Advanced HRT-Controller Aimed at Optimising Nitrogen Recovery by Microalgae: Application in an Outdoor Flat-Panel Membrane Photobioreactor
Abstract
:1. Introduction
2. Materials and Methods
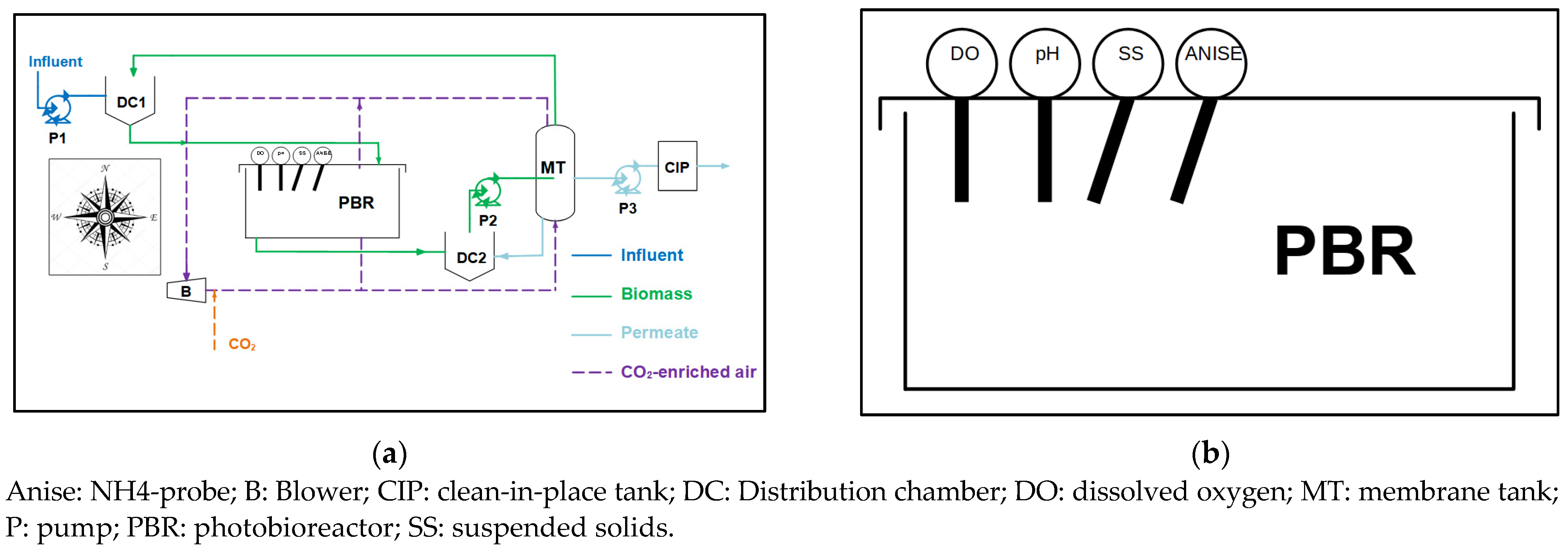
2.1. MPBR Pilot Plant
2.1.1. Instrumentation and Automation
2.1.2. Microalgae Substrate and Inoculum
2.1.3. Operation of the Pilot Plant
2.2. HRT Controller and Meteorological Model
2.2.1. Monitoring Parameters and HRT Controller Indexes
Dissolved Oxygen Standardised to 25 °C (DO25)
HRT_I1 Index
2.2.2. Auxiliar Meteorological Model for the HRT Controller
2.2.3. Initial HRT Controller
2.2.4. Hourly HRT Controller
2.2.5. Comparison with Fixed-HRT Calculation
2.3. Sampling and Methods
3. Results and Discussion
3.1. Obtaining the Control Parameters
3.2. Calibration of PAR Prediction
3.3. HRT Control
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| AnMBR | Anaerobic membrane bioreactor |
| ATU | Allylthiourea |
| BP | Biomass productivity |
| BP:SS | Biomass productivity normalised by suspended solids |
| BP:SS_YD | Biomass productivity normalised by suspended solids of the previous day |
| c | Centre of a Gaussian membership function |
| CAPEX | Capital expenditures |
| (cos ν)N | Cosine of angle ν normalised to 0–1 range |
| DO | Dissolved oxygen at culture temperature |
| DOsat | Dissolved oxygen concentration at saturation |
| DO25 | Dissolved oxygen standardised to 25 °C |
| DO25sat | Dissolved oxygen concentration at saturation at 25 °C |
| DO25′ | First derivative of the variation of DO25 |
| DO25′:SS | DO25′ normalised by SS (monitored by the sensor) |
| DO25sl | Slope of the relation DO25 vs. PAR |
| DO25sl_YD | Slope of the relation DO25 vs. PAR of the previous day |
| DO25sl:SS | DO25sl standardised with suspended solids |
| DO25sl:SS_YD | DO25sl standardised with suspended solids of the previous day |
| HRAP | High-rate algal pond |
| HRT | Hydraulic retention time |
| HRT0 | Initial hydraulic retention time |
| HRT_I1 | Index combining average SS from previous day with today average predicted PAR |
| L | Large |
| LN | Large Negative |
| LP | Large Positive |
| M | Medium |
| MPBR | Membrane photobioreactor |
| NH4 | Ammonium |
| NLR | Nitrogen loading rate |
| NO2 | Nitrite |
| NO3 | Nitrate |
| NRR | Nitrogen recovery rate |
| NRR_AV | Average nitrogen recovery rate |
| NRR:SS | Nitrogen recovery rate normalised by SS |
| NRR:SS_YD | Nitrogen recovery rate normalised by SS of the previous day |
| N:P | Nitrogen-phosphorus molar ratio |
| OPEX | Operational expenditures |
| ORP | Oxidation-reduction potential |
| P | Phosphorus |
| PAR | Daily average photosynthetically active radiation |
| PAR_AV | Average PAR |
| PAR_MAX | Daily maximum photosynthetically active radiation |
| PAR_MA60_FB | PAR moving average for the last 60 min |
| PAR_MA60_FW | PAR predicted from the model as moving average for the next 60 min |
| PAR_TDA_AV | PAR predicted from the model as today daily average |
| PBR | Photobioreactor |
| pH’ | First derivate of pH data dynamics |
| S | Small |
| SD | Standard deviation |
| SCADA | Supervisory control and data acquisition |
| SN | Small Negative |
| SP | Small Positive |
| SRT | Solid retention time |
| SS | Suspended solids |
| SS_AV | Average suspended solids |
| SS_YD_AV | Daily average of the suspended solids of the previous day |
| TCC | Total cloud cover |
| Temp | Temperature |
| TSN | Total soluble nitrogen in the effluent |
| UF-MT | Ultrafiltration membrane tank |
| WRRF | Water resource recovery facility |
| WSP | Waste stabilisation pond |
| WWTP | Wastewater treatment plant |
| XL | Extra Large |
| XS | Extra Small |
| ZE | Zero |
| α | Azimuth angle of PBR surface exposed to light |
| αs | Azimuth angle |
| β | Slope angle of PBR surface exposed to sun light |
| γs | Solar altitude angle |
| γs _MAX | Daily maximum solar altitude angle |
| δ | Declination |
| ΔDO25 | Variation of DO25 with time |
| ΔDO25_YD | Daily variation of DO25 of the previous day |
| ΔHRT | Controller output, i.e., the difference between the previous HRT and the following HRT |
| ΔPAR | Variation of PAR with time |
| ΔPAR_YD | Daily variation of PAR of the previous day |
| λ | Longitude |
| μ | Fuzzy membership value using a Gaussian membership function |
| σ | Amplitude of a Gaussian membership function |
| ν | Angle of incidence between sun and normal to PBR surface |
| φ | Latitude |
| ω | Hour angle |
References
- Paddock, M.B. Microalgae Wastewater Treatment: A Brief History. Preprints 2019. [Google Scholar] [CrossRef] [Green Version]
- Seco, A.; Aparicio, S.; González-Camejo, J.; Jiménez-Benítez, A.; Mateo, O.; Mora, J.F.; Noriega-Hevia, G.; Sanchis-Perucho, P.; Serna-García, R.; Zamorano-López, N.; et al. Resource recovery from sulphate-rich sewage through an innovative anaerobic-based water resource recovery facility (WRRF). Water Sci. Technol. 2018, 78, 1925–1936. [Google Scholar] [CrossRef]
- Hasport, N.; Krahe, D.; Kuchendorf, C.M. The potential impact of an implementation of microalgae-based wastewater treatment on the energy balance of a municipal wastewater treatment plant in Central Europe. Bioresour. Technol. 2022, 347, 126695. [Google Scholar] [CrossRef]
- Oswald, W.J.; Gotaas, H.B. Photosynthesis in sewage treatment. Trans. Am. Soc. Civ. Eng. 1957, 122, 73–97. [Google Scholar] [CrossRef]
- Wallace, J.; Champagne, P.; Hall, G. Time series relationships between chlorophyll-a, dissolved oxygen, and pH in three facultative wastewater stabilization ponds. Environ. Sci. Water Res. Technol. 2016, 2, 1032–1040. [Google Scholar] [CrossRef]
- Abis, K.L.; Mara, D.D. Primary facultative ponds in the UK: The effect of operational parameters on performance and algal populations. Water Sci. Technol. 2005, 51, 61–67. [Google Scholar] [CrossRef]
- Faleschini, M.; Esteves, J.L.; Camargo Valero, M.A. The Effects of Hydraulic and Organic Loadings on the Performance of a Full-Scale Facultative Pond in a Temperate Climate Region (Argentine Patagonia). Water Air Soil Pollut. 2012, 223, 2483–2493. [Google Scholar] [CrossRef]
- González-Camejo, J.; Ferrer, J.; Seco, A.; Barat, R. Outdoor microalgae-based urban wastewater treatment: Recent advances, applications, and future perspectives. Wiley Interdiscip. Rev. Water 2021, 8, e1518. [Google Scholar] [CrossRef]
- Inostroza, C.; Solimeno, A.; García, J.; Fernández-Sevilla, J.M.; Acién, F.G. Improvement of real-scale raceway bioreactors for microalgae production using Computational Fluid Dynamics (CFD). Algal Res. 2021, 54, 102207. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef]
- Morillas-España, A.; Lafarga, T.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; Rodríguez-Miranda, E.; Gómez-Serrano, C.; Gonzalez-López, C.V. Year-long evaluation of microalgae production in wastewater using pilot-scale raceway photobioreactors: Assessment of biomass productivity and nutrient recovery capacity. Algal Res. 2021, 60, 102500. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of Nutrients from Wastewaters Using Microalgae. Front. Sustain. Food Syst. 2018, 2, 59. [Google Scholar] [CrossRef] [Green Version]
- Acién, F.G.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef]
- Priyadharshini, S.D.; Babu, P.S.; Manikandan, S.; Subbaiya, R.; Govarthanan, M.; Karmegam, N. Phycoremediation of wastewater for pollutant removal: A green approach to environmental protection and long-term remediation. Environ. Pollut. 2021, 290, 117989. [Google Scholar] [CrossRef]
- Assunção, J.; Malcata, F.X. Enclosed “non-conventional” photobioreactors for microalga production: A review. Algal Res. 2020, 52, 102107. [Google Scholar] [CrossRef]
- Barbera, E.; Sforza, E.; Grandi, A.; Bertucco, A. Uncoupling solid and hydraulic retention time in photobioreactors for microalgae mass production: A model-based analysis. Chem. Eng. Sci. 2020, 218, 115578. [Google Scholar] [CrossRef]
- Gao, F.; Cui, W.; Xu, J.P.; Li, C.; Jin, W.H.; Yang, H.L. Lipid accumulation properties of Chlorella vulgaris and Scenedesmus obliquus in membrane photobioreactor (MPBR) fed with secondary effluent from municipal wastewater treatment plant. Renew. Energy 2019, 136, 671–676. [Google Scholar] [CrossRef]
- González-Camejo, J.; Jiménez-Benítez, A.; Ruano, M.V.; Robles, A.; Barat, R.; Ferrer, J. Optimising an outdoor membrane photobioreactor for tertiary sewage treatment. J. Environ. Manag. 2019, 245, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Le-Clech, P.; Henderson, R.K. Assessment of membrane photobioreactor (MPBR) performance parameters and operating conditions. Water Res. 2018, 138, 169–180. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, S.; Jiménez-Benítez, A.; Pachés, M.; Ruano, M.V.; Borrás, L.; Barat, R.; Seco, A. Improving membrane photobioreactor performance by reducing light path: Operating conditions and key performance indicators. Water Res. 2020, 172, 115518. [Google Scholar] [CrossRef] [Green Version]
- Raeisossadati, M.; Moheimani, N.R.; Parlevliet, D. Luminescent solar concentrator panels for increasing the efficiency of mass microalgal production. Renew. Sustain. Energy Rev. 2019, 101, 47–59. [Google Scholar] [CrossRef]
- Pawlowski, A.; Guzmán, J.L.; Berenguel, M.; Acién, F.G. Control system for pH in Raceway Photobioreactors based on Wiener Models. IFAC-PapersOnLine 2019, 52, 928–933. [Google Scholar] [CrossRef]
- Robles, A.; Capson-Tojo, G.; Galés, A.; Ruano, M.V.; Sialve, B.; Ferrer, J.; Steyer, J.P. Microalgae-bacteria consortia in high-rate ponds for treating urban wastewater: Elucidating the key state indicators during the start-up period. J. Environ. Manag. 2020, 261, 110244. [Google Scholar] [CrossRef]
- Foladori, P.; Petrini, S.; Andreottola, G. Evolution of real municipal wastewater treatment in photobioreactors and microalgae-bacteria consortia using real-time parameters. Chem. Eng. J. 2018, 345, 507–516. [Google Scholar] [CrossRef]
- Hossain, S.M.Z.; Sultana, N.; Jassim, M.S.; Coskuner GHazin, L.M.; Razzak, S.A.; Hossain, M.M. Soft-computing modeling and multiresponse optimization for nutrient removal process from municipal wastewater using microalgae. J. Water Process Eng. 2022, 45, 102490. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Nishshanka, G.K.S.H.; Sakarika, M.; Nimarshana, P.H.V.; Ariyadasa, T.U.; Kornaros, M. Artificial neural network (ANN) approach to optimize cultivation conditions of microalga Chlorella vulgaris in view of biodiesel production. Biochem. Eng. J. 2021, 173, 108072. [Google Scholar] [CrossRef]
- De-Luca, R.; Trabuio, M.; Barolo, M.; Bezzo, F. Microalgae growth optimization in open ponds with uncertain weather data. Comput. Chem. Eng. 2018, 117, 410–419. [Google Scholar] [CrossRef]
- De-Luca, R.; Bezzo, F.; Béchet, Q.; Bernard, O. Meteorological Data-Based Optimal Control Strategy for Microalgae Cultivation in Open Pond Systems. Complexity 2019, 2019, 4363895. [Google Scholar] [CrossRef]
- Lage, S.; Toffolo, A.; Gentili, F.G. Microalgal growth, nitrogen uptake and storage, and dissolved oxygen production in a polyculture based open pond fed with municipal wastewater in northern Sweden. Chemosphere 2021, 276, 130122. [Google Scholar] [CrossRef]
- González-Camejo, J.; Robles, A.; Seco, A.; Ferrer, J.; Ruano, M.V. On-line monitoring of photosynthetic activity based on pH data to assess microalgae cultivation. J. Environ. Manag. 2020, 276, 111343. [Google Scholar] [CrossRef]
- Vijayaraghavan, G.; Jayalakshmi, M. A Quick Review on Applications of Fuzzy Logic in WasteWater Treatment. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 421–425. [Google Scholar]
- Robles, A.; Capson-Tojo, G.; Ruano, M.V.; Latrille, E.; Steyer, J.-P. Development and pilot-scale validation of a fuzzy-logic control system for optimization of methane production in fixed-bed reactors. J. Process Control 2018, 68, 96–104. [Google Scholar] [CrossRef]
- Ruano, M.V.; Ribes, J.; Seco, A.; Ferrer, J. An advanced control strategy for biological nutrient removal in continuous systems based on pH and ORP sensors. Chem. Eng. J. 2012, 183, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, O.S.; Uthayakumar, H.; Kumaresan, K. Modeling of carbon dioxide fixation rate by micro algae using hybrid Artificial Intelligence and Fuzzy Logic methods and optimization by Genetic Algorithm. Res. Sq. 2021; pre-printed. [Google Scholar] [CrossRef]
- Nassef, A.M.; Sayed, E.T.; Rezk, H.; Abdelkareem, M.A.; Rodriguez, C.; Olabi, A.G. Fuzzy-modeling with Particle Swarm Optimization for enhancing the production of biodiesel from Microalga. Energy Sources Part A Recov. Util. Environ. Eff. 2019, 41, 2094–2103. [Google Scholar] [CrossRef]
- Naeini, M.A.; Zandieh, M.; Najafi, S.E.; Sajadi, S.M. Analyzing the development of the third-generation biodiesel production from microalgae by a novel hybrid decision-making method: The case of Iran. Energy 2020, 195, 116895. [Google Scholar] [CrossRef]
- García, J.; Ortiz, A.; Álvarez, E.; Belohlav, V.; García-Galán, M.J.; Díez-Montero, R.; Álvarez, J.A.; Uggetti, E. Nutrient removal from agricultural run-off in demonstrative full scale tubular photobioreactors for microalgae growth. Ecol. Eng. 2018, 120, 513–521. [Google Scholar] [CrossRef] [Green Version]
- Iasimone, F.; Panico, A.; De Felice, V.; Fantasma, F.; Iorizzi, M.; Pirozzi, F. Effect of light intensity and nutrients supply on microalgae cultivated in urban wastewater: Biomass production, lipids accumulation and settleability characteristics. J. Environ. Manag. 2018, 223, 1078–1085. [Google Scholar] [CrossRef]
- Pawlowski, A.; Frenández, I.; Guzmán, J.L.; Berenguel, M.; Acién, F.G.; Dormido, S. Event-based selective control strategy for raceway reactor: A simulation study. IFAC-PapersOnLine 2016, 49, 478–483. [Google Scholar] [CrossRef]
- Ras, M.; Steyer, J.P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Biotechnol. 2013, 12, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Viruela, A.; Robles, A.; Durán, F.; Ruano, M.V.; Barat, R.; Ferrer, J.; Seco, A. Performance of an outdoor membrane photobioreactor for resource recovery from anaerobically treated sewage. J. Clean. Prod. 2018, 178, 665–674. [Google Scholar] [CrossRef]
- Singh, V.; Mishra, V. Evaluation of the effects of input variables on the growth of two microalgae classes during wastewater treatment. Water Res. 2022, 213, 118165. [Google Scholar] [CrossRef]
- Collao, J.; Morales-Amaral, M.M.; Acién-Fernández, F.G.; Bolado-Rodríguez, S.; Fernandez-Gonzalez, N. Effect of operational parameters, environmental conditions, and biotic interactions on bacterial communities present in urban wastewater treatment photobioreactors. Chemosphere 2021, 284, 131271. [Google Scholar] [CrossRef]
- González-Camejo, J.; Barat, R.; Aguado, D.; Ferrer, J. Continuous 3-year outdoor operation of a flat-panel membrane photobioreactor to treat effluent from an anaerobic membrane bioreactor. Water Res. 2020, 169, 115238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Mishra, V. Exploring the effects of different combinations of predictor variables for the treatment of wastewater by microalgae and biomass production. Biochem. Eng. J. 2021, 174, 108129. [Google Scholar] [CrossRef]
- Ruano, M.V.; Ribes, J.; Seco, A.; Ferrer, J. Low cost-sensors as a real alternative to on-line nitrogen analysers in continuous systems. Water Sci. Technol. 2009, 60, 3261–3268. [Google Scholar] [CrossRef]
- Durán, F.; Robles, A.; Giménez, J.B.; Ferrer, J.; Ribes, J.; Serralta, J. Modeling the anaerobic treatment of sulfate-rich urban wastewater: Application to AnMBR technology. Water Res. 2020, 184, 116133. [Google Scholar] [CrossRef]
- González-Camejo, J.; Barat, R.; Pachés, M.; Murgui, M.; Ferrer, J.; Seco, A. Wastewater Nutrient Removal in a Mixed Microalgae-bacteria Culture: Effect of Light and Temperature on the Microalgae-bacteria Competition. Environ. Technol. 2018, 39, 503–515. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, S.; Pachés, M.; Borrás, L.; Seco, A. Comprehensive assessment of the microalgae-nitrifying bacteria competition in microalgae-based wastewater treatment systems: Relevant factors, evaluation methods and control strategies. Algal Res. 2022, 61, 102563. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Page, J. Chapter IIA-1—The Role of Solar-Radiation Climatology in the Design of Photovoltaic Systems. In Practical Handbook of Photovoltaics, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 573–643. ISBN 9780123859341. [Google Scholar] [CrossRef]
- Larsen, P.M. Industrial application of fuzzy logic control. Int. J. Man.-Mach. Stud. 1980, 12, 3–10. [Google Scholar] [CrossRef]
- Mendel, J.M. Fuzzy logic systems for engineering: A tutorial. Proc. IEEE 1995, 83, 345–376. [Google Scholar] [CrossRef] [Green Version]
- American Public Health Association (APHA); American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2012. [Google Scholar]
- Otondo, A.; Kokabian, B.; Stuart-Dahl, S.; Gude, V.G. Energetic evaluation of wastewater treatment using microalgae Chlorella vulgaris. J. Environ. Chem. Eng. 2018, 6, 3213–3222. [Google Scholar] [CrossRef]
- Rossi, S.; Sforza, E.; Pastore, M.; Bellucci, M.; Casagli, F.; Marazzi, F.; Ficara, E. Photo-respirometry to shed light on microalgae-bacteria consortia—A review. Rev. Environ. Sci. Biotechnol. 2020, 19, 43–72. [Google Scholar] [CrossRef]
- González-Camejo, J.; Viruela, A.; Ruano, M.V.; Barat, R.; Seco, A.; Ferrer, J. Effect of light intensity, light duration and photoperiods in the performance of an outdoor photobioreactor for urban wastewater treatment. Algal Res. 2019, 40, 101511. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, A.; Ruano, M.V.; Borrás, L.; Barat, R.; Ferrer, J. Effect of ambient temperature variations on an indigenous microalgae-nitrifying bacteria culture dominated by Chlorella. Bioresour. Technol. 2019, 290, 121788. [Google Scholar] [CrossRef]
- Lehmuskero, A.; Chauton, M.S.; Boström, T. Light and photosynthetic microalgae: A review of cellular- and molecular-scale optical processes. Prog. Oceanogr. 2018, 168, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Martínez, C.; Mairet, F.; Bernard, O. Theory of turbid microalgae cultures. J. Theor. Biol. 2018, 456, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Shoener, B.D.; Schramm, S.M.; Béline, F.; Bernard, O.; Martínez, C.; Plósz, B.G.; Snowling, S.; Steyer, J.P.; Valverde-Pérez, B.; Wágner, D.; et al. Microalgae and cyanobacteria modeling in water resource recovery facilities: A critical review. Water Res. X 2019, 2, 100024. [Google Scholar] [CrossRef]
- Mantovani, M.; Marazzi, F.; Fornaroli, R.; Bellucci, M.; Ficara, E.; Mezzanotte, V. Outdoor pilot-scale raceway as a microalgae-bacteria sidestream treatment in a WWTP. Sci. Total Environ. 2020, 710, 135583. [Google Scholar] [CrossRef]









| Parameter | Unit | Mean ± SD |
|---|---|---|
| NH4-N | mg N·L−1 | 53.7 ± 2.2 |
| NO2-N + NO3-N | mg N·L−1 | 0.5 ± 0.7 |
| P | mg P·L−1 | 5.2 ± 0.4 |
| N:P | molar ratio | 23.3 ± 1.6 |
| Parameter | Unit | Mean ± SD |
|---|---|---|
| Average solar PAR | µmol·m−2·s−1 | 275 ± 112 |
| Average maximum solar PAR | µmol·m−2·s−1 | 1218 ± 388 |
| Average Temperature | °C | 18.6 ± 1.5 |
| Average DO | mg O2·L−1 | 10.7 ± 0.5 |
| SRT | d | 2.25 ± 0.01 |
| HRT | d | 1.8 ± 0.4 |
| NLR | g N·day−1 | 7.3 ± 2.0 |
| Inference Rules: |
|---|
| IF HRT_I1 is S and DO25sl_YD is S, THEN HRT0 is XS |
| IF HRT_I1 is M and DO25sl_YD is S, THEN HRT0 is S |
| IF HRT_I1 is S and DO25sl_YD is M, THEN HRT0 is S |
| IF HRT_I1 is S and DO25sl_YD is L, THEN HRT0 is M |
| IF HRT_I1 is M and DO25sl_YD is M, THEN HRT0 is M |
| IF HRT_I1 is L and DO25sl_YD is S, THEN HRT0 is M |
| IF HRT_I1 is M and DO25sl_YD is L, THEN HRT0 is L |
| IF HRT_I1 is L and DO25sl_YD is M, THEN HRT0 is L |
| IF HRT_I1 is L and DO25sl_YD is L, THEN HRT0 is XL |
| Inference Rules: |
|---|
| IF PAR_60_FW is S and DO25′:SS is LN, THEN ΔHRT is LP |
| IF PAR_60_FW is M and DO25′:SS is LN, THEN ΔHRT is LP |
| IF PAR_60_FW is S and DO25′:SS is SN, THEN ΔHRT is LP |
| IF PAR_60_FW is L and DO25′:SS is LN, THEN ΔHRT is SP |
| IF PAR_60_FW is M and DO25′:SS is SN, THEN ΔHRT is SP |
| IF PAR_60_FW is S and DO25′:SS is SN, THEN ΔHRT is ZE |
| IF PAR_60_FW is L and DO25′:SS is SN, THEN ΔHRT is ZE |
| IF PAR_60_FW is M and DO25′:SS is ZE, THEN ΔHRT is ZE |
| IF PAR_60_FW is S and DO25′:SS is SP, THEN ΔHRT is ZE |
| IF PAR_60_FW is L and DO25′:SS is ZE, THEN ΔHRT is SN |
| IF PAR_60_FW is M and DO25′:SS is SP, THEN ΔHRT is SN |
| IF PAR_60_FW is S and DO25′:SS is LP, THEN ΔHRT is SN |
| IF PAR_60_FW is L and DO25′:SS is SP, THEN ΔHRT is LN |
| IF PAR_60_FW is M and DO25′:SS is LP, THEN ΔHRT is LN |
| IF PAR_60_FW is L and DO25′:SS is LP, THEN ΔHRT is LN |
| Parameter | HRT Controller | Fixed HRT (1.25 days) |
|---|---|---|
| NRR·NLR−1 (%) [1–55 days] | 66.0 ± 13.8 | 50.0 ± 11.9 |
| NRR·NLR−1 (%) [46–55 days] | 73.8 ± 5.1 | 50.8 ± 4.7 |
| NST (mgN·L−1) [1–55 days] | 19.0 ± 5.4 | 27.1 ± 5.0 |
| NST (mgN·L−1) [46–55 days] * | 13.4 ± 1.9 | 25.9 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Sánchez, J.F.; González-Camejo, J.; Seco, A.; Ruano, M.V. Advanced HRT-Controller Aimed at Optimising Nitrogen Recovery by Microalgae: Application in an Outdoor Flat-Panel Membrane Photobioreactor. ChemEngineering 2022, 6, 24. https://doi.org/10.3390/chemengineering6020024
Mora-Sánchez JF, González-Camejo J, Seco A, Ruano MV. Advanced HRT-Controller Aimed at Optimising Nitrogen Recovery by Microalgae: Application in an Outdoor Flat-Panel Membrane Photobioreactor. ChemEngineering. 2022; 6(2):24. https://doi.org/10.3390/chemengineering6020024
Chicago/Turabian StyleMora-Sánchez, Juan Francisco, Josué González-Camejo, Aurora Seco, and María Victoria Ruano. 2022. "Advanced HRT-Controller Aimed at Optimising Nitrogen Recovery by Microalgae: Application in an Outdoor Flat-Panel Membrane Photobioreactor" ChemEngineering 6, no. 2: 24. https://doi.org/10.3390/chemengineering6020024
APA StyleMora-Sánchez, J. F., González-Camejo, J., Seco, A., & Ruano, M. V. (2022). Advanced HRT-Controller Aimed at Optimising Nitrogen Recovery by Microalgae: Application in an Outdoor Flat-Panel Membrane Photobioreactor. ChemEngineering, 6(2), 24. https://doi.org/10.3390/chemengineering6020024








