What Is the Impact of Leaky Sewers on Groundwater Contamination in Urban Semi-Confined Aquifers? A Test Study Related to Fecal Matter and Personal Care Products (PCPs)
Abstract
:1. Introduction
2. Materials and Methods
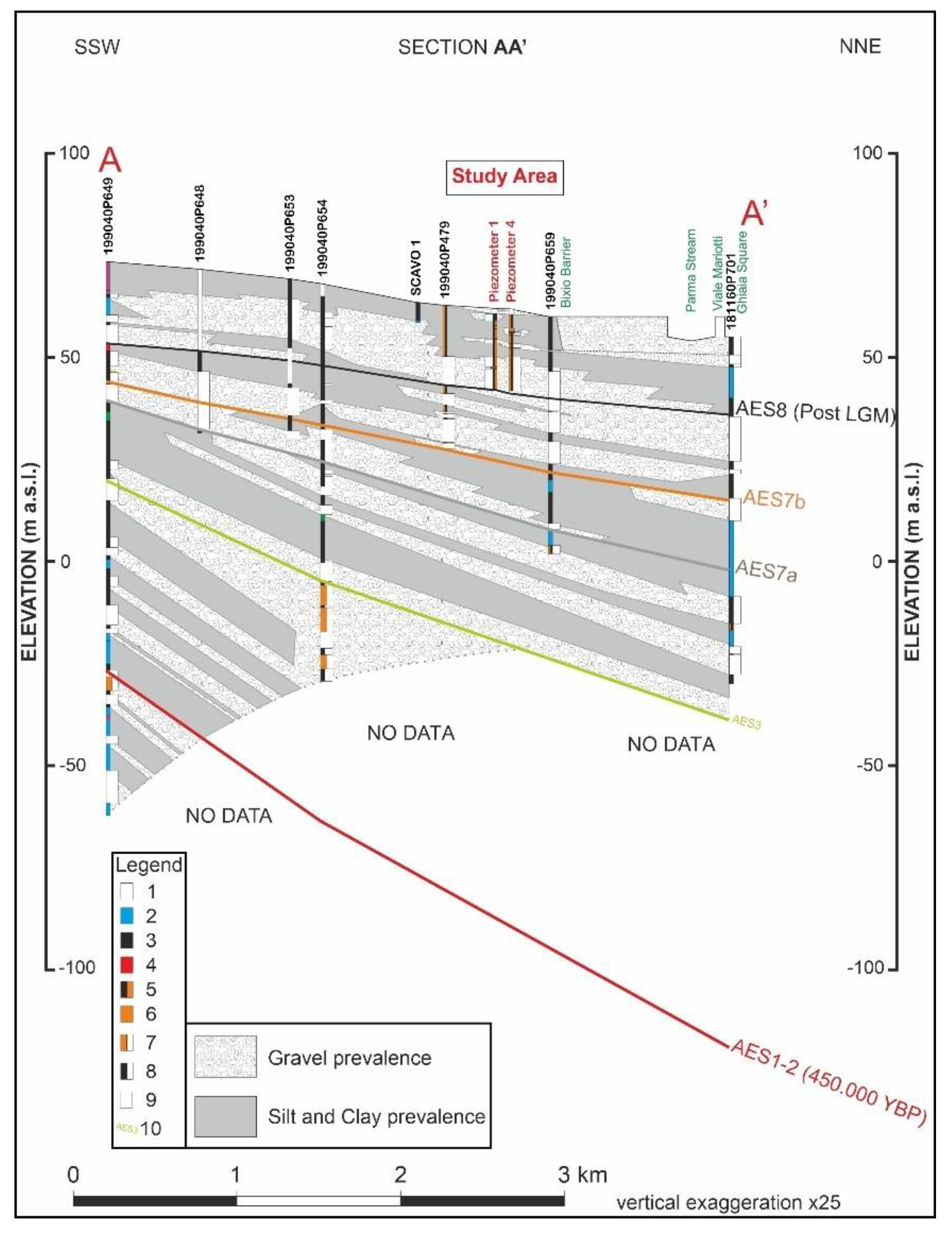
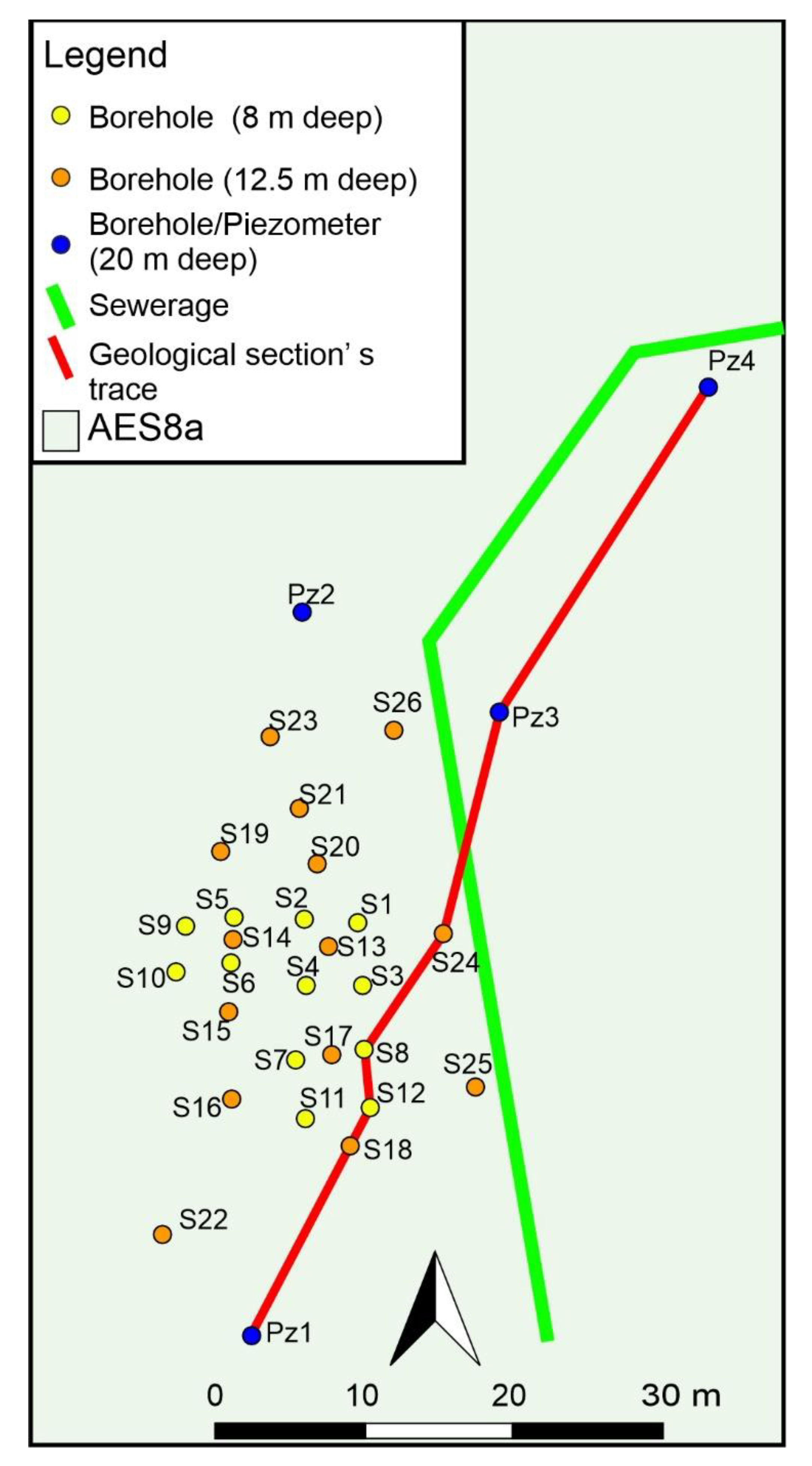
2.1. Study Area
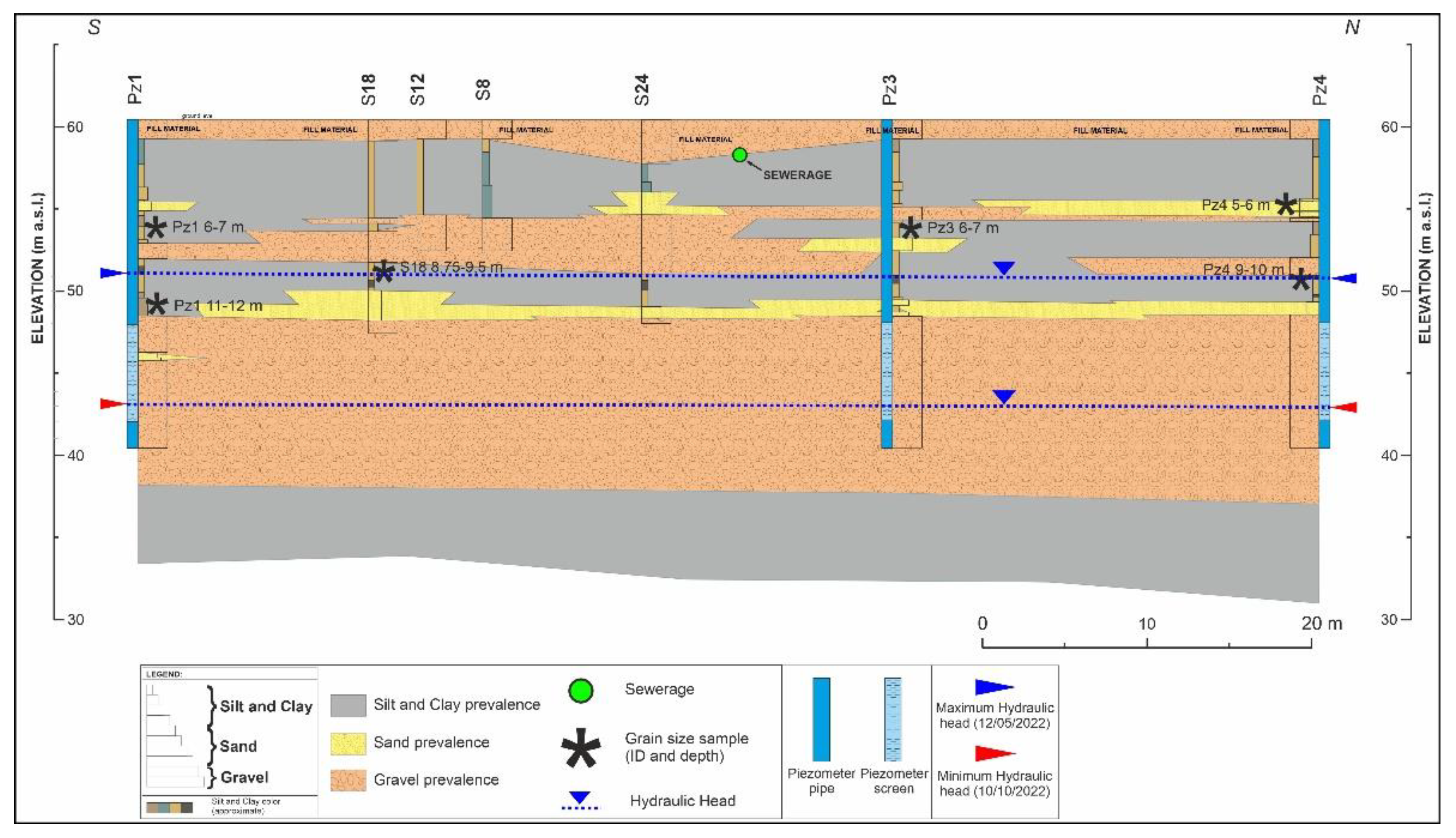
Geological and Hydrogeological Investigations
2.2. Water Sampling and Analyses
2.3. Chemical Analyses
2.4. Next-Generation Sequencing (NGS) for Bacterial Community Analyses
2.5. Microbiological Investigations (Fecal Indicators)
3. Results
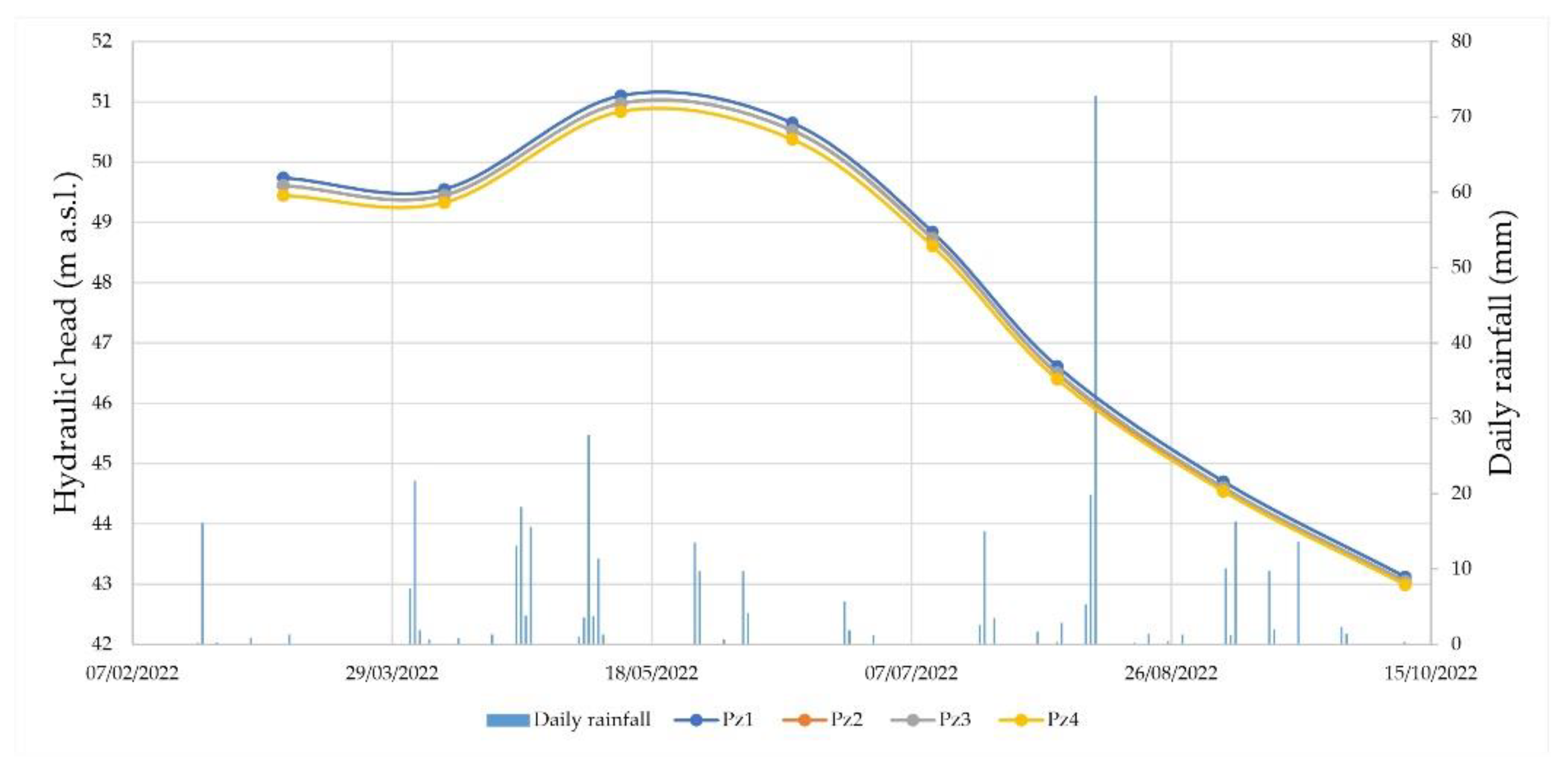
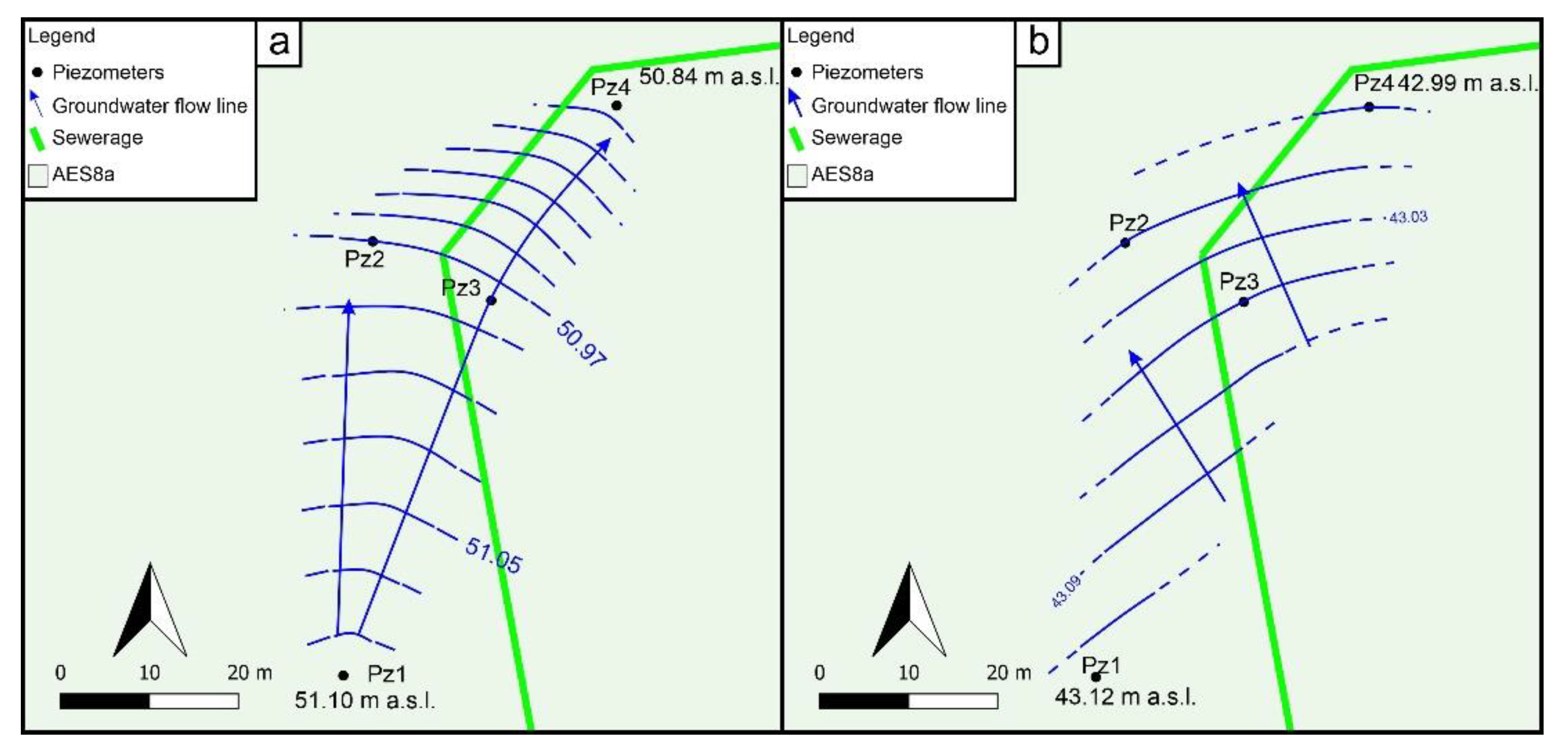
3.1. Geological, Hydrogeological, and Hydrochemical Investigations
3.2. Microbiological and Biomolecular Investigations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Echart, J.; Ghebremichael, K.; Khatri, K.; Mutikanga, H.; Sempewo, J.; Tsegaye, S.; Vairavamoorthy, K. The Future of Water in African Cities: Why Waste Water? Integrated Urban Water Management, Background Report; World Bank: Washington, DC, USA, 2012. [Google Scholar]
- Archundia, D.; Duwig, C.; Spadini, L.; Uzu, G.; Guédron, S.; Morel, M.C.; Cortez, R.; Ramos Ramos, O.; Chincheros, J.; Martins, J.M.F. How uncontrolled urban expansion increases the contamination of the Titicaca Lake Basin (El Alto, La Paz, Bolivia). Water Air Soil Pollut. 2017, 228, 44. [Google Scholar] [CrossRef]
- Hayzoun, H.; Garnier, C.; Durrieu, G.; Lenoble, V.; Le Poupon, C.; Angeletti, B.; Ouammou, A.; Mounier, S. Organic carbon, and major and trace element dynamic and fate in a large river subjected to poorly-regulated urban and industrial pressures (Sebou River, Morocco). Sci. Total Environ. 2015, 502, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Mottes, C.; Lesueur-Jannoyer, M.; Charlier, J.-B.; Carles, C.; Guéné, M.; Le Bail, M.; Malézieux, E. Hydrological and pesticide transfer modeling in a tropical volcanic watershed with the WATPPASS model. J. Hydrol. 2015, 529, 909–927. [Google Scholar] [CrossRef]
- Schaider, L.A.; Ackerman, J.M.; Rudel, R.A. Septic systems as sources of organic wastewater compounds in domestic drinking water wells in a shallow sand and gravel aquifer. Sci. Total Environ. 2016, 547, 470–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, L.; Gzyl, G.; Mazzon, P.; Łabaj, P.; Frączek, R.; Alberti, L. Stochastic Particle Tracking Application in Different Urban Areas in Central Europe: The Milano (IT) and Jaworzno (PL) Case Study to Secure the Drinking Water Resources. Sustainability 2021, 13, 10291. [Google Scholar] [CrossRef]
- Mester, T.; Szabó, G.; Balla, D. Assessment of shallow groundwater purification processes after the construction of a municipal sewerage network. Water 2021, 13, 1946. [Google Scholar] [CrossRef]
- Vollertsen, J.; Nielsen, L.; Blicher, T.D.; Hvitved-Jacobsen, T.; Nielsen, A.H. A sewer process model as planning and management tool-hydrogen sulfide simulation at catchment scale. Water Sci. Technol. 2011, 64, 348–354. [Google Scholar] [CrossRef]
- Barone, L.; Pilotti, M.; Valerio, G.; Balistrocchi, M.; Milanesi, L.; Chapra, S.C.; Nizzoli, D. Analysis of the residual nutrient load from a combined sewer system in a watershed of a deep Italian lake. J. Hydrol. 2019, 571, 202–213. [Google Scholar] [CrossRef]
- Pikaar, I.; Sharma, K.R.; Hu, S.; Gernjak, W.; Keller, J.; Yuan, Z. Reducing sewer corrosion through integrated urban water management. Science 2014, 345, 812–814. [Google Scholar] [CrossRef]
- Selvakumar, A.; Field, R.; Burgess, E.; Amick, R. Exfiltration in Sanitary Sewer Systems in the US. Urban Water J. 2010, 1, 227–234. [Google Scholar] [CrossRef]
- Karpf, C.; Krebs, P. Modelling of Groundwater Infiltration into Sewer Systems. Urban Water J. 2013, 10, 221–229. [Google Scholar] [CrossRef]
- Davies, J.P.; Clarke, B.A.; Whiter, J.T.; Cunningham, R.J. Factors Influencing the Structural Deterioration and Collapse of Rigid Sewer Pipes. Urban Water 2001, 3, 73–89. [Google Scholar] [CrossRef]
- Ellis, J.B.; Revitt, D.M.; Lister, P.; Willgress, C.; Buckley, A. Experimental Studies of Sewer Exfiltration. Water Sci. Technol. 2003, 47, 61–67. [Google Scholar] [CrossRef]
- Gokhale, S.; Graham, J.A. A New Development in Locating Leaks in Sanitary Sewers. Tunn. Undergr. Space Technol. 2004, 19, 85–96. [Google Scholar] [CrossRef]
- Wakida, F.T.; Lerner, D.N. Non-agricultural Sources of Groundwater Nitrate: A Review and Case Study. Water Res. 2005, 39, 3–16. [Google Scholar] [CrossRef]
- Ana, E.; Bauwens, W.; Pessemier, M.; Thoeye, C.; Smolders, S.; Boonen, I.; de Gueldre, G. An Investigation of the Factors Influencing Sewer Structural Deterioration. Urban Water J. 2009, 6, 303–312. [Google Scholar] [CrossRef]
- López-Serna, R.; Jurado, A.; Vázquez-Suñé, E.; Carrera, J.; Petrović, M.; Barceló, D. Occurrence of 95 Pharmaceuticals and Transformation Products in Urban Groundwaters Underlying the Metropolis of Barcelona, Spain. Environ. Pollut. 2013, 174, 305–315. [Google Scholar] [CrossRef]
- Lee, D.G.; Roehrdanz, P.R.; Feraud, M.; Ervin, J.; Anumol, T.; Jia, A.; Park, M.; Tamez, C.; Morelius, E.W.; Gardea-Torresdey, J.L.; et al. Wastewater Compounds in Urban Shallow Groundwater Wells Correspond to Exfiltration Probabilities of Nearby Sewers. Water Res. 2015, 85, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Roehrdanz, P.R.; Feraud, M.; Lee, D.G.; Means, J.C.; Snyder, S.A.; Holden, P.A. Spatial Models of Sewer Pipe Leakage Predict the Occurrence of Wastewater Indicators in Shallow Urban Groundwater. Environ. Sci. Technol. 2017, 51, 1213–1223. [Google Scholar] [CrossRef]
- Laakso, T.; Kokkonen, T.; Mellin, I.; Vahala, R. Sewer Condition Prediction and Analysis of Explanatory Factors. Water 2018, 10, 1239. [Google Scholar] [CrossRef]
- Jurado, A.; Mastroianni, N.; Vázquez-Suñé, E.; Carrera, J.; Tubau, I.; Pujades, E.; Postigo, C.; de Aida, M.L.; Barcelo, D. Drugs of Abuse in Urban Groundwater. A Case Study: Barcelona. Sci. Total Environ. 2012, 424, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, Sources and Fate of Pharmaceuticals and Personal Care Products in the Groundwater: A Review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Alfiya, Y.; Dubowski, Y.; Friedler, E. Diurnal Patterns of Micropollutants Concentrations in Domestic Greywater. Urban Water J. 2018, 15, 399–406. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M. Environmentally-related Contaminants of High Concern: Potential Sources and Analytical Modalities for Detection, Quantification, and Treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Rusiniak, P.; Kmiecik, E.; Wątor, K.; Duda, R.; Bugno, R. Pharmaceuticals and personal care products in the urban groundwater–preliminary monitoring (case study: Kraków, Southern Poland). Urban Water J. 2021, 14, 364–374. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; Díaz-Cruz, M.S.; Barceló, D. Introduction: Personal Care Products in the Aquatic Environment. In Personal Care Products in the Aquatic Environment, The Handbook of Environmental Chemistry 36; Díaz-Cruz, M.S., Barceló, D., Eds.; Series Editors: Damià Barceló, Andrey G. Kostianoy; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-18808-9. ISSN 1867-979X. [Google Scholar]
- Xianzhi, P.; Weihui, O.; Chunwei, W.; Zhifang, W.; Qiuxin, H.; Jiabin, J.; Jianhua, T. Occurrence and ecological potential of pharmaceuticals and personal care products in groundwater and reservoirs in the vicinity of municipal landfills in China. Sci. Total Environ. 2014, 490, 889–898. [Google Scholar] [CrossRef]
- Mosaddeghi, M.R.; Mahboubi, A.A.; Zandsalimi, S.; Unc, A. Influence of organic waste type and soil structure on the bacterial filtration rates in unsaturated intact soil columns. J. Environ. Manag. 2009, 90, 730–739. [Google Scholar] [CrossRef]
- Naclerio, G.; Fardella, G.; Marzullo, G.; Celico, F. Filtration of Bacillus subtilis and Bacillus cereus spores in a pyroclastic topsoil, carbonate Apennines, southern Italy. Colloids Surf. B Biointerfaces 2009, 70, 25–28. [Google Scholar] [CrossRef]
- Safadoust, A.; Mahboubi, A.A.; Mosaddeghi, M.R.; Gharabaghi, B.; Voroney, P.; Unc, A.; Khodakaramian, G. Significance of physical weathering of two-texturally dierent soils for the saturated transport of Escherichia coli and bromide. J. Environ. Manag. 2012, 107, 147–158. [Google Scholar] [CrossRef]
- Moradi, A.; Mosaddeghi, M.R.; Chavoshi, E.; Safadoust, A.; Soleimani, M. Effect of Crude Oil-Induced Water Repellency on Transport of Escherichia coli and Bromide Through Repacked and Physically-Weathered Soil Columns. Environ. Pollut. 2019, 255, 113230. [Google Scholar] [CrossRef]
- Bucci, A.; Petrella, E.; Naclerio, G.; Gambatese, S.; Celico, F. Bacterial migration through low-permeability fault zones in compartmentalised aquifer systems: A case study in Southern Italy. Int. J. Speleol. 2014, 43, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, P.; Petrella, E.; Bucci, A.; Salvioli-Mariani, E.; Chelli, A.; Sanangelantoni, A.M.; Raimondo, M.; Quagliarini, A.; Celico, F. Studying Hydraulic Interconnections in Low-Permeability Media by Using Bacterial Communities as Natural Tracers. Water 2020, 12, 1795. [Google Scholar] [CrossRef]
- Zanini, A.; Petrella, E.; Sanangelantoni, A.M.; Angelo, L.; Ventosi, B.; Viani, L.; Rizzo, P.; Remelli, S.; Bartoli, M.; Bolpagni, R.; et al. Groundwater characterization from an ecological and human perspective: An interdisciplinary approach in the Functional Urban Area of Parma. Italy Rendiconti Lincei. Sci. Fis. Nat. 2019, 30, 93–108. [Google Scholar] [CrossRef]
- Severini, E.; Ducci, L.; Sutti, A.; Robottom, S.; Sutti, S.; Celico, F. River–Groundwater Interaction and Recharge Effects on Microplastics Contamination of Groundwater in Confined Alluvial Aquifers. Water 2022, 14, 1913. [Google Scholar] [CrossRef]
- Di Dio, G.; Martini, A.; Lasagna, S.; Zanzucchi, G. Illustrative Notes of the Geological Map of Italy at 1:50,000 scale Sheet 199 Parma Sud. Geological, Seismic, and Soil Service of the Emilia-Romagna Region, APAT-Geological Service of Italy; S.EL.CA: Florence, Italy, 2005. [Google Scholar]
- Ricci Lucchi, F.; Colalongo, M.L.; Cremonini, G.; Gasperi, G.; Iaccarino, S.; Papani, G.; Raffi, S.; Rio, D. Evoluzione Sedimentaria e Paleogeografia Nel Margine Appenninico. In Guida alla Geologia del Margine Appenninico Padano; Cremonini, G., Ricci Lucchi, F., Eds.; Guida Geol. Reg. S.G.I.: Bologna, Italy, 1982; pp. 17–46. [Google Scholar]
- Di Dio, G.; Lasagna, S.; Preti, D.; Sagne, M. Carta geologica dei depositi quaternari della provincia di Parma. Il Quat. 1997, 10, 443–450. [Google Scholar]
- Di Dio, G. Applicazione di Concetti e Metodi della Stratigrafia Fisica alla Ricerca di Risorse Idriche nel Sottosuolo della Pianura Emiliano-Romagnola. Serie 3a; Giornale di Geologia: Bologna, Italy, 1998; Volume 60, pp. 35–39. [Google Scholar]
- Regione Emilia-Romagna & ENI-AGIP. Riserve Idriche Sotterranee della Regione Emilia-Romagna; Di Dio, G., Ed.; S.EL.CA.: Firenze, Italy, 1998. [Google Scholar]
- Cherry, J.; Parker, B.; Bradbury, K.; Eaton, T.; Gotkowitz, M.; Hart, D.; Borchardt, M.A. Contaminant Transport through Aquitards: A State of the Science Review; International Water Association: London, UK, 2006; pp. 16–26. ISBN 1583214984. [Google Scholar]
- Storti, F.; Balsamo, F. Particle size distributions by laser diffraction: Sensitivity of granular matter strength to analytical operating procedures. Solid Earth 2010, 1, 25–48. [Google Scholar] [CrossRef] [Green Version]
- Bucci, A.; Naclerio, G.; Allocca, V.; Celico, P.; Celico, F. Potential use of microbial community investigations to analyse hydrothermal systems behaviour: The case of Ischia Island, Southern Italy. Hydrol. Process. 2011, 25, 1866–1873. [Google Scholar] [CrossRef]
- Bucci, A.; Petrella, E.; Naclerio, G.; Allocca, V.; Celico, F. Microorganisms as contaminants and natural tracers: A 10-year research in some carbonate aquifers (southern Italy). Environ. Earth Sci. 2015, 74, 173–184. [Google Scholar] [CrossRef]
- Bucci, A.; Petrella, E.; Celico, F.; Naclerio, G. Use of molecular approaches in hydrogeological studies: The case of carbonate aquifers in southern Italy. Hydrogeol. J. 2017, 25, 1017–1031. [Google Scholar] [CrossRef]
- Milani, C.; Hevia, A.; Foroni, E.; Duranti, S.; Turroni, F.; Lugli, G.A.; Margolles, A. Assessing the fecal microbiota: An optimized ion torrent 16S rRNA gene-based analysis protocol. PLoS ONE 2013, 8, e68739. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Huttley, G.A. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Petrella, E.; Naclerio, G.; Falasca, A.; Bucci, A.; Capuano, P.; de Felice, V.; Celico, F. Non-permanent shallow halocline in a fractured carbonate aquifer, southern Italy. J. Hydrol. 2009, 373, 267–272. [Google Scholar] [CrossRef]
- Petrella, E.; Celico, F. Mixing of water in a carbonate aquifer, southern Italy, analysed through stable isotope investigations. Int. J. Speleol. 2013, 42, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Hernàndez-Diaz, R.; Petrella, E.; Bucci, A.; Naclerio, G.; Feo, A.; Sferra, G.; Celico, F. Integrating hydrogeological and microbiological data and modelling to characterize the hydraulic features and behaviour of coastal carbonate aquifers: A case in western Cuba. Water 2019, 11, 1989. [Google Scholar] [CrossRef] [Green Version]
- Chelli, A.; Francese, R.; Petrella, E.; Carri, A.; Quagliarini, A.; Segalini, A.; Celico, F. A multi-parameter field monitoring system to investigate the dynamics of large earth slides–earth flows in the Northern Apennines, Italy. Eng. Geol. 2020, 275, 105780. [Google Scholar] [CrossRef]
- Allocca, V.; Celico, F.; Petrella, E.; Marzullo, G.; Naclerio, G. The role of land use and environmental factors on microbial pollution of mountainous limestone aquifers. Environ. Geol. 2008, 55, 277–283. [Google Scholar] [CrossRef]
- Celico, F.; Capuano, P.; de Felice, V.; Naclerio, G. Hypersaline groundwater genesis assessment through a multidisciplinary approach: The case of Pozzo del Sale spring (southern Italy). Hydrogeol. J. 2008, 16, 1441–1451. [Google Scholar] [CrossRef]
- Bucci, A.; Allocca, V.; Naclerio, G.; Capobianco, G.; Divino, F.; Fiorillo, F.; Celico, F. Winter survival of microbial contaminants in soil: An in situ verification. J. Environ. Sci. 2015, 27, 131–138. [Google Scholar] [CrossRef]
- Takeuchi, M.; Hamana, K.; Hiraishi, A. Proposal of the genus Sphingomonas sensu stricto and three new genera, Sphingobium, Novosphingobium and Sphingopyxis, on the basis of phylogenetic and chemotaxonomic analyses. Int. J. Syst. Evol. Microbiol. 2001, 51, 1405–1417. [Google Scholar] [CrossRef]
- Hahn, M.W.; Kasalický, V.; Jezbera, J.; Brandt, U.; Jezberova, J.; Šimek, K. Limnohabitans curvus gen. nov. sp. nov. a planktonic bacterium isolated from a freshwater lake. Int. J. Syst. Evol. Microbiol. 2010, 60, 1358–1365. [Google Scholar] [CrossRef]
- White, D.C.; Sutton, S.D.; Ringelberg, D.B. The genus Sphingomonas: Physiology and ecology. Curr. Opin. Biotechnol. 1996, 7, 301–306. [Google Scholar] [CrossRef]
- La Scola, B.; Birtles, R.J.; Mallet, M.N.; Raoult, D. Massilia timonae gen. nov. sp. nov. isolated from blood of an immunocompromised patient with cerebellar lesions. J. Clin. Microbiol. 1998, 36, 2847–2852. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Verma, H.; Haider, S.; Bajaj, A.; Sood, U.; Ponnusamy, K.; Nagar, S.; Shakarad, M.N.; Negi, R.K.; Singh, Y.; et al. Comparative genomic analysis reveals habitat-specific genes and regulatory hubs within the genus Novosphingobium. MSystems 2017, 2, e00020-17. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.P.O.; Mot, R.D.; Speingael, D. Draft genome sequence of the carbofuran-mineralizing Novosphingobium sp. strain KN65.2. Genome Announc. 2015, 3, e00764-15. [Google Scholar] [CrossRef] [Green Version]
- Saxena, A.; Anand, S.; Dua, A.; Sangwan, N.; Khan, F.; Lal, R. Novosphingobium lindaniclasticum sp. nov. a hexachlorocyclohexane (HCH)- degrading bacterium isolated from an HCH dumpsite. Int. J. Syst. Evol. Microbiol. 2013, 63, 2160–2167. [Google Scholar] [CrossRef] [Green Version]
- Pearce, S.L.; Oakeshott, J.G.; Pandey, G. Insights into ongoing evolution of the hexachlorocyclohexane catabolic pathway from comparative genomics of ten Sphingomonadaceae strains. G3 Genes Genomes Genet. 2015, 5, 1081–1094. [Google Scholar] [CrossRef] [Green Version]
- Ohta, Y.; Nishi, S.; Hasegawa, R.; Hatada, Y. Combination of six enzymes of a marine Novosphingobium converts the stereoisomers of -O-4 lignin model dimers into the respective monomers. Sci. Rep. 2015, 5, 15105. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Petrillo, M.; Cantiello, P.; Naso, B.; Cozzuto, L.; Notomista, E.; Paolella, G.; Di Donato, A.; Salvatore, F. De novo sequencing and assembly of the whole genome of Novosphingobium sp. strain PP1Y. J. Bacteriol. 2011, 193, 384. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.P.; Wang, B.J.; Liu, Y.H.; Liu, S.J. Novosphingobium taihuense sp. nov. a novel aromatic-compound-degrading bacterium isolated from Taihu Lake, China. Int. J. Syst. Evol. Microbiol. 2005, 55, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Tiirola, M.A.; Busse, H.J.; Kämpfer, P.; Männistö, M.K. Novosphingobium lentum sp. nov. a psychrotolerant bacterium from a polychlorophenol bioremediation process. Int. J. Syst. Evol. Microbiol. 2005, 55, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.H.; Kwon, K.K.; Kang, J.-H.; Jung, H.-B.; Kim, S.-J. Novosphingobium pentaromativorans sp. nov. a high-molecular-mass polycyclic aromatic hydrocarbon-degrading bacterium isolated from estuarine sediment. Int. J. Syst. Evol. Microbiol. 2004, 54, 1483–1487. [Google Scholar] [CrossRef] [Green Version]
- Lal, R.; Dogra, C.; Malhotra, S.; Sharma, P.; Pal, R. Diversity, distribution and divergence of lin genes in hexachlorocyclohexane-degrading sphingomonads. Trends Biotechnol. 2006, 24, 121–130. [Google Scholar] [CrossRef]
- Hegedüs, B.; Kós, P.B.; Bende, G.; Bounedjoum, N.; Maróti, G.; Laczi, K.; Rákhely, G. Starvation-and xenobiotic-related transcriptomic responses of the sulfanilic acid-degrading bacterium, Novosphingobium resinovorum SA1. Appl. Microbiol. Biotechnol. 2018, 102, 305–318. [Google Scholar] [CrossRef]
- Ryan, M.P.; Adley, C.C. Sphingomonas paucimobilis: A persistent Gram-negative nosocomial infectious organism. J. Hosp. Infect. 2010, 75, 153–157. [Google Scholar] [CrossRef]
- Collado, L.; Figueras, M.J. Taxonomy, epidemiology, and clinical relevance of the genus Arcobacter. Clin. Microbiol. Rev. 2011, 24, 174–192. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, H.; Komagata, K. An attempt at grouping of the genus Pseudomonas. J. Gen. Appl. Microbiol. 1963, 9, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Shah, H.N.; Collins, D.M. Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int. J. Syst. Evol. Microbiol. 1990, 40, 205–208. [Google Scholar] [CrossRef]
- Bouvet, P.J.; Grimont, P.A. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov. Acinetobacter haemolyticus sp. nov. Acinetobacter johnsonii sp. nov. and Acinetobacter junii sp. nov. and emended descriptions of Acinetobacter calcoaceticus and Acinetobacter lwoffii. Int. J. Syst. Evol. Microbiol. 1986, 36, 228–240. [Google Scholar] [CrossRef] [Green Version]
- Ramees, T.P.; Dhama, K.; Karthik, K.; Rathore, R.S.; Kumar, A.; Saminathan, M.; Tiwari, R.; Malik, Y.S.; Singh, R.K. Arcobacter: An emerging food-borne zoonotic pathogen, its public health concerns and advances in diagnosis and control-A comprehensive review. Vet Q 2017, 37, 136–161. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [Green Version]
- Walker, M.J.; Barnett, T.C.; McArthur, J.D.; Cole, J.N.; Gillen, C.M.; Henningham, A.; Sriprakash, K.S.; Sanderson-Smith, M.L.; Nizet, V. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin. Microbiol. Rev. 2014, 27, 264–301. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.P.; Sevjahova, L.; Gorman, R.; White, S. The Emergence of the Genus Comamonas as Important Opportunistic Pathogens. Pathogens 2022, 11, 1032. [Google Scholar] [CrossRef]
- Songmei, L.; Yuanyuan, Z.; Juan, D.; Jianhua, L.; Mei, Y. Influence of streptomyces on the Corrosion Behavior of Steel A3 in Thiobacillus ferrooxidans Media. Acta Chim. Sin. 2010, 68, 67–74. [Google Scholar]
- Stanaszek-Tomal, E.; Fiertak, M. Biological corrosion in the sewage system and the sewage treatment plant. Procedia Eng. 2016, 161, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Hagedorn, C.; Harwood, V.J.; Blanch, A. Microbial Source Tracking: Methods, Applications, and Case Studies; 2011th ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2011; ISBN 1489991301. [Google Scholar]
- Kreader, C.A. Design and evaluation of Bacteroides DNA probes for the specific detection of human fecal pollution. Appl. Environ. Microbiol. 1995, 61, 1171–1179. [Google Scholar] [CrossRef] [Green Version]
- Sghir, A.; Gramet, G.; Suau, A.; Rochet, V.; Pochart, P.; Dore, J. Quantification of bacterial groups within human faecal flora by oligoneocletide probe hybridization. Appl. Environ. Microbiol. 2000, 66, 2263–2266. [Google Scholar] [CrossRef] [Green Version]
- Benevides, L.; Burman, S.; Martin, R.; Robert, V.; Thomas, M.; Miquel, S.; Soares, S. New insights into the diversity of the genus Faecalibacterium. Front. Microbiol. 2017, 8, 1790. [Google Scholar] [CrossRef] [PubMed]
- Merkova, M.; Zalesak, M.; Ringlova, E.; Julinova, M.; Ruzicka, J. Degradation of the surfactant Cocamidopropyl betaine by two bacterial strains isolated from activated sludge. Int. Biodeterior. Biodegrad. 2018, 127, 236–240. [Google Scholar] [CrossRef]
- Prousek, J. Fenton chemistry in biology and medicine. PureAppl. Chem. 2007, 79, 2325–2338. [Google Scholar] [CrossRef]
- Mackul’ak, T.; Mosný, M.; Grabic, R.; Golovko, O.; Koba, O.; Birošová, L. Fenton-like reaction: A possible way to efficiently remove illicit drugs and pharmaceuticals from wastewater. Environ. Toxicol. Pharmacol. 2015, 39, 483–488. [Google Scholar] [CrossRef]
- Blott, S.J.; Pye, K. Particle size distribution analysis of sand-sized particles by laser diffraction: An experimental investigation of instrument sensitivity and the effects of particle shape. Sedimentology 2006, 53, 671–685. [Google Scholar] [CrossRef]





| Sample Name | Mean Diameter (µm) | Mode (µm) | Span | Clay (%) | Silt (%) | Sand (%) |
|---|---|---|---|---|---|---|
| PZ1 6–7 m | 31.99 ± 1.97 | 28.32 ± 14.03 | 5.44 ± 0.16 | 18.09 | 64.9 | 17.01 |
| PZ1 11–12 m | 20.81 ± 0.79 | 8.62 ± 0.04 | 4.73 ± 0.1 | 21.7 | 71.04 | 7.26 |
| PZ3 6–7 m | 16.18 ± 0.61 | 7.18 ± 0.02 | 4.82 ± 0.13 | 28.1 | 67.08 | 4.82 |
| PZ4 5–6 m | 57.06 ± 2.05 | 75.2 ± 4.15 | 2.69 ± 0.05 | 5.97 | 56.67 | 37.36 |
| PZ4 9–10 m | 48.68 ± 2.74 | 57.51 ± 5.55 | 3.97 ± 0.07 | 10.46 | 60.69 | 28.85 |
| S18 8.75–9.5 m | 62.43 ± 3.91 | 78.62 ± 5.22 | 2.83 ± 0.03 | 5.98 | 54.32 | 39.7 |
| Sample | Parameter (CAS) | Technique | Analytical Method | Detection Limit | Results | Unit |
|---|---|---|---|---|---|---|
| IN wastewater | 2-Phenoxyethanol (122-99-6) | GC-MS | In-house | 0.2 | <0.2 | mg/L |
| Disodium EDTA (139-33-3) | GC-MS | ISO 16588 | 1 | 53 | μg/L | |
| PDMS calculated as polydimethylsiloxane with a viscosity of 1000 mPa.s (63148-62-9) | HPLC-RI | In-house | 100 | <100 | mg/L | |
| Pz3 groundwater | 2-Phenoxyethanol (122-99-6) | GC-MS | In-house | 0.2 | <0.2 | mg/L |
| Disodium EDTA (139-33-3) | GC-MS | ISO 16588 | 1 | <1 | μg/L | |
| PDMS calculated as polydimethylsiloxane with a viscosity of 1000 mPa.s (63148-62-9) | HPLC-RI | In-house | 100 | <100 | mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ducci, L.; Rizzo, P.; Pinardi, R.; Solfrini, A.; Maggiali, A.; Pizzati, M.; Balsamo, F.; Celico, F. What Is the Impact of Leaky Sewers on Groundwater Contamination in Urban Semi-Confined Aquifers? A Test Study Related to Fecal Matter and Personal Care Products (PCPs). Hydrology 2023, 10, 3. https://doi.org/10.3390/hydrology10010003
Ducci L, Rizzo P, Pinardi R, Solfrini A, Maggiali A, Pizzati M, Balsamo F, Celico F. What Is the Impact of Leaky Sewers on Groundwater Contamination in Urban Semi-Confined Aquifers? A Test Study Related to Fecal Matter and Personal Care Products (PCPs). Hydrology. 2023; 10(1):3. https://doi.org/10.3390/hydrology10010003
Chicago/Turabian StyleDucci, Laura, Pietro Rizzo, Riccardo Pinardi, Augusto Solfrini, Alessandro Maggiali, Mattia Pizzati, Fabrizio Balsamo, and Fulvio Celico. 2023. "What Is the Impact of Leaky Sewers on Groundwater Contamination in Urban Semi-Confined Aquifers? A Test Study Related to Fecal Matter and Personal Care Products (PCPs)" Hydrology 10, no. 1: 3. https://doi.org/10.3390/hydrology10010003
APA StyleDucci, L., Rizzo, P., Pinardi, R., Solfrini, A., Maggiali, A., Pizzati, M., Balsamo, F., & Celico, F. (2023). What Is the Impact of Leaky Sewers on Groundwater Contamination in Urban Semi-Confined Aquifers? A Test Study Related to Fecal Matter and Personal Care Products (PCPs). Hydrology, 10(1), 3. https://doi.org/10.3390/hydrology10010003









