Abstract
The objective of this study is to map, describe, and identify “water treatment using catalysts and/or nanomaterials” and their derivable aspects. A comprehensive search was conducted in academic databases such as WoS and Scopus, following the PRISMA methodology, to identify relevant studies published between 2010 and 2024. Inclusion and exclusion criteria were applied to select articles that address both experimental and theoretical aspects of photocatalysis in wastewater treatment. The methodology is developed through exploratory data analysis and the use of the Tree of Science algorithm. The first results indicate the roots, in which it is possible to gain knowledge of the environment for the implementation of a photoreactor it uses as a photocatalyst agent. A total of 94 relevant articles were identified. The results show that most studies focus on the degradation of organic pollutants using TiO2 as a photocatalyst. Additionally, there has been a significant increase in the number of publications and citations in recent years, indicating growing interest in this field. Then, in the trunk, some more solid ideas in terms of basic concepts, techniques and possible variations for the application of knowledge and development of future research related to the initial topic are indicated. Finally, through the leaves, new modifications and combinations of the photocatalytic materials are obtained, in search of improving their performance in terms of reduction in water contaminants. From the above, centrality in photocatalysis is identified as an alternative for water remediation using different photocatalysts. It is concluded that the total citation network contains, within the most important nodes, articles of high interest in the community, such as those authored by Zhang, Xiaofei; Nezamzadeh-Ejhieh, Alireza; or Li, Jingyi, from countries in the Middle East and the Asian continent, justified not only by the research capabilities of these countries, but also by the needs and problems that these regions face in terms of water scarcity. Future work indicates the need for and interest in improving various characteristics such as photocatalytic performance, the number of cycles that the material supports, and its reduction capacity in the presence of high concentrations of contaminants, with the intention of maximizing the benefits of its applicability in water treatment.
1. Introduction
Currently, there is a growing global problem due to the contamination of water sources, as a consequence of the presence of contaminants of various kinds such as emerging biological contaminants and heavy metals that generate great environmental, health, and economic impacts [1,2,3,4]. This contamination is generated by crude oil spills [5], poor disposal of waste [6], biological contamination transferred by maritime traffic, illegal refinery, and dyes used in the textile industry, among others, where many of these problems can be solved through scientific and technological research and development.
From the R + D + I system, the implementation of treatment technologies that reduce the number of contaminants present in water resources is generated. Currently there are different alternatives to treat various contaminants such as organic waste [7,8,9,10], pollutants in petroleum [11,12], heavy metals [13,14,15], microbiological contaminants found in bodies of water [16,17] and drug treatments [18,19,20]. However, many of these treatment systems are conventional and are not efficient due to the structural complexity or relative stability of many of these types of contaminants. This makes it necessary to develop and implement new modern treatment systems that combine conventional processes with physicochemical processes that seek the chemical transformation of contaminants. One of these treatments is heterogeneous photocatalysis, which belongs to the so-called advanced oxidation processes. (AOPs) [21,22,23,24].
For this process, different materials with suitable properties to act as catalysts are used, such as, for example, TiO2 [25,26,27] ZnO [28,29], CdO [30], SnO2 [31], iron oxides [32,33,34,35], and WO3 [25,36] ZnS [37]. Many of these materials have the ability to be excited with not very high energy light, absorbing part of the radiation of the solar spectrum that reaches the surface of the Earth (λ > 310 nm), which increases the interest in the possible use of sunshine. The most researched photocatalysts so far are broadband semiconductor metal oxides and especially TiO2, which is the most commonly used semiconductor in these types of processes, because it is low cost, non-toxic, insoluble in water and has a high chemical stability that makes it capable of producing electronic transitions by absorption of light in the closest ultraviolet (UV), allowing a reduction in the forbidden energy bandwidth (GAP) or by incorporating ions to improve the conductivity at active sites.
Taking this context into account, this article addresses the treatment of contaminated water using catalysts and nanomaterials. This treatment is carried out through a scientometric review, due to a large amount of research that has been carried out on this topic and in order to analyze the publications with the most impact in the Web of Science (WoS) and Scopus databases. The Tree of Science (ToS) is a methodological and systematic tool that, through a process analogous to that undertaken by trees in their development, classifies articles and the type of information provided by them into an initial, pioneering, and supportive group like the roots of the tree; into another central, strong, and highly relevant group like the trunk; and finally presents the branches of the tree, which represent the group of articles that bring novelty to the topic and set new trends and research lines around the treated subject, yet are supported and nourished by the work developed in the other components of the tree. This approach is widely used and versatile across different fields of knowledge, applicable in thematic areas such as education, marketing, administration, health, entrepreneurship, and natural sciences. The utility and versatility of ToS have been supported by previous research [38,39,40,41].
The ToS was applied to review the contributions of this topic over time and, finally, a cluster analysis was performed to study articles in different subareas. The rest of the article is organized as follows: first, a methodological part, where the process of selecting the published works is explained; this is followed by the results, showing the documents that are part of the root, trunk and leaves; and finally, the conclusion.
2. Methodology
In order to understand the scientific structure of the research field, a scientometric analysis was carried out in 2024, using Web of Science (WoS) (from 2004 to 2024) and Scopus (from 2004 to 2023). To identify the relevant articles in the remediation of contaminated water through physicochemical treatments, the search was divided into four parts according to the sections of the process: for the application part, the search commands ((remov* OR decomposition OR reducti* OR degrad* OR mineralization) were used; in the process variables, the search commands used were (chemical AND oxygen AND demand OR cod); for the synthesis method part, the search command was photocatal*; and finally, for the alternative solution, the search commands were (nanoparticles AND TiO2). It should be noted that, to execute these search parameters, it was necessary to merge and measure these databases through the development of exploratory data analysis (EDA) using scientometric tools such as Bibliometrix, thus facilitating the grouping of relevant data and references, as well as a complete description of the recent studies that allows a more efficient development of bibliometric analysis around the variable studied.
Table 1 outlines the primary parameters used. For example, to identify articles related to the role of the academic as a researcher, particular search terms were used in the title, abstract and keywords. WoS’s performance was 470 entries, while Scopus produced 167 entries. Although WoS returns a greater number of entries, it is still essential to incorporate Scopus due to the inclusion of 63 documents that are not present in WoS. The ‘bibliometrix’ and ‘tosr’ packages were used to consolidate data from both databases.

Table 1.
Search parameters. * Note 104 duplicates.
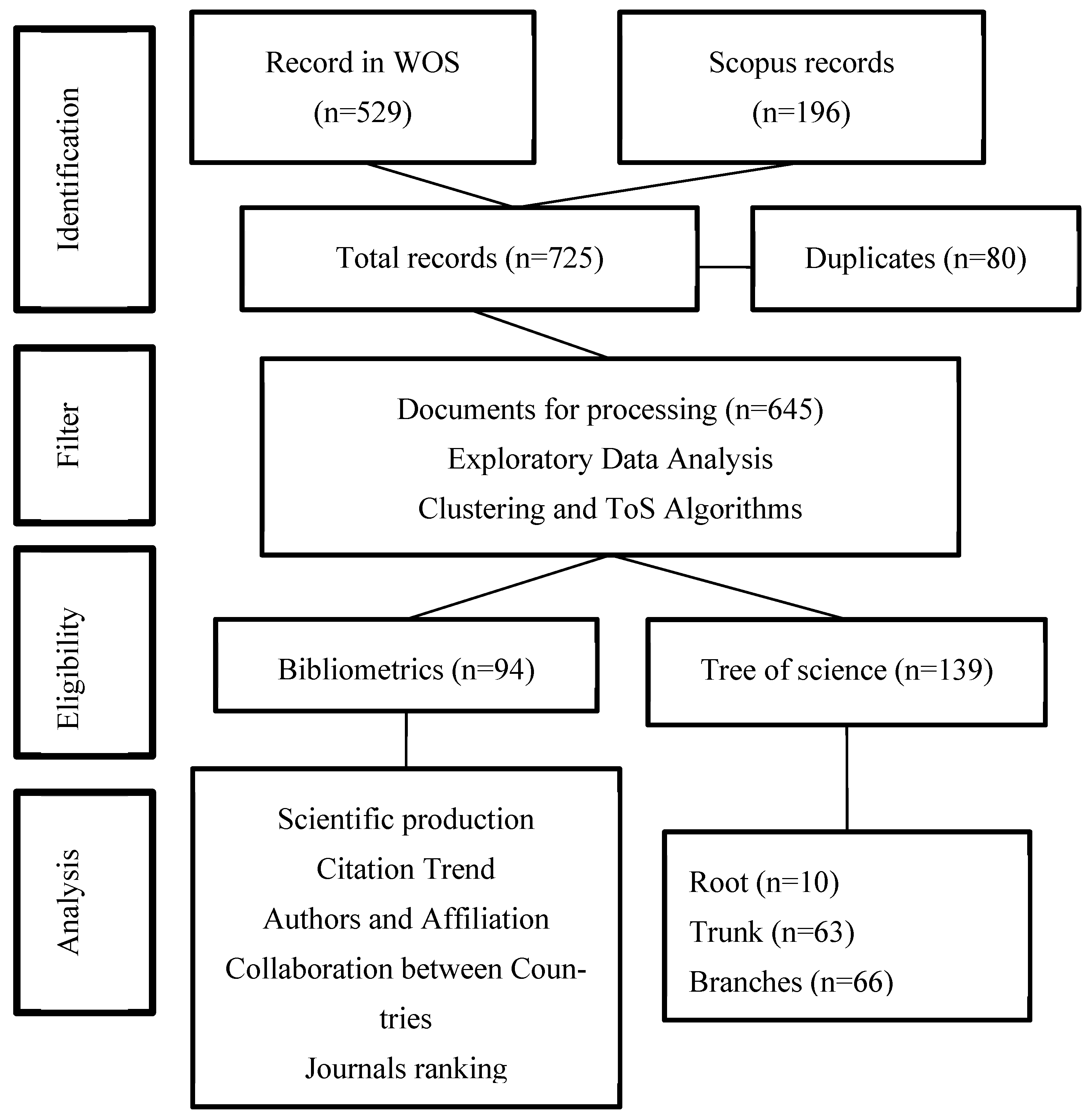
Figure 1 shows the general process to select the most relevant literature on the topic. Next, the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flow proposed by Liberati et al. (2009) [42], where the refinement process of the documents to reach a highly useful and convenient sample for the analysis included in this article is described.
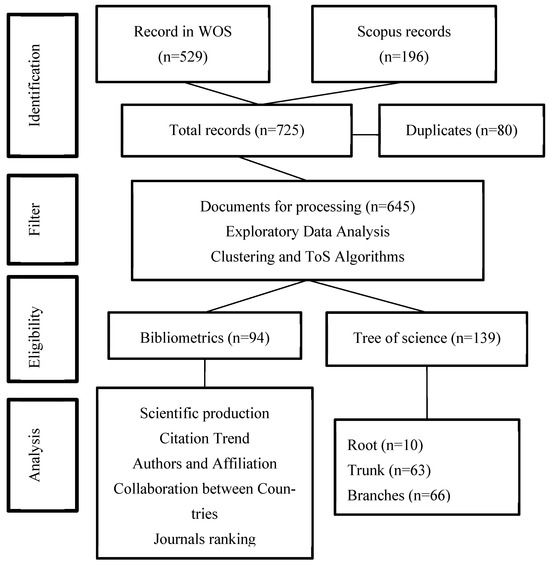
Figure 1.
PRISMA Flow. Source: authors’ construction based on Liberati et al. (2009) [42].
The network has 881 nodes (articles) and 2962 edges (links), and 60 relevant articles using in-degree and page rank metrics were identified. Then, 28 articles related to this research topic were manually selected. After removing duplicate documents, two authors reviewed the documents to identify reviews and unrelated documents to remove them from the list. A total of 22 articles not related to the main topic and 53 reviews were found. Unrelated documents were removed from this step because they collect documents that create new knowledge, but reviews do not generate new knowledge. Finally, there is a data set with 91,087 coded articles. The coded process is explained below.
3. Results
This section will provide a general overview of annual scientific production, production by country, authorship analysis, and journal analysis.
3.1. Scientometric Mapping and Scientific Production
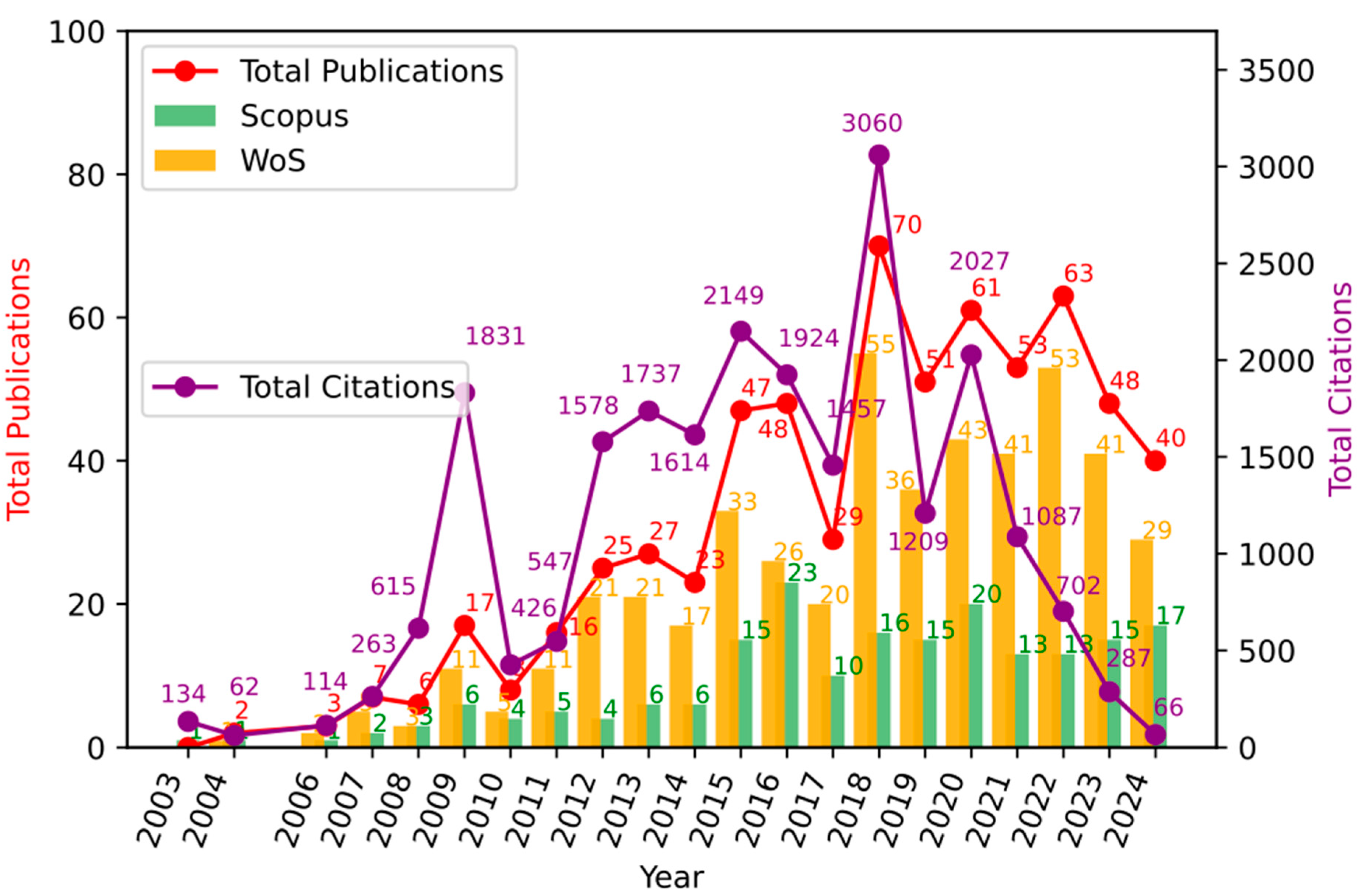
Figure 2 shows the articles published per year in both Web of Science (WoS) and Scopus, as well as an analysis of total publications per year and total citations. In order to understand the different moments of the development of the topic over time [43,44], at least three stages that stand out for the productivity and its corresponding citation are shown in Figure 2, as follows:
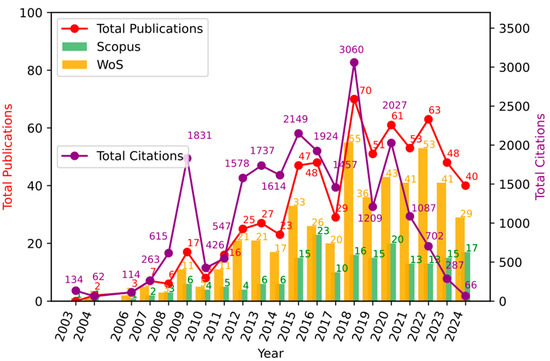
Figure 2.
Annual trends in publications and citations in photocatalysis for wastewater treatment. Measurement of scientific production. Source: authors’ construction based on AED.
3.1.1. (2000–2007) Initial Growth Phase
The initial growth phase, which goes from 2000 to 2007, is marked by the influential article by Chen et al. (2003) [45] that reports a study centered around the implementation of a photoreactor that uses nanostructured TiO2 materials as a photocatalyst agent, which, combined with an electrolysis system, allowed good performance in terms of a reduction in rhodamine 6G as a water contaminant. This article has had a substantial impact on the academic community, obtaining the highest number of citations in this phase with 130 mentions in other documents, and marking a trend or thematic segment in this chronological phase of the research development in this bibliometric report, since the majority of interactions carried out occurred around the application of TiO2 as a catalyst in water decontamination processes, thus evaluating different treatments that modify the base material already mentioned to improve its performance and photocatalytic behavior. Another notable contribution comes from Chen et al. (2004) [46] with 60 citations and from Li et al. (2007) [47] with 56 citations, who develop research regarding the decontamination and treatment of water contaminated with organic and inorganic products through the implementation of photocatalytic techniques developed with TiO2 base materials and enhanced by electrochemical processes with good performance. This phase represents a period of origin and thematic development, which justifies the low production of articles and perhaps the low citation of these in future works, due to their lack of academic maturity. However, they behave as a seed for the future development of research and academic production related to photocatalytic processes mediated by TiO2 as a base semiconductor.
3.1.2. (2008–2014) Thematic Development Phase
This second phase, between 2008 and 2014, presents a time period with much stronger growth in terms of scientific production than that presented in the first stage. Likewise, the research topic was reaching greater maturity, laying even more solid foundations in terms of basic concepts, techniques, and possible variations for the application of knowledge and development of future research related to the initial topic. The previous statements are supported by the fact that it is in this stage where the years with the highest number of citations are found, in relation to the number of publications carried out, as can be seen in Figure 2. In this chronological stage, the work developed by Maeda et al. (2009) [48] is found, which corresponds to the most cited article in this bibliometric report, with 648 citations. It addresses research related to photocatalytic processes mediated by a semiconductor material different from titanium dioxide, titanium nitride, and graphitic carbon, whose band gap is energetically lower than that of TiO2, and when doped with platinum or rhodium oxide, increases the performance and stability in the oxidation–reduction processes of water to obtain O2 or H2. Starting from the research reported in previously mentioned publications, the works published at this stage explore the application of different semiconductors for photocatalytic applications in water treatment, as conducted by Subash et al. (2013) [49], who synthesized a co-doped zirconium structure with silver and zinc oxide, trying to take advantage of the energetic interactions carried out between its valence and conduction bands, to favor photocatalytic processes of reduction in the contaminant Reactive Red 120 as a water contaminant; other relevant researches could be found in [50,51,52,53,54]. Better performances than those presented by other commercial materials were found, showing relevant and important results for the academic community, which led to it being cited 284 times by other authors.
3.1.3. (2015–2022) High Productivity Phase
In this stage, maximum productivity and thematic maturity are reached. The years with the greatest number of publications occur, which results in the years with the greatest number of citations. However, at this moment, the impact of these publications is lower, if the data from the existing relationship between the number of publications and the number of citations associated with them is analyzed. Nonetheless, this does not imply that, in the future, the number of citations of these articles will not increase, since they will be the basis and starting point for new and innovative research related to this field of knowledge.
At this stage, research reports predominate, in which the photocatalytic properties of materials used previously are studied and evaluated, but applied to new reduction processes or with modifications that aim at improving their response in the implemented processes. Likewise, the growing interest and relevance that the application of photocatalytic techniques has gained in the reduction in contaminants from the pharmaceutical industry is observed, taking into account that the articles with the greatest impact and with the greatest number of citations in this chronological stage of thematic development are referring to this topic. Such is the case of W-K. Jo [55] and T.S. Natarajan (2015) [56] who evaluated the impact of the morphology of TiO2-based nanostructured materials in interaction with graphitic carbon nitride for the reduction in isoniazid. They determined that the titanium dioxide nanotube morphology increases the surface area of the material and, at the same time, the photocatalytic response of the composite material. The studies carried out by Ahmadi et al. (2017) [57] and Deng et al. (2017) [58], are in this same thematic line. They report two different research projects focused on the treatment of water contaminated with tetracycline, some using a dioxide titanium structure as photocatalytic material with multi-walled carbon nanotubes. The others, using the AgIn5S8 structure with silver nanoparticles deposited on its surface to form a photocatalytic nanohybrid, obtained positive results in terms of photocatalytic reduction in the pharmaceutical contaminant; other relevant published information can be found in [55,56,57,58]. The reported documents report 269, 244, and 213 citations, respectively.
Figure 2 shows the annual distribution of publications and their respective citations from 2010 to 2024. Each bar represents the number of publications per year, while the superimposed line indicates the total citations received by these publications until the date of the search. This graph illustrates both the evolution of academic interest in the field and the cumulative impact of research over time.
3.2. Production by Countries
Country analysis is becoming a common scientometric technique for identifying the most productive places in the world on a specific topic. It is important to understand the dynamics of scientific production, and the quality and impact of research in the countries. This study shows the production (number of articles), the quality (according to Scimago metrics), and the impact (citations received) of research in a country. In addition, a collaboration network is created to understand the communities generated through interactions between researchers.
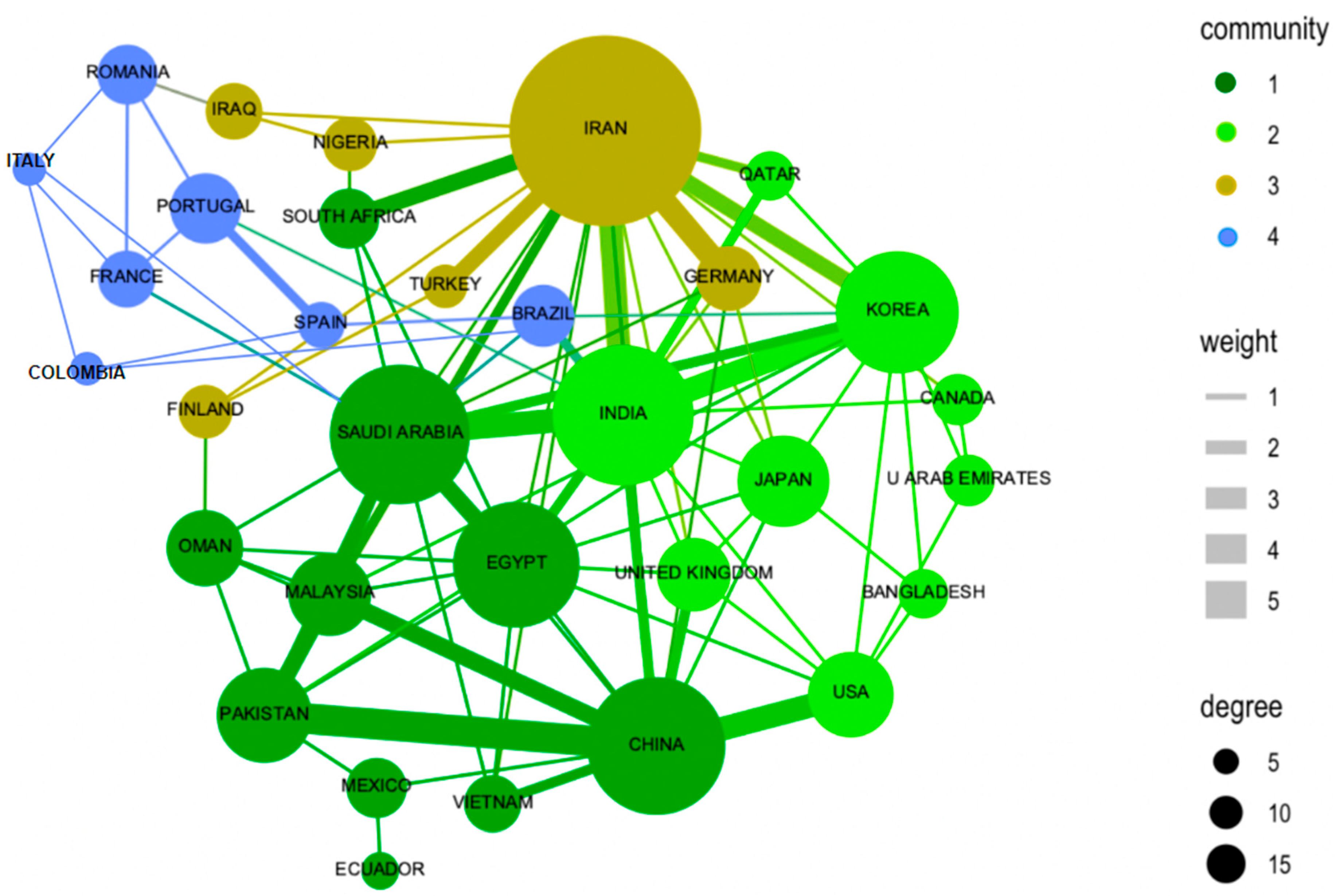
There are 45 countries that research and publish on the topic of photocatalysis as an alternative for water remediation. The first 10 are listed in Table 2. This list is organized according to the number of publications that represent the production of each country regarding the total number of records obtained in this search. These 10 countries represent 74.8% of scientific production on the subject, with Iran, India, and China being the countries that have the most significant contributions. This behavior can be explained because one of the most reported articles in these countries deals with the treatment of pharmaceutical wastewater with nanomaterials, especially contaminants such as tetracycline, binary mixture of dyes and 4-nitrophenol, presenting a high relationship with the proposed topic. In China, a behavior similar to what happens in Iran is reflected, where pharmaceutical waste has a great impact, but in this case conductive and semiconductor materials are used in order to evaluate the chemical reduction of oxygen, which is one of the response variables evaluated in the most cited articles in this country [45,58,59].

Table 2.
Most productive countries.
Finally, from the 14.53% of scientific production on the subject in India, according to the three articles with the most impact [45,60,61], it can be deduced that the main wastes to be intervened are those derived from tannery, which have been treated using nanomaterials that guarantee highly efficient, active, and reusable solar use. In the same line, other papers related to this subject can be found in [59,62,63].
The inter-relationships between countries and authors have been analyzed to understand the dynamics of collaboration in the field of photocatalysis applied to wastewater treatment. These inter-relationships are presented in Figure 3, which shows the co-authorship networks and international collaborations, respectively. Figure 3 illustrates the connections between authors who have collaborated on publications within this field. Nodes in the network represent authors, while the lines between them indicate co-authorships in scientific articles. The density of connections and the size of nodes are determined by the number of collaborations and the centrality of the author in the network, respectively. Figure 3 presents four subgroups of countries, and the inset figure of clusters by size shows a similar size among the four clusters. The internal figure of nodes and links over time shows the interaction between new countries and new relationships over time.
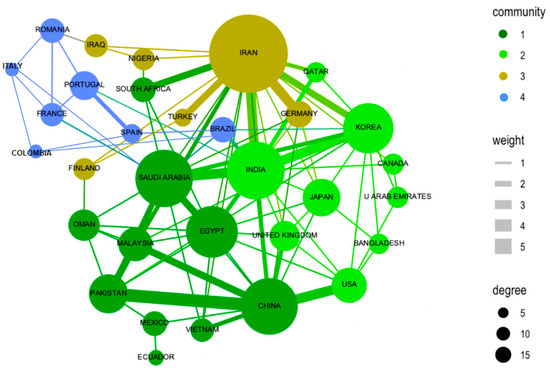
Figure 3.
Collaboration between countries. Source: authors’ construction based on Biblioshiny and AED.
3.3. Production by Authors
Table 3 details the scientific production by authors in the field of photocatalysis for wastewater treatment, highlighting the main contributors and their impacts on research. It provides essential analysis of how individuals and groups have influenced trends and developments in this area. In the analysis of the production by authors (see Table 3) it is possible to notice that Nezamzadeh is the most relevant author in the topic addressed by this review. He holds number one in a number of publications and citations by other prominent authors in this field of knowledge. As an example of his work, the articles published in 2013 [64,65] are highlighted, in which he reports a pair of research works where he synthesized and characterized structures of nano-zeolite doped with nanostructured copper oxide, and cyclonoptilolite zeolite doped with copper sulfide. He evaluated their photocatalytic behavior in the reduction in methylene blue and rhodamine B for the first material, and methyl Orange and green bromocresol, one of the largest water pollutants from the textile industry. These research works allowed for a conclusion that the synthesized materials have photocatalytic potential for decontamination applications of industrial wastewater with the presence of dyes, and that the temperature and drying process of the material have an effect on the capacity to reduce contaminants.

Table 3.
Top production by author. Source: authors’ construction based on RStudio, Biblioshiny, and EDA.
Nezamzadeh continued his research on the topic of photocatalytic materials, and in 2014, he published another of his research works [66] in which, in a process similar to previous research, he synthesized a photocatalytic material and evaluated its performance in the reduction in water pollutants. On this occasion, he used a zeolite cyclonoptilolite structure doped with zinc oxide for the photoreduction of 4-nitrophenol, obtaining good results in terms of photocatalytic capacity and demonstrating the influence on the contaminant reduction process of parameters such as pH, the addition of electron acceptors, and the concentration of the contaminant in the aqueous medium to be treated. Furthermore, from the point of view of the impact of his publications (H-Index), Nezamzadeh also takes first place thanks to the fact that the number of publications carried out by this author is also accompanied by a significant number of citations.
Another relevant author with a high research impact on the topic related to this review is Krishnakumar, who, together with Subash, has a large number of citations and a high rate of co-authorships and research collaborations as well as published scientific articles. These authors have developed high-impact research that has already accumulated a significant number of citations. Among them, different works stand out in which they synthesized photocatalytic materials based on zinc and silver oxide nanostructures, doped with other metallic materials that generate modifications and energetic interactions in the photocatalyst, thus improving its performance in terms of reducing dyes present in wastewater from different industries [45,67,68]. In 2012, they synthesized a structure in which they added bromine to its base material and evaluated its performance in the reduction in the contaminant black acid-1. In 2013, they added zirconium to its base structure and evaluated its photocatalytic capacity in the reduction in the red reagent 120. In 2014, they synthesized zinc oxide by co-opting it with silver and gold, to be used as a photocatalyst in the mineralization of methylene blue. Finally, they concluded from their research that the addition of these metals to its base structure improves the performance of the photocatalyst in the reduction in contaminants, and also that factors such as the concentration of the contaminant in the aqueous medium can modify the performance of the mineralization process.
The approach to the key authors of research on the topic of this document can be obtained from Table 3, by identifying highly cited and collaborative communities. Two communities of research collaboration between highly related authors can be identified through citations in their works.
Community number one (olive color) appears as the larger in terms of the number of authors and impact with at least eight members. In this community, there are hegemonic surnames of recognized authors such as Wang and Li, with a high degree of impact on the community and who are highly connected even with other classic and seminal authors such as Zhang and Chen who have the highest degree of impact and belong to community 2 (lime green).
3.4. Production by Journals
In Table 4, regarding the publication of articles related to research on the topic addressed in this review, an important group of journals was found that concentrates a high number of publications associated with photocatalytic processes. Among the most relevant journals is DESALINATION AND WATER TREATMENT, a journal focused on the research and application of technologies related to water treatment, integrated water management, water reuse, wastewater, and topics related to environmental and energy problems. However, despite having the largest number of publications on the subject of the review, this journal, according to SRJ, is located in Q3 with an H-index of 83 (2009–2023), an impact factor of 1.0 (2023), and a CiteScore of 2.2. It has the most publications on the topic discussed. In this sense, the JOURNAL OF HAZARDOUS MATERIALS, according to SRJ, is located in Q1 with an H-index of 352 (1975–2024), and an impact factor of 12.2 (2023), and a CiteScore of 25.4. This journal can be cited, which occupies the highest position in terms of impact factor, and the second position in terms of the number of publications related to the topic under study. This is a journal that focuses on topics related to the areas of environmental science and engineering through the publication of complete research papers, review articles, and research perspectives that allow dangers and risks to be reduced. Finally, the CHEMICAL ENGINEERING JOURNAL, according to SRJ, is located in Q1 with an H-index of 309 (1992–2023), an impact factor of 13.3 (2023), and a CiteScore of 21.7. This journal can be mentioned, which is a journal focused on topics related to catalysis, chemical reaction engineering, environmental chemical engineering, ecological and sustainable science and engineering, and novel materials. Although this journal does not have the greatest number of publications or H-index, it does present the greatest impact factor among the journals that top the list of greatest bibliographic production on the related topic.

Table 4.
Ranking of journals.
3.5. Tree of Science (ToS)
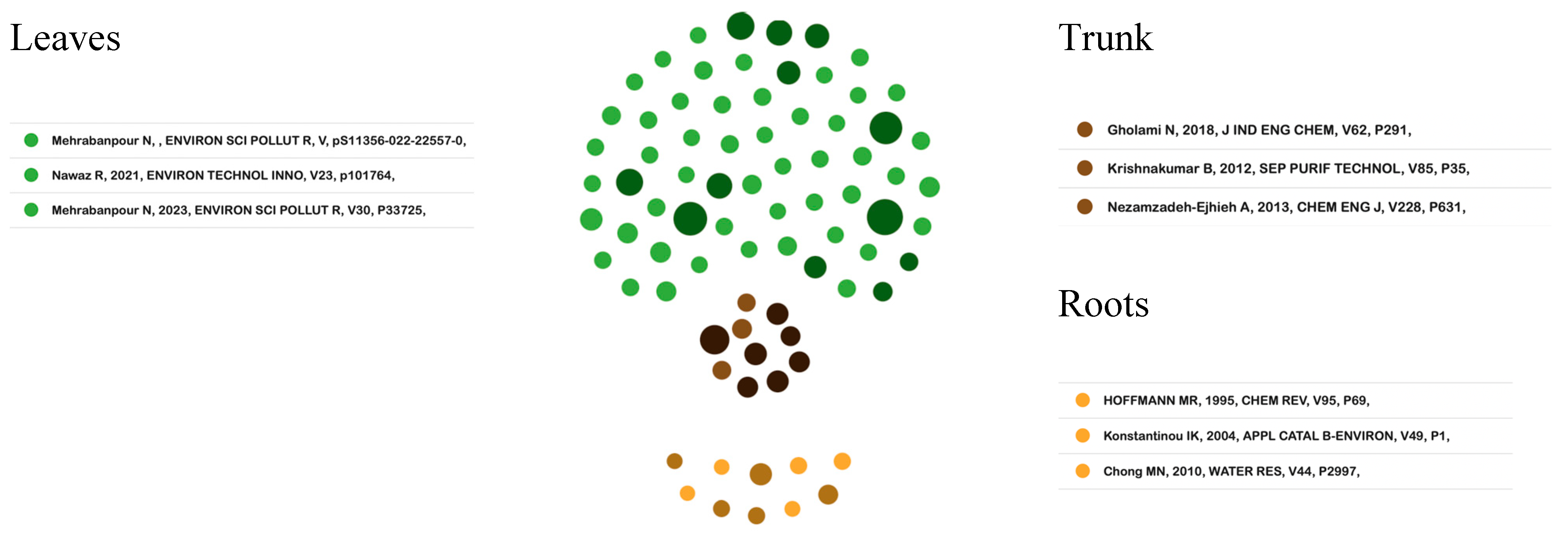
Figure 4 provides a graphical representation of the application of the literature recommendation algorithm (ToS) [41]. Specifically, for the field of photocatalytic processes, the development of the metaphor allows for identifying the thematic trends that are popular in the academic community. In this way, an exploration of the thematic branches proposed in the leaves, as recent studies, is developed. These connect with the documents that highlight the structure of the field coming from the trunk and in turn are connected with the foundational articles from the roots.
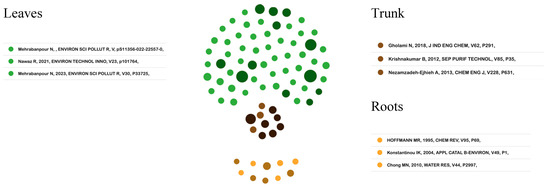
Figure 4.
Tree of Science. Source: authors’ construction based on AED and ToS [68,69,70,71,72,73,74,75,76].
3.5.1. Classic Documents (Root)
The first results allow for identifying that the study and development around photocatalytic processes has attracted the attention of the research community, due to the diversity of applications that this physicochemical process has in the contemporary needs of humanity. Semiconductors appear as the preferred type of material to mediate photocatalytic processes, due to their properties and optoelectronic characteristics [76]. Photocatalytic processes are currently studied and applied in fields such as water decontamination, greenhouse gas reduction, organic compound elimination, and carbon dioxide methanation [64,66,73].
TiO2 is a semiconductor widely used to mediate photocatalytic processes due to its morphological, optical, and energetic properties. In this sense, studies are found, such as the one carried out by Konstantinou and Albanis (2004) [75], in which the behavior of TiO2 as a photocatalyst in the reduction in textile dyes was evaluated, identifying the influence on the process of parameters such as pH, concentration of catalyst, the concentration of the substrate, and the presence of electron acceptors such as hydrogen peroxide and ammonium persulfate, in addition to molecular oxygen. Likewise, Konstantinou and Albanis (2004) [75], studied the reactions and intermediate products in the photocatalytic reaction for pesticide removal, in which the evaluation of byproducts and toxicity measurements were the key actions to evaluate the overall process.
Although TiO2 presents good photocatalytic behavior due to its electronic and energetic properties, an effective relationship between its rutile and anatase phases and high surface area presents certain limitations for its use with sunlight because the prohibited energy of its band is greater than the energy that the visible range of radiation can provide. However, alternatives have been implemented such as the functionalization of nanometric-scale TiO2 with other materials. The studies developed by Pelaez et al. (2012) [77] and Asahi et al. (2001) [78] are an example of the alternative mentioned above. In these works, the best efficiency in terms of the photocatalytic properties of nitrogen doped with TiO2 nanoparticles was demonstrated, compared to pure TiO2 when mediating water decontamination processes.
3.5.2. Structural Documents (Trunk)
The study of photocatalytic materials has had great relevance in the field of technological research, mainly due to their application in water-cleaning and purification processes. In this sense, within the most relevant articles and works on this topic, studies can be found that evaluate performance, effectiveness, physicochemical processes, and different parameters that can influence the application of a variety of semiconductor materials as photocatalytic materials in water-cleaning processes and contaminant removal.
Nezamzadeh-Ejhieh and Salimi, (2010) [79] developed a work in which the efficiency of an aqueous CuO/X zeolite solution is evaluated in terms of photo-reduction of o-phenylenediamine (OPD), which is a contaminating aromatic amine, used as a component of polymers, drugs, glucose biosensing, as a mediator, and as a substrate for dyeing natural and synthetic fibers, including textiles. Furthermore, in search of counteracting the rapid recombination of electron–hole pairs in the photocatalytic material, they analyzed the effect of adding hydrogen peroxide and potassium bromate. They determined that the CuO/X zeolite, in the presence of ultraviolet light, presents a better degradation rate of o-phenylenediamine compared to pure CuO or pure zeolite X, thanks to the electronic and energetic interaction generated between these materials. Likewise, they verified the positive influence of hydrogen peroxide or potassium bromate on the photo-reduction of o-phenylenediamine, by increasing the number of electrons trapped by zeolite X, the generation of radicals, and other oxidizing species.
Subsequently, Nezamzadeh-Ejhieh and Karimi-Shamsabadi, (2013) [68] continued researching using the same photocatalytic material, CuO/X zeolite, for the reduction in other chemical contaminants, methylene blue and rhodamine B. Similar results to those found with o-phenylenediamine were obtained in this research, in which the CuO/X zeolite, in the presence of hydrogen peroxide or potassium bromate, presents good performance in the reduction in contaminants, as well as a depletion of less than 5% after four catalysis cycles. Likewise, they found a direct relationship between the drying temperature of the photocatalyst and its capacity to reduce contaminants, concluding that, at 200 °C, it presents better catalytic behavior.
Continuing with the same thematic trend around research in which semiconductor materials are intended to be used as catalysts in water-cleaning and decontamination processes, Krishnakumar et al. (2012) [73] carried out research in which they synthesized zinc oxide doped with silver bromide (AgBr-ZnO) through a simultaneous precipitation process from zinc oxalate and silver bromide. This material was used as a photocatalyst in the degradation of the Acid Black dye, in a process assisted by ultraviolet light, demonstrating better behavior in terms of reduction capacity than commercial materials such as ZnO. In addition, the parameters that influence the reduction process were analyzed. For example, they determined that the pH with the best results in the reduction of the dye was 12, and that, with an amount of 4g per liter, better results were also obtained. In this study it was found that the degradation of the dye occurs mainly on the surface of the synthesized catalyst material.
Finally, but with the same thematic approach, is the study developed by Subash et al. (2013) [49], in which they developed an active and reusable solar catalyst, Zr-loaded Ag-ZnO, for the degradation of the Reactive Red 120 dye. (RR 120). The catalyst was prepared by precipitation–thermal decomposition and was compared with other catalysts doped with a single metal and commercial catalysts, finding better performance for this material.
It can then be concluded that the strong, robust, and leading part of the research in this thematic field has focused on the study of the synthesis of compound semiconductor materials with photocatalytic properties that can be used in the purification and cleaning processes of water contaminated with dyes and other chemical elements from the industrial sector.
3.5.3. Emerging Trends Documents (Leaves and Branches)
In this section, where there are studies and publications that mark the most current and innovative trends in terms of the thematic section that is being analyzed, research in which a general overview is made of the conditions of the photocatalytic processes and possible materials to be used for the treatment of contaminated water during industrial processes that involve palm oil can be found. Nawaz et al. (2021) [70] developed a review article that seeks to collect information about how problems regarding inefficient sunlight or visible light can be used in photocatalytic processes, in the incomplete recovery of the photocatalysts after treatment, in the lack of understanding of the photocatalysis process, as well as in the parametric optimization of this process used for water purification, using the specific removal rate (mg of COD removed/g of catalyst h) to quantitatively compare the results obtained by different researchers.
Likewise, different research works, in which the synthesis, characterization, and evaluation of the photocatalytic behavior of some compound semiconductor materials in the cleaning and purification processes of contaminated water from the textile, pharmaceutical, and hospital industries, among others, are studied and compared. Such is the case of Suresh et al. (2014) [80] and Suresh et al. (2016) [81] who, through a simple microwave combustion process, were able to first synthesize zirconium oxide nanoparticles on an activated carbon structure. Then, they synthesized nanoparticles of nickel oxide on the same structure, to evaluate their potential as a catalyst in the ultraviolet light photo-reduction process of wastewater contaminated with dyes from the textile industry. It was demonstrated that the coordinated combination of the oxygen vacancy sites, the structural defects of the nanoparticles supported on the activated carbon, together with the electronic and energetic properties that these composite materials exhibit, and along with the presence of oxygen on the surface of the activated carbon, has led to long-lasting light absorption and delayed charge recombination.
In this same sense are the works carried out by Hemmatpour et al. (2022) [82] and Mehrabanpour et al. (2023) [69], who develop their research around the synthesis of cadmium sulfide nanoparticles coupled with bismuth vanadate nanoparticles and lead sulfide, respectively, in order to evaluate their properties as photocatalysts in the reduction in contaminated water. Hemmatpour et al. (2022) [82] evaluated their material in the reduction in contaminated water with Eriochrome Black T dye, determining better photocatalytic behavior in the compound than in the components separately from their matter. It was also found that for ratios from 3–4 to 1 of BiVO4 and CdS, there is a greater performance in reducing the contaminant. For its part, Mehrabanpour et al. (2023) [69] evaluated their material supported by clinoptilolite in a reduction in water contaminated by cefotaxime from the pharmacological or hospital industry; it showed enhanced photocatalytic activity compared to pure materials measured through the decrease in the chemical oxygen demand of the treated waters.
A study similar to the previous one, which intended to reduce contaminants from the pharmacological or hospital industry, was carried out by Yousefi et al. (2021) [83] in which they synthesized and characterized SnO2 and CuO nanoparticles through a hydrothermal process and coupled mechanically. These nanoparticles were implemented as a photocatalyst in the process of reducing water contaminated with phenazopyridine, varying the relationship between the molar concentration of its components, finding greater efficiency for the following conditions: Cpp: 5.5 ppm at pH = 7; irradiation time: 180 min; catalyst dose: 0.8 g/L. The efficiency of the process was measured by the variation in the intensity of the main peak of the HPLC measurement carried out on contaminated water, before and after treatment.
In addition to the studies mentioned above, the documents that report research in which titanium dioxide is used in different presentations to develop photocatalysis processes stand out. Wang et al. (2020) [84], for example, carried out a research in which they reviewed existing advances around the design and methods to improve the performance and possible application of electro-photocatalysis devices for the treatment of contaminated water at an industrial level, evaluating possible variations that modify the interfacial interaction of the materials, in addition to the energetic and electronic conditions that facilitate the catalytic process, such as the reduction in the gap of the materials, or the shift of the maximum absorption of radiation to wavelengths within the visible range.
There is also the research carried out by Kaur et al. (2018) [85] in which, to treat water contaminated with the benzimidazole fungicide Carbendazim, different ceramic materials were synthesized in the form of beads immobilized with titanium dioxide and titanium dioxide doped with iron. Five materials were used to make the ceramic beads: cement, white cement, plaster of Paris, clay, and sand mixed with Portland cement. After the ceramic beads were obtained, the TiO2 and TiO2-Fe particles were immobilized on them through heat fixation, and they were placed in a flat plate reactor. It was found that the ceramic material increased the surface area of the catalyst, significantly benefiting the photocatalysis process. In addition, the Fe-TiO2-coated clay beads had maximum photoactivity under sunlight 87 ± 1% average. The high stability of the materials was also observed, supporting up to 40 cycles of photocatalysis.
In line with the trend in terms of the most frequent applications of catalytic materials in this bibliographic review, the use of composite or TiO2-based materials for the purification or cleaning processes of water contaminated by dyes or products from the textile industry was also found. Natarajan et al. (2013) [86] utilized titanium oxide-based nanomaterials with tubular shapes, with bismuth doping, through sol–gel and hydrothermal methods. This composite nanomaterial was used as a mediator in the photo-degradation of the Rhodamine B dye, demonstrating the following: high effectiveness in terms of reducing this contaminant compared to pure titanium oxide; an increase in the surface area with the addition of bismuth, explained by the change in firm from titanium oxide nanoparticles to titanium nanotubes; and a complete reduction in the contaminant after 3 h of reaction.
Likewise, the research developed by Zarrin and Heshmatpour, (2020) [87] and Bashir et al. (2021) [88] was found, in which they synthesize TiO2-based materials combined with different materials in order to modify their electronic structure, energy behavior, surface area, and consequently its photocatalytic capacity in reducing water contaminated by dyes. Zarrin and Heshmatpour, (2020) [87] on the other hand, designed nanohybrids of graphene dioxide doped with different additives such as niobium oxide, tin (IV) oxide (SnO2), reduced graphene oxide, and ceramics (SiO2/Fe3O4/ZrO2), through the hydrothermal and sol–gel methods already mentioned in other research works. Moreover, Bashir et al. (2021) [88] added nickel (Ni) and praseodymium (Pr3+) ions to titanium dioxide, generating a decrease to almost half of the initial size of the material particles, which benefits the catalytic processes that titanium can develop, by increasing its total surface area. Finally, both research works obtained encouraging results regarding the photoreduction of the organic dyes crystal violet and methyl orange for the materials synthesized by Zarrin and Heshmatpour, (2020) [87], and of the reactive blue-19, reactive orange-13, and reactive blue-13 dyes, for which reductions of 99.0%, 70.0% and 85.0% were obtained, respectively, by the material synthesized by Bashir et al. (2021) [88].
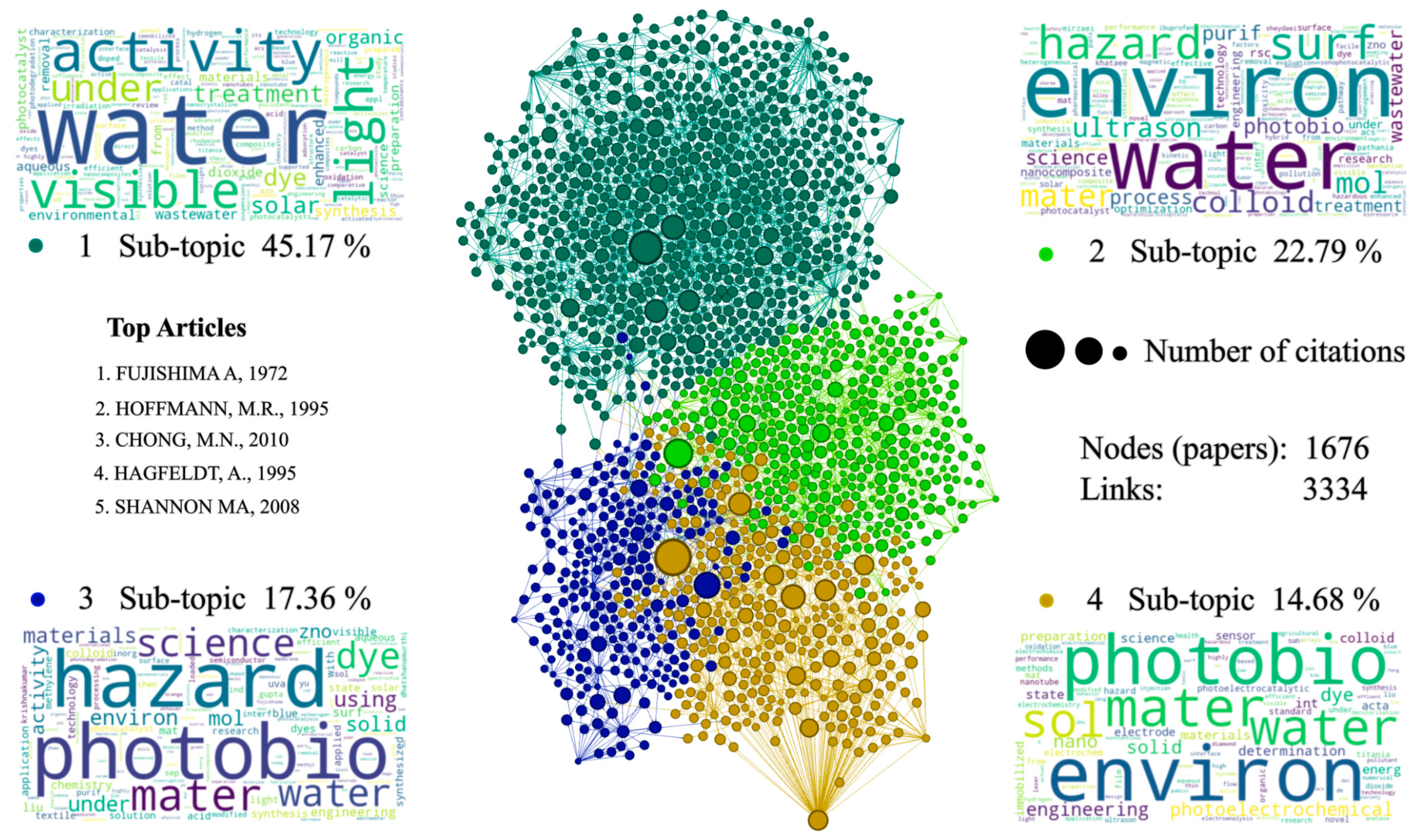
In the section corresponding to the branches of the tree or thematic lines (see Figure 5), four communities can be found that are related through citations and references between articles. These communities can be classified into four topics with relevant key words, such as water, hazard, photobio, and environmental. Among the articles from all the communities, the document published by Fujishima and Honda (1971) [89] stands out. This article corresponds to community 4; it is the article with the highest number of historical citations on the topic covered. It addresses the thematic basis of the phenomenon of photo-generation of the electron–hole pair in semiconductors such as TiO2, without the need to imprint a potential difference in the electronic conduction process. Another research that needs to be highlighted, due to its number of citations and general relevance to the communities, corresponds to the work presented by Hoffmann et al. (1995) [74] belonging to community 1, who developed a very complete report in which different topics are related to the application of semiconductors, such as TiO2 as catalysts. In this article, they discuss topics that include generalities and theoretical bases of photocatalysis in semiconductors, photocatalytic properties of quantum semiconductors, modifications to semiconductors in search of improving their photocatalytic properties, doping with nanomaterials, important process variables in catalytic reactions, and a general review of the reactors implemented to develop photocatalysis with a semiconductor. This is an article of utmost importance and a mandatory reference for anyone carrying out research on this topic.
With great relevance to the level of interactions and citations, the research article carried out by Shannon et al. (2008) [90] is found in community 2. They develop a thematic review regarding the techniques, treatments, and alternatives developed or put into practice for the treatment of contaminated water, a topic closely related to one of the main applications of semiconductor materials with photocatalytic characteristics studied in this bibliometric analysis. As the main exponent of community 3 is the work by Neppolian (2002) [91], who carried out a very consistent study of the topic around which this bibliographic review and analysis is developed. In this article, a comparison is made in terms of photoreduction performance between TiO2 and other semiconductors such as ZnO, ZrO2, and WO3, which also present photocatalytic characteristics, but none with better results than TiO2. In order to analyze this behavior, the materials were tested as catalysts in the reduction process of three dyes from the textile industry: yellow reagent 17, blue reagent 4 and red reagent 2, all in the presence of solar irradiation. In this research, greater photo-degradation is reported for the red reactive dye, in addition to an optimal pH for the process ranging between 11 and 13, and ideal catalyst concentrations of m 8 × 10−4 to 1.2 × 10−3 M for the yellow reagent, 4.16 × 10−4 to 1.25 × 10−3 M for the red reagent, and 1 × 10−4 to 5 × 10−4 M for the blue reagent.
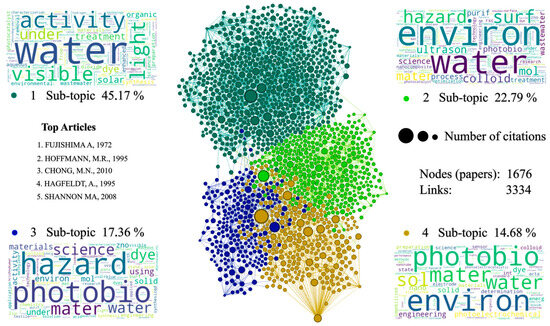
Figure 5.
Citation network. Source: Authors’ construction based on Gephi and RStudio [74,76,89,90,92].
Figure 5.
Citation network. Source: Authors’ construction based on Gephi and RStudio [74,76,89,90,92].
4. Conclusions
A bibliographic review was developed that allows the scientific community to obtain an idea of the current state and trajectory of research on the topic related to this article. For this purpose, it all started with a seed that provided results in two scientific databases, Web of Science and Scopus, yielding a series of results that were analyzed using ‘bibliometrix’ and ‘tosr’. From the results, an evolutionary trend in the subject was identified based on the root documents, which address topics related to basic concepts of the photocatalytic process as an alternative for the treatment of contaminated water, transcending the application of structures based on titanium dioxide with different doping, for its application mainly in the reduction in water contaminated with dyes and dyes from the textile industry, to finally reach a final stage in which new modifications and combinations in photocatalytic materials are researched, with the aim of improving their performance in terms of reducing water contaminants.
Likewise, the thematic–chronological maturation process was evident, which was divided into three stages, from 2000 to 2007, from 2008 to 2014, and from 2015 to 2024. These stages are divided into an initial period of low production but a high number of citations for the few published articles that served as a theoretical basis and starting point for future research; followed by a period in which, although the number of publications increased, the increase in citations associated with these documents was substantially greater; and concluding with a stage in which the increase in scientific production around this topic was much greater, but the relationship between the number of publications and the number of citations was lower. Moreover, it was possible to demonstrate the important contributions in terms of scientific production from countries in the Middle East and the Asian continent, justified not only by the research capabilities of these countries, but also by the needs and problems faced by these regions regarding water scarcity.
All this research production allows for noticing a marked trend in future research, towards studying the synthesis and application of new combinations of photocatalytic materials or different treatments to materials that already work, to improve their performance and diversify the water contaminants that can be treated with these photocatalysts.
Author Contributions
J.M.M.-V.: interpreted, conceptualized, corrected, and completed the final manuscript, L.M.E.-C.: interpreted, conceptualized, corrected, and completed the final manuscript, D.A.T.-C.: writing—original draft, review and editing, S.A.-R.: writing—original draft, review and editing, E.R.-P.: provided technical corrections and suggestions for the final manuscript and K.J.C.-D.: writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out using internal resources of the laboratory and with the support of the National University of Colombia.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank Universidad Nacional de Colombia, Manizales Campus for the financial support of Minciencias within the framework of the program “Convocatoria nacional para el fortalecimiento de la investigación, la creación y la innovación en las sedes de presencia nacional de la Universidad Nacional de Colombia 2019–2021 (Sede Tumaco)”, and D.A. Torres-Ceron thanks to Universidad Nacional de Colombia, Manizales Campus for the financial support of the “Implementación de tecnologías limpias para el tratamiento de superficies para el sector de la galvanotecnia, con énfasis en la gestión de residuos y eficiencia hídrica y ambiental para la industria en Caldas, Código BPIN: 2021000100388”.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Jimenez-Relinque, E.; Lee, S.F.; Plaza, L.; Castellote, M. Synergetic Adsorption–Photocatalysis Process for Water Treatment Using TiO2 Supported on Waste Stainless Steel Slag. Environ. Sci. Pollut. Res. 2022, 29, 39712–39722. [Google Scholar] [CrossRef] [PubMed]
- Kocijan, M.; Ćurković, L.; Gonçalves, G.; Podlogar, M. The Potential of RGO@TiO2 Photocatalyst for the Degradation of Organic Pollutants in Water. Sustainability 2022, 14, 12703. [Google Scholar] [CrossRef]
- Shehab, M.A.; Sharma, N.; Valsesia, A.; Karacs, G.; Kristály, F.; Koós, T.; Leskó, A.K.; Nánai, L.; Hernadi, K.; Németh, Z. Preparation and Photocatalytic Performance of TiO2 Nanowire-Based Self-Supported Hybrid Membranes. Molecules 2022, 27, 2951. [Google Scholar] [CrossRef] [PubMed]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The Photocatalytic Process in the Treatment of Polluted Water. Chem. Pap. 2023, 77, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Benitez, C. Contaminación de Crudo Llegó Hasta Imbilí En Tumaco. Available online: https://www.diariodelsur.com.co/contaminacion-de-crudo-llego-hasta-imbili-en-tumaco/ (accessed on 28 August 2024).
- Estrada Loaiza, J. Análisis Técnico Económico de Alternativas Para El Procesamiento de Los Residuos Solidos de La Truchicultura En Belmira, Antioquia, Con Énfasis En El Ensilaje Biológico. Master’s Thesis, Universidad de Antioquia, Medellín, Colombia, 2022. [Google Scholar]
- Barkul, R.P.; Patil, M.K.; Patil, S.M.; Shevale, V.B.; Delekar, S.D. Sunlight-Assisted Photocatalytic Degradation of Textile Effluent and Rhodamine B by Using Iodine Doped TiO2 Nanoparticles. J. Photochem. Photobiol. A Chem. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Rincón, G.J.; La Motta, E.J. A Fluidized-Bed Reactor for the Photocatalytic Mineralization of Phenol on TiO2-Coated Silica Gel. Heliyon 2019, 5, e01966. [Google Scholar] [CrossRef]
- Mahajan, M.R.; Ramachandran, K.; Sathyamurthy, R.; Geetha, B.T.; Sathish, T.; Anderson, A.; Rajasimman, M.; Saravanan, R.; Ghfar, A.A.; Dragoi, E.-N. Annealed Titanium Dioxide Nanomaterials for Rapid Hydrogen Production and Rhodamine-B Degradation. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Alahiane, S.; Sennaoui, A.; Sakr, F.; Dinne, M.; Qourzal, S.; Assabbane, A. Synchronous Role of Coupled Adsorption-Photocatalytic Degradation of Direct Red 80 with Nanocrystalline TiO2-Coated Non-Woven Fibres Materials in a Static Batch Photoreactor. Groundw. Sustain. Dev. 2020, 11, 100396. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Yu, S.; Wen, X.; Wu, D. Highly Efficient Solar-Light-Driven Photocatalytic Degradation of Pollutants in Petroleum Refinery Wastewater on Hierarchically-Structured Copper Sulfide (CuS) Hollow Nanocatalysts. Sep. Purif. Technol. 2022, 284, 120254. [Google Scholar] [CrossRef]
- Haolat, J.O.; George, A.; Issa Suleiman, M.; Berthod, M.; Wang, K. UV-TiO2 Treatment of the Cooling Water of an Oil Refinery. J. Water Process Eng. 2018, 26, 176–181. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green Synthesis of TiO2 Nanoparticles from Syzygium Cumini Extract for Photo-Catalytic Removal of Lead (Pb) in Explosive Industrial Wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Amaya-Roncancio, S.; Torres-Ceron, D.A.; Velasquez-Tamayo, J.P.; Mercado, D.F.; Arellano-Ramírez, I.D.; Restrepo-Parra, E. Experimental and Theoretical Study of Cr(VI) Photoreduction and Adsorption onto SO42−-Doped TiO2 Obtained by Plasma Electrolytic Oxidation. Mater. Today Chem. 2023, 31, 101620. [Google Scholar] [CrossRef]
- Vargas-Villanueva, S.; Velásquez-Tamayo, J.P.; Torres-Cerón, D.A.; Mercado, D.F.; Torres-Palma, R.A.; Riassetto, D.; Riva, J.S.; Amaya-Roncancio, S.; Castilla-Acevedo, S.F.; Restrepo-Parra, E. Impact of the Duty Cycle on the Morphology and Photocatalytic Properties of S-TiO2 Obtained by Plasma Electrolytic Oxidation to Treat Real Electroplating Wastewater Contaminated with Cr6+. J. Environ. Chem. Eng. 2023, 11, 110246. [Google Scholar] [CrossRef]
- Cherni, Y.; Messaoud, M.; Ben, S.-B.O.; Salhi, R.; Elleuch, R.; Kasmi, M.; Chatti, A.; Trabelsi, I.; Elleuch, L. A Sustainable Nanobioremediation Approach for Tunisian Landfill Leachate Using Ag/Fe Co-Doped TiO2 Nanoparticles Combined with Saccharomyces Cerevisiae. Euro-Mediterr. J. Environ. Integr. 2023, 8, 287–302. [Google Scholar] [CrossRef]
- Godvin Sharmila, V.; Rajesh Banu, J.; Gunasekaran, M.; Angappane, S.; Yeom, I.T. Nano-layered TiO2 for Effective Bacterial Disintegration of Waste Activated Sludge and Biogas Production. J. Chem. Technol. Biotechnol. 2018, 93, 2701–2709. [Google Scholar] [CrossRef]
- Ghosh, S.; Harsha, N.V.M.S.; Singh, S.P.; Shriwastav, A. Simultaneous Removal of Ciprofloxacin and Disinfection from Wastewater by Combined Photocatalytic Reactor (PCR) and Membrane Bioreactor (MBR) System. J. Environ. Chem. Eng. 2023, 11, 110855. [Google Scholar] [CrossRef]
- Mohammadi Nezhad, A.; Talaiekhozani, A.; Mojiri, A.; Sonne, C.; Cho, J.; Rezania, S.; Vasseghian, Y. Photocatalytic Removal of Ceftriaxone from Wastewater Using TiO2/MgO under Ultraviolet Radiation. Environ. Res. 2023, 229, 115915. [Google Scholar] [CrossRef]
- Mingmongkol, Y.; Polnok, A.; Phuinthiang, P.; Channei, D.; Ratananikom, K.; Nakaruk, A.; Khanitchaidecha, W. Photocatalytic Degradation Mechanism of the Pharmaceutical Agent Salbutamol Using the Mn-Doped TiO2 Nanoparticles Under Visible Light Irradiation. ACS Omega 2023, 8, 17254–17263. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and Application of Titanium Dioxide Photocatalysis for Energy, Decontamination and Viral Disinfection: A Review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef]
- Ji, H.; Ni, J.; Zhao, D.; Liu, W. Application of Titanate Nanotubes for Photocatalytic Decontamination in Water: Challenges and Prospects. ACS EST Eng. 2022, 2, 1015–1038. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A.; Chauhan, N.S.; Tahir, M.; Kumari, K.; Mittal, A.; Kumar, N. TiO2/Bi2O3/PANI Nanocomposite Materials for Enhanced Photocatalytic Decontamination of Organic Pollutants. Inorg. Chem. Commun. 2022, 146, 110093. [Google Scholar] [CrossRef]
- Wei, D.; Wu, J.; Wang, Y.; Zhong, J.; Li, D.; Jin, X.; Wu, Y.; Chen, P.; Liu, H.; Lv, W.; et al. Dual Defect Sites of Nitrogen Vacancy and Cyano Group Synergistically Boost the Activation of Oxygen Molecules for Efficient Photocatalytic Decontamination. Chem. Eng. J. 2023, 462, 142291. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Mahadik, M.A.; Moholkar, A.V.; Bhosale, C.H. Photoelectrocatalytic Degradation of Oxalic Acid Using WO3 and Stratified WO3/TiO2 Photocatalysts under Sunlight Illumination. Ultrason. Sonochem. 2017, 35, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Joy, S.; Rastogi, G.; Sankaranarayanan, K. Photocatalytic Degradation of Phenol Using Ionic Liquid Stabilized TiO2 Nanoparticles. Mater. Res. Express 2019, 6, 115059. [Google Scholar] [CrossRef]
- Su, H.; Gong, Y.; Lou, H.; Pang, Y.; Yang, D.; Gao, D.; Qiu, X. The Open Core-Shell TiO2@ZnIn2S4 Step-Scheme Heterojunction to Enhance Mass Transfer and Light Utilization for Efficient Photocatalytic Performance. J. Clean. Prod. 2023, 419, 138034. [Google Scholar] [CrossRef]
- Vignesh, K.; Suganthi, A.; Rajarajan, M.; Sara, S.A. Photocatalytic Activity of AgI Sensitized ZnO Nanoparticles under Visible Light Irradiation. Powder Technol. 2012, 224, 331–337. [Google Scholar] [CrossRef]
- Bahamonde Soria, R.; Chinchin, B.D.; Arboleda, D.; Zhao, Y.; Bonilla, P.; Van der Bruggen, B.; Luis, P. Effect of the Bio-Inspired Modification of Low-Cost Membranes with TiO2:ZnO as Microbial Fuel Cell Membranes. Chemosphere 2022, 291, 132840. [Google Scholar] [CrossRef]
- Upadhyay, G.K.; Rajput, J.K.; Pathak, T.K.; Swart, H.C.; Purohit, L.P. Photoactive CdO:TiO2 Nanocomposites for Dyes Degradation under Visible Light. Mater. Chem. Phys. 2020, 253, 123191. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ahmed, A.I.; Mannaa, M.A. Preparation and Characterization of SnO2 Doped TiO2 Nanoparticles: Effect of Phase Changes on the Photocatalytic and Catalytic Activity. J. Sci. Adv. Mater. Devices 2019, 4, 400–412. [Google Scholar] [CrossRef]
- Espinosa, J.C.; Catalá, C.; Navalón, S.; Ferrer, B.; Álvaro, M.; García, H. Iron Oxide Nanoparticles Supported on Diamond Nanoparticles as Efficient and Stable Catalyst for the Visible Light Assisted Fenton Reaction. Appl. Catal. B 2018, 226, 242–251. [Google Scholar] [CrossRef]
- Theerakarunwong, C.D.; Phothi, R. Community Refinery Wastewater Photodegradation by Fe-Doped TiO2 Films. Water Air Soil Pollut. 2018, 229, 231. [Google Scholar] [CrossRef]
- Nogueira, V.; Lopes, I.; Rocha-Santos, T.A.P.; Gonçalves, F.; Pereira, R. Treatment of Real Industrial Wastewaters through Nano-TiO2 and Nano-Fe2O3 Photocatalysis: Case Study of Mining and Kraft Pulp Mill Effluents. Environ. Technol. 2018, 39, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lee, B.-K. Structure and Activity of TiO2/FeO Co-Doped Carbon Spheres for Adsorptive-Photocatalytic Performance of Complete Toluene Removal from Aquatic Environment. Appl. Catal. A Gen. 2016, 523, 272–282. [Google Scholar] [CrossRef]
- Subash, B.; Krishnakumar, B.; Pandiyan, V.; Swaminathan, M.; Shanthi, M. Synthesis and Characterization of Novel WO3 Loaded Ag–ZnO and Its Photocatalytic Activity. Mater. Res. Bull. 2013, 48, 63–69. [Google Scholar] [CrossRef]
- Kolo, L.; Firdaus, F.; Taba, P.; Zakir, M.; Soekamto, N.H. Selectivity of the New Catalyst ZnO-MCM-48-CaO in Esterification of Calophyllum inophyllum Oil. Automot. Exp. 2022, 5, 217–229. [Google Scholar] [CrossRef]
- Benavides-Sánchez, E.A.; Castro-Ruíz, C.A.; Brand Narváez, M.A. El Emprendimiento de Base Tecnológica y Su Punto de Encuentro Con La Convergencia Tecnocientífica: Una Revisión a Partir Del Algoritmo Tree of Science. Rev. CEA 2023, 9, e2153. [Google Scholar] [CrossRef]
- Landínez Martínez, D.A.; Montoya Arenas, D.A. Políticas de Salud Pública Para La Prevención y El Tratamiento de La Enfermedad Vascular Cerebral: Una Revisión Sistemática Por Medio de La Metodología ToS (Tree of Science). Med. UPB 2019, 38, 129–139. [Google Scholar] [CrossRef]
- Patrus, R.; Silva, V.T.O. e A Organização de Uma Revisão de Literatura Por Meio Da Tree of Science (Árvore Da Ciência): Um Exemplo Sobre a Avaliação Da Pós-Graduação. Avaliação Rev. Avaliação Educ. Super. 2019, 24, 68–88. [Google Scholar] [CrossRef]
- Zuluaga, M.; Robledo, S.; Arbelaez-Echeverri, O.; Osorio-Zuluaga, G.A.; Duque-Méndez, N. Tree of Science-ToS: A Web-Based Tool for Scientific Literature Recommendation. Search Less, Research More! Issues Sci. Technol. Librariansh. 2022, 100, 2696. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- A., A.M.G.; Robledo, S.; Zuluaga, M. Topic Modeling: Perspectives from a Literature Review. IEEE Access 2023, 11, 4066–4078. [Google Scholar] [CrossRef]
- Sun, L.; Wu, L.; Qi, P. Global Characteristics and Trends of Research on Industrial Structure and Carbon Emissions: A Bibliometric Analysis. Environ. Sci. Pollut. Res. 2020, 27, 44892–44905. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, M.; Zhang, L.; Zhang, J.; Jin, L. Application of Nano TiO2 towards Polluted Water Treatment Combined with Electro-Photochemical Method. Water Res 2003, 37, 3815–3820. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, M.; Zhang, J.; Ying, X.; Jin, L. Photocatalytic Degradation of Organic Wastes by Electrochemically Assisted TiO2 Photocatalytic System. J. Environ. Manag. 2004, 70, 43–47. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Li, L.; Xian, Y.; Jin, L. Fabrication of TiO2/Ti Electrode by Laser-Assisted Anodic Oxidation and Its Application on Photoelectrocatalytic Degradation of Methylene Blue. J. Hazard. Mater. 2007, 139, 72–78. [Google Scholar] [CrossRef]
- Maeda, K.; Wang, X.; Nishihara, Y.; Lu, D.; Antonietti, M.; Domen, K. Photocatalytic Activities of Graphitic Carbon Nitride Powder for Water Reduction and Oxidation under Visible Light. J. Phys. Chem. C 2009, 113, 4940–4947. [Google Scholar] [CrossRef]
- Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. Highly Efficient, Solar Active, and Reusable Photocatalyst: Zr-Loaded Ag–ZnO for Reactive Red 120 Dye Degradation with Synergistic Effect and Dye-Sensitized Mechanism. Langmuir 2013, 29, 939–949. [Google Scholar] [CrossRef]
- Tachikawa, T.; Choi, J.R.; Fujitsuka, M.; Majima, T. Photoinduced Charge-Transfer Processes on MOF-5 Nanoparticles: Elucidating Differences between Metal-Organic Frameworks and Semiconductor Metal Oxides. J. Phys. Chem. C 2008, 112, 14090–14101. [Google Scholar] [CrossRef]
- Ghasemi, S.; Rahimnejad, S.; Setayesh, S.R.; Rohani, S.; Gholami, M.R. Transition Metal Ions Effect on the Properties and Photocatalytic Activity of Nanocrystalline TiO2 Prepared in an Ionic Liquid. J. Hazard. Mater. 2009, 172, 1573–1578. [Google Scholar] [CrossRef]
- Shivaraju, H.P.; Sajan, C.P.; Rungnapa, T.; Kumar, V.; Ranganathaiah, C.; Byrappa, K. Photocatalytic Treatment of Organic Pollutants in Textile Effluent Using Hydrothermally Prepared Photocatalytic Composite. Mater. Res. Innov. 2010, 14, 80–86. [Google Scholar] [CrossRef]
- Fang, T.; Yang, C.; Liao, L. Photoelectrocatalytic Degradation of High COD Dipterex Pesticide by Using TiO2/Ni Photo Electrode. J. Environ. Sci. 2012, 24, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Khairy, M.; Zakaria, W. Effect of Metal-Doping of TiO2 Nanoparticles on Their Photocatalytic Activities toward Removal of Organic Dyes. Egypt. J. Pet. 2014, 23, 419–426. [Google Scholar] [CrossRef]
- Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Effective Removal of Organic Pollutants Using GeO2/TiO2 Nanoparticle Composites under Direct Sunlight. Mater. Chem. Front. 2018, 2, 741–751. [Google Scholar] [CrossRef]
- Jo, W.-K.; Natarajan, T.S. Influence of TiO2 Morphology on the Photocatalytic Efficiency of Direct Z-Scheme g-C3N4/TiO2 Photocatalysts for Isoniazid Degradation. Chem. Eng. J. 2015, 281, 549–565. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ramezani Motlagh, H.; Jaafarzadeh, N.; Mostoufi, A.; Saeedi, R.; Barzegar, G.; Jorfi, S. Enhanced Photocatalytic Degradation of Tetracycline and Real Pharmaceutical Wastewater Using MWCNT/TiO2 Nano-Composite. J. Environ. Manag. 2017, 186, 55–63. [Google Scholar] [CrossRef]
- Deng, F.; Zhao, L.; Luo, X.; Luo, S.; Dionysiou, D.D. Highly Efficient Visible-Light Photocatalytic Performance of Ag/AgIn5S8 for Degradation of Tetracycline Hydrochloride and Treatment of Real Pharmaceutical Industry Wastewater. Chem. Eng. J. 2018, 333, 423–433. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, G.; Yu, W.; He, W.; Wang, Q.; Zhong, B.; Wang, Q.; Liao, S.; Li, R.; Chen, F.; et al. Molecular Characterization of Human Echinococcosis in Sichuan, Western China. Acta Trop. 2019, 190, 45–51. [Google Scholar] [CrossRef]
- Sheikh, M.U.D.; Naikoo, G.A.; Thomas, M.; Bano, M.; Khan, F. Solar-Assisted Photocatalytic Reduction of Methyl Orange Azo Dye over Porous TiO2 Nanostructures. New J. Chem. 2016, 40, 5483–5494. [Google Scholar] [CrossRef]
- Shokri, A.; Mahanpoor, K.; Soodbar, D. Evaluation of a Modified TiO2 (GO–B–TiO2) Photo Catalyst for Degradation of 4-Nitrophenol in Petrochemical Wastewater by Response Surface Methodology Based on the Central Composite Design. J. Environ. Chem. Eng. 2016, 4, 585–598. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Lee, J.Y.; Bajaj, H.C.; Jo, W.-K.; Tayade, R.J. Synthesis of Multiwall Carbon Nanotubes/TiO2 Nanotube Composites with Enhanced Photocatalytic Decomposition Efficiency. Catal. Today 2017, 282, 13–23. [Google Scholar] [CrossRef]
- Saikia, L.; Bhuyan, D.; Saikia, M.; Malakar, B.; Dutta, D.K.; Sengupta, P. Photocatalytic Performance of ZnO Nanomaterials for Self Sensitized Degradation of Malachite Green Dye under Solar Light. Appl. Catal. A Gen. 2015, 490, 42–49. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green Synthesis of TiO2 Nanoparticles Using Leaf Extract of Jatropha Curcas L. for Photocatalytic Degradation of Tannery Wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Tu, S.; Ning, Z.; Duan, X.; Zhao, X.; Chang, L. Efficient Electrochemical Hydrogen Peroxide Generation Using TiO2/RGO Catalyst and Its Application in Electro-Fenton Degradation of Methyl Orange. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129657. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Khorsandi, S. Photocatalytic Degradation of 4-Nitrophenol with ZnO Supported Nano-Clinoptilolite Zeolite. J. Ind. Eng. Chem. 2014, 20, 937–946. [Google Scholar] [CrossRef]
- Liu, K.; Yang, Y.; Sun, F.; Liu, Y.; Tang, M.; Chen, J. Rapid Degradation of Congo Red Wastewater by Rhodopseudomonas Palustris Intimately Coupled Carbon Nanotube-Silver Modified Titanium Dioxide Photocatalytic Composite with Sodium Alginate. Chemosphere 2022, 299, 134417. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Karimi-Shamsabadi, M. Decolorization of a Binary Azo Dyes Mixture Using CuO Incorporated Nanozeolite-X as a Heterogeneous Catalyst and Solar Irradiation. Chem. Eng. J. 2013, 228, 631–641. [Google Scholar] [CrossRef]
- A Mehrabanpour, N.; Nezamzadeh-Ejhieh, A.; Ghattavi, S. Cefotaxime Degradation by the Coupled Binary CdS-PbS: Characterization and the Photocatalytic Process Kinetics. Environ. Sci. Pollut. Res. 2022, 30, 33725–33736. [Google Scholar] [CrossRef]
- Nawaz, R.; Kait, C.F.; Chia, H.Y.; Isa, M.H.; Huei, L.W.; Sahrin, N.T.; Khan, N. Countering Major Challenges Confronting Photocatalytic Technology for the Remediation of Treated Palm Oil Mill Effluent: A Review. Environ. Technol. Innov. 2021, 23, 101764. [Google Scholar] [CrossRef]
- Mehrabanpour, N.; Nezamzadeh-Ejhieh, A.; Ghattavi, S. The Boosted Photocatalytic Effects of a Zeolite Supported CdS towards an Antibiotic Model Pollutant: A Brief Kinetics Study. Environ. Sci. Pollut. Res. 2023, 30, 5089–5102. [Google Scholar] [CrossRef]
- Gholami, N.; Ghasemi, B.; Anvaripour, B.; Jorfi, S. Enhanced Photocatalytic Degradation of Furfural and a Real Wastewater Using UVC/TiO2 Nanoparticles Immobilized on White Concrete in a Fixed-Bed Reactor. J. Ind. Eng. Chem. 2018, 62, 291–301. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Subash, B.; Swaminathan, M. AgBr–ZnO–An Efficient Nano-Photocatalyst for the Mineralization of Acid Black 1 with UV Light. Sep. Purif. Technol. 2012, 85, 35–44. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Kontos, A.G.; de la Cruz, A.A.; O’shea, K.; Dunlop, P.S.M.; Byrne, J.A.; Dionysiou, D.D. A Comparative Study on the Removal of Cylindrospermopsin and Microcystins from Water with NF-TiO2-P25 Composite Films with Visible and UV–Vis Light Photocatalytic Activity. Appl. Catal. B Environ. 2012, 121–122, 30–39. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Salimi, Z. Heterogeneous Photodegradation Catalysis of O-Phenylenediamine Using CuO/X Zeolite. Appl. Catal. A Gen. 2010, 390, 110–118. [Google Scholar] [CrossRef]
- Suresh, P.; Vijaya, J.J.; Kennedy, L.J. Photocatalytic Degradation of Textile-Dyeing Wastewater by Using a Microwave Combustion-Synthesized Zirconium Oxide Supported Activated Carbon. Mater. Sci. Semicond. Process. 2014, 27, 482–493. [Google Scholar] [CrossRef]
- Suresh, P.; Vijaya, J.J.; Balasubramaniam, T.; John Kennedy, L. Synergy Effect in the Photocatalytic Degradation of Textile Dyeing Waste Water by Using Microwave Combustion Synthesized Nickel Oxide Supported Activated Carbon. Desal. Water Treat. 2016, 57, 3766–3781. [Google Scholar] [CrossRef]
- Hemmatpour, P.; Nezamzadeh-Ejhieh, A.; Ershadi, A. A Brief Study on the Eriochrome Black T Photodegra-dation Kinetic by CdS/BiVO4 Coupled Catalyst. Mater. Res. Bull. 2022, 151, 111830. [Google Scholar] [CrossRef]
- Yousefi, A.; Nezamzadeh-Ejhieh, A.; Mirmohammadi, M. The Coupled CuO-SnO2 Catalyst: Characterization and the Photodegradation Kinetics towards Phenazopyridine. Environ. Technol. Innov. 2021, 22, 101496. [Google Scholar] [CrossRef]
- Wang, Y.; Zu, M.; Zhou, X.; Lin, H.; Peng, F.; Zhang, S. Designing Efficient TiO2-Based Photoelectrocatalysis Systems for Chemical Engineering and Sensing. Chem. Eng. J. 2020, 381, 122605. [Google Scholar] [CrossRef]
- Kaur, T.; Sraw, A.; Wanchoo, R.K.; Toor, A.P. Solar Assisted Degradation of Carbendazim in Water Using Clay Beads Immobilized with TiO2 & Fe Doped TiO2. Sol. Energy 2018, 162, 45–56. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Enhanced Photocatalytic Activity of Bismuth-Doped TiO2 Nanotubes under Direct Sunlight Irradiation for Degradation of Rhodamine B Dye. J. Nano Res. 2013, 15, 1669. [Google Scholar] [CrossRef]
- Zarrin, S.; Heshmatpour, F. Facile Preparation of New Nanohybrids for Enhancing Photocatalytic Activity toward Removal of Organic Dyes under Visible Light Irradiation. J. Phys. Chem. Sol. 2020, 140, 109271. [Google Scholar] [CrossRef]
- Bashir, A.; Rafique, U.; Bashir, R.; Jamil, S.; Bashir, F.; Sultan, M.; Mubeen, M.; Mehmood, Z.; Iqbal, A.; Akhter, Z. Synthesis and Comparative Evaluation of Optical and Electrochemical Properties of Ni+2 and Pr+3 Ions Co-Doped Mesoporous TiO2 Nanoparticles with Undoped Titania. Appl. Nanosci. 2021, 11, 2397–2413. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and Technology for Water Purification in the Coming Decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Neppolian, B. Solar/UV-Induced Photocatalytic Degradation of Three Commercial Textile Dyes. J. Hazard. Mater. 2002, 89, 303–317. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Graetzel, M. Light-Induced Redox Reactions in Nanocrystalline Systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).