An Electrochemical Characterisation of Silica–Zirconia Oxide Nanostructured Materials for Fuel Cells
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of Si-ZrO2 Nanoparticles
2.3. Characterisation
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. The High-Resolution Transmission Electron Microscopy
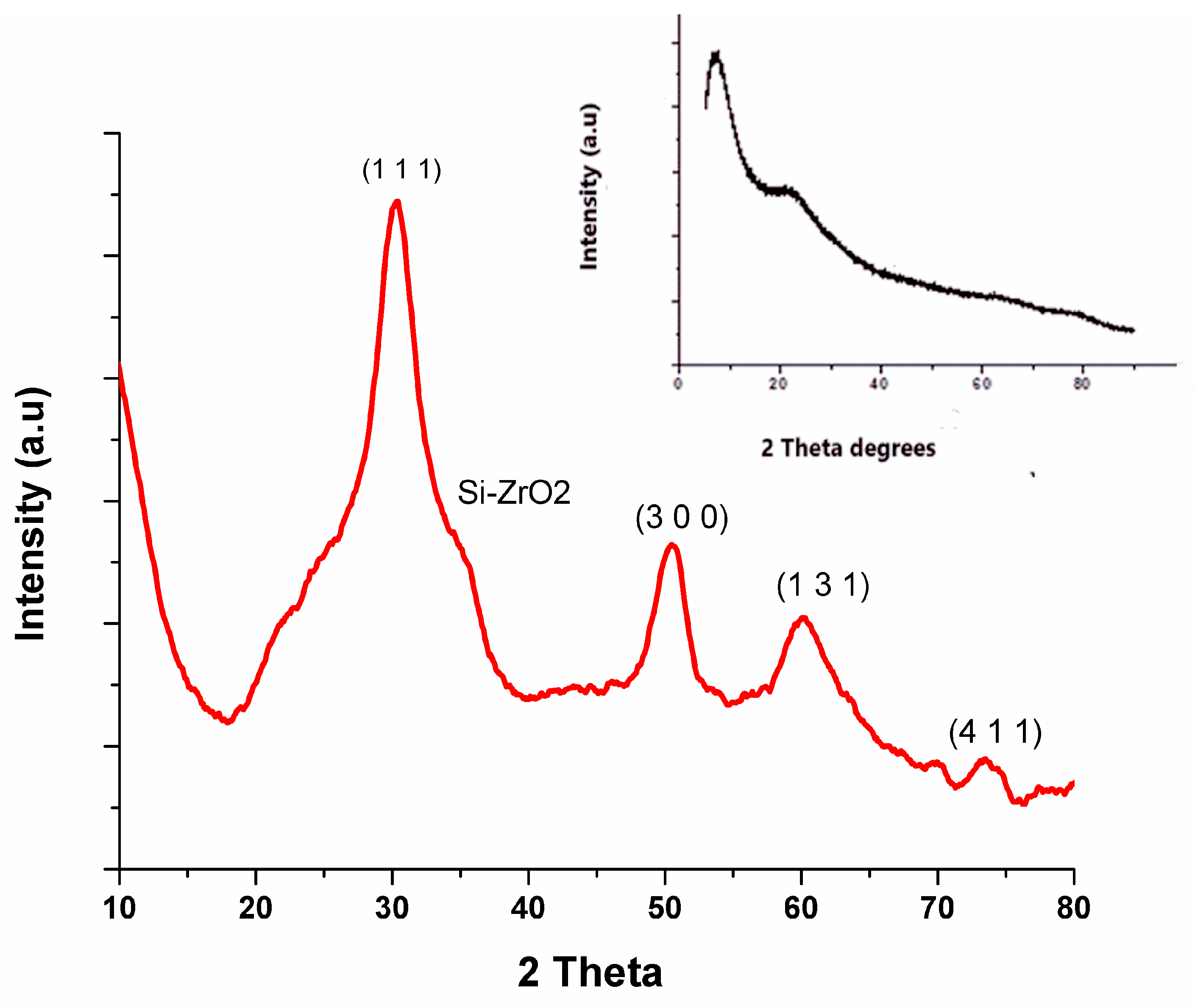
3.2. Structural Analysis
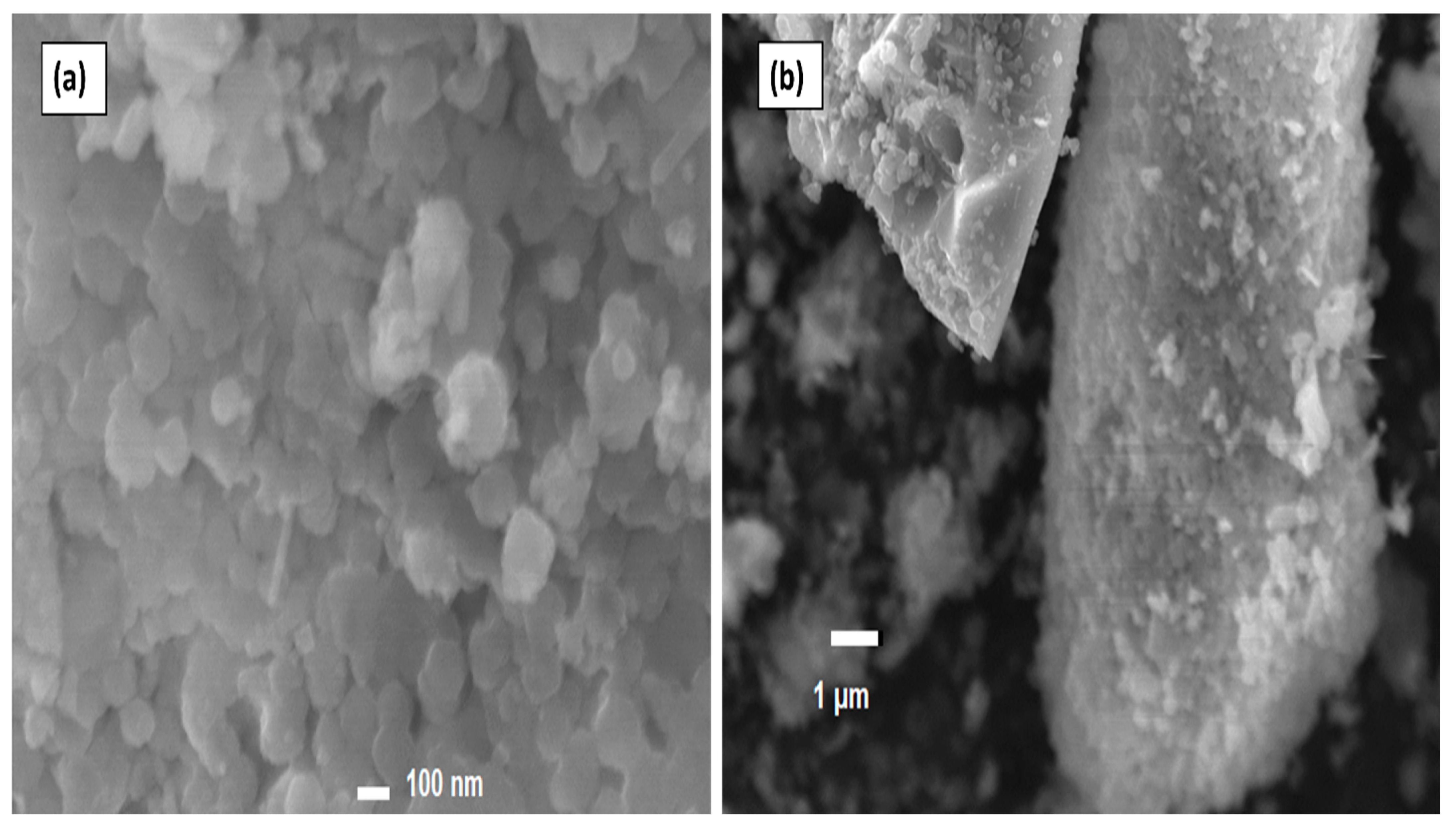
3.3. Scanning Electron Microscopy (SEM) Analysis
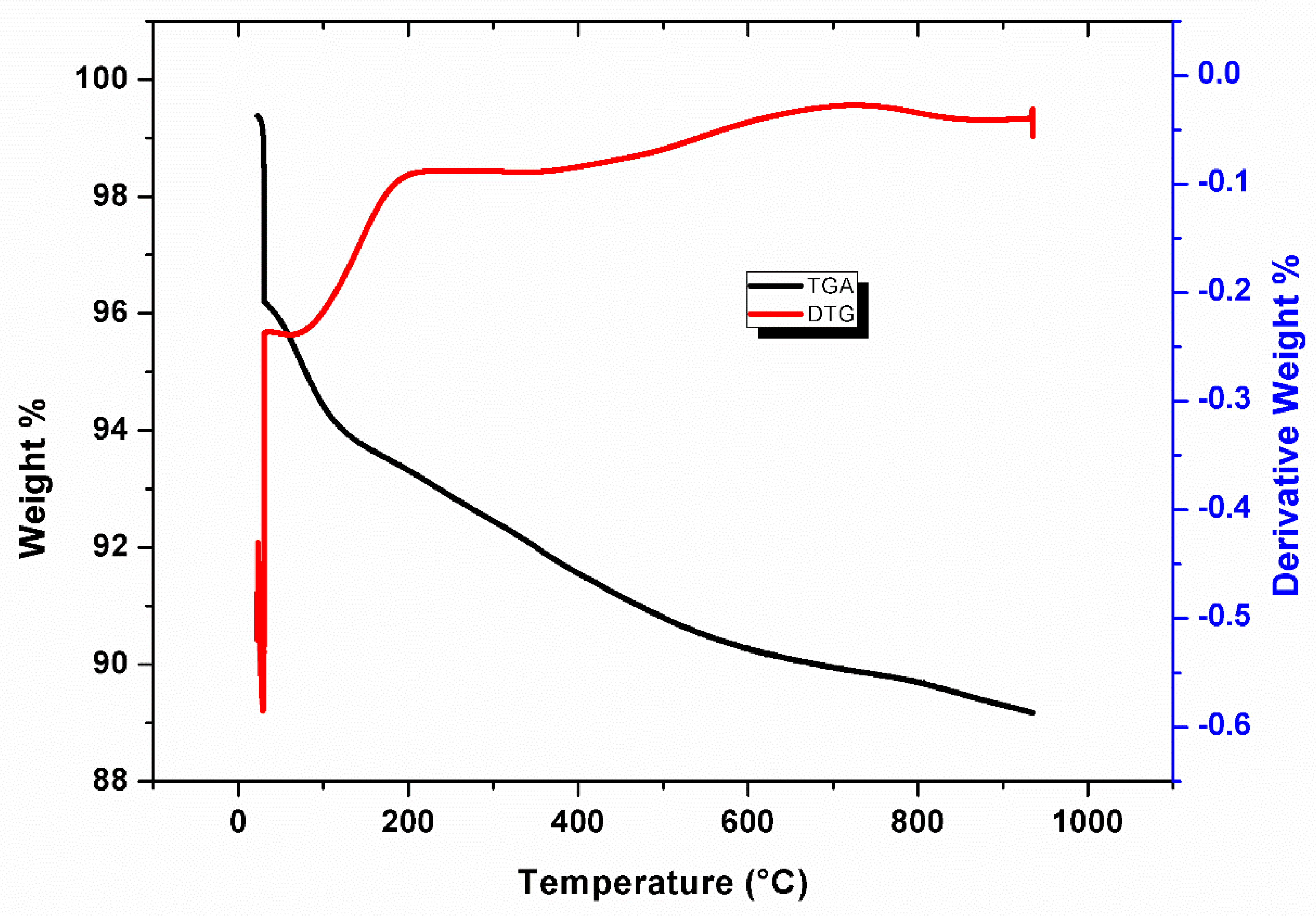
3.4. TGA and Derivative Thermo-Gravimetric (DTG)
3.5. FTIR Spectrum of ZrO2 Nanoparticles
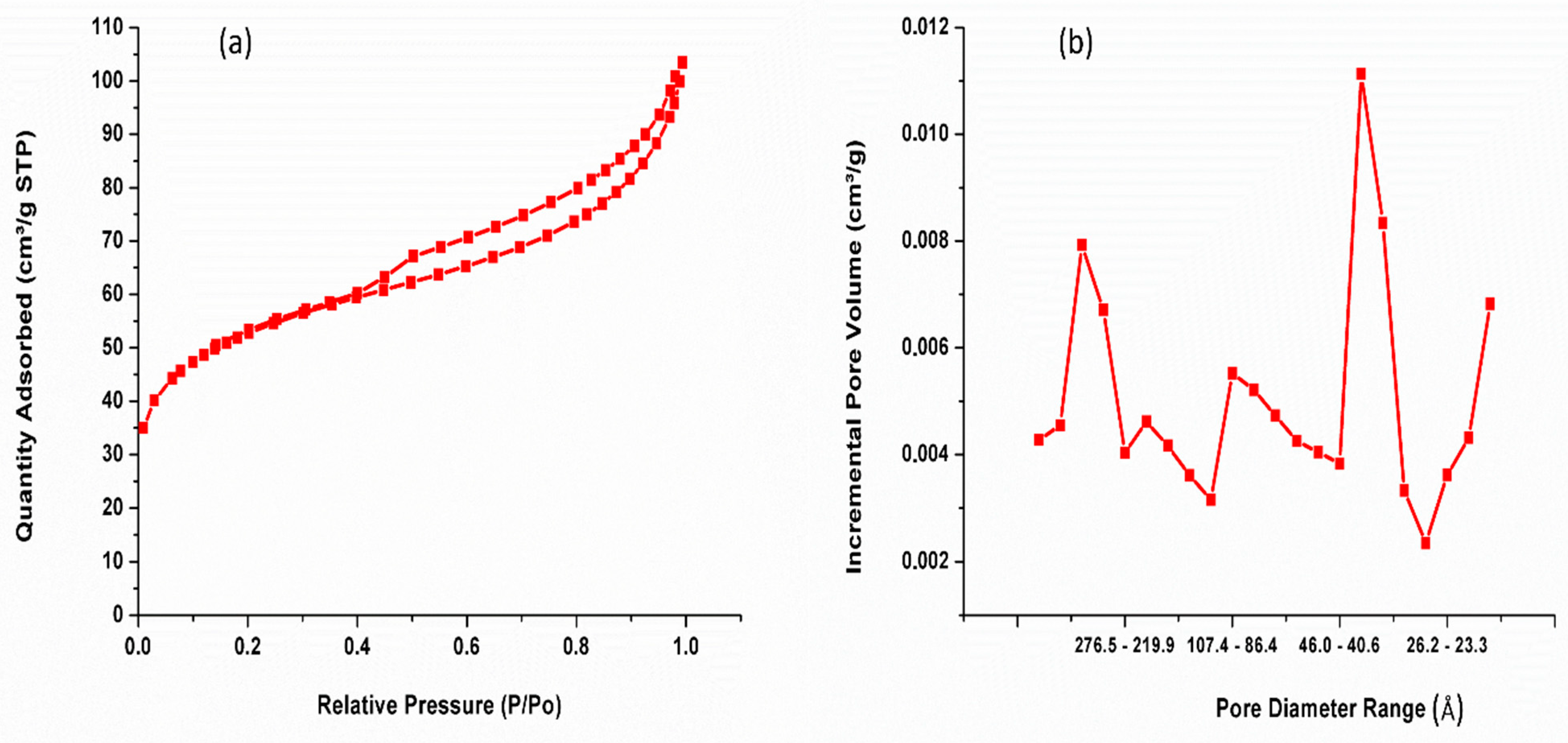
3.6. Nitrogen Adsorption–Desorption
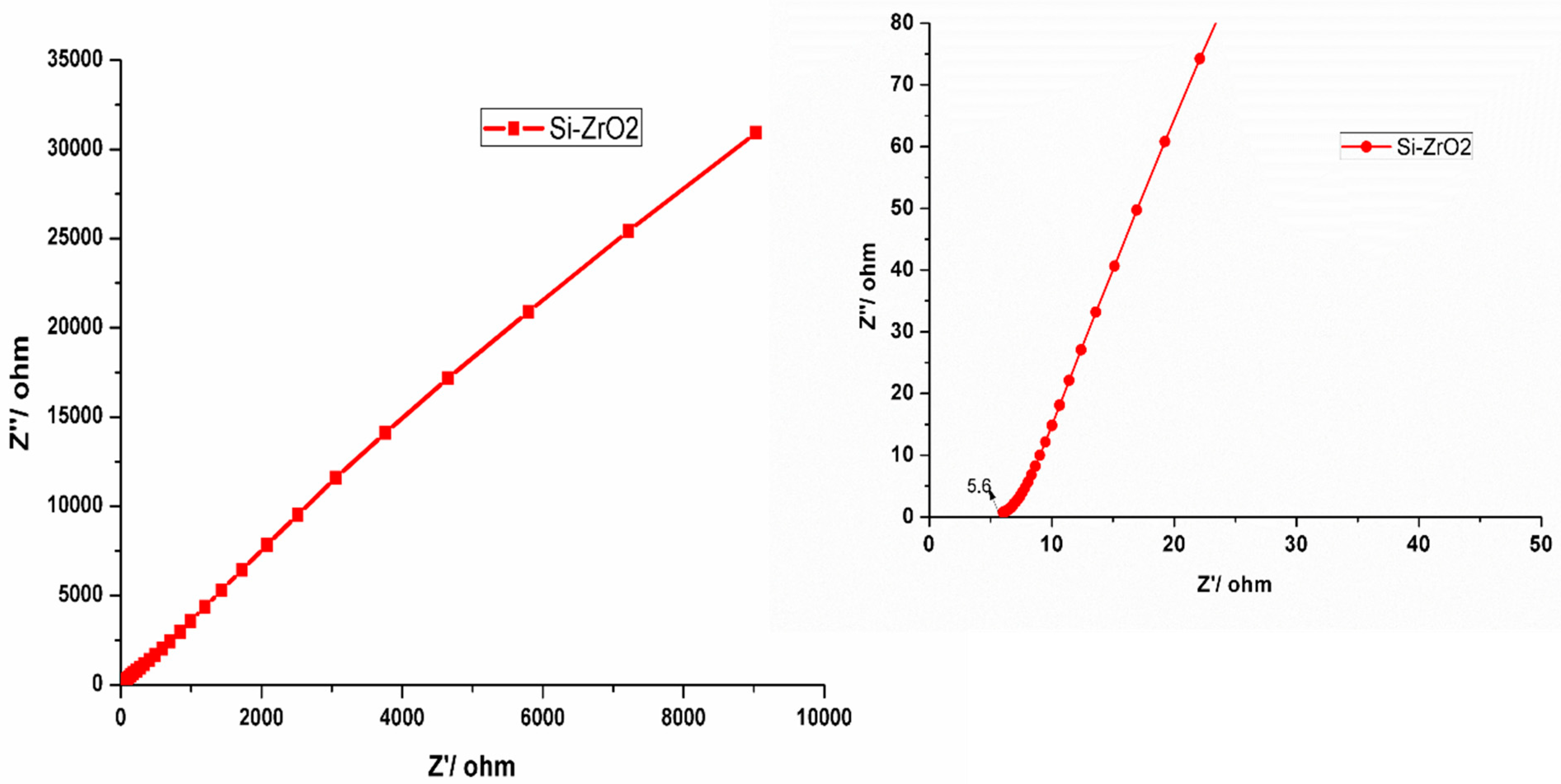
3.7. Electrochemical Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anjum, S.; Ishaque, S.; Fatima, H.; Farooq, W.; Hano, C.; Abbasi, B.H.; Anjum, I. Emerging Applications of Nanotechnology in Healthcare Systems: Grand Challenges and Perspectives. Pharmaceuticals 2021, 14, 707. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, B.; Sathyanath, S.K.M.; Chang, J.; Yu, J.; Leifer, K.; Engqvist, H.; Li, Q.; Xia, W. Microstructure of rapidly-quenched ZrO2-SiO2 glass-ceramics fabricated by container-less aerodynamic levitation technology. J. Am. Ceram. Soc. 2023, 106, 2635–2651. [Google Scholar] [CrossRef]
- Khan, S.; Mansoor, S.; Rafi, Z.; Kumari, B.; Shoaib, A.; Saeed, M.; Alshehri, S.; Ghoneim, M.M.; Rahamathulla, M.; Hani, U. A review on nanotechnology: Properties, applications, and mechanistic insights of cellular uptake mechanisms. J. Mol. Liq. 2022, 348, 118008. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S. Precise calculation of crystallite size of nanomaterials: A review. J. Alloys Compd. 2023, 968, 171914. [Google Scholar] [CrossRef]
- Lin, H.; Yin, C.; Mo, A. Zirconia Based Dental Biomaterials: Structure, Mechanical Properties, Biocompatibility, Surface Modification, and Applications as Implant. Front. Dent. Med. 2021, 2, 689198. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of nanotechnology in medical field: A brief review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Ballem, M.A.; Córdoba, J.M.; Odén, M. Mesoporous silica templated zirconia nanoparticles. J. Nanopart. Res. 2011, 13, 2743–2748. [Google Scholar] [CrossRef]
- Raj, S.; Hattori, M.; Ozawa, M. Ag-doped ZrO2 nanoparticles prepared by hydrothermal method for efficient diesel soot oxidation. Mater. Lett. 2019, 234, 205–207. [Google Scholar] [CrossRef]
- Hidayat, D.; Syoufian, A.; Utami, M.; Wijaya, K. Synthesis and Application of Na2O/ZrO2 Nanocomposite for Microwave-assisted Transesterification of Castor Oil. ICS Phys. Chem. 2021, 1, 26. [Google Scholar] [CrossRef]
- Bansal, P.; Kaur, N.; Prakash, C.; Chaudhary, G.R. ZrO2 nanoparticles: An industrially viable, efficient and recyclable catalyst for synthesis of pharmaceutically significant xanthene derivatives. Vacuum 2018, 157, 9–16. [Google Scholar] [CrossRef]
- Sengupta, P.; Bhattacharjee, A.; Maiti, H.S. Zirconia: A Unique Multifunctional Ceramic Material. Trans. Indian Inst. Met. 2019, 72, 1981–1998. [Google Scholar] [CrossRef]
- Verdi, C.; Karsai, F.; Liu, P.; Jinnouchi, R.; Kresse, G. Thermal transport and phase transitions of zirconia by on-the-fly machine-learned interatomic potentials. npj Comput. Mater. 2021, 7, 156. [Google Scholar] [CrossRef]
- Chitoria, A.K.; Mir, A.; Shah, M. A review of ZrO2 nanoparticles applications and recent advancements. Ceram. Int. 2023, 49, 32343–32358. [Google Scholar] [CrossRef]
- Matt, S.B.; Raghavendra, S.; Shivanna, M.; Sidlinganahalli, M.; Siddalingappa, D.M. Electrochemical Detection of Paracetamol by Voltammetry Techniques Using Pure Zirconium Oxide Nanoparticle Based Modified Carbon Paste Electrode. J. Inorg. Organomet. Polym. Mater. 2021, 31, 511–519. [Google Scholar] [CrossRef]
- Yilmaz, S.; Cobaner, S.; Yalaz, E.; Horri, B.A. Synthesis and Characterization of Gadolinium-Doped Zirconia as a Potential Electrolyte for Solid Oxide Fuel Cells. Energies 2022, 15, 2826. [Google Scholar] [CrossRef]
- Gorbova, E.; Tzorbatzoglou, F.; Molochas, C.; Chloros, D.; Demin, A.; Tsiakaras, P. Fundamentals and Principles of Solid-State Electrochemical Sensors for High Temperature Gas Detection. Catalysts 2021, 12, 1. [Google Scholar] [CrossRef]
- Min, H.K.; Kim, Y.W.; Kim, C.; Ibrahim, I.A.; Han, J.W.; Suh, Y.W.; Jung, K.D.; Park, M.B.; Shin, C.H. Phase transformation of ZrO2 by Si incorporation and catalytic activity for isopropyl alcohol dehydration and dehydrogenation. Chem. Eng. J. 2022, 428, 131766. [Google Scholar] [CrossRef]
- Ahmadi, F.; Sodagar-Taleghani, A.; Ebrahimnejad, P.; Moghaddam, S.P.H.; Ebrahimnejad, F.; Asare-Addo, K.; Nokhodchi, A. A review on the latest developments of mesoporous silica nanoparticles as a promising platform for diagnosis and treatment of cancer. Int. J. Pharm. 2022, 625, 122099. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tong, Z.; Qiao, Y.; Yang, Z.; Yue, S.; Li, X.; Su, D.; Ji, H. High thermal stability of SiO2–ZrO2 aerogels using solvent-thermal aging. J. Solid State Chem. 2020, 291, 121624. [Google Scholar] [CrossRef]
- Sigwadi, R.; Mokrani, T.; Dhlamini, M. The synthesis, characterization and electrochemical study of zirconia oxide nanoparticles for fuel cell application. Phys. B Condens. Matter 2020, 581, 411842. [Google Scholar] [CrossRef]
- Sigwadi, R.; Dhlamini, M.S.; Mokrani, T.; Nonjola, P. Effect of Synthesis Temperature on Particles Size and Morphology of Zirconium Oxide Nanoparticle. J. Nano Res. 2017, 50, 18–31. [Google Scholar] [CrossRef]
- Dong, W.-S.; Lin, F.-Q.; Liu, C.-L.; Li, M.-Y. Synthesis of ZrO2 nanowires by ionic-liquid route. J. Colloid Interface Sci. 2009, 333, 734–740. [Google Scholar] [CrossRef]
- Kocjan, A.; Logar, M.; Shen, Z. The agglomeration, coalescence and sliding of nanoparticles, leading to the rapid sintering of zirconia nanoceramics. Sci. Rep. 2017, 7, 2541. [Google Scholar] [CrossRef]
- Klinger, M. More features, more tools, more CrysTBox. J. Appl. Crystallogr. 2017, 50, 1226–1234. [Google Scholar] [CrossRef]
- Lestari, N.D.; Nurlaila, R.; Muwwaqor, N.F.; Pratapa, S. Synthesis of high-purity zircon, zirconia, and silica nanopowders from local zircon sand. Ceram. Int. 2019, 45, 6639–6647. [Google Scholar]
- Mostafa, N.Y.; Zaki, Z.; Mohsen, Q.; Alotaibi, S.; El-moemen, A.A.; Amin, M.A. Carboxylate-assisted synthesis of highly-defected monoclinic zirconia nanoparticles. J. Mol. Struct. 2020, 1214, 128232. [Google Scholar] [CrossRef]
- Soontaranon, S.; Limphirat, W.; Pratapa, S. XRD, WAXS, FTIR, and XANES studies of silica-zirconia systems. Ceram. Int. 2019, 45, 15660–15670. [Google Scholar]
- Xu, L.; Lei, H.; Ding, Z.; Chen, Y.; Ding, R.; Kim, T. Preparation of the rod-shaped SiO2@C abrasive and effects of its microstructure on the polishing of zirconia ceramics. Powder Technol. 2022, 395, 338–347. [Google Scholar] [CrossRef]
- Saravanan, S.; Dubey, R. Synthesis of SiO2 nanoparticles by sol-gel method and their optical and structural properties. Romanian J. Inf. Sci. Technol. 2020, 23, 105–112. [Google Scholar]
- Lv, X.; Yuan, L.; Rao, C.; Wu, X.; Qing, X.; Weng, X. Structure and near-infrared spectral properties of mesoporous silica for hyperspectral camouflage materials. Infrared Phys. Technol. 2023, 129, 104558. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Zhu, Y.; An, D.; Gao, W.; Wang, Z.; Ma, Y.; Wang, Z. A sustainable route for the preparation of activated carbon and silica from rice husk ash. J. Hazard. Mater. 2011, 186, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, Y.; Li, S.; Zhong, L.; Wang, J.; Chen, Y. High-surface-area mesoporous silica-yttria-zirconia ceramic materials prepared by coprecipitation method—The role of silicon. Ceram. Int. 2022, 48, 21951–21960. [Google Scholar] [CrossRef]
- Bumajdad, A.; Nazeer, A.A.; Al Sagheer, F.; Nahar, S.; Zaki, M.I. Controlled Synthesis of ZrO2 Nanoparticles with Tailored Size, Morphology and Crystal Phases via Organic/Inorganic Hybrid Films. Sci. Rep. 2018, 8, 3695. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.V.; Babu, B.; Reddy, I.N.; Shim, J. Synthesis and characterization of pure tetragonal ZrO2 nanoparticles with enhanced photocatalytic activity. Ceram. Int. 2018, 44, 6940–6948. [Google Scholar] [CrossRef]
- Khalid, S.; Cao, C.; Wang, L.; Zhu, Y. Microwave Assisted Synthesis of Porous NiCo2O4 Microspheres: Application as High Performance Asymmetric and Symmetric Supercapacitors with Large Areal Capacitance. Sci. Rep. 2016, 6, 22699. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.-P.; Wu, Z.-S.; Chen, L.; Lu, H.; Zheng, Y.-R.; Weidner, T.; Feng, X.; Landfester, K.; Crespy, D. Precursor-controlled and template-free synthesis of nitrogen-doped carbon nanoparticles for supercapacitors. RSC Adv. 2015, 5, 50063–50069. [Google Scholar] [CrossRef]




| Sample ID | Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| Si-ZrO2 | 185 | 0.14 |
| ZrO2 | 162 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigwadi, R.; Mokrani, T.; Nemavhola, F. An Electrochemical Characterisation of Silica–Zirconia Oxide Nanostructured Materials for Fuel Cells. ChemEngineering 2025, 9, 26. https://doi.org/10.3390/chemengineering9020026
Sigwadi R, Mokrani T, Nemavhola F. An Electrochemical Characterisation of Silica–Zirconia Oxide Nanostructured Materials for Fuel Cells. ChemEngineering. 2025; 9(2):26. https://doi.org/10.3390/chemengineering9020026
Chicago/Turabian StyleSigwadi, Rudzani, Touhami Mokrani, and Fulufhelo Nemavhola. 2025. "An Electrochemical Characterisation of Silica–Zirconia Oxide Nanostructured Materials for Fuel Cells" ChemEngineering 9, no. 2: 26. https://doi.org/10.3390/chemengineering9020026
APA StyleSigwadi, R., Mokrani, T., & Nemavhola, F. (2025). An Electrochemical Characterisation of Silica–Zirconia Oxide Nanostructured Materials for Fuel Cells. ChemEngineering, 9(2), 26. https://doi.org/10.3390/chemengineering9020026







