, F.; Hamdi, M.; Boisset-Helbert, C. Evaluation of the Efficiency of Ethanol Precipitation and Ultrafiltration on the Purification and Characteristics of Exopolysaccharides Produced by Three Lactic Acid Bacteria. BioMed Res. Int. 2018, 2018, 1896240. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Rudra, S.G.; Mazumder, K.; Nigam, V.; Bandopadhyay, R. Structural and Functional Properties of Exopolysaccharide Excreted by a Novel Bacillus anthracis (Strain PFAB2) of Hot Spring Origin. Indian J. Microbiol. 2018, 58, 39–50. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.; Reis, M.A. Engineering aspects of microbial exopolysaccharide production. Bioresour. Technol. 2017, 245, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Osemwegie, O.O.; Adetunji, C.O.; Ayeni, E.A.; Adejobi, O.I.; Arise, R.O.; Nwonuma, C.O.; Oghenekaro, A.O. Exopolysaccharides from bacteria and fungi: Current status and perspectives in Africa. Heliyon 2020, 6, e04205. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Okoro, O.V.; Nie, L.; Petri, D.F.S.; Shavandi, A. Protein-Based 3D Biofabrication of Biomaterials. Bioengineering 2021, 8, 48. [Google Scholar] [CrossRef]
- Shavandi, A.; Hosseini, S.; Okoro, O.V.; Nie, L.; Eghbali Babadi, F.; Melchels, F. 3D Bioprinting of Lignocellulosic Biomaterials. Adv. Healthc. Mater. 2020, 9, 2001472. [Google Scholar] [CrossRef] [PubMed]
- Donot, F.; Fontana, A.; Baccou, J.C.; Schorr-Galindo, S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohydr. Polym. 2012, 87, 951–962. [Google Scholar] [CrossRef]
- Hivechi, A.; Milan, P.B.; Modabberi, K.; Amoupour, M.; Ebrahimzadeh, K.; Gholipour, A.R.; Sedighi, F.; Amini, N.; Bahrami, S.H.; Rezapour, A.; et al. Synthesis and Characterization of Exopolysaccharide Encapsulated PCL/Gelatin Skin Substitute for Full-Thickness Wound Regeneration. Polymers 2021, 13, 854. [Google Scholar] [CrossRef]
- Mahapatra, S.; Banerjee, D. Fungal Exopolysaccharide: Production, Composition and Applications. Microbiol. Insights 2013, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, C.; Roy, R.; Tribedi, P.; Ghosh, A.; Ghosh, A. Chapter 11-Biofertilizers as substitute to commercial agrochemicals. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 263–290. [Google Scholar]
- Zisu, B.; Shah, N.P. Effects of pH, Temperature, Supplementation with Whey Protein Concentrate, and Adjunct Cultures on the Production of Exopolysaccharides by Streptococcus thermophilus 1275. J. Dairy Sci. 2003, 86, 3405–3415. [Google Scholar] [CrossRef] [Green Version]
- Torres, C.A.V.; Antunes, S.; Ricardo, A.R.; Grandfils, C.; Alves, V.D.; Freitas, F.; Reis, M.A.M. Study of the interactive effect of temperature and pH on exopolysaccharide production by Enterobacter A47 using multivariate statistical analysis. Bioresour. Technol. 2012, 119, 148–156. [Google Scholar] [CrossRef]
- Hereher, F.; ElFallal, A.; Abou-Dobara, M.; Toson, E.; Abdelaziz, M.M. Cultural optimization of a new exopolysaccharide producer “Micrococcus roseus”. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 632–639. [Google Scholar] [CrossRef]
- Imran, M.Y.M.; Reehana, N.; Jayaraj, K.A.; Ahamed, A.A.P.; Dhanasekaran, D.; Thajuddin, N.; Alharbi, N.S.; Muralitharan, G. Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20. Int. J. Biol. Macromol. 2016, 93, 731–745. [Google Scholar] [CrossRef]
- Ermiş, E.; Poyraz, E.; Dertli, E.; Yılmaz, M.T. Optimization of exopolysaccharide production of Lactobacillus brevis E25 using RSM and characterization. Sakarya Üniversitesi Fen Bilimleri Enstitüsü Dergisi 2020, 24, 151–160. [Google Scholar] [CrossRef]
- Hamidi, M.; Gholipour, A.R.; Delattre, C.; Sesdighi, F.; Seveiri, R.M.; Pasdaran, A.; Kheirandish, S.; Pierre, G.; Kozani, P.S.; Kozani, P.S. Production, characterization and biological activities of exopolysaccharides from a new cold-adapted yeast: Rhodotorula mucilaginosa sp. GUMS16. Int. J. Biol. Macromol. 2020, 151, 268–277. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mohammadifar, M.A.; Mortazavian, A.M.; Rouhi, M.; Ghasemi, J.B.; Delshadian, Z. Extraction optimization of pepsin-soluble collagen from eggshell membrane by response surface methodology (RSM). Food Chem. 2016, 190, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Okoro, O.V.; Sun, Z.; Birch, J. Experimental evaluation of a polystyrene sulphonic acid resin catalyst in the hydrolysis of low-grade lipids from the meat processing industry. Biomass Bioenergy 2018, 116, 49–59. [Google Scholar] [CrossRef]
- Roosta, M.; Ghaedi, M.; Asfaram, A. Simultaneous ultrasonic-assisted removal of malachite green and safranin O by copper nanowires loaded on activated carbon: Central composite design optimization. RSC Adv. 2015, 5, 57021–57029. [Google Scholar] [CrossRef]
- Nie, L.; Chang, P.; Liang, S.; Hu, K.; Hua, D.; Liu, S.; Sun, J.; Sun, M.; Wang, T.; Okoro, O.V.; et al. Polyphenol rich green tea waste hydrogel for removal of copper and chromium ions from aqueous solution. Clean. Eng. Technol. 2021, 4, 100167. [Google Scholar] [CrossRef]
- Pavlova, K.; Koleva, L.; Kratchanova, M.; Panchev, I. Production and characterization of an exopolysaccharide by yeast. World J. Microbiol. Biotechnol. 2004, 20, 435–439. [Google Scholar] [CrossRef]
- Pavlova, K.; Panchev, I.; Krachanova, M.; Gocheva, M. Production of an exopolysaccharide by Antarctic yeast. Folia Microbiol. 2009, 54, 343. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.W. Biosynthesis of Microbial Exopolysaccharides. In Advances in Microbial Physiology; Rose, A.H., Morris, J.G., Eds.; Academic Press: Cambridge, MA, USA, 1982; Volume 23, pp. 79–150. [Google Scholar]
- Wang, Y.-C.; Lin, F.-Y.; Hsu, T.-H. Effects of Nitrogen from Different Sources on Mycelial Biomass and Polysaccharide Production and Pellet Morphology in Submerged Cultures of Grifola frondosa. BioResources 2021, 16, 2937–2952. [Google Scholar] [CrossRef]
- Valentino, F.; Karabegovic, L.; Majone, M.; Morgan-Sagastume, F.; Werker, A. Polyhydroxyalkanoate (PHA) storage within a mixed-culture biomass with simultaneous growth as a function of accumulation substrate nitrogen and phosphorus levels. Water Res. 2015, 77, 49–63. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Shi, Y.-P.; Gong, X.-Y. Effects of C/N in the substrate on the simultaneous production of polyhydroxyalkanoates and extracellular polymeric substances by Haloferax mediterranei via kinetic model analysis. RSC Adv. 2017, 7, 18953–18961. [Google Scholar] [CrossRef] [Green Version]
- Braun, M.; Altan, H.; Beck, S.J.A.E. Using regression analysis to predict the future energy consumption of a supermarket in the UK. Appl. Energy 2014, 130, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Okoro, O.V.; Sun, Z.; Birch, J.J.S. Catalyst-free biodiesel production methods: A comparative technical and environmental evaluation. Sustainability 2018, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Goh, K.K.T. Isolation and Characterisation of Bacterial Exopolysaccharide Produced by Lactobacillus Delbrueckii Subsp Bulgaricus NCFB 2483 and Sphingomonas Elodea ATCC31461. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 30 July 2004. [Google Scholar]
- Seesuriyachan, P.; Kuntiya, A.; Techapun, C. Exopolysaccharide production by Lactobacillus confusus TISTR 1498 using coconut water as an alternative carbon source: The effect of peptone, yeast extract and beef extract. Sonklanakarin J. Sci. Technol. 2011, 33, 379. [Google Scholar]
- Cho, D.H.; Chae, H.J.; Kim, E.Y. Synthesis and characterization of a novel extracellular polysaccharide by Rhodotorula glutinis. Appl. Biochem. Biotechnol. 2001, 95, 183–193. [Google Scholar] [CrossRef]
- Lo, Y.M.; Argin-Soysal, S.; Hsu, C.-H. Chapter 22-Bioconversion of Whey Lactose into Microbial Exopolysaccharides. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 559–583. [Google Scholar]
- Kaditzky, S.; Vogel, R.F. Optimization of exopolysaccharide yields in sourdoughs fermented by Lactobacilli. Eur. Food Res. Technol. 2008, 228, 291. [Google Scholar] [CrossRef]
- Ryan, P.; Ross, R.; Fitzgerald, G.; Caplice, N.; Stanton, C. Sugar-coated: Exopolysaccharide producing lactic acid bacteria for food and human health applications. Food Funct. 2015, 6, 679–693. [Google Scholar] [CrossRef]
- Maalej, H.; Hmidet, N.; Boisset, C.; Buon, L.; Heyraud, A.; Nasri, M. Optimization of exopolysaccharide production from Pseudomonas stutzeri AS22 and examination of its metal-binding abilities. J. Appl. Microbiol. 2015, 118, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, J.S.; Kee, N.L.A.; Frost, C.L.; Pletschke, B.I. Extracellular polysaccharide production in Bacillus licheniformis SVD1 and its immunomodulatory effect. BioResources 2012, 7, 4976–4993. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Paul, A.K. Optimization of Cultural Conditions for Production of Extracellular Polymeric Substances (EPS) by Serpentine Rhizobacterium Cupriavidus pauculus KPS 201. J. Polym. 2013, 2013, 692374. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, J.; Dou, W.; Shi, J.; Xu, Z. Enhancing the production of a novel exopolysaccharide by Bacillus mucilaginosus CGMCC5766 Using Statistical experiment design. Trop. J. Pharm. Res. 2013, 12, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Nagananthini, G.; Rajapriya, S.; Arivuvel, P.S. Extraction And Optimization Of Extracellular Polysaccharide Production In Spirulina Platensis MK 343101. Int. J. Sci. Technol. Res. 2020, 9, 1–5. [Google Scholar]
- Vinothini, G.; Latha, S.; Arulmozhi, M.; Dhanasekaran, D. Statistical optimization, physio-chemical and bio-functional attributes of a novel exopolysaccharide from probiotic Streptomyces griseorubens GD5. Int. J. Biol. Macromol. 2019, 134, 575–587. [Google Scholar] [CrossRef]
- Wang, B.; Song, Q.; Zhao, F.; Han, Y.; Zhou, Z. Production optimization, partial characterization and properties of an exopolysaccharide from Lactobacillus sakei L3. Int. J. Biol. Macromol. 2019, 141, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.; Yousef, N. Optimization of production and intrinsic viscosity of an exopolysaccharide from a high yielding Virgibacillus salarius BM02: Study of its potential antioxidant, emulsifying properties and application in the mixotrophic cultivation of Spirulina platensis. Int. J. Biol. Macromol. 2020, 149, 552–561. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Advances in production and simplified methods for recovery and quantification of exopolysaccharides for applications in food and health1. J. Dairy Sci. 2016, 99, 3229–3238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okoro, O.V.; Sun, Z. The characterisation of biochar and biocrude products of the hydrothermal liquefaction of raw digestate biomass. Biomass Convers. Biorefin. 2020. [Google Scholar] [CrossRef]

| Parameters | Coded and Actual Values for the Levels in the Experimental Design | ||||
|---|---|---|---|---|---|
| Low axial | Low | Center | High | High axial | |
| Levels | −2 | −1 | 0 | +1 | +2 |
| pH value, p (dimensionless) | 0.64 | 2 | 4 | 6 | 7.36 |
| Sucrose concentration, S (g/100 mL) | 0 | 1 | 3 | 5 | 6.36 |
| Ammonium sulfate concentration, A (g/100 mL) | 1.27 | 4 | 8 | 12 | 14.73 |
| Runs | Coded Values of Parameters | Actual Values of Parameters | Response | ||||
|---|---|---|---|---|---|---|---|
| p | S (g/100 mL) | A (g/100 mL) | p | S (g/100 mL) | A (g/100 mL) | YEPS (g/100 mL) | |
| 1 | −1.68 | 0 | 0 | 0.64 | 3 | 8 | 2.59 |
| 2 | 0 | 0 | 0 | 4 | 3 | 8 | 8.13 |
| 3 | −1 | 1 | 1 | 2 | 5 | 12 | 10.09 |
| 4 | 0 | 0 | −1.68 | 4 | 3 | 1.27 | 0.19 |
| 5 | −1 | −1 | 1 | 2 | 1 | 12 | 11.54 |
| 6 | 0 | 0 | 0 | 4 | 3 | 8 | 7.23 |
| 7 | 1 | 1 | 1 | 6 | 5 | 12 | 10.64 |
| 8 | 0 | 0 | 0 | 4 | 3 | 8 | 7.00 |
| 9 | −1 | 1 | −1 | 2 | 5 | 4 | 2.83 |
| 10 | −1 | −1 | −1 | 2 | 1 | 4 | 2.93 |
| 11 | 0 | 0 | 0 | 4 | 3 | 8 | 7.53 |
| 12 | 0 | 0 | 0 | 4 | 3 | 8 | 6.84 |
| 13 | 0 | 1.68 | 0 | 4 | 6.36 | 8 | 7.38 |
| 14 | 1 | −1 | −1 | 6 | 1 | 4 | 2.93 |
| 16 | 1.68 | 0 | 0 | 7.36 | 3 | 8 | 7.99 |
| 17 | 1 | 1 | −1 | 6 | 5 | 4 | 3.23 |
| 18 | 1 | −1 | 1 | 6 | 1 | 12 | 11.64 |
| 19 | 0 | 0 | 0 | 4 | 3 | 8 | 7.91 |
| 20 | 0 | 0 | 1.68 | 4 | 3 | 14.73 | 13.05 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 9 | 226.52 | 25.17 | 24.96 | 0.00 | ** |
| p | 1 | 7.52 | 7.52 | 7.46 | 0.02 | ** |
| S | 1 | 1.16 | 1.16 | 1.15 | 0.31 | * |
| A | 1 | 210.51 | 210.51 | 208.80 | 0.00 | ** |
| p2 | 1 | 6.54 | 6.54 | 6.48 | 0.03 | ** |
| S2 | 1 | 0.91 | 0.91 | 0.90 | 0.37 | * |
| A2 | 1 | 0.67 | 0.67 | 0.66 | 0.44 | * |
| p × S | 1 | 0.09 | 0.09 | 0.09 | 0.77 | * |
| p × A | 1 | 0.01 | 0.01 | 0.01 | 0.93 | * |
| A × S | 1 | 0.88 | 0.88 | 0.87 | 0.38 | * |
| YEPS, (g/100 mL) (Predicted. Yield) | YEPS, (g/100 mL) (Exp. Yield) | Relative Absolute Error |
|---|---|---|
| 14.83 | 13.48 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okoro, O.V.; Gholipour, A.R.; Sedighi, F.; Shavandi, A.; Hamidi, M. Optimization of Exopolysaccharide (EPS) Production by Rhodotorula mucilaginosa sp. GUMS16. ChemEngineering 2021, 5, 39. https://doi.org/10.3390/chemengineering5030039
Okoro OV, Gholipour AR, Sedighi F, Shavandi A, Hamidi M. Optimization of Exopolysaccharide (EPS) Production by Rhodotorula mucilaginosa sp. GUMS16. ChemEngineering. 2021; 5(3):39. https://doi.org/10.3390/chemengineering5030039
Chicago/Turabian StyleOkoro, Oseweuba Valentine, Amir Reza Gholipour, Faezeh Sedighi, Amin Shavandi, and Masoud Hamidi. 2021. "Optimization of Exopolysaccharide (EPS) Production by Rhodotorula mucilaginosa sp. GUMS16" ChemEngineering 5, no. 3: 39. https://doi.org/10.3390/chemengineering5030039
APA StyleOkoro, O. V., Gholipour, A. R., Sedighi, F., Shavandi, A., & Hamidi, M. (2021). Optimization of Exopolysaccharide (EPS) Production by Rhodotorula mucilaginosa sp. GUMS16. ChemEngineering, 5(3), 39. https://doi.org/10.3390/chemengineering5030039