Building a Code-Based Model to Describe Syngas Production from Biomass
Abstract
1. Introduction
- Includes the creation of a kinetic gasification model.
- Universally applicable for all biomass types.
- Contains all important reactor types (fixed bed and fluidized bed reactor).
- Includes essential process parameters and substance mass flows.
- Contains enough reactions with reaction rates derived from scientific studies.
- Allows integration into the DWSIM process simulator.
- Validation with experimental data from the literature is feasible.
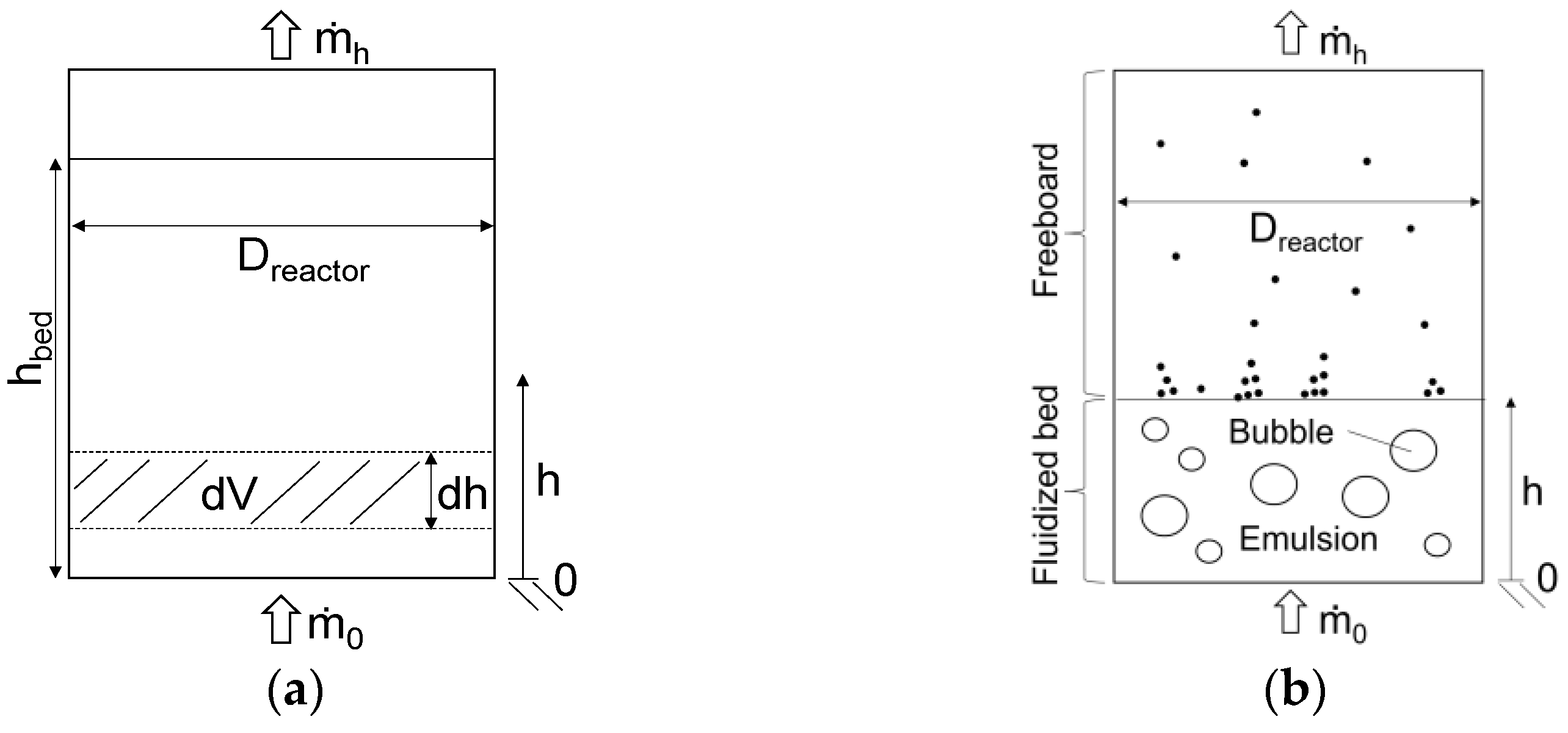
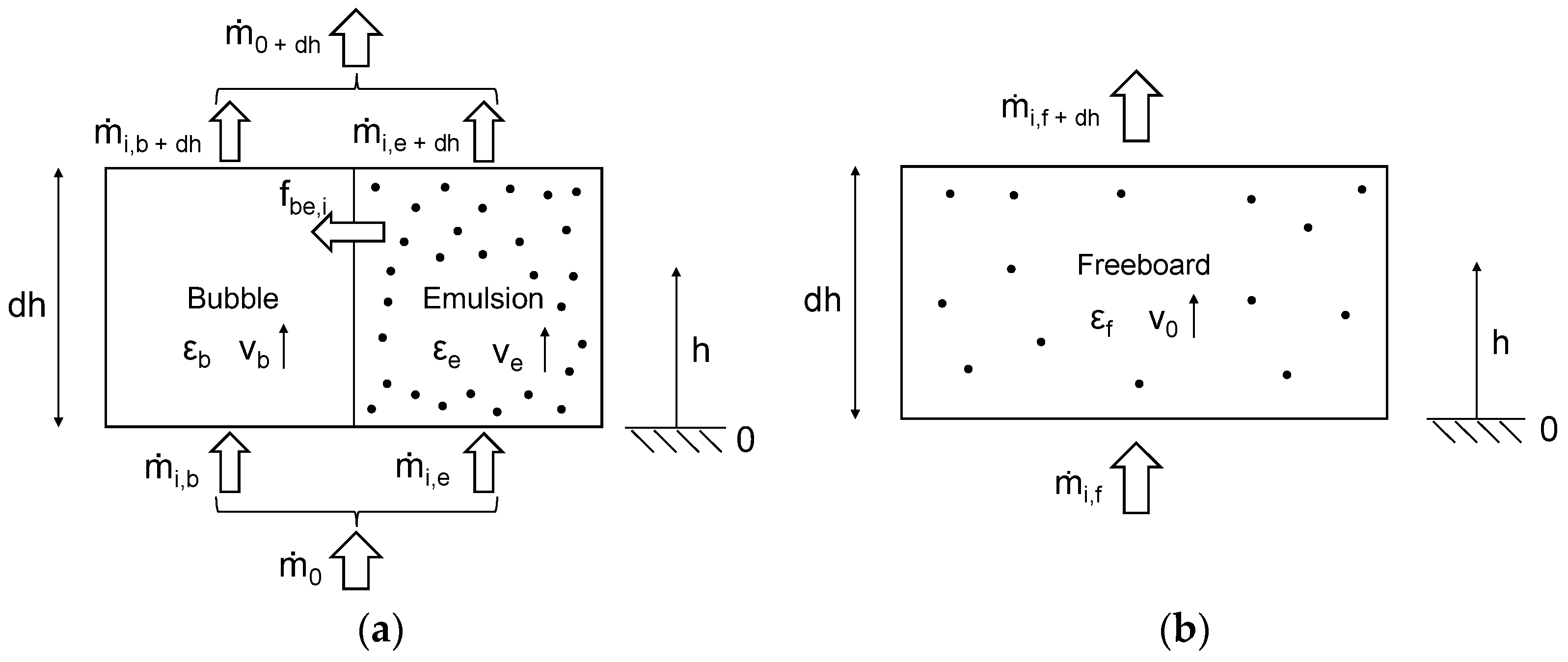
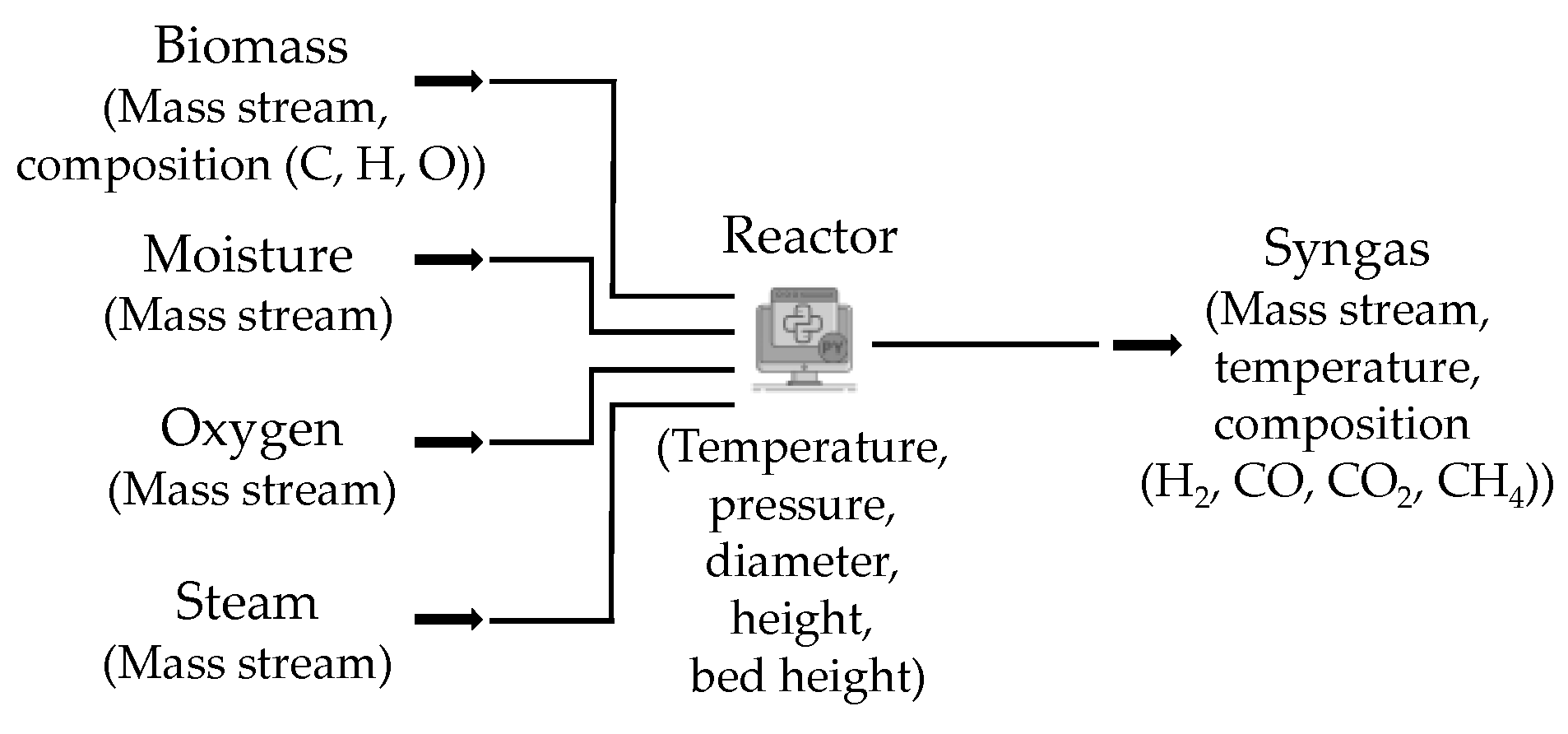
2. Modeling Method
- All gaseous components have ideal behavior.
- Char consists entirely of carbon.
- Tar is a mixture of condensable organics. It is represented by ethyne (C2H2).
- The pressure is uniform in the reactor. No pressure losses take place.
- Particle abrasion, agglomeration, and fragmentation are neglected.
- The reactor is considered ideal. Dispersion effects are neglected.
- Mass stream of incoming biomass.
- Biomass specification into the mass fractions of carbon (C), hydrogen (H), and oxygen (O).
- Moisture content of incoming biomass.
- Mass stream of added oxygen.
- Mass stream of added steam.
- Process temperature and pressure.
- Reactor dimensions: diameter, total height, and bed height.
3. Results
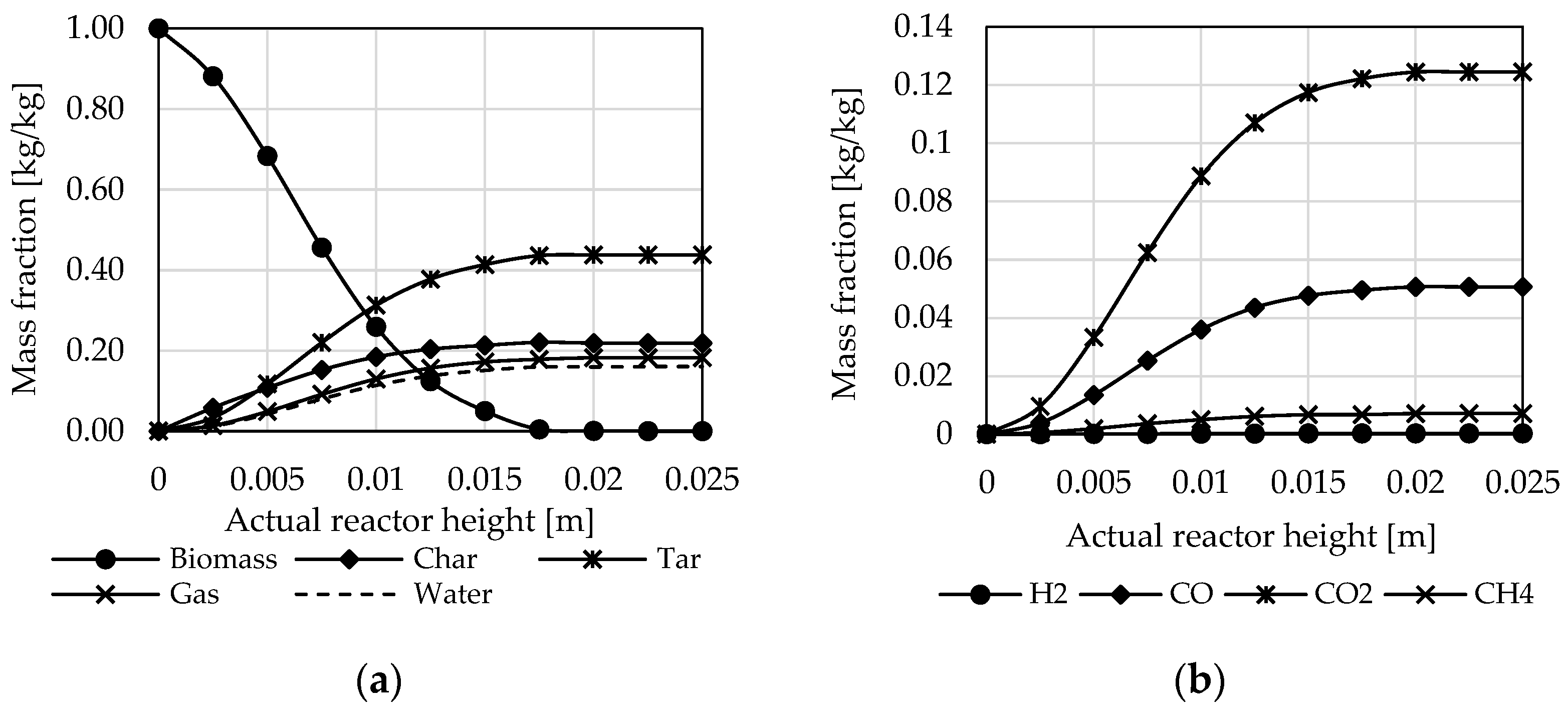
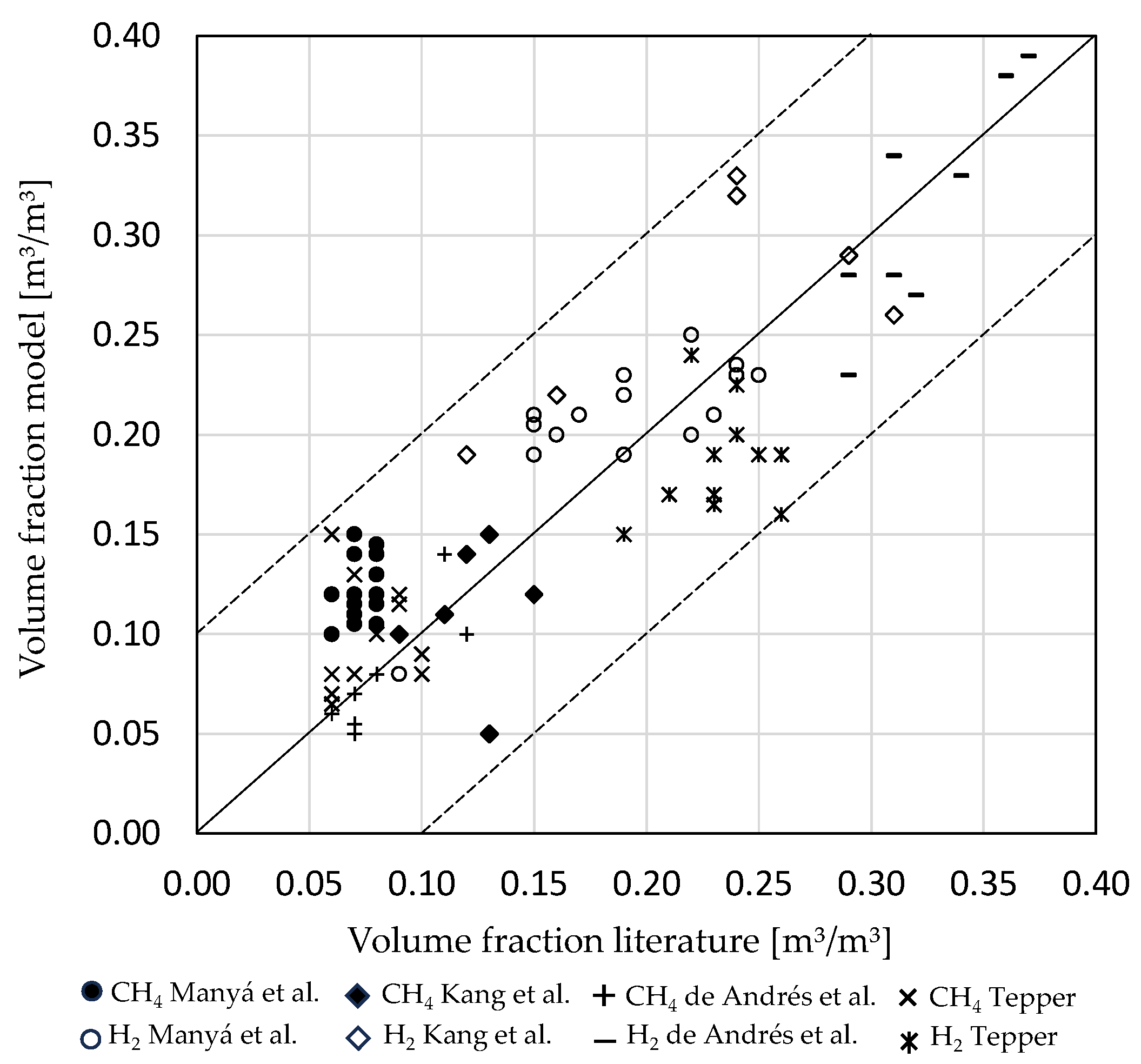
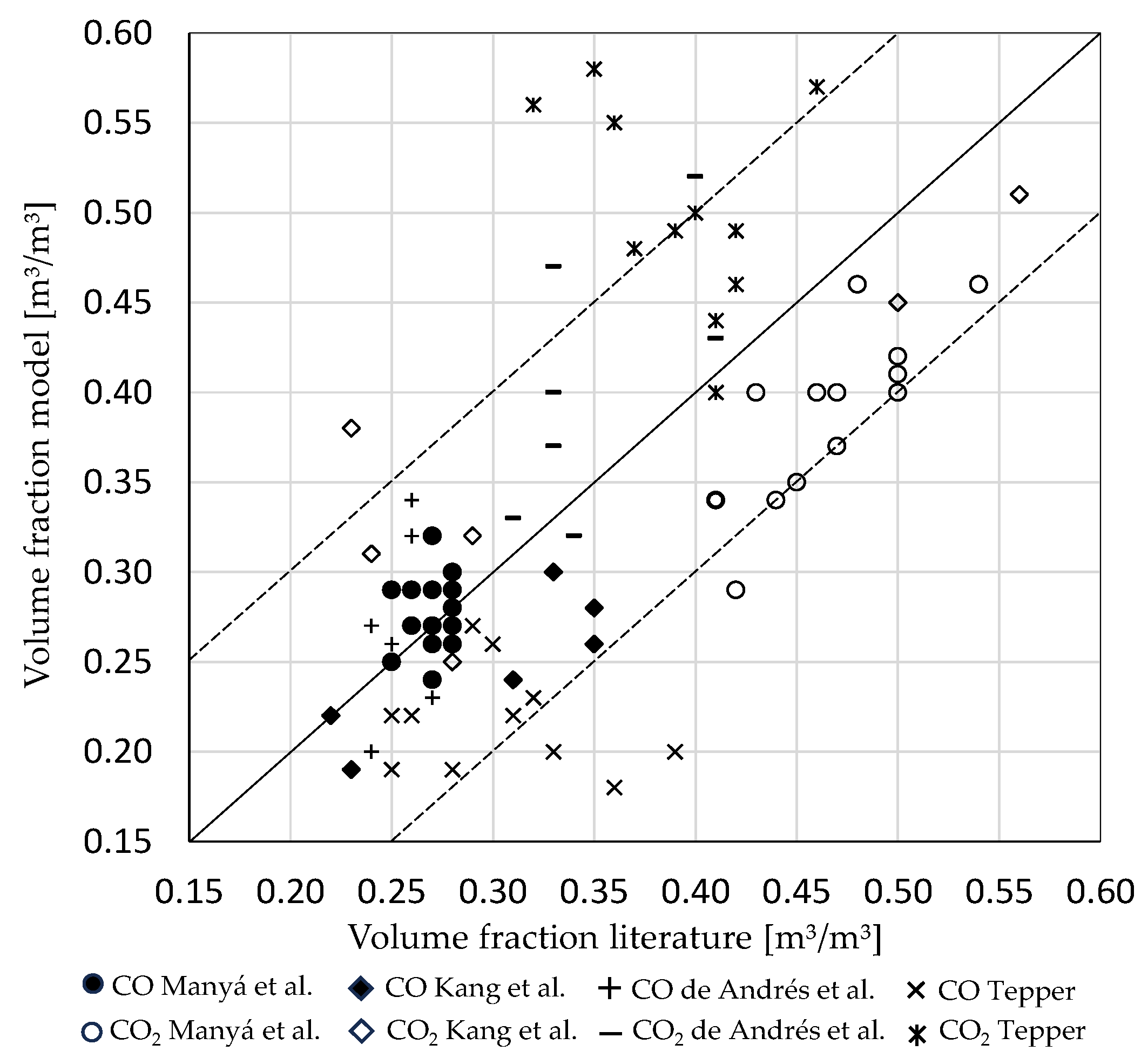
3.1. Influence of Biomass Composition
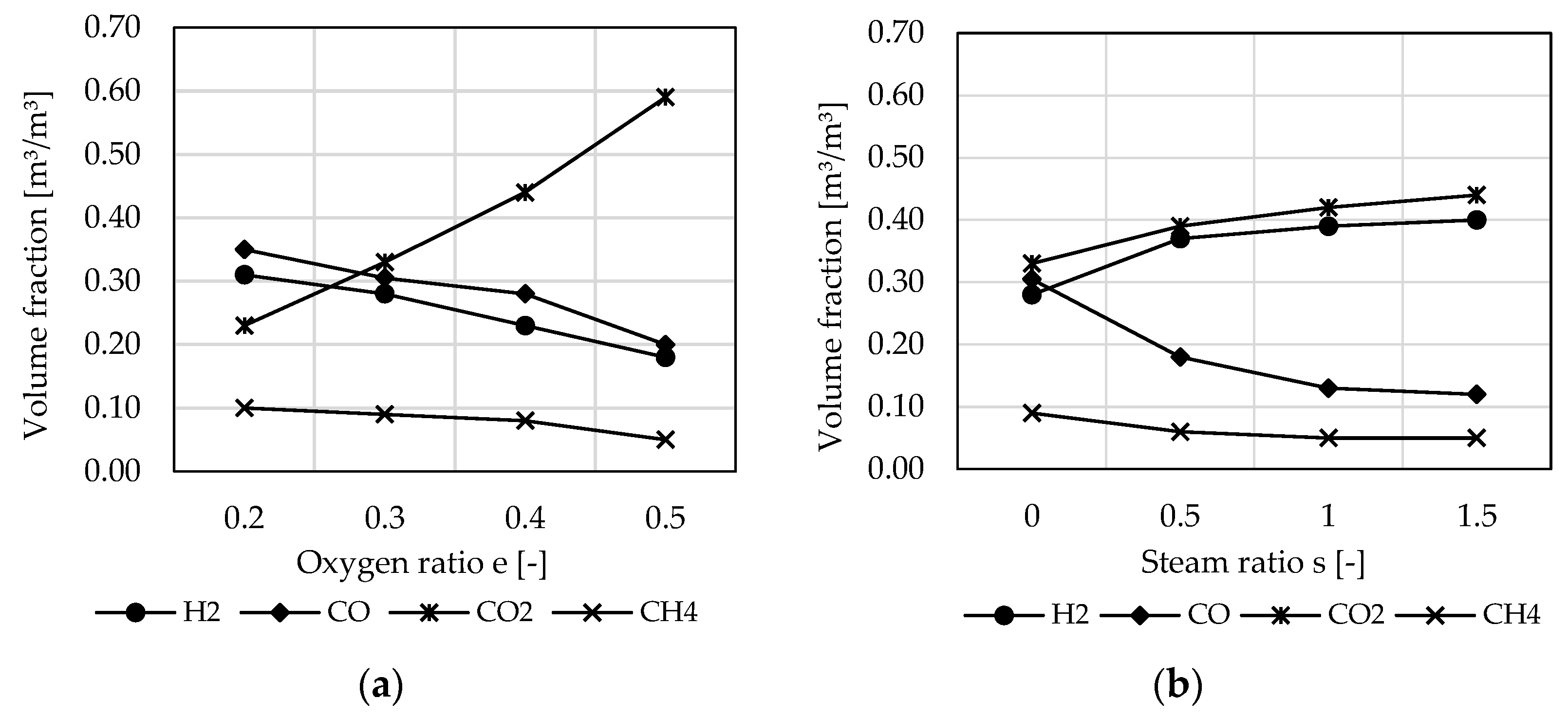
3.2. Influence of Incoming Mass Stream Ratios
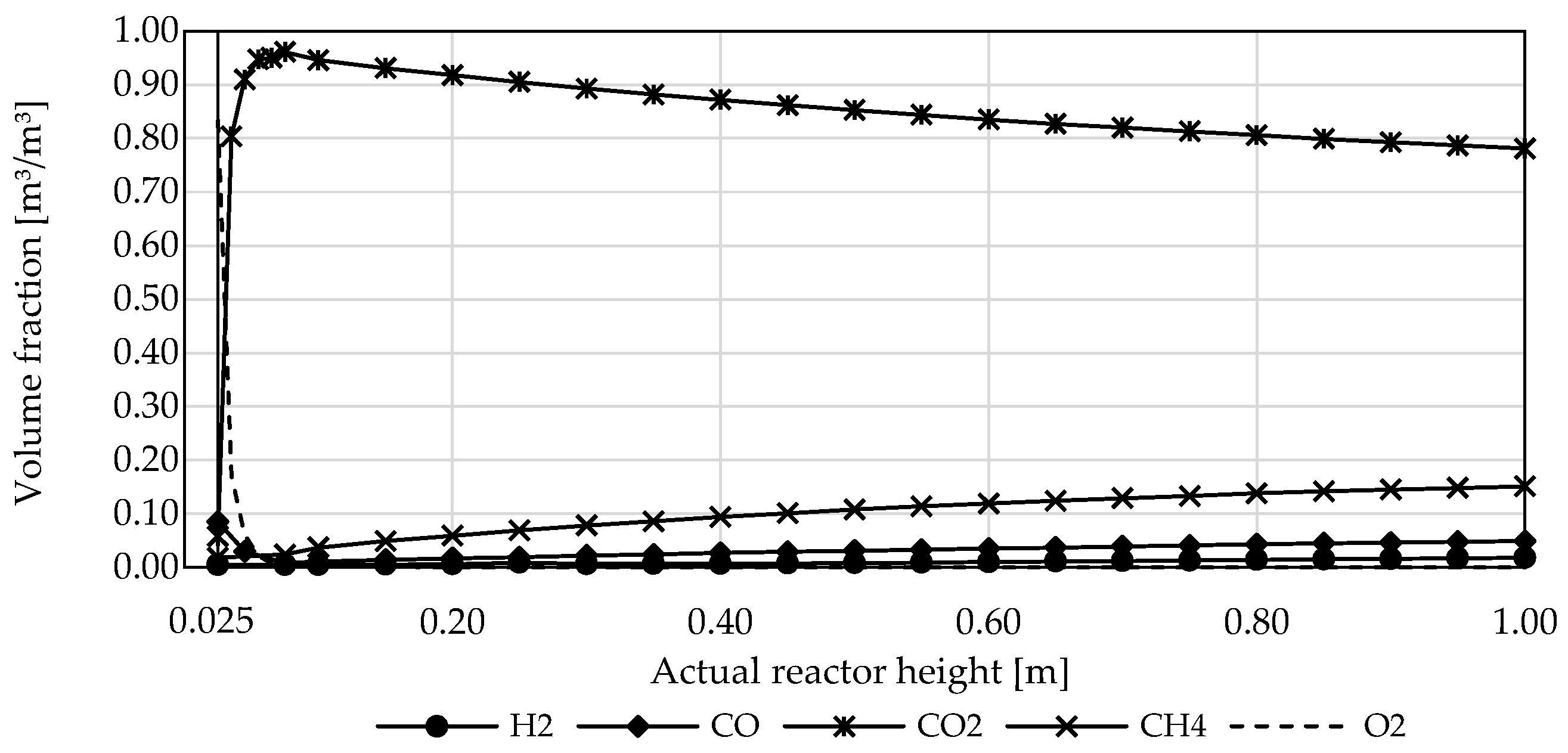
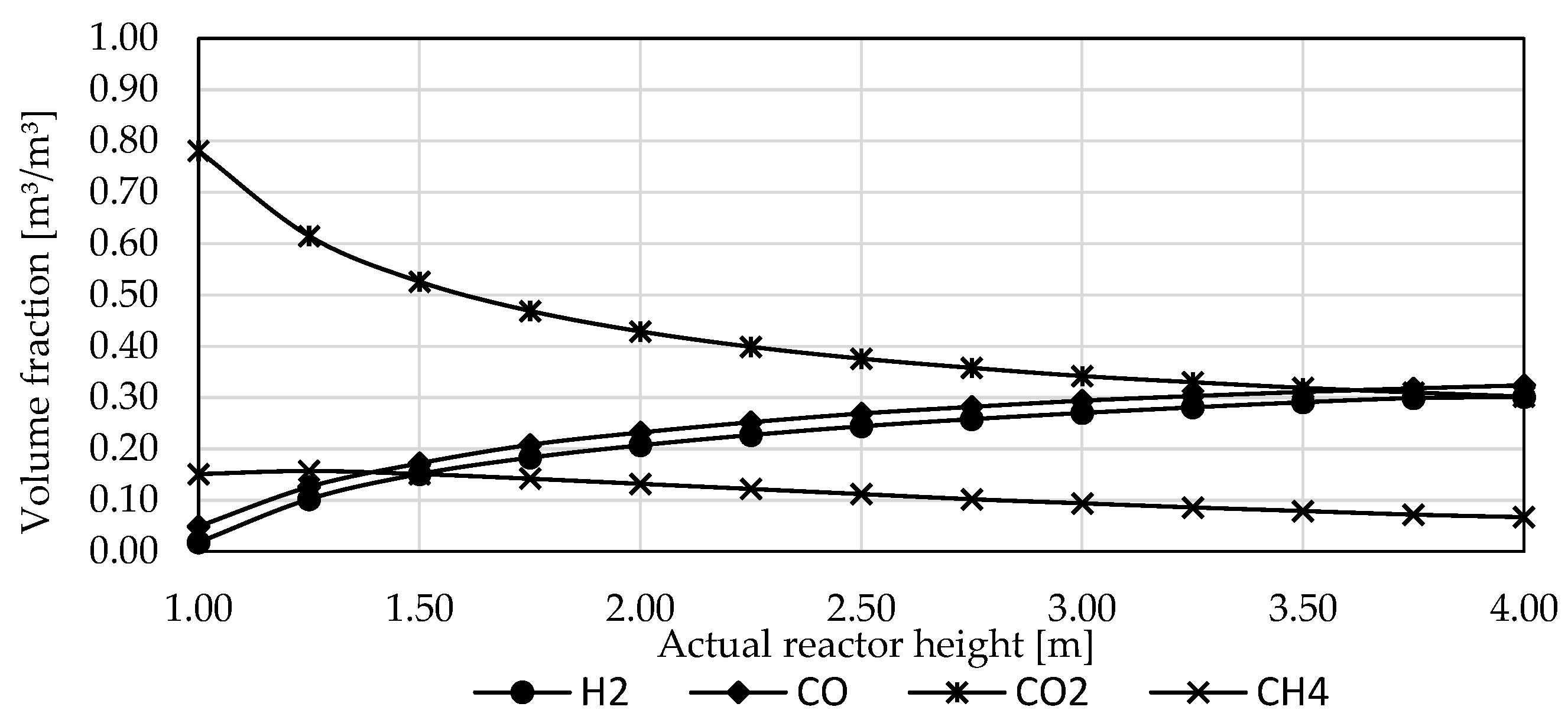
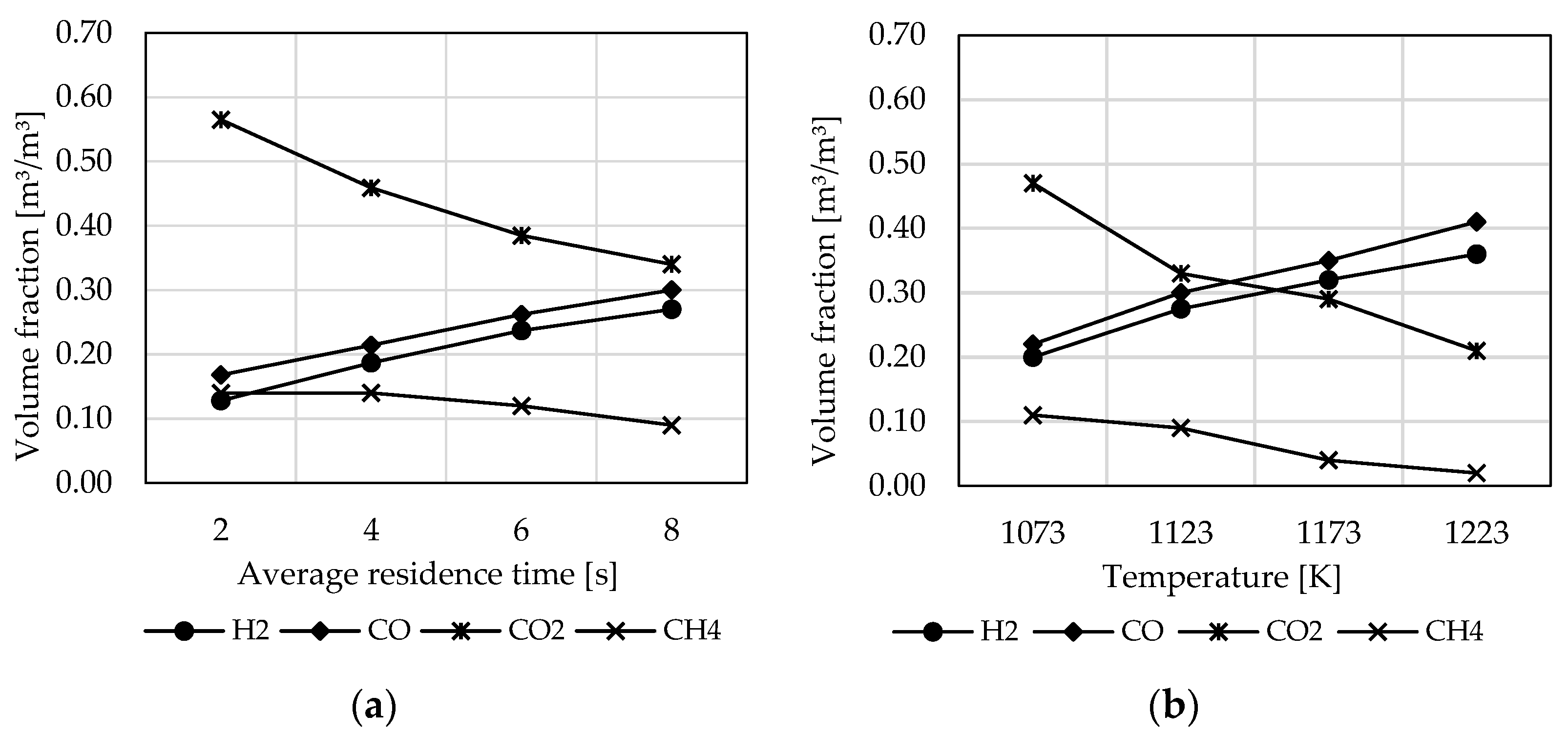
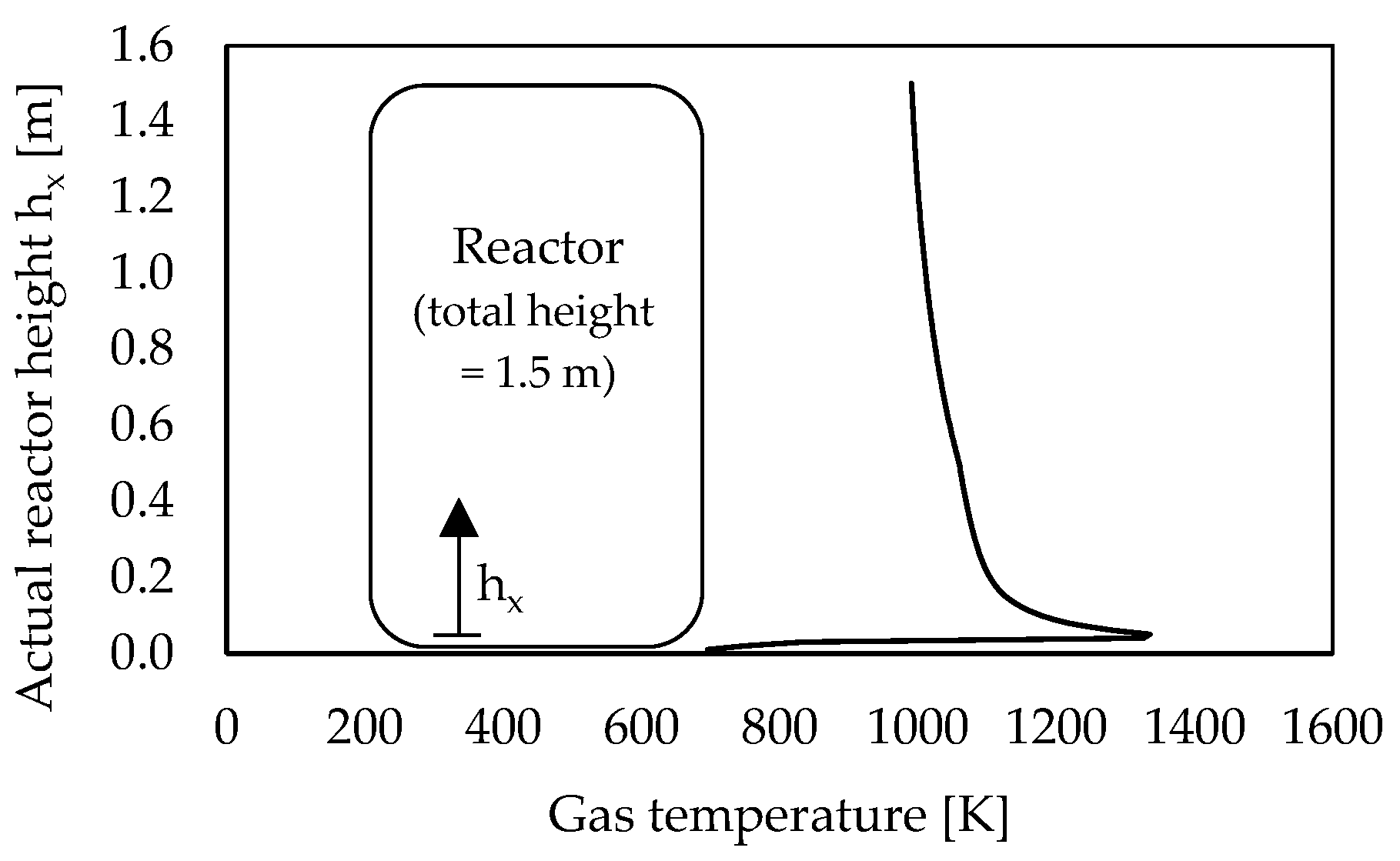
3.3. Influence of Reactor Design and Process Parameters
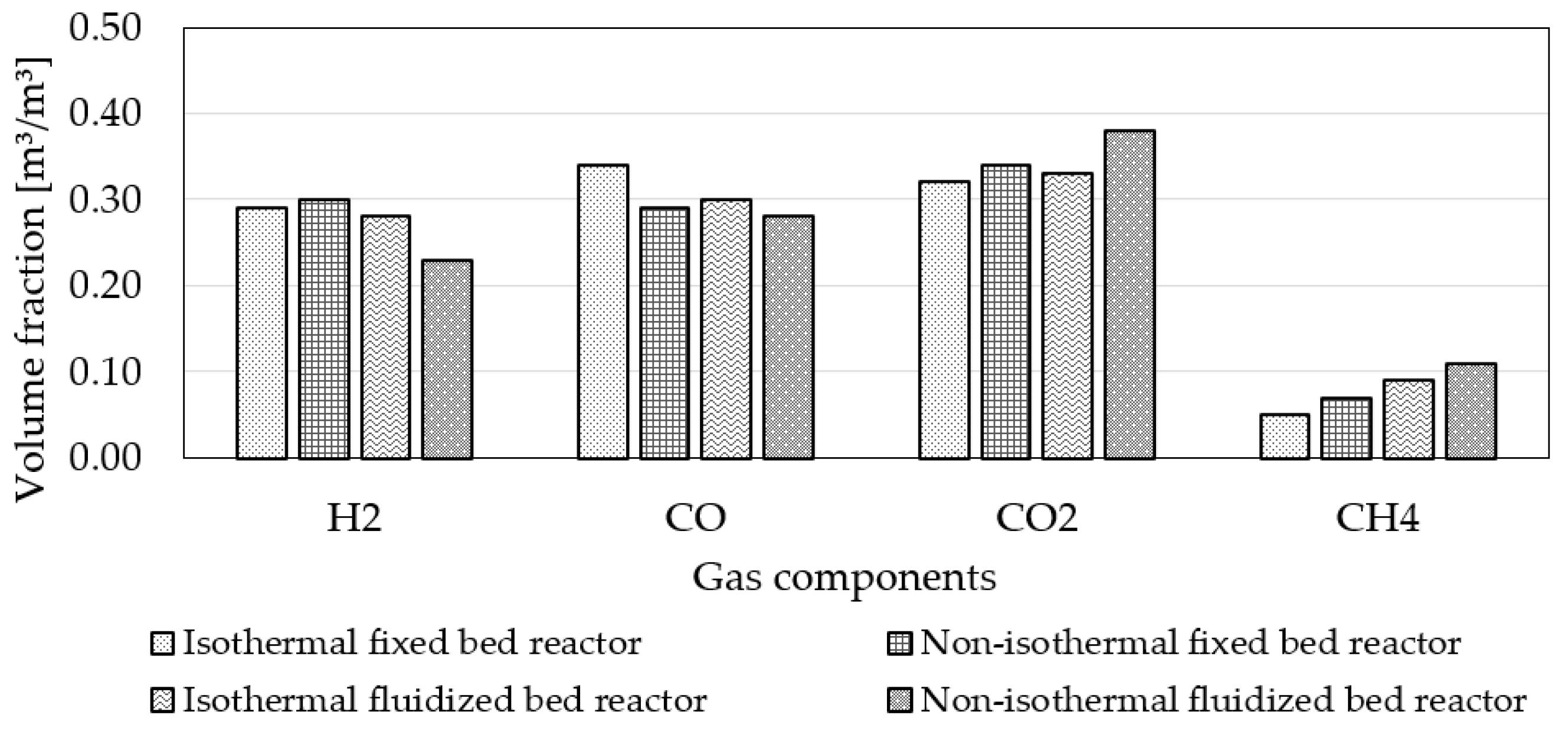
3.4. Influence of Reactor Type
4. Discussion
- Selection of the reactions: Only eleven reactions are considered due to the simplified approach. However, more reactions are known to influence the syngas composition.
- Substance data are estimated and show inaccuracies.
- Reaction kinetics: Different kinetic data exist for the same chemical equations, which show significant differences in the reaction rate.
- Considered substances: The primary substances are biomass, char, tar, water, oxygen, hydrogen, carbon monoxide, carbon dioxide, and methane. Char is assumed to consist entirely of carbon. Tar is a complex mixture of different aromatics but is considered in the model to be simplified as ethyne. If tar is described by other substances such as ethane or benzene or defined by a composition of different aromatics, this has a direct influence on the tar reactions R3 and R9. Carbon monoxide, hydrogen, and methane proportions change and influence the equilibrium reactions R10 and R11.
- In some cases, process parameters, such as the average gas residence time in the reactor, are assumed.
- Ideal assumptions: The heat and mass balance equations are based on ideal reactor models. Real occurring effects, like dispersion effects, are neglected.
- Catalysts: The fluidized bed consists of biomass and char particles. The use of a catalyst in the fluidized bed is neglected. In practice, a solid inert material or a catalyst is added, influencing the reactions.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations/Nomenclature
| Initial mass flow [kg/s] | |
| Mass flow after height h [kg/s] | |
| Mass flow (component i) in bubble phase [kg/s] | |
| Mass flow (component i) in emulsion phase [kg/s] | |
| Mass flow (component i) in freeboard [kg/s] | |
| Volume stream gas [m3/s] | |
| Reaction enthalpy [J/mol] | |
| Pre-exponential factor for reaction kinetics | |
| Cross-sectional area reactor [m2] | |
| Volume-related specific surface [1/m] | |
| C | Concentration [mol/m3] |
| Heat capacity gas [J/(kg∙K)] | |
| Particle diameter [m] | |
| Initial particle diameter [m] | |
| Reactor diameter [m] | |
| e | Oxygen ratio |
| Activation energy [J/(mol)] | |
| Mass transfer (component i) between bubble and emulsion phases [kg/(m3∙s)] | |
| h | Height [m] |
| k | Reaction constant |
| Effective reaction constant | |
| Equilibrium constant | |
| Overall heat transfer coefficient between gas and particle bed [W/(m2∙K)] | |
| Overall heat transfer coefficient between gas and reactor wall [W/(m2∙K)] | |
| P | Pressure [Pa] |
| Partial pressure of component i [Pa] | |
| R | Ideal gas constant [J/(mol∙K)] |
| r | Reaction rate [mol/(m3∙s)] |
| Reaction rate for homogeneous reactions (component i) in bubble phase [kg/(m3∙s)] | |
| Reaction rate for homogeneous reactions (component i) emulsion phase [kg/(m3∙s)] | |
| Reaction rate for homogeneous reactions (component i) in freeboard [kg/(m3∙s)] | |
| Reaction rate for heterogeneous reactions (component i) in emulsion phase [kg/(m3∙s)] | |
| Reaction rate for heterogeneous reactions (component i) in emulsion phase [kg/(m3∙s)] | |
| Molar reaction rate (component i) [mol/(m3∙s)] | |
| t | Time [s] |
| T | Temperature [K] |
| Temperature at particle surface [K] | |
| Temperature at wall [K] | |
| Superficial velocity in the reactor [m/s] | |
| V | Volume [m3] |
| Mass fraction (component i) | |
| Mass fraction (element e) of component i | |
| X | Turnover |
| Mass transfer coefficient [m/s] | |
| Void fraction bubble phase | |
| Void fraction emulsion phase | |
| Void fraction freeboard | |
| External efficiency factor | |
| Internal efficiency factor | |
| Particle efficiency factor | |
| Pore efficiency factor | |
| Density gas [kg/m3] | |
| Average gas residence time [s] |
Appendix A
| Author | Temperature [K] | Oxygen Ratio [-] | Volume Fraction Experimental Results [-] | Volume Fraction Modeled [-] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | CH4 | H2 | CO | CO2 | CH4 | |||
| Manyá et al. [33] | ||||||||||
| 1 | 1123 | 0.244 | 0.24 | 0.27 | 0.41 | 0.08 | 0.23 | 0.29 | 0.34 | 0.14 |
| 2 | 1123 | 0.307 | 0.19 | 0.26 | 0.47 | 0.08 | 0.22 | 0.29 | 0.37 | 0.12 |
| 3 | 1123 | 0.349 | 0.16 | 0.27 | 0.50 | 0.07 | 0.20 | 0.27 | 0.42 | 0.11 |
| 4 | 1123 | 0.238 | 0.23 | 0.27 | 0.42 | 0.07 | 0.25 | 0.32 | 0.29 | 0.15 |
| 5 | 1123 | 0.286 | 0.19 | 0.28 | 0.45 | 0.08 | 0.23 | 0.30 | 0.35 | 0.13 |
| 6 | 1123 | 0.328 | 0.15 | 0.28 | 0.50 | 0.07 | 0.21 | 0.28 | 0.40 | 0.12 |
| 7 | 1123 | 0.341 | 0.15 | 0.28 | 0.50 | 0.07 | 0.21 | 0.27 | 0.41 | 0.12 |
| 8 | 1123 | 0.297 | 0.17 | 0.28 | 0.47 | 0.08 | 0.21 | 0.27 | 0.40 | 0.12 |
| 9 | 1123 | 0.245 | 0.24 | 0.25 | 0.44 | 0.07 | 0.23 | 0.29 | 0.34 | 0.14 |
| 10 | 1123 | 0.291 | 0.23 | 0.27 | 0.43 | 0.07 | 0.21 | 0.27 | 0.40 | 0.12 |
| 11 | 1123 | 0.343 | 0.19 | 0.27 | 0.41 | 0.07 | 0.23 | 0.29 | 0.34 | 0.14 |
| 12 | 1123 | 0.242 | 0.25 | 0.27 | 0.41 | 0.07 | 0.23 | 0.29 | 0.34 | 0.14 |
| 13 | 1123 | 0.293 | 0.22 | 0.26 | 0.46 | 0.06 | 0.20 | 0.27 | 0.40 | 0.12 |
| 14 | 1123 | 0.338 | 0.15 | 0.25 | 0.54 | 0.07 | 0.19 | 0.25 | 0.46 | 0.11 |
| Kang et al. [34] | ||||||||||
| 1 | 1073 | 0.2 | 0.31 | 0.31 | 0.23 | 0.15 | 0.26 | 0.24 | 0.38 | 0.12 |
| 2 | 1130 | 0.2 | 0.24 | 0.33 | 0.29 | 0.13 | 0.33 | 0.30 | 0.32 | 0.05 |
| 3 | 1073 | 0.1 | 0.24 | 0.35 | 0.28 | 0.13 | 0.32 | 0.28 | 0.25 | 0.15 |
| 4 | 1073 | 0.15 | 0.29 | 0.35 | 0.24 | 0.12 | 0.29 | 0.26 | 0.31 | 0.14 |
| 5 | 1073 | 0.25 | 0.16 | 0.22 | 0.50 | 0.11 | 0.22 | 0.22 | 0.45 | 0.11 |
| 6 | 1073 | 0.3 | 0.12 | 0.23 | 0.56 | 0.09 | 0.19 | 0.19 | 0.51 | 0.10 |
| de Andrés et al. [35] | ||||||||||
| 1 | 1073 | 0.2 | 0.32 | 0.25 | 0.31 | 0.11 | 0.27 | 0.26 | 0.33 | 0.14 |
| 2 | 1123 | 0.2 | 0.34 | 0.26 | 0.27 | 0.12 | 0.33 | 0.34 | 0.23 | 0.10 |
| 3 | 1123 | 0.3 | 0.31 | 0.26 | 0.34 | 0.09 | 0.28 | 0.32 | 0.32 | 0.08 |
| 4 | 1123 | 0.4 | 0.29 | 0.24 | 0.41 | 0.06 | 0.23 | 0.27 | 0.43 | 0.07 |
| 5 | 1073 | 0.3 | 0.29 | 0.23 | 0.40 | 0.08 | 0.28 | 0.13 | 0.52 | 0.08 |
| 6 | 1123 | 0.3 | 0.36 | 0.24 | 0.33 | 0.07 | 0.38 | 0.20 | 0.37 | 0.05 |
| 7 | 1073 | 0.3 | 0.31 | 0.22 | 0.39 | 0.07 | 0.34 | 0.10 | 0.47 | 0.08 |
| 8 | 1123 | 0.3 | 0.37 | 0.23 | 0.33 | 0.07 | 0.39 | 0.14 | 0.40 | 0.05 |
| Tepper [13] | ||||||||||
| 1 | 1100 | 0.32 | 0.22 | 0.29 | 0.41 | 0.08 | 0.24 | 0.27 | 0.40 | 0.10 |
| 2 | 1118 | 0.38 | 0.24 | 0.30 | 0.41 | 0.06 | 0.23 | 0.26 | 0.44 | 0.07 |
| 3 | 1099 | 0.40 | 0.24 | 0.32 | 0.39 | 0.06 | 0.20 | 0.23 | 0.49 | 0.08 |
| 4 | 1045 | 0.33 | 0.26 | 0.28 | 0.40 | 0.06 | 0.16 | 0.19 | 0.50 | 0.15 |
| 5 | 1069 | 0.33 | 0.25 | 0.25 | 0.42 | 0.07 | 0.19 | 0.22 | 0.46 | 0.13 |
| 6 | 1080 | 0.37 | 0.26 | 0.26 | 0.42 | 0.07 | 0.19 | 0.22 | 0.46 | 0.13 |
| 7 | 1100 | 0.47 | 0.23 | 0.25 | 0.46 | 0.06 | 0.17 | 0.19 | 0.57 | 0.07 |
| 8 | 1072 | 0.35 | 0.23 | 0.31 | 0.37 | 0.09 | 0.19 | 0.22 | 0.48 | 0.12 |
| 9 | 1096 | 0.45 | 0.21 | 0.33 | 0.36 | 0.10 | 0.17 | 0.20 | 0.55 | 0.08 |
| 10 | 1078 | 0.45 | 0.19 | 0.36 | 0.35 | 0.10 | 0.15 | 0.18 | 0.58 | 0.09 |
| 11 | 1095 | 0.45 | 0.23 | 0.39 | 0.32 | 0.07 | 0.17 | 0.20 | 0.56 | 0.08 |
References
- Pawlik, V. Verteilung der Weltweiten Energieerzeugung nach Energieträger im Jahr 2022. Statista 2024. Available online: https://de.statista.com/statistik/daten/studie/167998/umfrage/weltweiter-energiemix-nach-energietraeger (accessed on 2 September 2024).
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction, 2nd ed.; Elsevier: Chennai, India, 2013. [Google Scholar]
- Gómez-Barea, A.; Leckner, B. Modeling of biomass gasification in fluidized bed. Prog. Energy Combust. Sci. 2010, 36, 444–509. [Google Scholar] [CrossRef]
- Baruah, D.; Baruah, D.C. Modeling of biomass gasification: A review. Renew. Sustain. Energy Rev. 2014, 39, 806–815. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Gerber, S.; Behrendt, F.; Oevermann, M. An Eulerian modeling approach of wood gasification in a bubbling fluidized bed reactor using char as bed material. Fuel 2010, 89, 2903–2917. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, L. CFD modeling of a fluidized bed sewage sludge gasifier for syngas. Asia-Pac. J. Chem. Eng. 2008, 3, 161–170. [Google Scholar] [CrossRef]
- Kinoshita, C.M.; Wang, Y.; Takahashi, P.K. Chemical Equilibrium Computations for Gasification of Biomass to Produce Methanol. Energy Sources 1991, 13, 361–368. [Google Scholar] [CrossRef]
- Li, X.; Grace, J.R.; Watkinson, A.P.; Lim, C.J.; Ergüdenler, A. Equilibrium modeling of gasification: A free energy minimization approach and its application to a circulating fluidized bed coal gasifier. Fuel 2001, 80, 195–207. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Pio, D.T.; Tarelho, L.A.C.; Sánchez, P.; Sanchez-Silva, L. Simulation of biomass gasification in bubbling fluidized bed reactor using Aspen Plus®. Energy Convers. Manag. 2021, 235, 113981. [Google Scholar] [CrossRef]
- Nikoo, M.B.; Mahinpey, N. Simulation of biomass gasification in fluidized bed reactor using ASPEN PLUS. Biomass. Bioenergy 2008, 32, 1245–1254. [Google Scholar] [CrossRef]
- Kunii, D.; Levenspiel, O.; Brenner, H. Fluidization Engineering, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Tepper, H. Zur Vergasung von Rest- und Abfallholz in Wirbelschichtreaktoren für Dezentrale Energieversorgungsanlagen. 2005. Available online: https://opendata.uni-halle.de//handle/1981185920/10692 (accessed on 3 June 2024).
- Pilar González-Vázquez, M.; Rubiera, F.; Pevida, C.; Pio, D.T.; Tarelho, L.A.C. Thermodynamic Analysis of Biomass Gasification Using Aspen Plus: Comparison of Stoichiometric and Non-Stoichiometric Models. Energies 2021, 14, 189. [Google Scholar] [CrossRef]
- Zhu, F.; Shen, L.; Xu, P.; Yuan, H.; Hu, M.; Qi, J.; Chen, J. Numerical Simulation of an Improved Updraft Biomass Gasifier Based on Aspen Plus. Int. J. Environ. Res. Public Health 2022, 19, 17089. [Google Scholar] [CrossRef]
- Tavares, R.; Monteiro, E.; Tabet, F.; Rouboa, A. Numerical investigation of optimum operating conditions for syngas and hydrogen production from biomass gasification using Aspen Plus. Renew. Energy 2020, 146, 1309–1314. [Google Scholar] [CrossRef]
- Azeez, K.T.A.; Poyilil, S.C.M.; Palatel, A. Aspen Plus Simulation of Biomass Gasification: A Comprehensive Model Incorporating Reaction Kinetics, Hydrodynamics and Tar Production. Process Integr. Optim. Sustain. 2022, 7, 255–268. [Google Scholar] [CrossRef]
- Arthur, J.R. Reactions between carbon and oxygen. Trans. Faraday Soc. 1951, 47, 164–178. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Wagenaar, B.M.; Prins, W.; van Swaaij, W.P.M. Flash pyrolysis kinetics of pine wood. Fuel Process Technol. 1993, 36, 291–298. [Google Scholar] [CrossRef]
- Adánez, J.; de Diego, L.F.; García-Labiano, F.; Abad, A.; Abanades, J.C. Determination of Biomass Char Combustion Reactivities for FBC Applications by a Combined Method. Ind. Eng. Chem. Res. 2001, 40, 4317–4323. [Google Scholar] [CrossRef]
- Smooth, L.D.; Smith, P.J. Coal Combustion and Gasification, 1st ed.; Springer: New York, NY, USA, 1985. [Google Scholar]
- Mitani, T.; Williams, F.A. Studies of cellular flames in hydrogen, oxygen, nitrogen mixtures. Combust. Flame 1980, 39, 169–190. [Google Scholar] [CrossRef]
- Groppi, G.; Tronconi, E.; Forzatti, P.; Berg, M. Mathematical modelling of catalytic combustors fuelled by gasified biomasses. Catal. Today 2000, 59, 151–162. [Google Scholar] [CrossRef]
- Dryer, F.L.; Glassman, I. High-temperature oxidation of CO and CH4. Symp. Int. Combust. 1973, 14, 987–1003. [Google Scholar] [CrossRef]
- Cozzani, V. Reactivity in Oxygen and Carbon Dioxide of Char Formed in the Pyrolysis of Refuse-Derived Fuel. Ind. Eng. Chem. Res. 2000, 39, 864–872. [Google Scholar] [CrossRef]
- Barrio, M.; Gøbel, B.; Rimes, H.; Henriksen, U.; Hustad, J.E.; Sørensen, L.H. Steam Gasification of Wood Char and the Effect of Hydrogen Inhibition on the Chemical Kinetics. In Progress in Thermochemical Biomass Conversion; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001; pp. 32–46. [Google Scholar] [CrossRef]
- Cozzani, V.; Nicolella, C.; Petarca, L.; Rovatti, M.; Tognotti, L. A Fundamental Study on Conventional Pyrolysis of a Refuse-Derived Fuel. Ind. Eng. Chem. Res. 1995, 34, 2006–2020. [Google Scholar] [CrossRef]
- Biba, V.; Macak, J.; Klose, E.; Malecha, J. Mathematical model for coal gasification under pressure. Ind. Eng. Chem. Process. Dev. 1978, 17, 92–98. [Google Scholar] [CrossRef]
- Yoon, H.; Wei, J.; Denn, M.M. A model for moving-bed coal gasification reactors. AIChE J. 1978, 24, 885–903. [Google Scholar] [CrossRef]
- Jones, W.P.; Lindstedt, R.P. Global reaction schemes for hydrocarbon combustion. Combust. Flame 1988, 73, 233–249. [Google Scholar] [CrossRef]
- Stephan, P.; Kabelac, S.; Kind, M.; Mewes, D.; Schaber, K.; Wetzel, T. VDI-Wärmeatlas: Fachlicher Träger VDI-Gesellschaft Verfahrenstechnik und Chemieingenieurwesen, 12th ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Manyà, J.J.; Aznar, M.; Sánchez, J.L.; Arauzo, J.; Murillo, M.B. Further Experiments on Sewage Sludge Air Gasification: Influence of the Nonstationary Period on the Overall Results. Ind. Eng. Chem. Res. 2006, 45, 7313–7320. [Google Scholar] [CrossRef]
- Kang, S.-W.; Dong, J.-I.; Kim, J.-M.; Lee, W.-C.; Hwang, W.-G. Gasification and its emission characteristics for dried sewage sludge utilizing a fluidized bed gasifier. J. Mater. Cycles Waste Manag. 2011, 13, 180–185. [Google Scholar] [CrossRef]
- de Andrés, J.M.; Narros, A.; Rodríguez, M.E. Air-steam gasification of sewage sludge in a bubbling bed reactor: Effect of alumina as a primary catalyst. Fuel Process Technol. 2011, 92, 433–440. [Google Scholar] [CrossRef]
| No. | Name | Stoichiometry |
|---|---|---|
| R1 | Devolatilization | |
| R2 | Char combustion | |
| [18] | ||
| R3 | Partial tar oxidation | |
| R4 | Hydrogen oxidation | |
| R5 | Methane oxidation | |
| R6 | Carbon monoxide oxidation | |
| R7 | Boudouard reaction | |
| R8 | Steam gasification | |
| R9 | Thermal tar cracking | |
| R10 | Water–gas shift reaction | |
| R11 | Methanation |
| No. | A0 | EA | Reaction Scheme | Source |
|---|---|---|---|---|
| R1 | - | - | [19] | |
| 150 | [20] | |||
| R2 | 68 | [21] | ||
| R3 | [22] 1 | |||
| R4 | 110 | [23,24] | ||
| R5 | 203 | [25] | ||
| R6 | 167 | [25] | ||
| R7 | 221 | [26] | ||
| R8 | 237 | [27] | ||
| R9 | 102 | [28] | ||
| R10 | 13 | [29] | ||
| [30] | ||||
| R11 | 125 | [31] 2 | ||
| [3] |
| Biomass Specification [kg/kg] 1 | Product Composition [kg/kg] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | Char | Tar | H2 | CO | CO2 | CH4 | H2O | |
| Cardoon | 0.43 | 0.04 | 0.44 | 0.22 | 0.42 | 0.0003 | 0.05 | 0.14 | 0.007 | 0.16 |
| Wheat straw | 0.49 | 0.05 | 0.41 | 0.21 | 0.49 | 0.0003 | 0.05 | 0.09 | 0.007 | 0.15 |
| Pine sawdust | 0.45 | 0.07 | 0.41 | 0.21 | 0.45 | 0.0003 | 0.05 | 0.06 | 0.007 | 0.22 |
| Coir pith | 0.44 | 0.05 | 0.43 | 0.22 | 0.44 | 0.0003 | 0.05 | 0.12 | 0.007 | 0.16 |
| Manyá et al. [33] | Kang et al. [34] | De Andrés et al. [35] | Tepper [13] | ||
|---|---|---|---|---|---|
| Reactor height [m] | 0.3 | 0.6 | 0.7 | 3.0 | |
| Reactor diameter [m] | 0.04 | 0.07 | 0.03 | 0.40 | |
| Volume stream [m3/h] | 0.18–0.3 | 1.1–1.4 1 | 0.20–0.26 | 110–270 | |
| Gas velocity [m/s] | 0.04–0.07 | 0.08–0.10 1 | 0.06–0.09 | 0.25–0.60 | |
| Residence time [s] | 4–7 | 6–8 1 | 7–10 | 5–12 | |
| Biomass composition | C [kg/kg] | 0.55 | 0.40 | 0.28 | 0.50 |
| H [kg/kg] | 0.07 | 0.06 | 0.05 | 0.07 | |
| O [kg/kg] | 0.28 | 0.47 | 0.62 | 0.42 | |
| Moisture [kg/kg] | 0.09 | 0.06 | 0.07 | 0.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinkmann, S.; Seyfang, B.C. Building a Code-Based Model to Describe Syngas Production from Biomass. ChemEngineering 2024, 8, 94. https://doi.org/10.3390/chemengineering8050094
Brinkmann S, Seyfang BC. Building a Code-Based Model to Describe Syngas Production from Biomass. ChemEngineering. 2024; 8(5):94. https://doi.org/10.3390/chemengineering8050094
Chicago/Turabian StyleBrinkmann, Simon, and Bernhard C. Seyfang. 2024. "Building a Code-Based Model to Describe Syngas Production from Biomass" ChemEngineering 8, no. 5: 94. https://doi.org/10.3390/chemengineering8050094
APA StyleBrinkmann, S., & Seyfang, B. C. (2024). Building a Code-Based Model to Describe Syngas Production from Biomass. ChemEngineering, 8(5), 94. https://doi.org/10.3390/chemengineering8050094






