Exploring Strategies for Copper Removal from Nickel Anolytes: A Review
Abstract
:1. Introduction
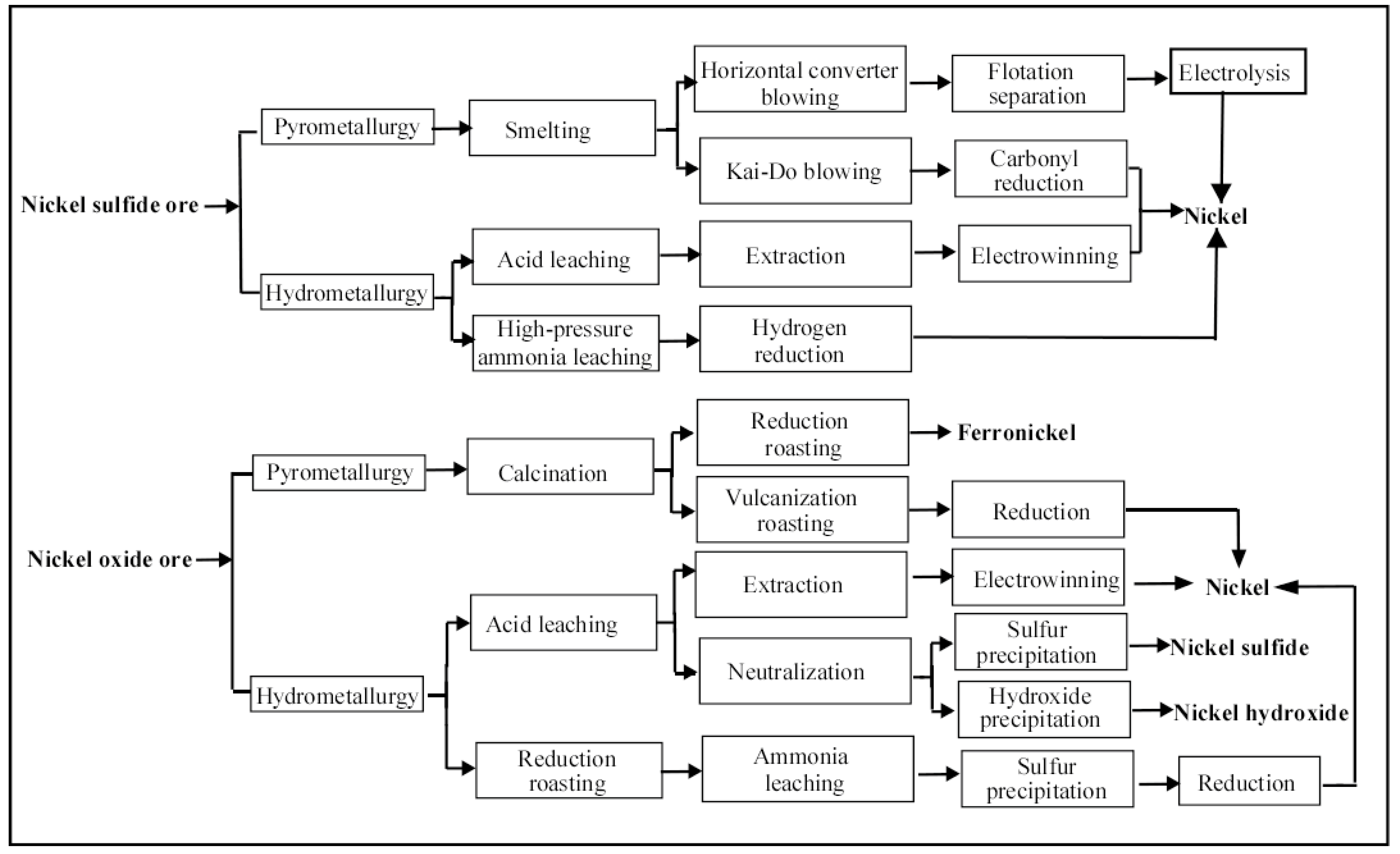
2. Nickel Production Process
2.1. Distribution of Nickel Ore
2.2. Primary Production Process of Nickel
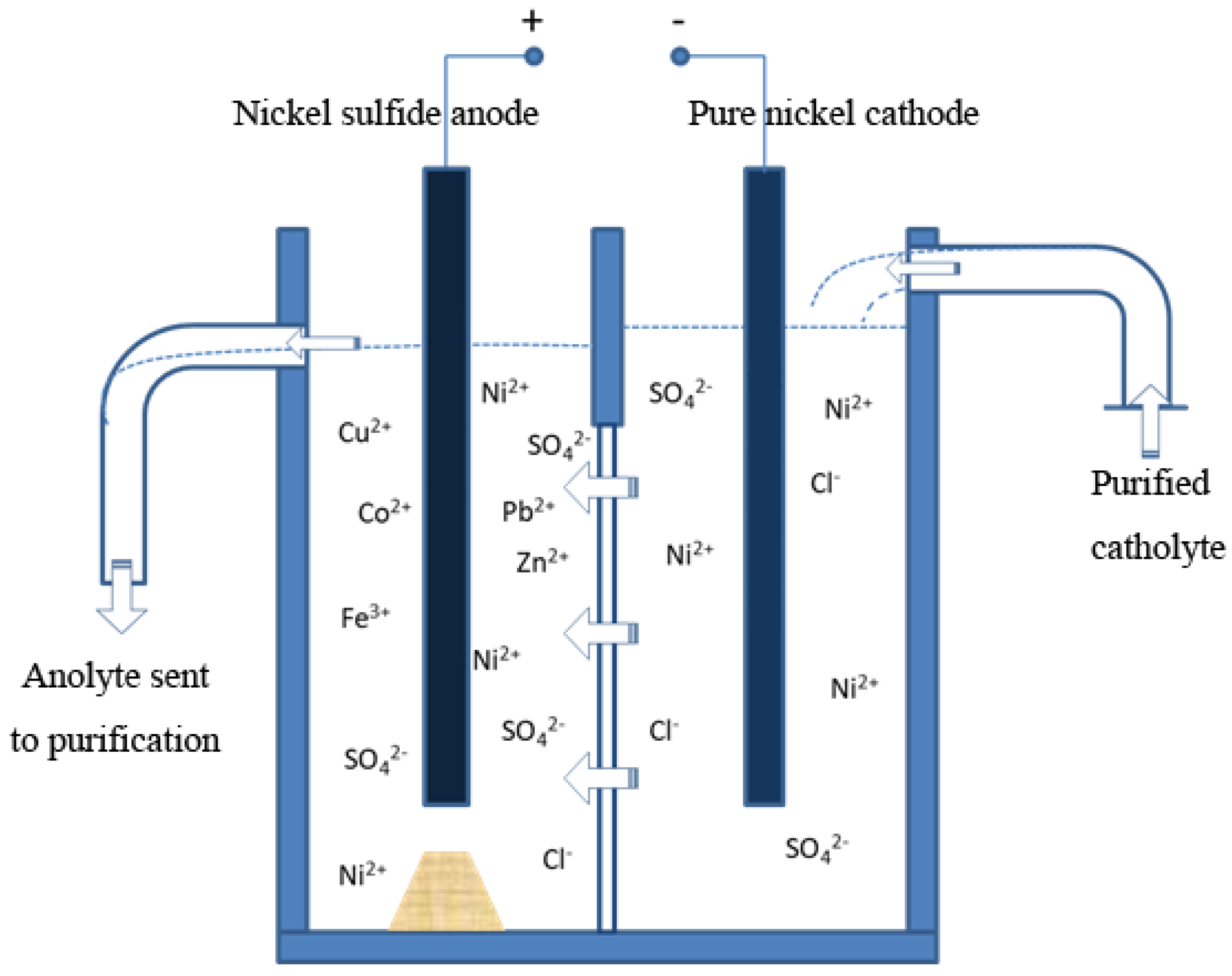
2.3. Electrolytic Refining Process of Nickel Sulfide
3. Removal of Copper from Nickel Electrolysis Anolyte
3.1. Potential-Based Separation Method
3.2. Chemical Precipitation Method
3.3. Solvent Extraction
3.4. Ion-Exchange Method
4. Extraction of Nickel Using Iminodiacetic Acid Chelating Resin
4.1. Chelating Resin Preparation and Character
4.2. Adsorption Mechanism
4.3. Practical Application of IDA Chelating Resin in Nickel Extraction
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tripathi, C.C.; Kumar, M.; Kumar, D. Atom beam sputtered Mo2C films as a diffusion barrier for copper metallization. Appl. Surf. Sci. 2009, 255, 3518–3522. [Google Scholar] [CrossRef]
- Kudelski, A.; Janik-Czachor, M.; Bukowska, J.; Dolata, M.; Szummer, A. Surface-enhanced Raman scattering (SERS) on copper electrodeposited under nonequilibrium conditions. J. Mol. Struct. 1999, 482–483, 245–248. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Y.; Yang, F.; Gao, L.; Sun, J. A symmetrical bi-electrode electrochemical technique for high-efficiency transfer of CVD-grown graphene. Nanotechnology 2014, 25, 145704. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhi, X.; Zhai, H.J. A facile approach to improve the electrochemical properties of polyaniline-carbon nanotube composite electrodes for highly flexible solid-state supercapacitors. Int. J. Hydrogen Energy 2018, 43, 18339–18348. [Google Scholar] [CrossRef]
- Cheng, Z.P.; Yang, Y.; Liu, X.D.; Yang, Y.L.; Li, F.S. Preparation of core-shell Cu/Al powders by displacement method. Acta Chim. Sin. 2007, 65, 81–85. [Google Scholar]
- Gibin, S.R.; Sivagurunathan, P. Synthesis and characterization of nickel cobalt ferrite (Ni1xCoxFe2O4) nano particles by co-precipitation method with citrate as chelating agent. J. Mater. Sci. Mater. Electron. 2016, 28, 1985–1996. [Google Scholar] [CrossRef]
- Chui, V.W.D.; Mok, K.W.; Ng, C.Y.; Luong, B.P.; Ma, K.K. Removal and recovery of copper(II), chromium(III), and nickel(II) from solutions using crude shrimp chitin packed in small columns. Environ. Int. 1996, 22, 463–468. [Google Scholar] [CrossRef]
- So, W.W.; Choe, S.; Chuang, R.; Lee, C.C. An effective diffusion barrier metallization process on copper. Thin Solid Films 2000, 376, 164–169. [Google Scholar] [CrossRef]
- Fei, J.; Wilcox, G.D. Electrodeposition of zinc–nickel compositionally modulated multilayer coatings and their corrosion behaviours. Surf. Coat. Technol. 2006, 200, 3533–3539. [Google Scholar] [CrossRef]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of heavy metals from acid mine drainage. Water Res. 2002, 36, 4757–4764. [Google Scholar] [CrossRef]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Electrochemical deposition of black nickel solar absorber coatings on stainless steel AISI316L for thermal solar cells. Sol. Energy Mater. Sol. Cells 2005, 87, 685–694. [Google Scholar]
- Xin, H. Recovery of Sulfur from Anode Slime of Nickel Sulfide Electrolysisby Vacuum Evaporation. J. Univ. Sci. Technol. Beijing 2002, 24, 410–413. [Google Scholar]
- Tang, J.; Steenari, B.M. Solvent extraction separation of copper and zinc from MSWI fly ash leachates. Waste Manag. 2015, 44, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.M.; Nikolaev, A.V.; Gindin, L.M.; Kheifez, V.L.; Volkov, L.V.; Maizlish, R.S. Solvent extraction removal of cobalt and other impurity elements from nickel electrolytes. Hydrometallurgy 1979, 4, 377–387. [Google Scholar] [CrossRef]
- Dai, K.; Liu, Y.P.; Hu, H.P.; Liu, S.J.; Cheng, Z.Y.; Chen, Q.Y. Removal of Copper from Nickel Anode Electrolyte by AMPY-1. Nonferrous Met. (Extr. Metall.) 2015, 10, 10–13. [Google Scholar]
- Burkin, A.R.; Preston, J.S. α-Substituted oxime extractants—II: Extraction of Cu(II), Ni(II), Co(II) and Fe(II) by aliphatic α-hydroxyiminoketones and α-dioximes. J. Inorg. Nucl. Chem. 1975, 37, 2187–2195. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chiang, C.L.; Chen, C.R. Removal of heavy metal ions by a chelating resin containing glycine as chelating groups. Sep. Purif. Technol. 2007, 54, 396–403. [Google Scholar] [CrossRef]
- Cardillo, G.; Orena, M.; Sandri, S.; Orena, M. Polymer Supported Reagents. Chromic Acid on Anion Exchange Resin. Synthesis of Aldehydes and Ketones from Allylic and Benzylic Halides. Tetrahedron Lett. 1976, 44, 3985–3986. [Google Scholar] [CrossRef]
- Tang, S.H.; Zhu, X.K.; Cai, J.J.; Li, S.Z.; He, X.X.; Wang, J.H. Chromatographic Separation of Cu, Fe and Zn using AG MP-1 Anion Exchange Resin for Isotope Determination by MC-ICPMS. Rock Miner. Anal. 2006, 1, 5–8. [Google Scholar]
- Vernon, F. Some aspects of ion exchange in copper hydrometallurgy. Hydrometallurgy 1979, 4, 147–157. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Li, M.A.; Na, B.Y.; Quan, P.H.; Jie, H.E.; Bassig, B.A.; Min, D.; Wei, Z.Y.; Zhang, Z.T.; Ning, C. A Retrospective Cohort Mortality Study in Jinchang, the Largest Nickel Production Enterprise in China. Biomed. Environ. Sci. BES 2014, 27, 567–571. [Google Scholar]
- Yang, A.M.; Bai, Y.N.; Pu, H.Q.; Zheng, T.Z.; Cheng, N. Prevalence of Metabolic Syndrome in Chinese Nickel-exposed Workers. Biomed. Environ. Sci. 2014, 27, 475–477. [Google Scholar] [PubMed]
- Ma, B.; Yang, W.J.; Li, Q.; Wang, C.Y.; Wang, H. Mineral Phase Analysis and Treatment Technological Selection of Magnesium-rich Nickel Oxide Ore. Nonferrous Met. (Extr. Metall.) 2016, 8, 1–5. [Google Scholar]
- Yang, Z.Q.; Wang, Y.Q.; Gao, Q.; Wu, S. Present Situation and Development Strategy and Key Technologies of China’s Nickel Resources Sustainable Development. Conserv. Util. Miner. Resour. 2016, 2, 58–69. [Google Scholar]
- Murofushi, A.; Otake, T.; Sanematsu, K.; Ya, K.Z.; Ito, A.; Kikuchi, R.; Sato, T. Mineralogical evolution of a weathering profile in the Tagaung Taung Ni laterite deposit: Significance of smectite in the formation of high-grade Ni ore in Myanmar. Miner. Depos. 2022, 57, 1107–1122. [Google Scholar] [CrossRef]
- Balbin, A.; Capilitan, J.; Taboada, E.; Tabañag, I.D. Characteristics of Nickel Laterite Mine Waste in Caraga Region, Philippines and Its Potential Utilization. Nat. Environ. Pollut. Technol. 2023, 22, 1267–1276. [Google Scholar] [CrossRef]
- Ober, J.A. Mineral Commodity Summaries 2017; U.S. Geological Survey: Reston, VA, USA, 2018. [Google Scholar]
- Yang, X.S.; Guo, Y.S.; Chen, B.Y.; Cui, Y.L.; Guo, X. The distribution and the exploration, development and utilization situation of the lateritic nickel ore resources in the world. Acta Geosci. Sin. 2013, 34, 193–201. [Google Scholar]
- Lu, C.Y.; Lu, X.G.; Zou, X.L.; Cheng, H.W.; Xu, Q. Current situation and utilization technology of nickel ore in China. Chin. J. Nat. 2015, 37, 269–277. [Google Scholar]
- Pandey, N.; Tripathy, S.K.; Patra, S.K.; Jha, G. Recent Progress in Hydrometallurgical Processing of Nickel Lateritic Ore. Trans. Indian Inst. Met. 2023, 76, 11–30. [Google Scholar] [CrossRef]
- Zhao, K.; Li, X.; Xue, M.; Jou, B.J.D.; Lee, W.C. Short-term forecasting through intermittent assimilation of data from Taiwan and Mainland China coastal radars for typhoon Meranti (2010) at landfall. J. Geophys. Res. 2012, 117, 1–20. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, S.; Yuan, Z.; Han, Y.; Ma, S. Study on Flotation Technology of Copper-nickel Sulfide Ore in North Korea. Metal Mine 2010, 4, 84–88. [Google Scholar]
- Tsymbulov, L.B.; Knyazev, M.V.; Tsemekhman, L.S. Oxide nickel ores smelting of ferronickel in two-zone Vaniukov Furnace. Can. Metall. Q. 2011, 50, 135–144. [Google Scholar] [CrossRef]
- Hirasawa, R.; Matsuoka, J.; Wakabayashi, K. Research for Factorial Effects on Leaching of Nickel Oxide Ore by Nitric Acid Solution. J. Min. Metall. Inst. Jpn. 1980, 96, 337–340. [Google Scholar]
- Zhou, H.P.; Li, Y.Q.; Lei, M.F.; Jing, X.U.; Wang, P.C.; Weng, C.J. New Beneficiation Technique for Certain Refractory Fine Copper-Nickel Sulfide Ore. Min. Metall. Eng. 2015, 35, 35–38. [Google Scholar]
- Wang, H.; Ye, Z.; Hao, F.; Yuan, H. Experimental Study on a Low-grade Copper-nickel Sulfide Ore. Metal Mine 2015, 8, 78–84. [Google Scholar]
- Dutton, M.D.; Vasiluk, L.; Ford, F.; Bellantino Perco, M.; Taylor, S.R.; Lopez, K.; Bolger, G.T.; Gopalapillai, Y.; Hale, B. Towards an exposure narrative for metals and arsenic in historically contaminated Ni refinery soils: Relationships between speciation, bioavailability, and bioaccessibility. Sci. Total Environ. 2019, 686, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Xu, Y.; Xu, Y. Development of nickel and progress of technology in China. Ming Metall. 1997, 6, 43–54. [Google Scholar]
- GB-6516-2010; Electrolytic nickel. National Nonferrous Metals Standardization Technical Committee: Beijing, China, 2010.
- Ilyas, N.; Ilyas, S.; Sajjad-Ur-Rahman, S.; Yousaf, S.; Zia, A.; Sattar, S. Removal of copper from an electroplating industrial effluent using the native and modified spirogyra. Water Sci. Technol. 2018, 78, 147–155. [Google Scholar] [CrossRef]
- Li, J.T.; Chen, A.L. Deep removal of copper from nickel electrolyte using manganese sulfide. Trans. Nonferrous Met. Soc. China 2015, 25, 3802–3807. [Google Scholar] [CrossRef]
- Liu, X.; He, Y.; Zhao, Z.; Chen, X. Study on removal of copper from nickel-copper mixed solution by membrane electrolysis. Hydrometallurgy 2018, 180, 153–157. [Google Scholar] [CrossRef]
- GB3102; Thermodynamics of Standard Solubility Product. National Nonferrous Metals Standardization Technical Committee: Beijing, China, 1996.
- Harumsa, K.; Kazuyuki, T. Nickel and Cobalt Refining at Niihama Nickel Refinery. J. Min. Inst. Jpn. 2010, 123, 678–681. [Google Scholar]
- Ton, S.J.; Neumann, K.T.; Nørby, P.; Skrydstrup, T. Nickel-Mediated Alkoxycarbonylation for Complete Carbon Isotope Replacement. J. Am. Chem. Soc. (ACS) 2021, 143, 17816–17824. [Google Scholar] [CrossRef] [PubMed]
- Yusem, G.; Pintauro, P.N.; Cheng, P.C.; An, W. Electrocatalytic hydrogenation of soybean oil in a radial flow-through Raney nickel powder reactor. J. Appl. Electrochem. 1996, 26, 989–997. [Google Scholar] [CrossRef]
- Rao, R.; Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Properties of Copper Chromite Catalysts in Hydrogenation Reactions. J. Catal. 1997, 171, 406–419. [Google Scholar] [CrossRef]
- Chen, X.; Chen, A.; Zhao, Z.; Liu, X.; Shi, Y.; Wang, D. Removal of Cu from the nickel electrolysis anolyte using nickel thiocarbonate. Hydrometallurgy 2013, 113, 106–110. [Google Scholar] [CrossRef]
- Zeng, Z.O.; Zeng, Z.Y.; Wu, H.R. Removal copper from an anolyte of nickel electrolysis by electrowinning. J. South China Univ. Technol. (Nat. Sci.) 1994, 22, 32–38. [Google Scholar]
- Kravchenko, T.A.; Polyanskiy, L.L.; Krysanov, V.A.; Zelensky, E.S.; Kalinitchev, A.I.; Höll, W.H. Chemical precipitation of copper from copper-zinc solutions onto selective sorbents. Hydrometallury 2009, 95, 141–144. [Google Scholar] [CrossRef]
- Su, R.J. Study on the Process of Copper Removal by Nickel Electrolysis Purification; Lanzhou University of Technology: Lanzhou, China, 2012; pp. 6–8. Available online: https://d.wanfangdata.com.cn/thesis/ChJUaGVzaXNOZXdTMjAyMzA5MDESCFkyMTA5ODU4GghzbG84b2JzNw%253D%253D (accessed on 22 November 2023).
- Zhai, X.J. Research on sulfur-containing active nickel. Nonferrous Min. Metall. 1998, 14, 31–33. [Google Scholar]
- Shu, Y.D.; Yan, X.X. Preparation of active anode slime for removing copper from nickel electrolyte. Nonferrous Met. (Smelt.) 1996, 5, 24–26. [Google Scholar]
- Gu, G.B.; Wu, X.M.; Cheng, F.; Zhang, Z.M.; Li, Y.Y. Application of ultrafine particles in chemical separation-research on the characteristics of activated nickel sulfide. J. South China Univ. Technol. (Nat. Sci.) 1996, 8, 96–100. [Google Scholar]
- Wang, M.; Yu, H.T.; Ma, X.Q.; Yao, Y.; Wang, L.; Liu, L.H.; Cao, K.; Liu, S.L.; Dong, C.; Zhao, B.M.; et al. Copper oxide-modified graphene anode and its application in organic photovoltaic cells. Opt. Express 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chen, X.Y.; Liu, X.H.; Zhao, Z.W. Removal of Cu from the nickel electrolysis anolyte using amorphous MnS. Hydrometallurgy 2014, 146, 149–153. [Google Scholar]
- Peng, B.; Fan, H.Y.; Gao, J.Z. Solid-liquid solvent extraction of metal ions. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2003, 10, 1–7. [Google Scholar]
- Fischer, C.; Wagner, H.; Bagreev, V.V. On the tri-n-octylammonium chloro complexes of Cu(II), Zn(II) and Co(II) in benzene solution. Polyhedron 1983, 2, 1141–1146. [Google Scholar] [CrossRef]
- Sarangi, K.; Parhi, P.K.; Padhan, E.; Palai, A.K.; Nathsarma, K.C.; Park, K.H. Separation of iron(III), copper(II) and zinc(II) from a mixed sulphate/chloride solution using TBP, LIX 84I and Cyanex 923. Sep. Purif. Technol. 2007, 55, 44–49. [Google Scholar] [CrossRef]
- Fang, W.U.; Jun, L. Study on Separation of Cu~(2+) from Nickle Sulphate Solution with Extractant M5640. J. Wuyi Univ. (Nat. Sci. Ed.) 2004, 18, 25–27. [Google Scholar]
- Avdibegović, D.; Barbier, E.; Jaklič, B.; Škapin, S.D.; Spreitzer, M.; Binnemans, K. Removal of copper and iron from ethanolic solutions by an anion exchange resin and its implication to rare-earth magnet recycling. Chemosphere 2023, 330, 138603. [Google Scholar] [CrossRef]
- Chen, A.L.; Qiu, G.Z.; Zhao, Z.W.; Sun, P.M.; Yu, R.L. Removal of copper from nickel anode electrolyte through ion exchange. Trans. Nonferrous Met. Soc. China 2009, 19, 253–258. [Google Scholar] [CrossRef]
- Ma, J.B.; He, B.L. The application of ion exchange resins in hydrometallurgy. Ion Exch. Adsorpt. 1993, 9, 250–260. [Google Scholar]
- Xiang, W.H.; Liu, Z. New Progress of Synthesis and Application Study of Specific Ion Exchanger. Guangxi Chem. Ind. 2003, 32, 16–22. [Google Scholar]
- Lou, F.; Dong, B.; Bi, Y.B.; Xie, J.C. Adsorption of metalcation by chelating resin. Technol. Water Treat. 2011, 37, 23–27. [Google Scholar]
- Wu, X.Y.; He, Y.Y.; Ping, L.H. Study on Chelating Resins Syntheses and Adsorption Properties of Thiourea Type Resins. J. Wuhan Univ. (Nat. Sci. Ed.) 1999, 45, 129–134. [Google Scholar]
- Zhuang, H.; Zhang, Z.; Jin, W.; Guo, L. Preparation and adsorption properties of crosslinked polyaminated chitosan chelating resin. Ion Exch. Adsorpt. 2001, 17, 507–514. [Google Scholar]
- Kong, L.F.; Wang, Z.P.; Zhang, X.C.; Yang, Y.Q. Synthesis and Application of Specific Ion Exchanger. Inn. Mong. Petrochem. Ind. 2006, 32, 19–21. [Google Scholar]
- Kuz’min, V.I.; Kuz’min, D.V. Sorption of nickel and copper from leach pulps of low-grade sulfide ores using Purolite S930 chelating resin. Hydrometallurgy 2014, 141, 76–81. [Google Scholar] [CrossRef]
- Noureddine, C.; Lekhmici, A.; Mubarak, M.S. Sorption properties of the iminodiacetate ion exchange resin, amberlite IRC-718, toward divalent metal ions. J. Appl. Polym. Sci. 2007, 107, 1316–1319. [Google Scholar] [CrossRef]
- Mendes, F.D.; Martins, A.H. Selective sorption of nickel and cobalt from sulphate solutions using chelating resins. Int. J. Miner. Process. 2004, 74, 359–371. [Google Scholar] [CrossRef]
- Dinu, M.V.; Dragan, E.S. Heavy metals adsorption on some iminodiacetate chelating resins as a function of the adsorption parameters. React. Funct. Polym. 2008, 68, 1346–1354. [Google Scholar] [CrossRef]
- Silva, R.M.P.; Manso, J.P.H.; Rodrigues, J.R.C.; Lagoa, R.J.L. A comparative study of alginate beads and an ion-exchange resin for the removal of heavy metals from a metal plating effluent. J. Environ. Sci. Health Part A 2008, 43, 1311–1317. [Google Scholar] [CrossRef]
- Agrawal, A.; Sahu, K. Separation and recovery of lead from a mixture of some heavy metals using Amberlite IRC 718 chelating resin. J. Hazard. Mater. 2006, 133, 299–303. [Google Scholar] [CrossRef]
- Valverde, J.L.; Lucas, A.D.; Carmona, M.; González, M.; Rodríguez, J.F. Equilibrium data of the exchange of Cu2+, Cd2+ and Zn2+ ions for H+ on the cationic exchanger Lewatit TP-207. J. Chem. Technol. Biotechnol. Biotechnol. 2010, 79, 1371–1375. [Google Scholar] [CrossRef]
- Jachuła, J.; Kołodyńska, D.; Hubicki, Z. Removal of Cd(II) and Pb(II) complexes with glycolic acid from aqueous solutions on different ion exchangers. Can. J. Chem. 2010, 88, 540–547. [Google Scholar] [CrossRef]
- Johnson, B.E.; Santschi, P.H.; Chuang, C.Y.; Otosaka, S.; Addleman, R.S.; Douglas, M.; Rutledge, R.D.; Chouyyok, W.; Davidson, J.D.; Fryxell, G.E.; et al. Collection of Lanthanides and Actinides from Natural Waters with Conventional and Nanoporous Sorbents. Environ. Sci. Technol. 2012, 46, 11251–11258. [Google Scholar] [CrossRef] [PubMed]
- Zainol, Z.; Nicol, M.J. Comparative study of chelating ion exchange resins for the recovery of nickel and cobalt from laterite leach tailings. Hydrometallurgy 2009, 96, 283–287. [Google Scholar] [CrossRef]
- Mendes, F.D.; Martins, A.H. Selective nickel and cobalt uptake from pressure sulfuric acid leach solutions using column resin sorption. Int. J. Miner. Process. 2005, 77, 53–63. [Google Scholar] [CrossRef]
- Korngold, E.; Belayev, N.; Aronov, L.; Titelman, S. Influence of complexing agents on the removal of metals from water by a cation exchanger. Desalination 2001, 133, 83–88. [Google Scholar] [CrossRef]
- Yebra-Biurrun, M.C.; Carro-Mariño, N. Flow injection flame atomic absorption determination of Cu, Mn and Zn partitioning in seawater by on-line room temperature sonolysis and minicolumn chelating resin methodology. Talanta 2010, 83, 425–430. [Google Scholar] [CrossRef]
- Nicolai, M.R.C.; Tousset, N.; Nicolai, Y. Trace metals analysis in estuarine and seawater by ICP-MS using on line preconcentration and matrix elimination with chelating resin. Talanta 1999, 50, 433–444. [Google Scholar] [CrossRef]



| Element | Nickel 1# (mol/L) | Nickel 0# (mol/L) |
|---|---|---|
| Ni | 1.19 | 1.19 |
| Cu | <4.72 × 10−5 | <4.72 × 10−6 |
| Fe | <7.14 × 10−5 | <5.36 × 10−6 |
| Co | <1.70 × 10−4 | <1.70 × 10−5 |
| Zn | <5.35 × 10−6 | <1.53 × 10−6 |
| Pb | <3.00 × 10−4 | <7.00 × 10−5 |
| Na+ | <1.96 | <1.96 |
| Cl− | >1.41 | >1.41 |
| H3BO3 | <9.7 × 10−2 | <9.7 × 10−2 |
| Cation | Ksp(S2−) | Ksp(OH−) |
|---|---|---|
| Cd2+ | 8.0 × 10−27 | 2.8 × 10−14 |
| Co2+ | 4.0 × 10−21 | 1.6 × 10−15 |
| Cu2+ | 6.0 × 10−36 | 1.3 × 10−20 |
| Fe2+ | 6.0 × 10−18 | 8.0 × 10−16 |
| Fe3+ | - | 3.0 × 10−39 |
| Mn2+ | 3.0 × 10−13 | 1.9 × 10−13 |
| Ni2+ | 2.0 × 10−26 | 2.0 × 10−15 |
| Pb2+ | 3 × 10−28 | 1.2 × 10−15 |
| Zn2+ | 1.6 × 10−24 | 3.0 × 10−17 |
| Chelating Resins | Functional Groups | Selectivity Order |
|---|---|---|
| Aminocarboxylic acid | —NC(H2COOH)2 | Fe3+ > Ni2+ > Cu2+ > Zn2+ |
| Iminodiacetic acid | —N(CH2COOH)2 | Cu2+ ≫ Ni2+ > Zn2+ > Fe2+ |
| Phosphoric acid | —PO(OH)2 | U4+ > Fe3+ ≥ UO22+ > Cu2+ |
| Polyamide | —CH2CH2NH— | Hg2+ > Cu2+ > Zn2+ > Ni2+ |
| Dithiocarboxylic acid | —CSSH | Ag+ > Cu2+ > Zn2+ > Mn2+ |
| Thiourea | —NC(NH2)S | Ag+ > Au3+ = Pd2+ > Hg2+ |
| Resin Type | Selectivity | PH | Max Capacity/mmol·g−1 |
|---|---|---|---|
| Amberlite IRC-748 | Cu2+ > Ni2+ > Co2+ | 5.0 | 1.060 |
| 4.0 | 1.252 | ||
| 1.0 | 2.000 | ||
| Amberlite IRC-718 | Fe2+ > Cu2+ > Zn2+ > Ni2+ | 5.0 | 2.250 |
| 4.0 | 0.95 | ||
| Lewatit TP-207 | Cu2+ > Zn2+ > Cd2+ | 5.0 | 0.87 |
| 4.0 | 1.38 | ||
| Chelex-100 | Cu2+ > Zn2+ > Cd2+ | 6.0 | 0.021 |
| 5.6 | 2.15 | ||
| Purolite S-930 | Cr3+ > Cu2+ > Ni2+> Zn2+ >Co2+ > Cd2+ > Fe2+ > Mn2+ | 4.0 | 0.89 |
| Lonac SR-5 | 1.0 | 1.25 | |
| Diaion CR-10 | 5.0 | 2.809 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Ju, K. Exploring Strategies for Copper Removal from Nickel Anolytes: A Review. ChemEngineering 2023, 7, 116. https://doi.org/10.3390/chemengineering7060116
Tang X, Ju K. Exploring Strategies for Copper Removal from Nickel Anolytes: A Review. ChemEngineering. 2023; 7(6):116. https://doi.org/10.3390/chemengineering7060116
Chicago/Turabian StyleTang, Xiaowei, and Kunyu Ju. 2023. "Exploring Strategies for Copper Removal from Nickel Anolytes: A Review" ChemEngineering 7, no. 6: 116. https://doi.org/10.3390/chemengineering7060116
APA StyleTang, X., & Ju, K. (2023). Exploring Strategies for Copper Removal from Nickel Anolytes: A Review. ChemEngineering, 7(6), 116. https://doi.org/10.3390/chemengineering7060116




