Enhanced Efficiency of CZTS Solar Cells with Reduced Graphene Oxide and Titanium Dioxide Layers: A SCAPS Simulation Study
Abstract
1. Introduction
2. Materials and Methods
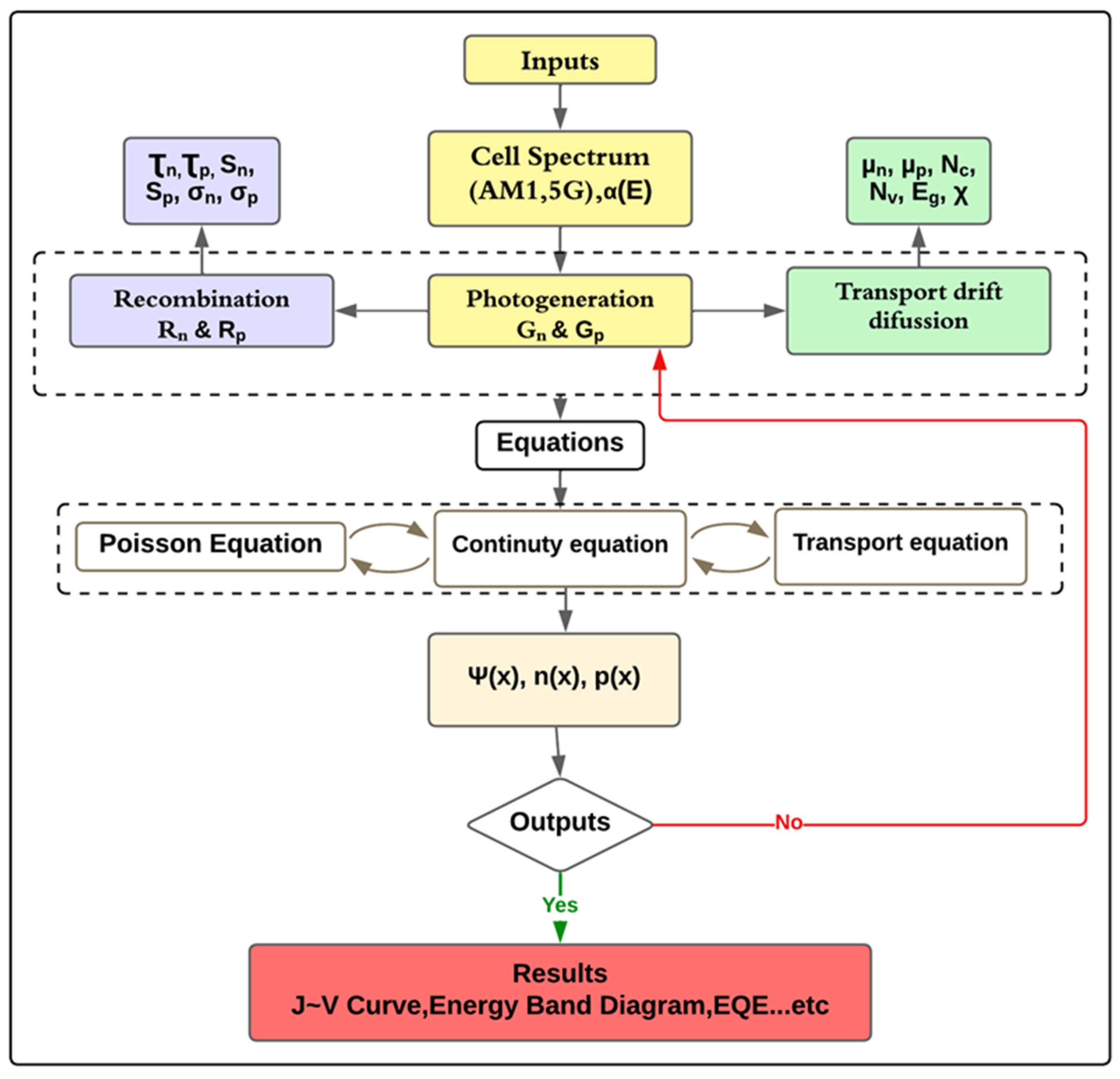
2.1. Simulation of the Proposed Configuration
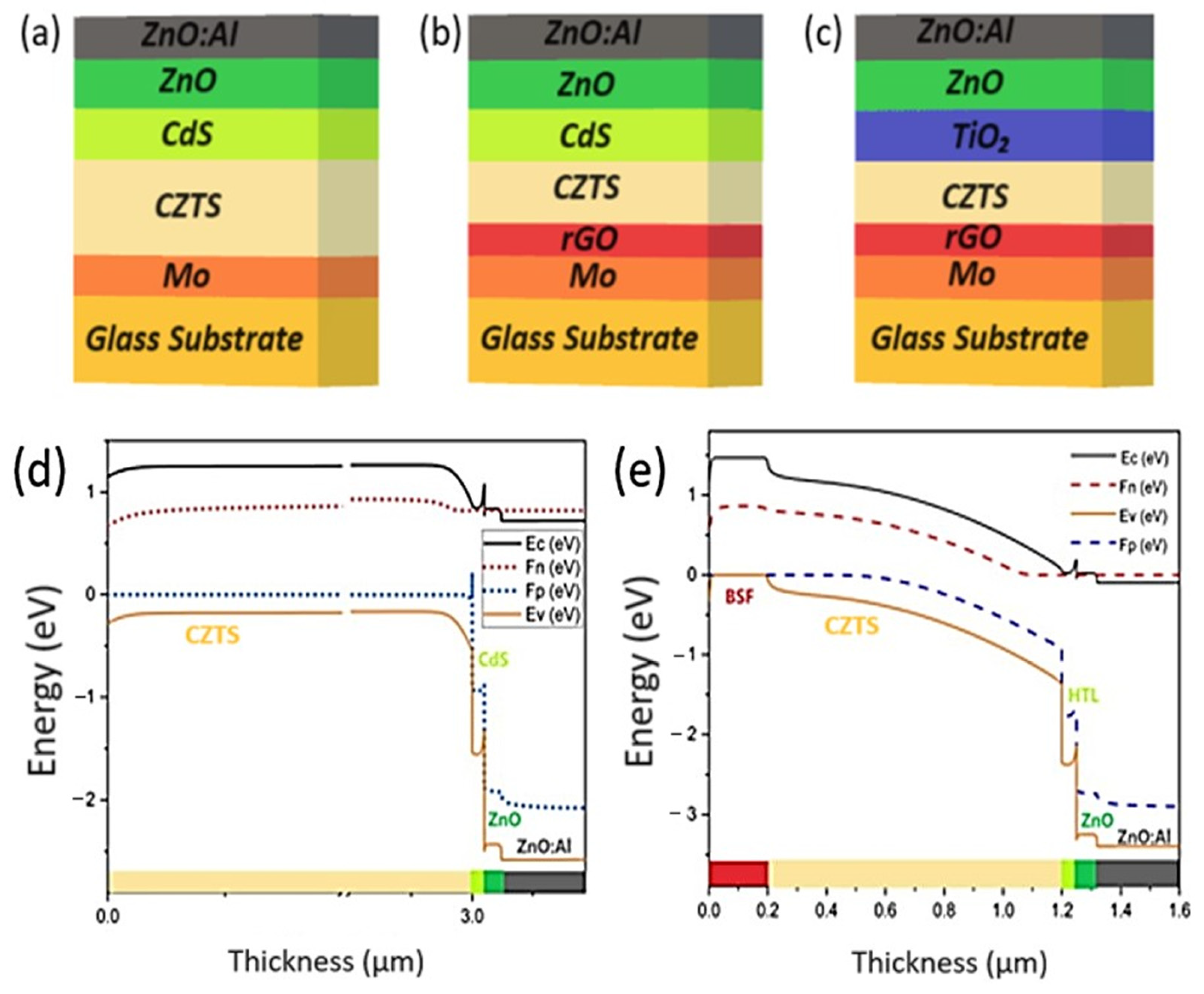
Configurations
- Conventional CZTS SC without BSF Layer.
- 2.
- CZTS SC with rGO BSF Layer.
- 3.
- CZTS SC with TiO2 Buffer Layer:
2.2. Material Parameters
3. Results
3.1. Part I: Conventional CZTS SC
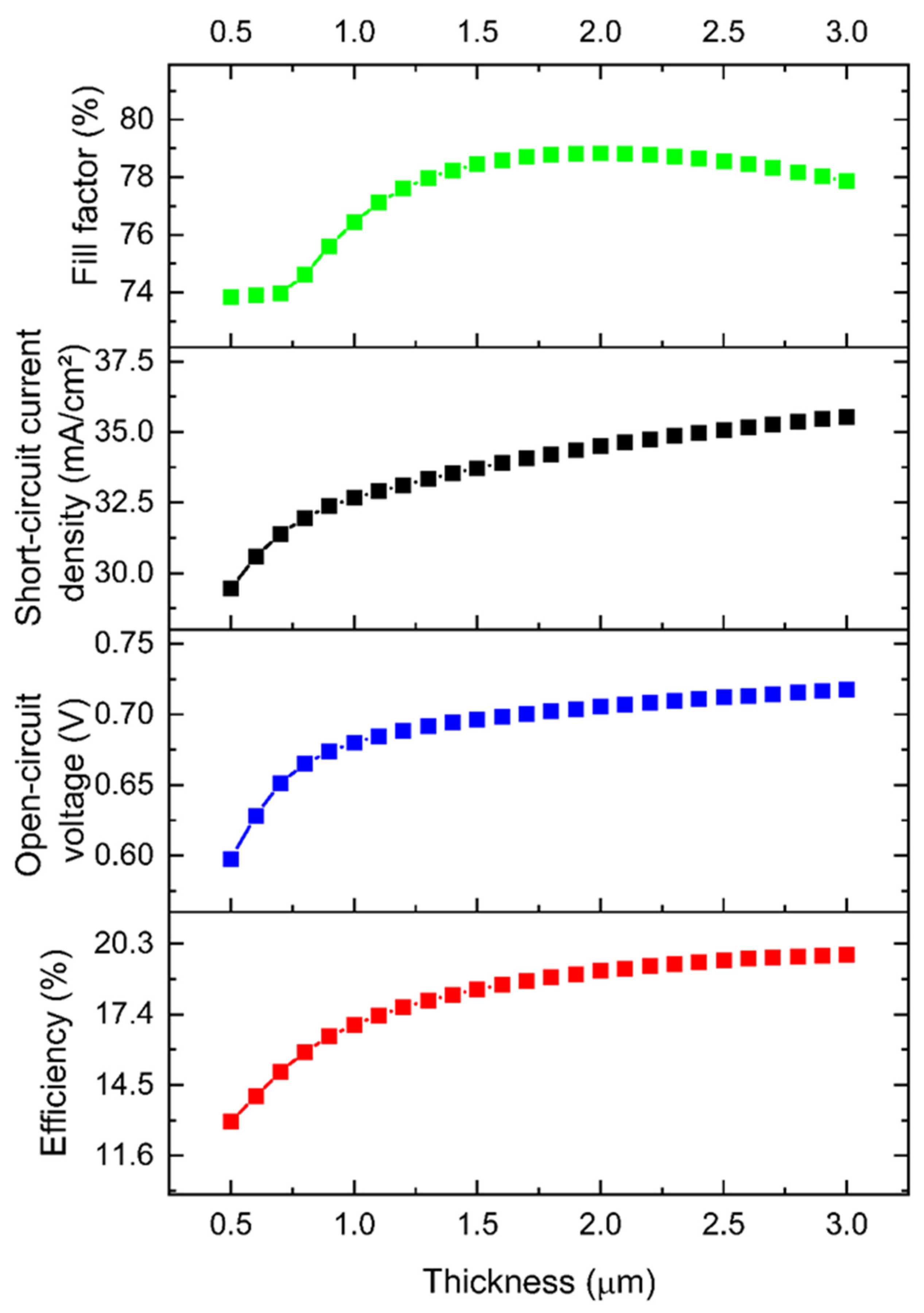
3.1.1. Thickness Optimization of the CZTS Absorber Layer
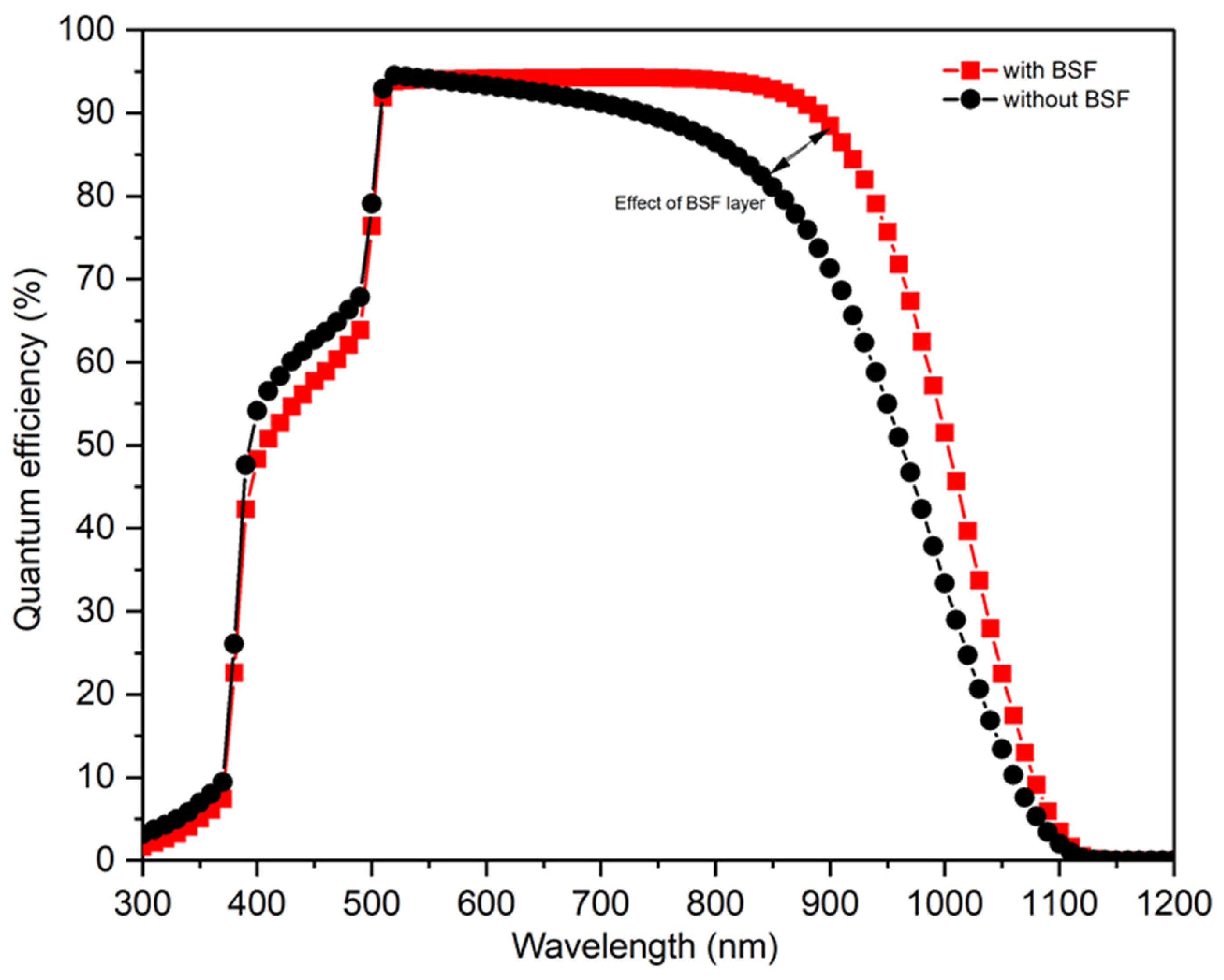
3.1.2. Quantum Efficiency of the CZTS Layer with Various Thicknesses
3.1.3. Current Flow (J) Related to Voltage (V)
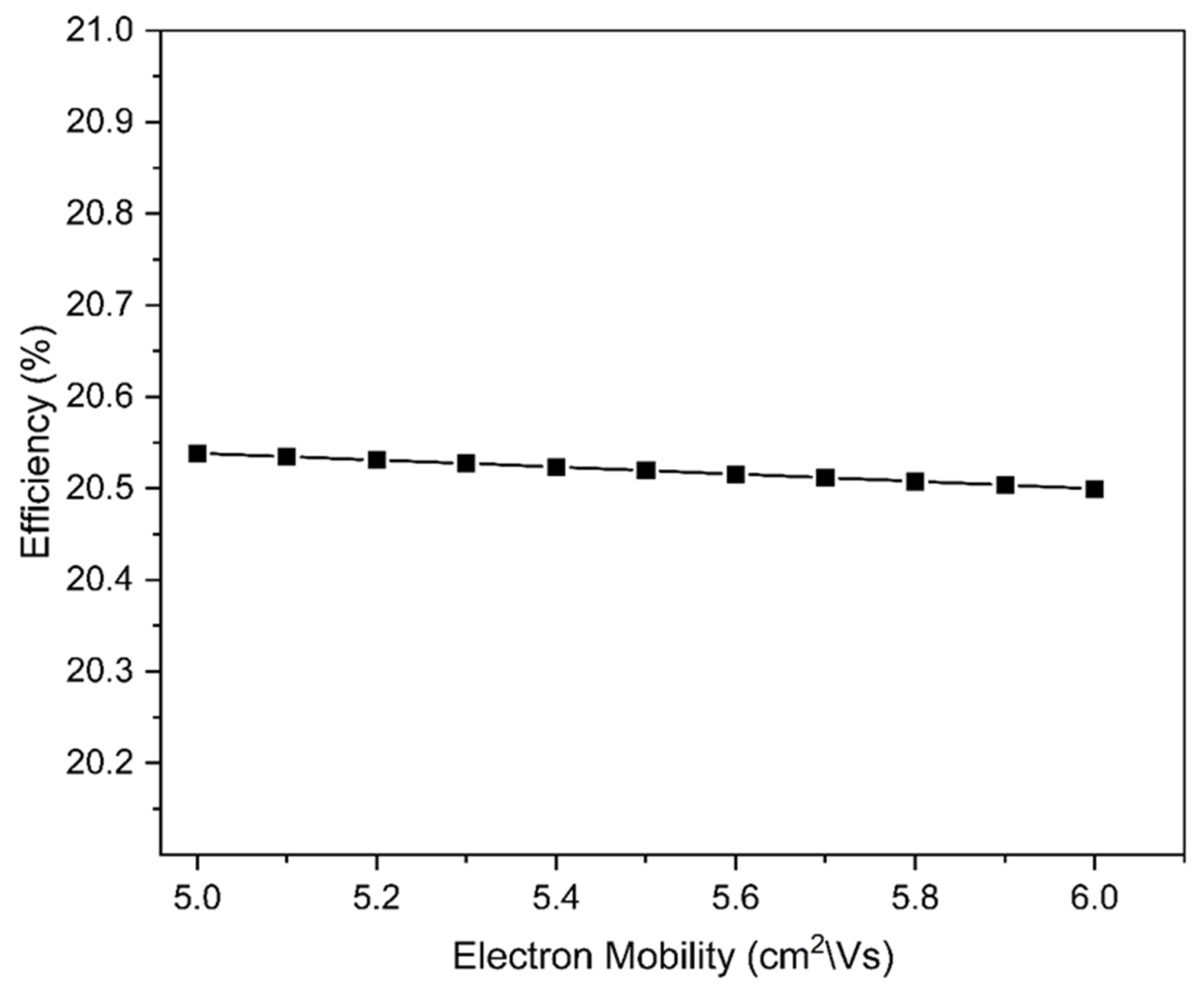
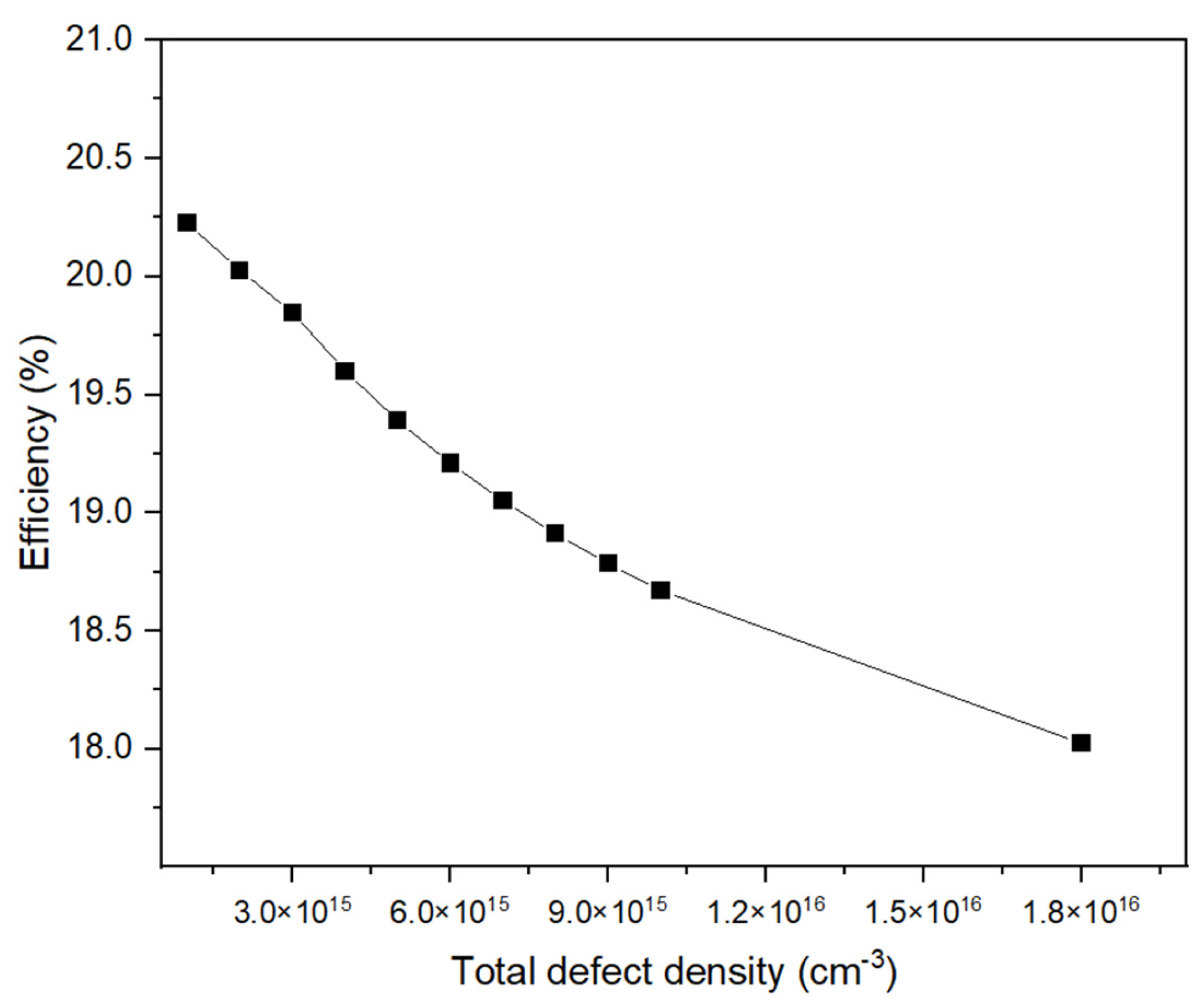
3.1.4. Impact of Electron Mobilities and Total Defect Density of the Absorber Layer
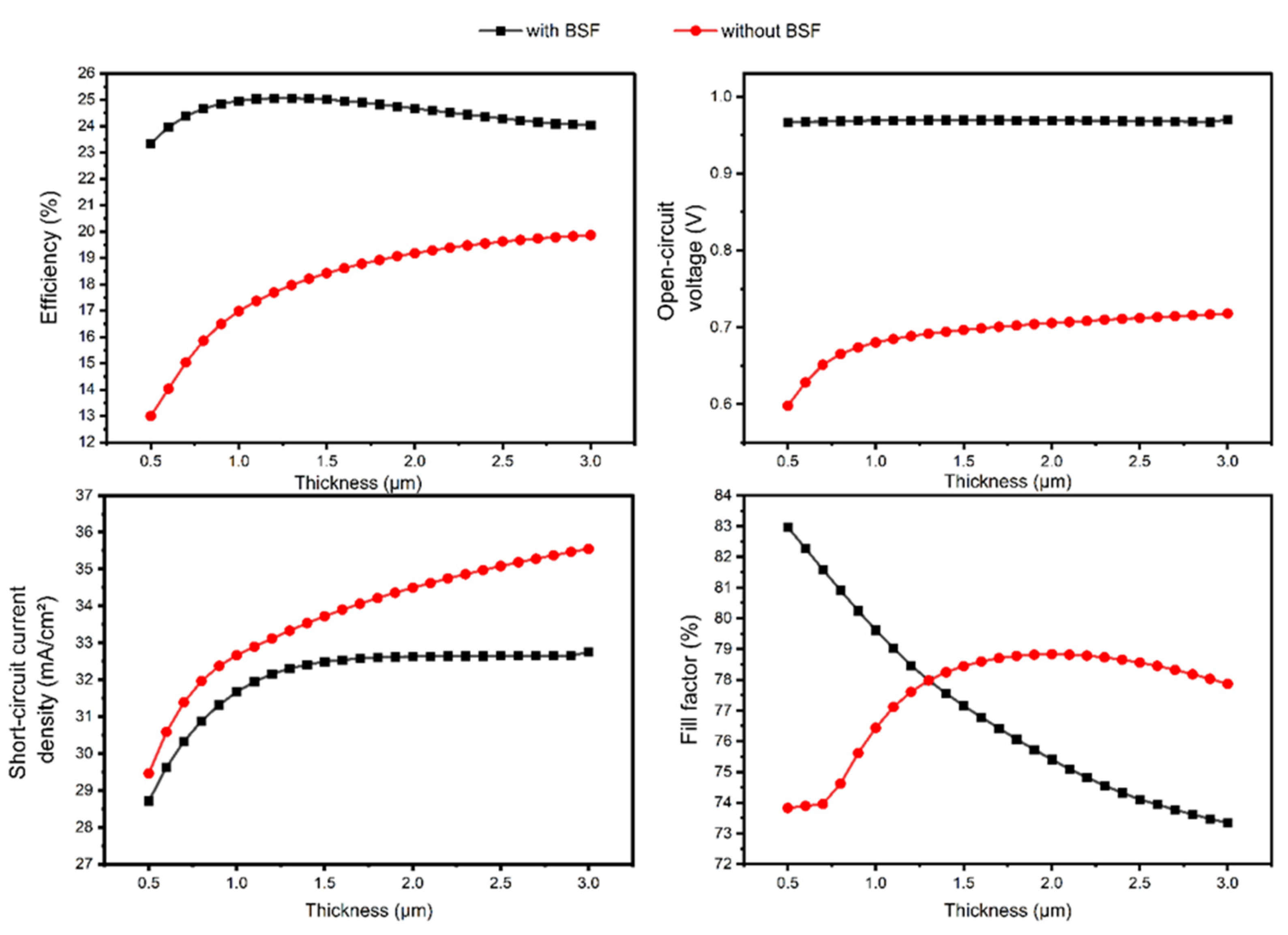
3.2. Part 2: CZTS with BSF Layer (rGO)
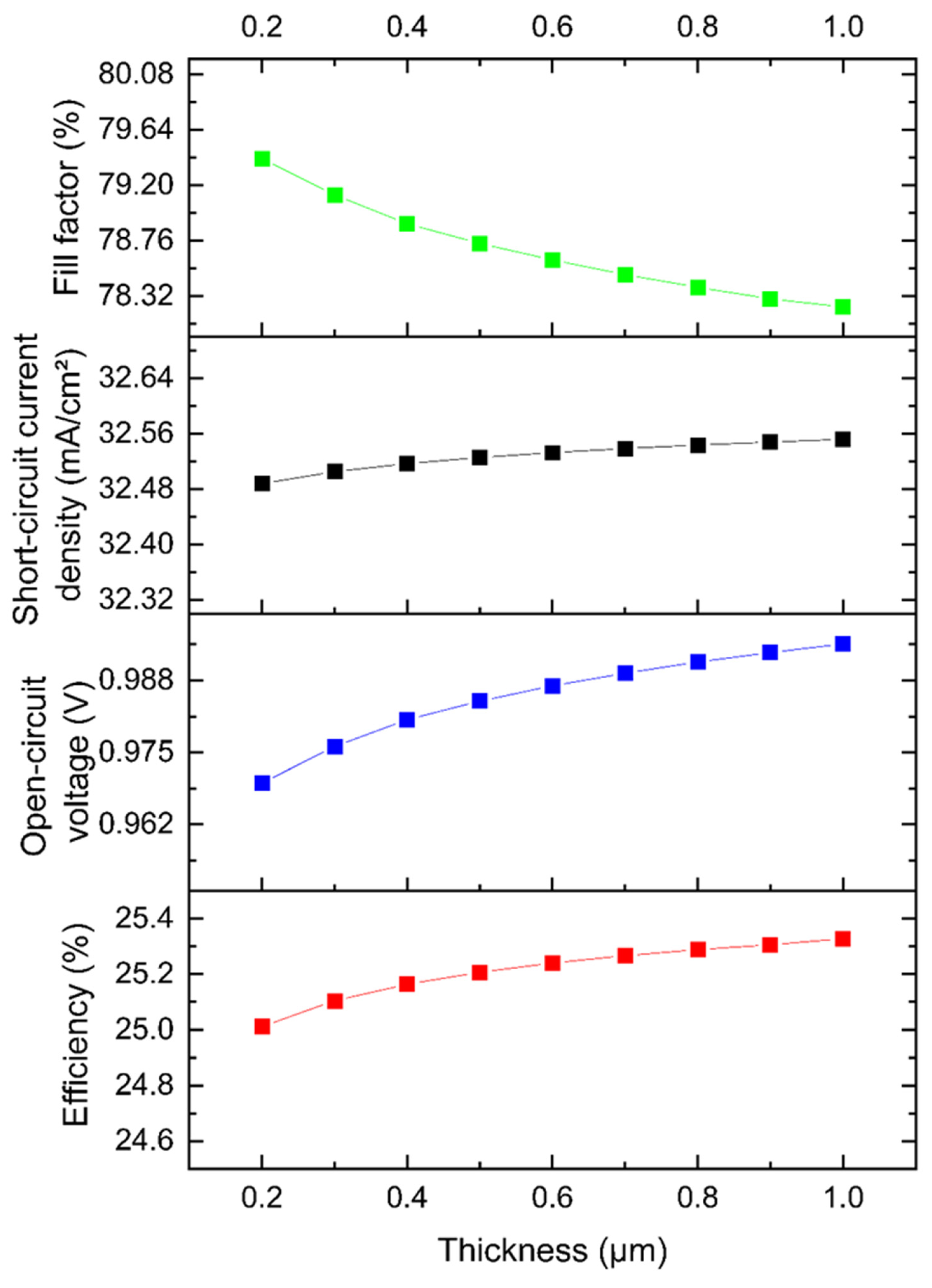
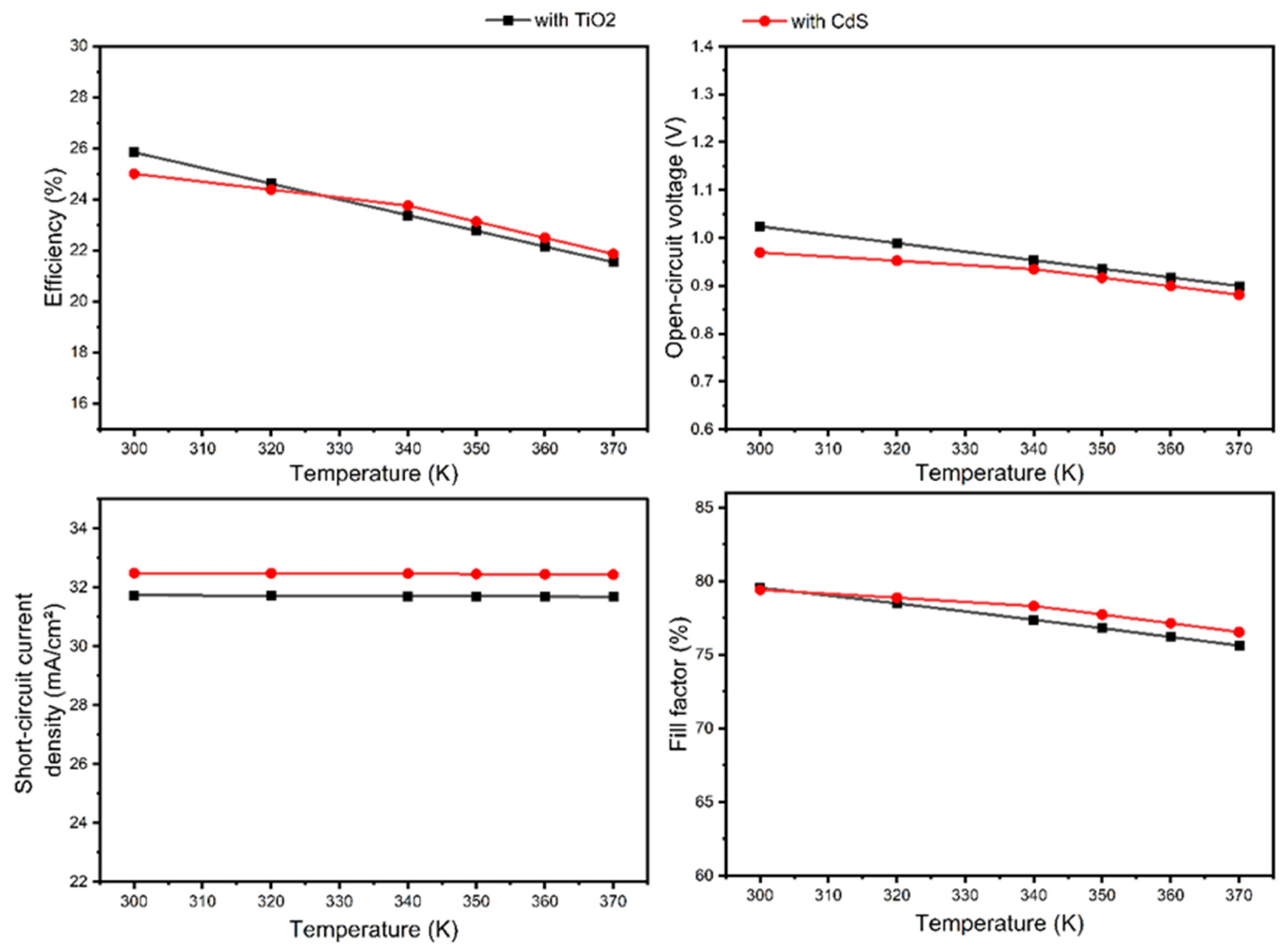
3.3. Part 3: CZTS SC with TiO2 Buffer Layer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CZTS | Copper zinc tin sulfide (commonly known as CZTS) |
| CdS | Cadmium sulfide |
| TiO2 | Titanium dioxide |
| ZnO | Zinc oxide |
| Al | Aluminuim |
| Mo | Molybdenum |
| ALD | Atomic layer deposition |
| CBD | Chemical bath deposition |
| SC | Solar cell |
| BSF | Back surface field |
| SCAPS | Solar Cell Capacitance Simulator |
| rGO | Reduced graphene oxide |
| CIGS | Copper, gallium, indium and selenium |
| VOC | Open Circuit current |
| Jsc | Short-circuit current density |
| FF | Fill factor |
| η | Efficiency |
| QE | Quantum efficiency |
| εᵣ(x) | Dielectric constant |
| ε0 | Permittivity of free space |
| n(x) | Concentration of carriers |
| Jn | Current density of electron |
| Jp | Current density of hole |
| e | Elementary charge |
| ψ | Electrostatic potential |
| ρ | Charge density |
| NA | Density of acceptors |
| ND | Density of acceptors and donors |
| G | Rate of producing electron–hole pairs |
| µn | Mobility of electrons |
| µp | Mobility of holes |
| ϕ | Electrostatic potential |
| Dn | Coefficient of electron diffusion |
| Dp | Coefficient of hole diffusion |
| J | Current density |
| V | Voltage |
References
- Yang, X.; Qin, X.; Yan, W.; Zhang, C.; Zhang, D.; Guo, B. Electronic Structure and Optical Properties of Cu2ZnSnS4 under Stress Effect. Crystals 2022, 12, 1454. [Google Scholar] [CrossRef]
- Soonmin, H.; Hardani Nandi, P.; Mwankemwa, B.S.; Malevu, T.D.; Malik, M.I. Overview on Different Types of Solar Cells: An Update. Appl. Sci. 2023, 13, 2051. [Google Scholar] [CrossRef]
- Vilanova, A.; Dias, P.; Lopes, T.; Mendes, A. The route for commercial photoelectrochemical water splitting: A review of large-area devices and key upscaling challenges. Chem. Soc. Rev. 2024, 53, 2388–2434. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Z.A.; Ahmad, T.; Ansari, M.Z. Chapter Nine—Copper zinc tin sulfide thin-film solar cells: An overview. In Photovoltaics Beyond Silicon; Solar Cell Engineering; Elsevier: Amsterdam, The Netherlands, 2024; pp. 303–322. [Google Scholar] [CrossRef]
- Tseberlidis, G.; Trifiletti, V.; Le Donne, A.; Frioni, L.; Acciarri, M.; Binetti, S. Kesterite solar-cells by drop-casting of inorganic sol–gel inks. Sol. Energy 2020, 208, 532–538. [Google Scholar] [CrossRef]
- Trifiletti, V.; Mostoni, S.; Butrichi, F.; Acciarri, M.; Binetti, S.; Scotti, R. Study of Precursor-Inks Designed for High-Quality Cu2ZnSnS4 Films for Low-Cost PV Application. ChemistrySelect 2019, 4, 4905–4912. [Google Scholar] [CrossRef]
- Panzeri, G.; Dell’Oro, R.; Trifiletti, V.; Parravicini, J.; Acciarri, M.; Binetti, S.; Magagnin, L. Copper electrodeposition onto zinc for the synthesis of kesterite Cu2ZnSnS4 from a Mo/Zn/Cu/Sn precursor stack. Electrochem. Commun. 2019, 109, 106580. [Google Scholar] [CrossRef]
- Islam, M.S.; Doroody, C.; Kiong, T.S.; Rahman, K.S.; Zuhdi, A.W.M.; Yap, B.K.; Alam, M.N.-E.; Amin, N. MoS2 thin film hetero-interface as effective back surface field in CZTS-based solar cells. Mater. Sci. Semicond. Process. 2024, 182, 108721. [Google Scholar] [CrossRef]
- Banerjee, A.; Sarkar, A.; Shukla, S.; Saxena, S.; Banerjee, A.; Guchhait, A.; Lawaniya, R.; Kumar, A.; Dalapati, G.K. Chapter 9—Sulfide and selenide-based flexible and semitransparent solar cells for building integrated photovoltaics. In Sulfide and Selenide Based Materials for Emerging Applications; Dalapati, G., Shun Wong, T., Kundu, S., Chakraborty, A., Zhuk, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 179–194. [Google Scholar] [CrossRef]
- Su’ait, M.S.; Sahudin, M.A.; Ludin, N.A.; Ahmad, A.; Rahman, M.Y.A.; Ahmoum, H.; Ataollahi, N.; Scardi, P. Potential of transition metal sulfides, Cu2ZnSnS4 as inorganic absorbing layers in dye-sensitized solar cells. J. Clean. Prod. 2023, 394, 136327. [Google Scholar] [CrossRef]
- Ataollahi, N.; Bazerla, F.; Malerba, C.; Chiappini, A.; Ferrari, M.; Di Maggio, R.; Scardi, P. Synthesis and post-annealing of Cu2ZnSnS4 absorber layers based on Oleylamine/1-dodecanethiol. Materials 2019, 12, 3320. [Google Scholar] [CrossRef]
- Yussuf, S.T.; Oranzie, M.; Cox, M.; Xia, R.; Admassie, S.; January, J.L.; Peng, X.; Iwuoha, E.I. Microwave-synthesized aluminum, gallium and indium-substituted stannite nanocrystal absorbers for solar cell applications. Mater. Today Sustain. 2024, 26, 100771. [Google Scholar] [CrossRef]
- Huang, C.H.; Chuang, W.J. Dependence of performance parameters of CdTe solar cells on semiconductor properties studied by using SCAPS-1D. Vacuum 2015, 118, 32–37. [Google Scholar] [CrossRef]
- Romeo, A.; Artegiani, E. CdTe-Based Thin Film Solar Cells: Past, Present and Future. Energies 2021, 14, 1684. [Google Scholar] [CrossRef]
- Buonomenna, M.G. Inorganic Thin-Film Solar Cells: Challenges at the Terawatt-Scale. Symmetry 2023, 15, 1718. [Google Scholar] [CrossRef]
- Ashagre, S.; Ogundele, A.K.; Ike, J.N.; Gebremichael, B.; Bekele, M.; Sharma, G.D.; Mola, G.T. Synergistic contribution of potassium sulfide doped with silver nanoparticles on the performance of thin film organic solar cells. J. Phys. Chem. Solids 2023, 177, 111290. [Google Scholar] [CrossRef]
- Keerthi Priya, T.; Deb, P.; Choudhury, A. Simulation of High Efficiency Hybrid FTO/TiO2/CH3NH3SnI3/RGO-Based Solar Cell Using SCAPS-1D. Optik 2024, 316, 172051. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, B.; Liu, Z.; Wu, J.; Arnold, S.; Shi, H.; Osterrieder, T.; Hauch, J.A.; Wu, Z.; Luo, J.; et al. Optimizing Perovskite Thin-Film Parameter Spaces with Machine Learning-Guided Robotic Platform for High-Performance Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2302594. [Google Scholar] [CrossRef]
- Ali, S.; Ahmad, K.; Khan, R.A.; Kumar, P. Simulation-Based Studies on FAGeI3-Based Lead (Pb2+)-Free Perovskite Solar Cells. Crystals 2025, 15, 135. [Google Scholar] [CrossRef]
- Alsalme, A.; Altowairqi, M.F.; Alhamed, A.A.; Khan, R.A. Optimization of Photovoltaic Performance of Pb-Free Perovskite Solar Cells via Numerical Simulation. Molecules 2023, 28, 224. [Google Scholar] [CrossRef]
- Yerezhep, D.; Omarova, Z.; Aldiyarov, A.; Shinbayeva, A.; Tokmoldin, N. IR Spectroscopic Degradation Study of Thin Organometal Halide Perovskite Films. Molecules 2023, 28, 1288. [Google Scholar] [CrossRef]
- Alsalme, A.; Alsaeedi, H. Twenty-Two Percent Efficient Pb-Free All-Perovskite Tandem Solar Cells Using SCAPS-1D. Nanomaterials 2023, 13, 96. [Google Scholar] [CrossRef]
- Siddique, A.; Islam, N.; Karmaker, H.; Iqbal, A.A.; Khan, A.A.M.; Islam, A.; Das, B.K. Numerical modelling and performance investigation of inorganic Copper-Tin-Sulfide (CTS) based perovskite solar cell with SCAPS-1D. Results Opt. 2024, 16, 100713. [Google Scholar] [CrossRef]
- Chee, A.K.W. On current technology for light absorber materials used in highly efficient industrial solar cells. Renew. Sustain. Energy Rev. 2023, 173, 113027. [Google Scholar] [CrossRef]
- Ahmoum, H.; Su’ait, M.S.; Ataollahi, N.; Mustaffa, M.U.S.; Boughrara, M.; Chelvanathan, P.; Sopian, K.; Li, G.; Kerouad, M.; Scardi, P.; et al. Suppressing the secondary phases via N2 preheating of Cu2ZnSnS4 thin films with the addition of oleylamine and/or 1-Dodecanethiol solvents. Inorg. Chem. Commun. 2021, 134, 109031. [Google Scholar]
- Shah, U.A.; Wang, A.; Irfan Ullah, M.; Ishaq, M.; Alam Shah, I.; Zeng, Y.; Abbasi, M.S.; Umair, M.A.; Farooq, U.; Liang, G.; et al. A Deep Dive into Cu2 ZnSnS4 (CZTS) Solar Cells: A Review of Exploring Roadblocks, Breakthroughs, and Shaping the Future. Small 2024, 20, 2310584. [Google Scholar] [CrossRef]
- Le Donne, A.; Trifiletti, V.; Binetti, S. New Earth-Abundant Thin Film Solar Cells Based on Chalcogenides. Front. Chem. 2019, 7, 297. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Zhuk, S.; Masudy-Panah, S.; Kushwaha, A.; Seng, H.L.; Chellappan, V.; Suresh, V.; Su, Z.; Batabyal, S.K.; Guchhait, A.; et al. Impact of molybdenum out diffusion and interface quality on the performance of sputter grown CZTS based solar cells. Sci. Rep. 2017, 7, 1350. [Google Scholar] [CrossRef]
- Tseberlidis, G.; Trifiletti, V.; Vitiello, E.; Vitiello, E.; Husien, A.H.; Husien, A.H.; Frioni, L.; Da Lisca, M.; Alvarez, J.; Acciarri, M.; et al. Band-Gap Tuning Induced by Germanium Introduction in Solution-Processed Kesterite Thin Films. ACS Omega 2022, 7, 23445–23456. [Google Scholar] [CrossRef]
- Lin, J.; Wu, X.; Xu, J.; Yang, Y. Inhibiting the formation of MoS2 between Cu2ZnSnS4 thin film and Mo(211) foil substrate by inserting a Mo(110) intermediate layer. Opt. Mater. 2022, 124, 111996. [Google Scholar] [CrossRef]
- Lin, J.; Xu, J.; Yang, Y. Numerical analysis of the effect of MoS2 interface layers on copper-zinc-tin-sulfur thin film solar cells. Optik 2020, 201, 163496. [Google Scholar] [CrossRef]
- Srivastava, M.; Banerjee, S.; Bairagi, S.; Singh, P.; Kumar, B.; Singh, P.; Kale, R.D.; Mulvihill, D.M.; Ali, S.W. Recent progress in molybdenum disulfide (MoS2) based flexible nanogenerators: An inclusive review. Chem. Eng. J. 2024, 480, 147963. [Google Scholar] [CrossRef]
- Haque, A.; Mamun, M.A.A.; Taufique, M.F.N.; Karnati, P.; Ghosh, K. Temperature Dependent Electrical Transport Properties of High Carrier Mobility Reduced Graphene Oxide Thin Film Devices. IEEE Trans. Semicond. Manuf. 2018, 31, 535–544. [Google Scholar] [CrossRef]
- Safian MT uddeen Umar, K.; Mohamad Ibrahim, M.N. Synthesis and scalability of graphene and its derivatives: A journey towards sustainable and commercial material. J. Clean. Prod. 2021, 318, 128603. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, P.; Zhao, F.J. Dietary cadmium exposure, risks to human health and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2022, 53, 939–963. [Google Scholar] [CrossRef]
- Tseberlidis, G.; Gobbo, C.; Trifiletti, V.; Di Palma, V.; Binetti, S. Cd-free kesterite solar cells: State-of-the-art and perspectives. Sustain. Mater. Technol. 2024, 41, e01003. [Google Scholar] [CrossRef]
- Tseberlidis, G.; Di Palma, V.; Trifiletti, V.; Frioni, L.; Valentini, M.; Malerba, C.; Mittiga, A.; Acciarri, M.; Binetti, S.O. Titania as Buffer Layer for Cd-Free Kesterite Solar Cells. ACS Mater. Lett. 2023, 5, 219–224. [Google Scholar] [CrossRef]
- Gobbo, C.; Di Palma, V.; Trifiletti, V.; Malerba, C.; Valentini, M.; Matacena, I.; Daliento, S.; Binetti, S.; Acciarri, M.; Tseberlidis, G. Effect of the ZnSnO/AZO Interface on the Charge Extraction in Cd-Free Kesterite Solar Cells. Energies 2023, 16, 4137. [Google Scholar] [CrossRef]
- Razzaq, Z.; Khalid, A.; Ahmad, P.; Farooq, M.; Khandaker, M.U.; Sulieman, A.; Rehman, I.U.; Shakeel, S.; Khan, A. Photocatalytic and Antibacterial Potency of Titanium Dioxide Nanoparticles: A Cost-Effective and Environmentally Friendly Media for Treatment of Air and Wastewater. Catalysts 2021, 11, 709. [Google Scholar] [CrossRef]
- Ge, S.; Sang, D.; Zou, L.; Yao, Y.; Zhou, C.; Fu, H.; Xi, H.; Fan, J.; Meng, L.; Wang, C. A Review on the Progress of Optoelectronic Devices Based on TiO2 Thin Films and Nanomaterials. Nanomaterials 2023, 13, 1141. [Google Scholar] [CrossRef]
- Cheng, X.; Gotoh, K.; Mochizuki, T.; Usami, N. Controllable Optical and Electrical Properties of Nb-Doped TiO2 Films by RF Sputtering. In Proceedings of the 2018 IEEE 7th World Conference on Photovoltaic Energy Conversion (WCPEC), Waikoloa, HI, USA, 10–15 June 2018; pp. 1986–1990. [Google Scholar] [CrossRef]
- Khan, H.; Shah, M.U.H. Modification strategies of TiO2 based photocatalysts for enhanced visible light activity and energy storage ability: A review. J. Environ. Chem. Eng. 2023, 11, 111532. [Google Scholar] [CrossRef]
- Houshmand, M.; Esmaili, H.; Zandi, M.H.; Gorji, N.E. Degradation and device physics modeling of TiO2/CZTS ultrathin film photovoltaics. Mater. Lett. 2015, 157, 123–126. [Google Scholar]
- Chang, H.; Sun, Z.; Saito, M.; Yuan, Q.; Zhang, H.; Li, J.; Wang, Z.; Fujita, T.; Ding, F.; Zheng, Z.; et al. Regulating Infrared Photoresponses in Reduced Graphene Oxide Phototransistors by Defect and Atomic Structure Control. ACS Nano 2013, 7, 6310–6320. [Google Scholar] [CrossRef] [PubMed]
- Abid Sehrawat, P.; Islam, S.S.; Mishra, P.; Ahmad, S. Reduced graphene oxide (rGO) based wideband optical sensor and the role of Temperature, Defect States and Quantum Efficiency. Sci. Rep. 2018, 8, 3537. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Lacey, S.D.; Xu, L.; Xie, H.; Li, T.; Danner, V.A.; Hu, L. Reduced graphene oxide film with record-high conductivity and mobility. Mater. Today 2018, 21, 186–192. [Google Scholar] [CrossRef]
- Mattson, E.C.; Johns, J.E.; Pande, K.; Bosch, R.A.; Cui, S.; Gajdardziska-Josifovska, M.; Weinert, M.; Chen, J.H.; Hersam, M.C.; Hirschmugl, C.J. Vibrational Excitations and Low-Energy Electronic Structure of Epoxide-Decorated Graphene. J. Phys. Chem. Lett. 2014, 5, 212–219. [Google Scholar] [CrossRef]
- Jeong, W.L.; Park, S.H.; Jho, Y.D.; Joo, S.K.; Lee, D.S. The Role of the Graphene Oxide (GO) and Reduced Graphene Oxide (RGO) Intermediate Layer in CZTSSe Thin-Film Solar Cells. Materials 2022, 15, 3419. [Google Scholar] [CrossRef]
- Shafi, M.A.; Bouich, A.; Fradi, K.; Guaita, J.M.; Khan, L.; Mari, B. Effect of deposition cycles on the properties of ZnO thin films deposited by spin coating method for CZTS-based solar cells. Optik 2022, 258, 168854. [Google Scholar] [CrossRef]
- Moghadam Ziabari, S.A.; Abdolahzadeh Ziabari, A.; Mousavi, S.J. Efficiency enhancement of thin-film solar cell by implementation of double-absorber and BSF layers: The effect of thickness and carrier concentration. J. Comput. Electron. 2022, 21, 675–683. [Google Scholar] [CrossRef]
- Nowsherwan, G.A.; Hussain, S.S.; Khan, M.; Haider, S.; Akbar, I.; Nowsherwan, N.; Ikram, S.; Ishtiaq, S.; Riaz, S.; Naseem, S. Role of graphene-oxide and reduced-graphene-oxide on the performance of lead-free double perovskite solar cell. Z. Für Naturforschung A 2022, 77, 1083–1098. [Google Scholar] [CrossRef]
- Zaidi, B.; Kerboub, A.; Bouarroudj, T.; Shekhar, C.; Mahmood, T.; Saeed, M. Enhancement of Performance of TiO2/Cu2O Solar Cells. J. Optoelectron. Adv. Mater. 2023, 25, 549–553. [Google Scholar]
- Liu, X.; Hu, Y. Investigation of TiO2 as the buffer layer in wide bandgap chalcopyrite solar cells using SCAPS. J. Mater. Sci. Mater. Electron. 2022, 33, 6253–6261. [Google Scholar] [CrossRef]
- Kumari, N.; Ingole, S. Enhancement of CZTS photovoltaic device performance with silicon at back-contact: A study using SCAPS-1D. Solar Energy 2022, 236, 301–307. [Google Scholar] [CrossRef]
- Gupta, G.K.; Dixit, A. Effect of precursor and composition on the physical properties of the low-cost solution processed Cu2ZnSnS4 thin film for solar photovoltaic application. J. Renew. Sustain. Energy 2017, 9, 013502. [Google Scholar] [CrossRef]
- Nisika Ghosh, A.; Kaur, K.; Bobba, R.S.; Qiao, Q.; Kumar, M. Engineering Cu2ZnSnS4 grain boundaries for enhanced photovoltage generation at the Cu2ZnSnS4/TiO2 heterojunction: A nanoscale investigation using Kelvin probe force microscopy. J. Appl. Phys. 2021, 130, 195301. [Google Scholar] [CrossRef]
- Nisika Kaur, K.; Arora, K.; Bobba, R.S.; Qiao, Q.; Kumar, M. Energy level alignment and nanoscale investigation of a TiO2/Cu-Zn-Sn-S interface for alternative electron transport layer in earth abundant Cu-Zn-Sn-S solar cells. J. Appl. Phys. 2019, 126, 193104. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Taghipour, N.; Peng, L.; Konstantatos, G. Ag-Refined Kesterite in Superstrate Solar Cell Configuration with 9.7% Power Conversion Efficiency. Adv. Funct. Mater. 2022, 32, 2205948. [Google Scholar] [CrossRef]
- Bencherif, H.; Dehimi, L.; Mahsar, N.; Kouriche, E.; Pezzimenti, F. Modeling and optimization of CZTS kesterite solar cells using TiO2 as efficient electron transport layer. Mater. Sci. Eng. B 2022, 276, 115574. [Google Scholar] [CrossRef]






| Parameters | Layer Material | |||||
|---|---|---|---|---|---|---|
| CZTS [44] | CdS [13] | ZnO [49] | Al:ZnO [50] | rGO [45,46,51] | TiO2 [37,52,53] | |
| Thickness (µm) | 0.5–3 | 0.05–0.15 | 0.07 | 0.35 | 0.2–1 | 0.05–0.15 |
| Bandgap (eV) | 1.43 | 2.4 | 3.4 | 3.4 | 1.09 | 3.2 |
| Electron affinity (eV) | 4.2 | 4.4 | 4.6 | 4.6 | 3.2 | 4.2 |
| Dielectric permittivity | 7 | 10 | 9 | 9 | 10 | 10 |
| CB density of states (cm−3) | 2.2·1018 | 2.2·1018 | 2.2·1018 | 2.2·1018 | 2.2·1018 | 2.2·1018 |
| VB density of states (cm−3) | 1.8·1019 | 1.8·1019 | 1.8·1019 | – | 2·1018 | 1.8·1019 |
| Electron/hole mobility (cm2V−1s−1) | 5–6/25 | 100/25 | 100/25 | 100/25 | 320/123 | 100/25 |
| Electron thermal velocity (cm/s) | 107 | 107 | 107 | 107 | – | 107 |
| Hole thermal velocity (cm/s) | 107 | 107 | 107 | 107 | – | 107 |
| Shallow uniform donor density ND (cm−3) | 0 | 1015 | 1015 | 1015 | 1015 | 1015 |
| Shallow uniform acceptor density NA (cm−3) | 1·1015–1.8·1016 | – | – | – | 2·1018 | – |
| Voc (v) | Jsc (mA\cm2) | FF (%) | Efficiency (%) | |
|---|---|---|---|---|
| CZTS/CdS | 0.70 | 33.72 | 78.44 | 18.42 |
| CZTS with BSF/CdS | 0.97 | 32.49 | 77.15 | 25.01 |
| CZTS/TiO2 | 1.02 | 32.65 | 77.06 | 25.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatihi, D.; Tseberlidis, G.; Trifiletti, V.; Binetti, S.; Isotta, E.; Scardi, P.; Kamal, A.; Adhiri, R.; Ataollahi, N. Enhanced Efficiency of CZTS Solar Cells with Reduced Graphene Oxide and Titanium Dioxide Layers: A SCAPS Simulation Study. ChemEngineering 2025, 9, 38. https://doi.org/10.3390/chemengineering9020038
Fatihi D, Tseberlidis G, Trifiletti V, Binetti S, Isotta E, Scardi P, Kamal A, Adhiri R, Ataollahi N. Enhanced Efficiency of CZTS Solar Cells with Reduced Graphene Oxide and Titanium Dioxide Layers: A SCAPS Simulation Study. ChemEngineering. 2025; 9(2):38. https://doi.org/10.3390/chemengineering9020038
Chicago/Turabian StyleFatihi, Dounia, Giorgio Tseberlidis, Vanira Trifiletti, Simona Binetti, Eleonora Isotta, Paolo Scardi, Abderrafi Kamal, R’hma Adhiri, and Narges Ataollahi. 2025. "Enhanced Efficiency of CZTS Solar Cells with Reduced Graphene Oxide and Titanium Dioxide Layers: A SCAPS Simulation Study" ChemEngineering 9, no. 2: 38. https://doi.org/10.3390/chemengineering9020038
APA StyleFatihi, D., Tseberlidis, G., Trifiletti, V., Binetti, S., Isotta, E., Scardi, P., Kamal, A., Adhiri, R., & Ataollahi, N. (2025). Enhanced Efficiency of CZTS Solar Cells with Reduced Graphene Oxide and Titanium Dioxide Layers: A SCAPS Simulation Study. ChemEngineering, 9(2), 38. https://doi.org/10.3390/chemengineering9020038










