Optimizing Photocatalytic Lead Removal from Wastewater Using ZnO/ZrO2: A Response Surface Methodology Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of the Simulated Pb Contaminated Wastewater
2.3. Preparation Composite
2.4. Batch Experimental Setup of the Photocatalytic Process
2.5. Optimization by Response Surface Methodology (RSM)
2.6. Characterization of the ZnO/ZrO2 Photocatalyst
3. Results and Discussion
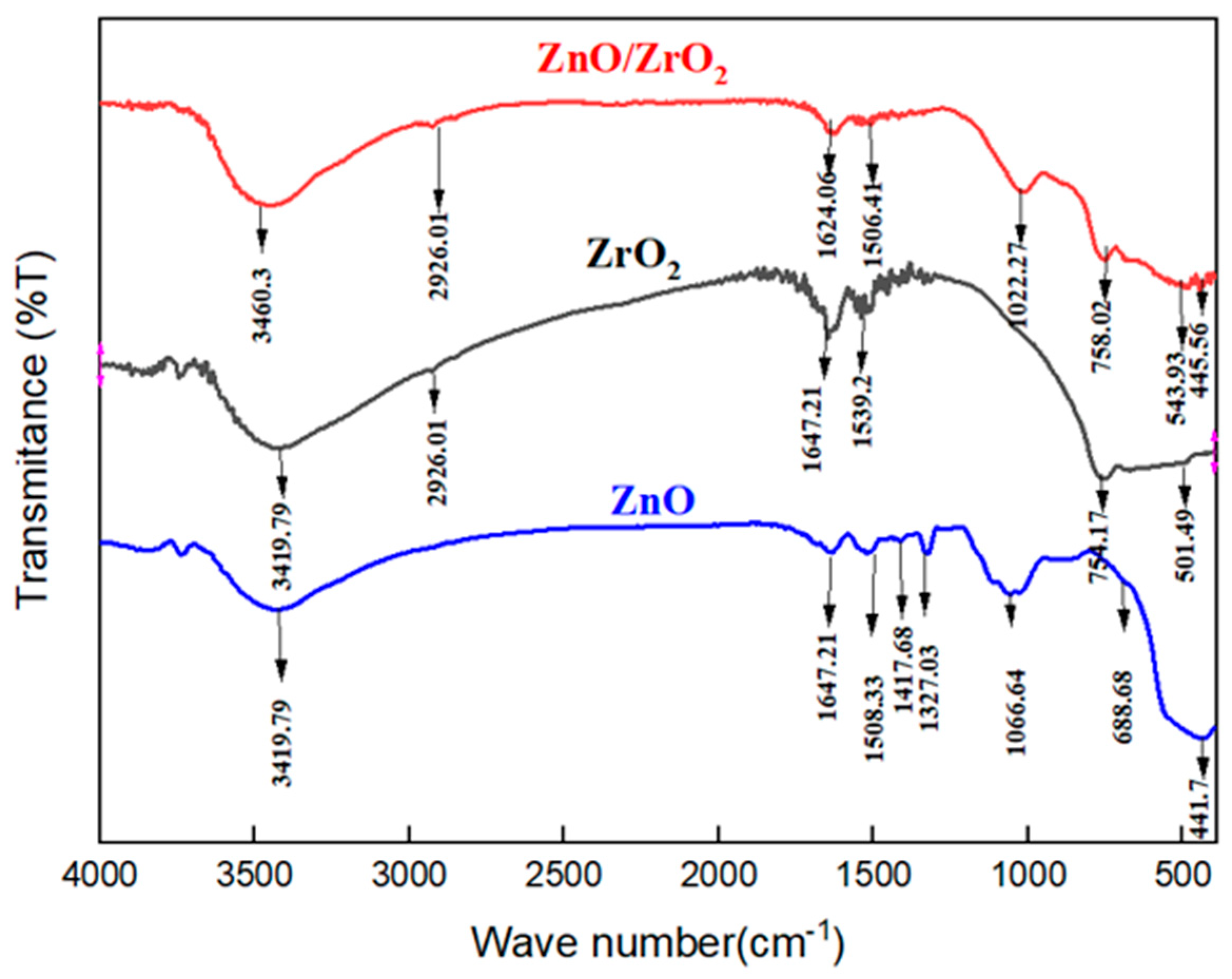
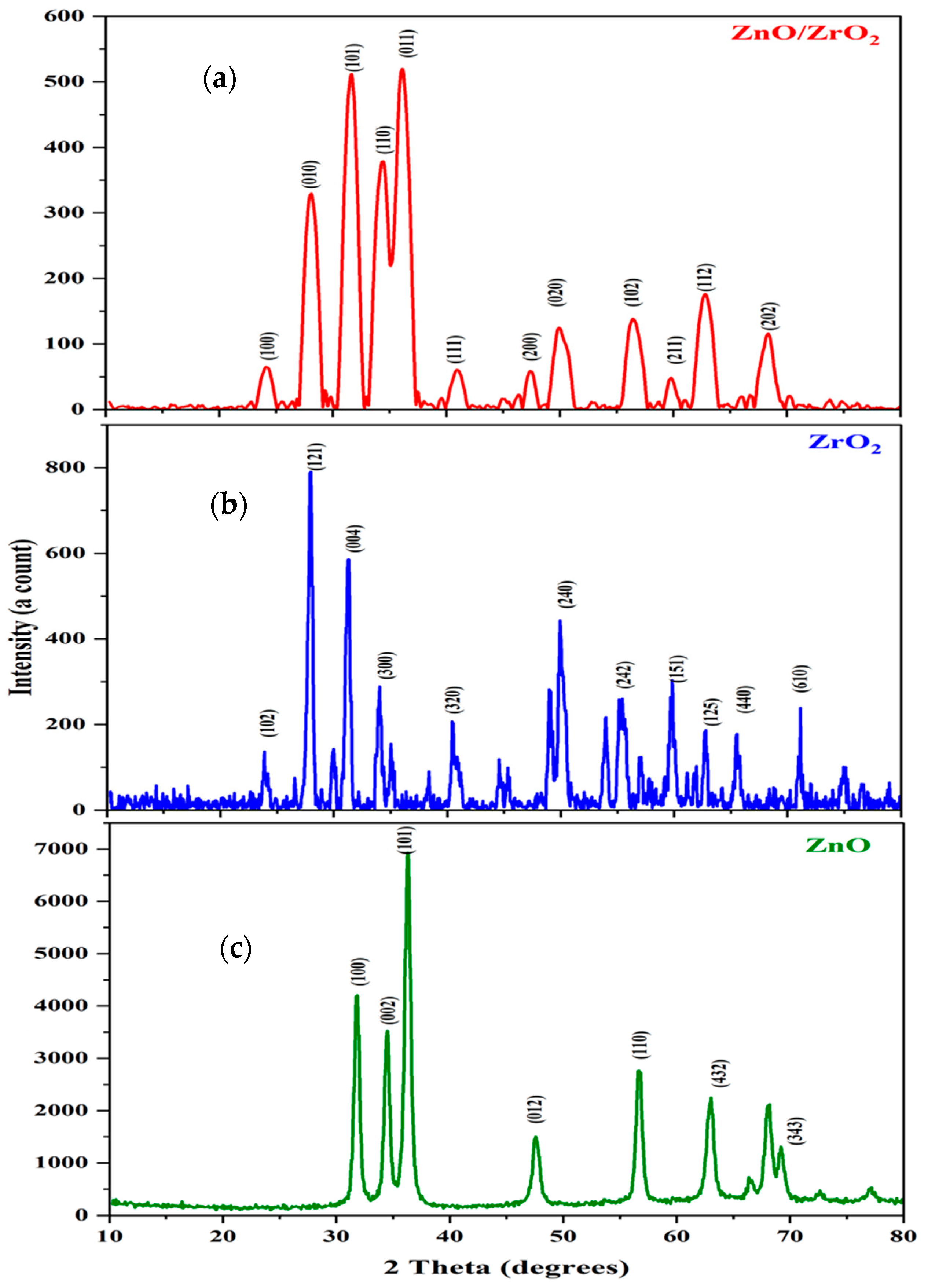
3.1. Characterization of the ZnO, ZrO2, and ZnO/ZrO2
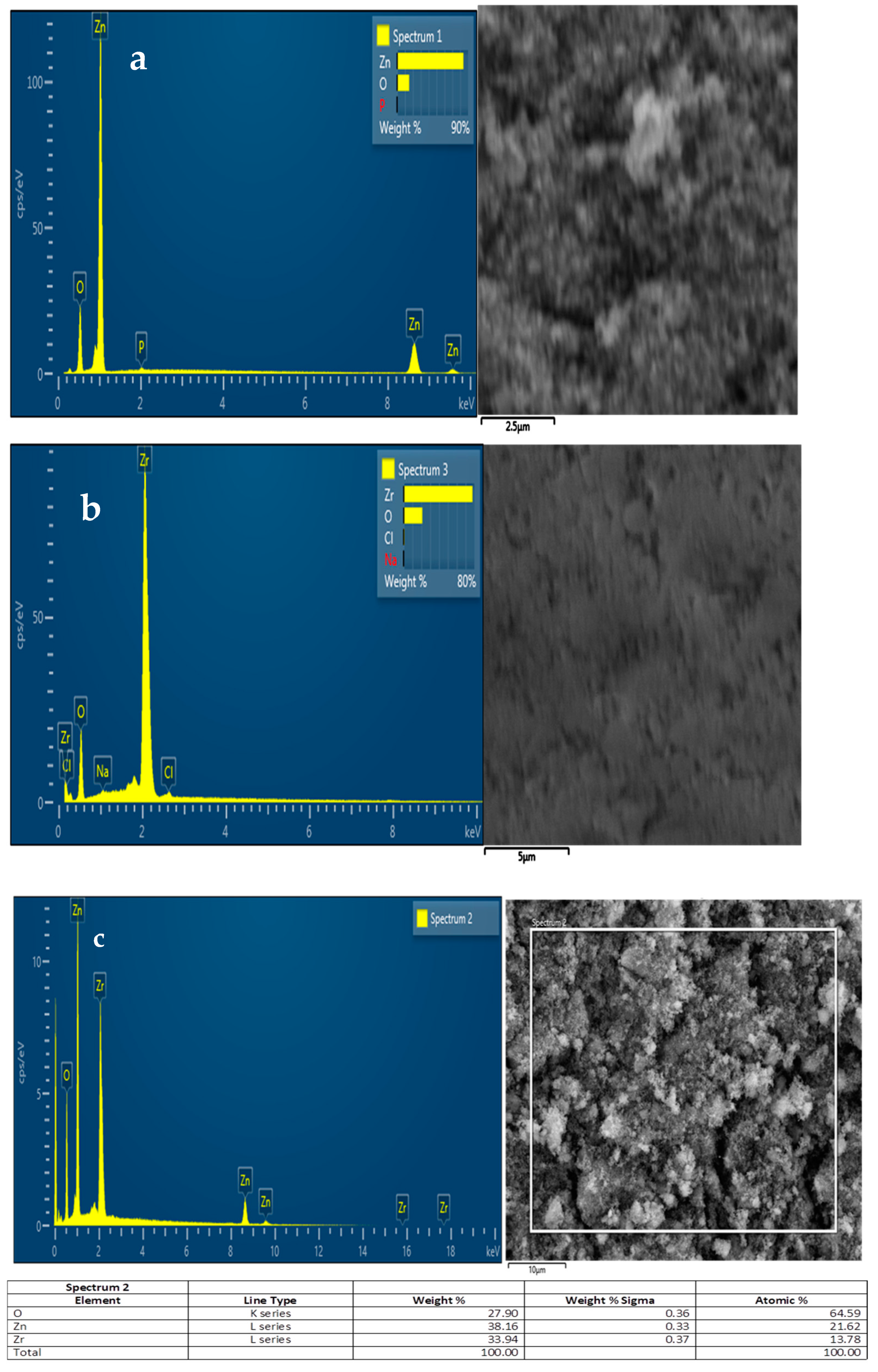
3.2. FESEM and EDS Analysis
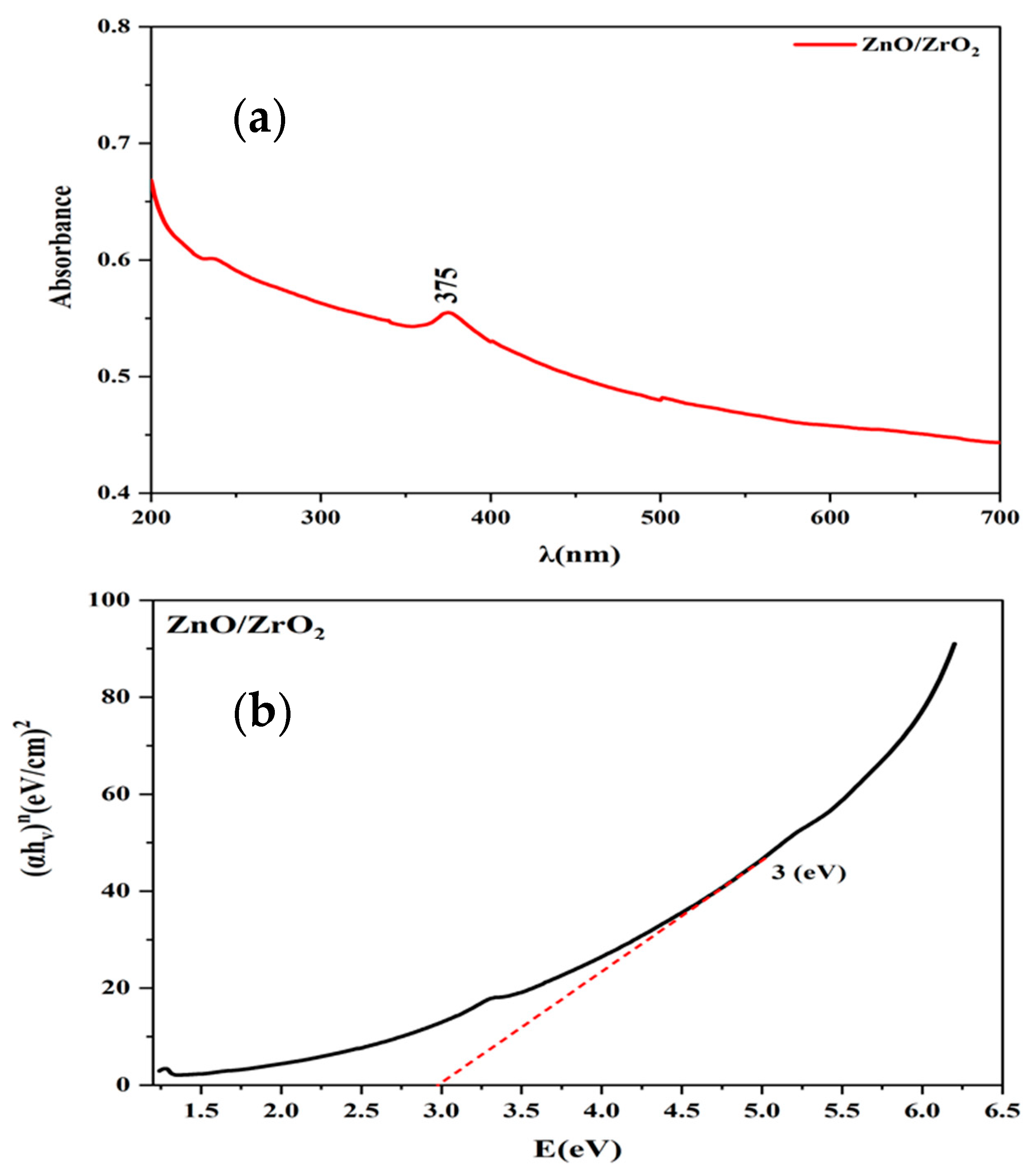
3.3. Energy Band Gap
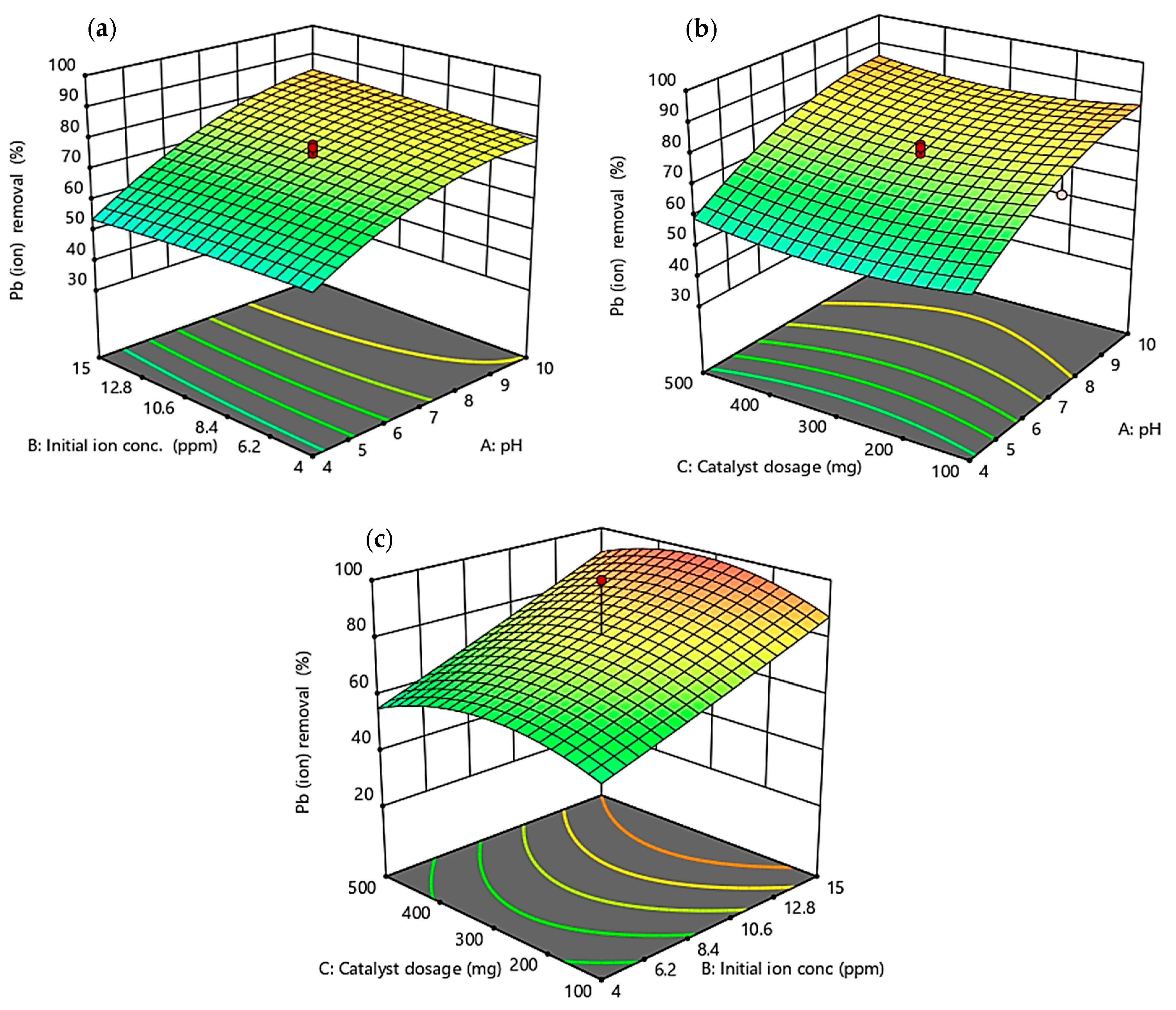
3.4. Response Surface Optimization
3.5. The Mechanism of Pb Removal from Wastewater
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ray, S.; Vashishth, R. From Water to Plate: Reviewing the Bioaccumulation of Heavy Metals in Fish and Unraveling Human Health Risks in the Food Chain. Emerg. Contam. 2024, 10, 100358. [Google Scholar] [CrossRef]
- Sable, H.; Singh, V.; Kumar, V.; Roy, A.; Pandit, S.; Kaur, K.; Rustagi, S.; Malik, S. Toxicological and bioremediation profiling of nonessential heavy metals (mercury, chromium, cadmium, aluminium) and their impact on human health: A review. Toxicol. Anal. Clin. 2024, in press. [Google Scholar] [CrossRef]
- Alsaffar, M.A.; Ghany, M.A.R.A.; Mageed, A.K.; AbdulRazak, A.A.; Ali, J.M.; Sukkar, K.A.; Ayodele, B.V. Effect of Textural Properties on the Degradation of Bisphenol from Industrial Wastewater Effluent in a Photocatalytic Reactor: A Modeling Approach. Appl. Sci. 2023, 13, 8966. [Google Scholar] [CrossRef]
- Raj, K.; Das, A.P. Lead pollution: Impact on environment and human health and approach for a sustainable solution. Environ. Chem. Ecotoxicol. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- Dave, D.M.; Yang, M. Lead in drinking water and birth outcomes: A tale of two water treatment plants. J. Health Econ. 2022, 84, 102644. [Google Scholar] [CrossRef] [PubMed]
- Alsaedi, S.S.; Mohammed, S.S.; Mahdi, A.E.; Shnain, Z.Y.; Sh Majdi, H.; AbdulRazak, A.A.; Alwasiti, A.A. Modeling spinel oxide based-photocatalytic degradation of organic pollutants from industrial wastewater. Chem. Eng. Commun. 2024, 211, 603–613. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K.; Oh, S. A critical review on lead removal from industrial wastewater: Recent advances and future outlook. J. Water Process. Eng. 2022, 45, 102518. [Google Scholar] [CrossRef]
- Ghorbani, M.; Seyedin, O.; Aghamohammadhassan, M. Adsorptive removal of lead (II) ion from water and wastewater media using carbon-based nanomaterials as unique sorbents: A review. J. Environ. Manage 2020, 254, 109814. [Google Scholar] [CrossRef] [PubMed]
- Dhokpande, S.R.; Deshmukh, S.M.; Khandekar, A.; Sankhe, A. A review outlook on methods for removal of heavy metal ions from wastewater. Sep. Purif. Technol. 2024, 350, 127868. [Google Scholar] [CrossRef]
- Fei, Y.; Hu, Y.H. Recent progress in removal of heavy metals from wastewater: A comprehensive review. Chemosphere 2023, 335, 139077. [Google Scholar] [CrossRef]
- Mishra, S.; Sundaram, B. A review of the photocatalysis process used for wastewater treatment. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Ali, H.M.; Arabpour Roghabadi, F.; Ahmadi, V. Solid-supported photocatalysts for wastewater treatment: Supports contribution in the photocatalysis process. Sol. Energy 2023, 255, 99–125. [Google Scholar] [CrossRef]
- Lee, D.-E.; Kim, M.-K.; Danish, M.; Jo, W.-K. State-of-the-art review on photocatalysis for efficient wastewater treatment: Attractive approach in photocatalyst design and parameters affecting the photocatalytic degradation. Catal. Commun. 2023, 183, 106764. [Google Scholar] [CrossRef]
- Hojjati-Najafabadi, A.; Farahbakhsh, E.; Gholamalian, G.; Feng, P.; Davar, F.; Aminabhavi, T.M.; Vasseghian, Y.; Kamyab, H.; Rahimi, H. Controllable synthesis of nanostructured flower-like cadmium sulfides for photocatalytic degradation of methyl orange under different light sources. J. Water Process. Eng. 2024, 59, 105002. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H.; Ammar, S.H.; Sabit, D.A.; Najim, A.A.; Radeef, A.Y.; Taher, A.G. The latest progress in the design and application of semiconductor photocatalysis systems for degradation of environmental pollutants in wastewater: Mechanism insight and theoretical calculations. Mater. Sci. Semicond. Process. 2024, 173, 108153. [Google Scholar] [CrossRef]
- Ceretta, M.B.; Vieira, Y.; Wolski, E.A.; Foletto, E.L.; Silvestri, S. Biological degradation coupled to photocatalysis by ZnO/polypyrrole composite for the treatment of real textile wastewater. J. Water Process. Eng. 2020, 35, 101230. [Google Scholar] [CrossRef]
- Jiad, M.M.; Abbar, A.H. Efficient wastewater treatment in petroleum refineries: Hybrid electro-fenton and photocatalysis (UV/ZnO) process. Chem. Eng. Res. Des. 2023, 200, 431–444. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Y.; Li, Q.; Xin, C.; Tian, Y.; Zhang, W.; Yu, X. Design of porous ZrO2 with well-tuned band structures and strong visible-light harvesting via Zn doping for enhanced visible-light photocatalysis. Chem. Eng. J. 2024, 481, 148489. [Google Scholar] [CrossRef]
- Gopal, V.; Harsha, S.; Selvaraj, A. Cow urine-based green synthesis of sunlight-responsive ZrO2-Bi2O3 and its application in photocatalysis of 2,4-Dichlorophenoxyactetic acid in aqueous solution—Kinetics, mechanisms and sustainability analysis. Catal. Commun. 2024, 187, 106869. [Google Scholar] [CrossRef]
- Maqbool, A.; Shahid, A.; Jahan, Z.; Niazi, M.B.K.; Inam, M.A.; Tawfeek, A.M.; Kamel, E.M.; Akhtar, M.S. Development of ZnO-GO-NiO membrane for removal of lead and cadmium heavy metal ions from wastewater. Chemosphere 2023, 338, 139622. [Google Scholar] [CrossRef]
- Karthik, P.; Ravichandran, S.; Prakash, N.; Mukkannan, A.; Rajesh, J. Evaluation of ZnO infused CA/PCL nanocomposites using potential wastewater treatment and invitro anticancer activity. Water Cycle 2024, 5, 121–130. [Google Scholar] [CrossRef]
- Geetha, M.; Vashisht, N.B.; Thanvir, S.; Roslan, N.C.; Mohamedzain, T.H.; Alfarwati, S.; Al-Lohedan, H.; Rajabathar, J.R.; Zaidi, S.A.; Sadasivuni, K.K. Multi-functional nanoscale ZrO2 catalysts for sustainable water treatment. Mater. Chem. Phys. 2024, 316, 129096. [Google Scholar] [CrossRef]
- Wahba, M.A.; Yakout, S.M.; Mohamed, W.A.A.; Galal, H.R. Remarkable photocatalytic activity of Zr doped ZnO and ZrO2/ZnO nanocomposites: Structural, morphological and photoluminescence properties. Mater. Chem. Phys. 2020, 256, 123754. [Google Scholar] [CrossRef]
- Majhool, A.K.; Sukkar, K.A.; Alsaffar, M.A. Combining α-Al2O3 Packing Material and a ZnO Nanocatalyst in an Ozonized Bubble Column Reactor to Increase the Phenol Degradation from Wastewater. Processes 2023, 11, 2416. [Google Scholar] [CrossRef]
- Majhool, A.K.; Sukkar, K.A.; Alsaffar, M.A.; Majdi, H.S. Integrated Process for High Phenol Removal from Wastewater Employing a ZnO Nanocatalyst in an Ozonation Reaction in a Packed Bubble Column Reactor. ChemEngineering 2023, 7, 112. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Photocatalytic activity of nanostructured ZnO–ZrO2 binary oxide using fluorometric method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 145, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.S.; Alsaffar, M.A.; AbdulRazak, A.A. One-step synthesis of magnetic fly ash composites for methylene blue removal: Batch and column study. Environ. Sci. Pollut. Res. 2023, 30, 124748–124766. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.; Chen, L.; Mao, X. Response surface methodology for process optimization in livestock wastewater treatment: A review. Heliyon 2024, 10, e30326. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Ahmadi, A.; Heidarzadeh, S.; Mokhtari, A.R.; Darezereshki, E.; Harouni, H.A. Optimization of heavy metal removal from aqueous solutions by maghemite (γ-Fe2O3) nanoparticles using response surface methodology. J. Geochem. Explor. 2014, 147, 151–158. [Google Scholar] [CrossRef]
- Maleki, S.; Karimi-Jashni, A.; Mousavifard, M. Removal of Ni(II) ions from wastewater by ion exchange resin: Process optimization using response surface methodology and ensemble machine learning techniques. J. Environ. Chem. Eng. 2024, 12, 112417. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. Lead (Pb) removal from contaminated water using constructed wetland planted with Scirpus grossus: Optimization using response surface methodology (RSM) and assessment of rhizobacterial addition. Chemosphere 2022, 291, 132952. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.K.; Pandey, L.M.; Uppaluri, R.V.S. Adsorptive removal of Zn, Fe, and Pb from Zn dominant simulated industrial wastewater solution using polyvinyl alcohol grafted chitosan variant resins. Chem. Eng. J. 2023, 459, 141563. [Google Scholar] [CrossRef]
- Biju, R.; Ravikumar, R.; Raghavan, J.R.V.; Indulal, C.R. Nanocomposite of zinc zirconia for better degradation of an organic dye. Bull. Mater. Sci. 2022, 45, 180. [Google Scholar] [CrossRef]
- Alsaffar, M.A.; Rashid, S.A.; Ayodele, B.V.; Hamidon, M.N.; Yasin, F.M.; Ismail, I.; Hosseini, S.; Babadi, F.E. Response Surface Optimization of Multilayer Graphene Growth on Alumina-Supported Bimetallic Cobalt–Nickel Substrate. Arab. J. Sci. Eng. 2020, 45, 7455–7465. [Google Scholar] [CrossRef]
- Pandey, J.; Shrivastava, V.; Nagarajan, R. Metastable Bi2Zr2O7 with Pyrochlore-like Structure: Stabilization, Oxygen Ion Conductivity, and Catalytic Properties. Inorg. Chem. 2018, 57, 13667–13678. [Google Scholar] [CrossRef] [PubMed]
- Aghabeygi, S.; Khademi-Shamami, M. ZnO/ZrO2 nanocomposite: Sonosynthesis, characterization and its application for wastewater treatment. Ultrason. Sonochem. 2018, 41, 458–465. [Google Scholar] [CrossRef]
- Khalili, S.; Chenari, H.M. The influence of ZrO2 addition on the morphological, structural, vibrational, and optical characteristics of composite fibers based on ZnO. Thin Solid Film. 2022, 741, 139031. [Google Scholar] [CrossRef]
- Haq, S.; Afsar, H.; Ali MBen Almalki, M.; Albogami, B.; Hedfi, A. Green Synthesis and Characterization of a ZnO-ZrO2 Heterojunction for Environmental and Biological Applications. Crystals 2021, 11, 1502. [Google Scholar] [CrossRef]
- Ban, T.; Sakai, T.; Ohya, Y. Synthesis of zinc oxide crystals with different shapes from zincate aqueous solutions stabilized with triethanolamine. Cryst. Res. Technol. 2007, 42, 849–855. [Google Scholar] [CrossRef]
- Reddy, C.h.V.; Babu, B.; Reddy, I.N.; Shim, J. Synthesis and characterization of pure tetragonal ZrO2 nanoparticles with enhanced photocatalytic activity. Ceram. Int. 2018, 44, 6940–6948. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Effect of annealing temperatures on properties of sol-gel grown ZnO-ZrO2 films. Cryst. Res. Technol. 2010, 45, 1154–1160. [Google Scholar] [CrossRef]
- Arote, S.A.; Pathan, A.S.; Hase, Y.V.; Bardapurkar, P.P.; Gapale, D.L.; Palve, B.M. Investigations on synthesis, characterization and humidity sensing properties of ZnO and ZnO-ZrO2 composite nanoparticles prepared by ultrasonic assisted wet chemical method. Ultrason. Sonochem. 2019, 55, 313–321. [Google Scholar] [CrossRef]
- Deepika, R.; Veerakumar, P. Microwave-assisted hydrothermal synthesis of ZnO@ZrO2 nanohybrid for biomedical and photocatalytic applications. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133574. [Google Scholar] [CrossRef]
- Fatimah, S.; Ragadhita, R.; Fitria, D.; Husaeni, A.; Bayu, A.; Nandiyanto, D. How to Calculate Crystallite Size from X-ray Diffraction (XRD) using Scherrer Method. ASEAN J. Sci. Eng. 2021, 2, 65–76. [Google Scholar] [CrossRef]
- Priyadarshini, R.; Titus, A.; Sahoo, S.; Muppala, C.; Ramkumar, G.; Anh Pham, Q.; Rubavathy, S.J.; Rajasimman, M.; Hojjati-Najafabadi, A. Deep learning for the encounter of inorganic nanomaterial for efficient photochemical hydrogen production. Int. J. Hydrogen Energy 2024, 52, 664–673. [Google Scholar] [CrossRef]


| Factor | Low | High |
|---|---|---|
| pH | 4 | 10 |
| Initial concentration (ppm) | 4 | 15 |
| ZnO/ZrO2 dosage (mg) | 100 | 500 |
| Run | A:pH | B: Initial Ion Conc | C:Dosage | Pb Removal |
|---|---|---|---|---|
| (ppm) | mg | % | ||
| 1 | 4 | 4 | 500 | 63.45 |
| 2 | 7 | 9.5 | 100 | 72.34 |
| 3 | 1.84344 | 9.5 | 300 | 36.23 |
| 4 | 10 | 4 | 100 | 82.12 |
| 5 | 4 | 4 | 100 | 55.34 |
| 6 | 10 | 15 | 500 | 88.21 |
| 7 | 10 | 4 | 100 | 83.23 |
| 8 | 7 | 9.5 | 300 | 75.23 |
| 9 | 7 | 9.5 | 643.77 | 86.23 |
| 10 | 7 | 9.5 | 300 | 71.13 |
| 11 | 7 | 9.5 | 300 | 73.13 |
| 12 | 7 | 9.5 | 300 | 74.21 |
| 13 | 10 | 15 | 100 | 94.23 |
| 14 | 7 | 9.5 | 300 | 76.32 |
| 15 | 7 | 18.9537 | 300 | 75.23 |
| 16 | 4 | 15 | 500 | 52.23 |
| 17 | 4 | 4 | 500 | 65.35 |
| 18 | 7 | 9.5 | 300 | 78.23 |
| 19 | 10 | 15 | 100 | 86.21 |
| 20 | 10 | 15 | 500 | 83.24 |
| 21 | 7 | 9.5 | 300 | 77.23 |
| 22 | 12.1566 | 9.5 | 300 | 80.12 |
| 23 | 4 | 4 | 100 | 57.45 |
| 24 | 7 | 0.046316 | 300 | 70.23 |
| 25 | 10 | 4 | 500 | 84.23 |
| 26 | 4 | 15 | 100 | 63.26 |
| 27 | 10 | 4 | 500 | 84.13 |
| 28 | 7 | 9.5 | 300 | 77.23 |
| 29 | 4 | 15 | 100 | 60.23 |
| 30 | 4 | 15 | 500 | 51.22 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 4750.91 | 9 | 527.88 | 57.05 | <0.0001 | Significant |
| A-pH | 3905.61 | 1 | 3905.61 | 422.09 | <0.0001 | |
| B-conc. | 6.7 | 1 | 6.7 | 0.7242 | 0.049 | |
| C-dose | 0.1463 | 1 | 0.1463 | 0.0158 | 0.9012 | |
| AB | 67.35 | 1 | 67.35 | 7.28 | 0.0138 | |
| AC | 0.2367 | 1 | 0.2367 | 0.0256 | 0.8745 | |
| BC | 144.25 | 1 | 144.25 | 15.59 | 0.0008 | |
| A2 | 532.71 | 1 | 532.71 | 57.57 | <0.0001 | |
| B2 | 6.86 | 1 | 6.86 | 0.7412 | 0.3995 | |
| C2 | 132.45 | 1 | 132.45 | 14.31 | 0.0012 | |
| Residual | 185.06 | 20 | 9.25 | |||
| Lack of Fit | 90.5 | 5 | 18.1 | 2.87 | 0.0516 | Not significant |
| Pure Error | 94.56 | 15 | 6.3 | |||
| Cor Total | 4935.97 | 29 |
| Number | pH | Conc | Dosage | Pb Removal | Desirability | |
|---|---|---|---|---|---|---|
| 1 | 7.448 | 12.029 | 103.783 | 81.696 | 1 | |
| 2 | 10 | 4 | 100 | 79.997 | 1 | |
| 3 | 10 | 15 | 500 | 84.787 | 1 | |
| 4 | 10 | 15 | 100 | 91.212 | 1 | Selected |
| 5 | 7 | 9.5 | 300 | 74.844 | 1 |
| Run Order | Actual Value | Predicted Value | Residual | Leverage | Internally Studentized Residuals | Externally Studentized Residuals | Cook’s Distance | Influence on Fitted Value DFFITS | Standard Order |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 63.45 | 63.23 | 0.2272 | 0.371 | 0.094 | 0.092 | 0.001 | 0.07 | 5 |
| 2 | 72.34 | 78.32 | −5.98 | 0.189 | −2.181 | −2.435 | 0.111 | −1.174 | 21 |
| 3 | 36.23 | 35.47 | 0.7565 | 0.536 | 0.365 | 0.357 | 0.015 | 0.384 | 17 |
| 4 | 82.12 | 80 | 2.13 | 0.391 | 0.896 | 0.891 | 0.052 | 0.714 | 10 |
| 5 | 55.34 | 57.15 | −1.81 | 0.391 | −0.763 | −0.755 | 0.037 | −0.605 | 9 |
| 6 | 88.21 | 84.79 | 3.42 | 0.371 | 1.419 | 1.458 | 0.119 | 1.12 | 8 |
| 7 | 83.23 | 80 | 3.23 | 0.391 | 1.363 | 1.394 | 0.119 | 1.118 | 2 |
| 8 | 75.23 | 74.84 | 0.3904 | 0.119 | 0.137 | 0.133 | 0 | 0.049 | 27 |
| 9 | 86.23 | 84.7 | 1.53 | 0.615 | 0.81 | 0.803 | 0.105 | 1.014 | 22 |
| 10 | 71.13 | 74.84 | −3.71 | 0.119 | −1.3 | −1.324 | 0.023 | −0.487 | 26 |
| 11 | 73.13 | 74.84 | −1.71 | 0.119 | −0.6 | −0.59 | 0.005 | −0.217 | 24 |
| 12 | 74.21 | 74.84 | −0.6326 | 0.119 | −0.222 | −0.216 | 0.001 | −0.08 | 23 |
| 13 | 94.23 | 91.21 | 3.02 | 0.391 | 1.272 | 1.293 | 0.104 | 1.036 | 4 |
| 14 | 76.32 | 74.84 | 1.48 | 0.119 | 0.518 | 0.508 | 0.004 | 0.187 | 25 |
| 15 | 75.23 | 73.93 | 1.3 | 0.536 | 0.629 | 0.619 | 0.046 | 0.666 | 20 |
| 16 | 52.23 | 54.22 | −1.99 | 0.371 | −0.826 | −0.819 | 0.04 | −0.629 | 15 |
| 17 | 65.34 | 63.23 | 2.12 | 0.371 | 0.878 | 0.873 | 0.045 | 0.67 | 13 |
| 18 | 78.23 | 74.84 | 3.39 | 0.119 | 1.187 | 1.2 | 0.019 | 0.442 | 29 |
| 19 | 86.21 | 91.21 | −5 | 0.391 | −2.107 | −2.328 | 0.285 | −1.866 | 12 |
| 20 | 83.24 | 84.79 | −1.55 | 0.371 | −0.641 | −0.631 | 0.024 | −0.485 | 16 |
| 21 | 77.23 | 74.84 | 2.39 | 0.119 | 0.836 | 0.83 | 0.009 | 0.305 | 28 |
| 22 | 80.12 | 81.37 | −1.25 | 0.536 | −0.603 | −0.593 | 0.042 | −0.638 | 18 |
| 23 | 57.45 | 57.15 | 0.2992 | 0.391 | 0.126 | 0.123 | 0.001 | 0.099 | 1 |
| 24 | 70.23 | 72.03 | −1.8 | 0.536 | −0.867 | −0.861 | 0.087 | −0.927 | 19 |
| 25 | 84.23 | 85.58 | −1.35 | 0.371 | −0.56 | −0.55 | 0.019 | −0.423 | 6 |
| 26 | 63.26 | 60.16 | 3.09 | 0.391 | 1.304 | 1.328 | 0.109 | 1.065 | 11 |
| 27 | 84.13 | 85.58 | −1.45 | 0.371 | −0.601 | −0.591 | 0.021 | −0.454 | 14 |
| 28 | 77.23 | 74.84 | 2.39 | 0.119 | 0.836 | 0.83 | 0.009 | 0.305 | 30 |
| 29 | 60.23 | 60.16 | 0.0722 | 0.391 | 0.03 | 0.03 | 0 | 0.024 | 3 |
| 30 | 51.22 | 54.22 | −3 | 0.371 | −1.244 | −1.263 | 0.091 | −0.97 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakir, H.A.; Alsaffar, M.A.; Mageed, A.K.; Sukkar, K.A.; Ghany, M.A.A. Optimizing Photocatalytic Lead Removal from Wastewater Using ZnO/ZrO2: A Response Surface Methodology Approach. ChemEngineering 2024, 8, 72. https://doi.org/10.3390/chemengineering8040072
Shakir HA, Alsaffar MA, Mageed AK, Sukkar KA, Ghany MAA. Optimizing Photocatalytic Lead Removal from Wastewater Using ZnO/ZrO2: A Response Surface Methodology Approach. ChemEngineering. 2024; 8(4):72. https://doi.org/10.3390/chemengineering8040072
Chicago/Turabian StyleShakir, Hiba Abduladheem, May Ali Alsaffar, Alyaa K. Mageed, Khalid A. Sukkar, and Mohamed A. Abdel Ghany. 2024. "Optimizing Photocatalytic Lead Removal from Wastewater Using ZnO/ZrO2: A Response Surface Methodology Approach" ChemEngineering 8, no. 4: 72. https://doi.org/10.3390/chemengineering8040072
APA StyleShakir, H. A., Alsaffar, M. A., Mageed, A. K., Sukkar, K. A., & Ghany, M. A. A. (2024). Optimizing Photocatalytic Lead Removal from Wastewater Using ZnO/ZrO2: A Response Surface Methodology Approach. ChemEngineering, 8(4), 72. https://doi.org/10.3390/chemengineering8040072







