Stable Isotopic Evidence of Paleorecharge in the Northern Gulf Coastal Plain (USA)
Abstract
1. Introduction
2. Materials and Methods
2.1. Isotope Systematics
2.2. Study Area Setting
2.2.1. Physiography, Climate, and Hydrogeology
2.2.2. Paleoclimate
2.3. Sampling and Laboratory Analyses
2.4. Modeling
3. Results
3.1. Water Analyses
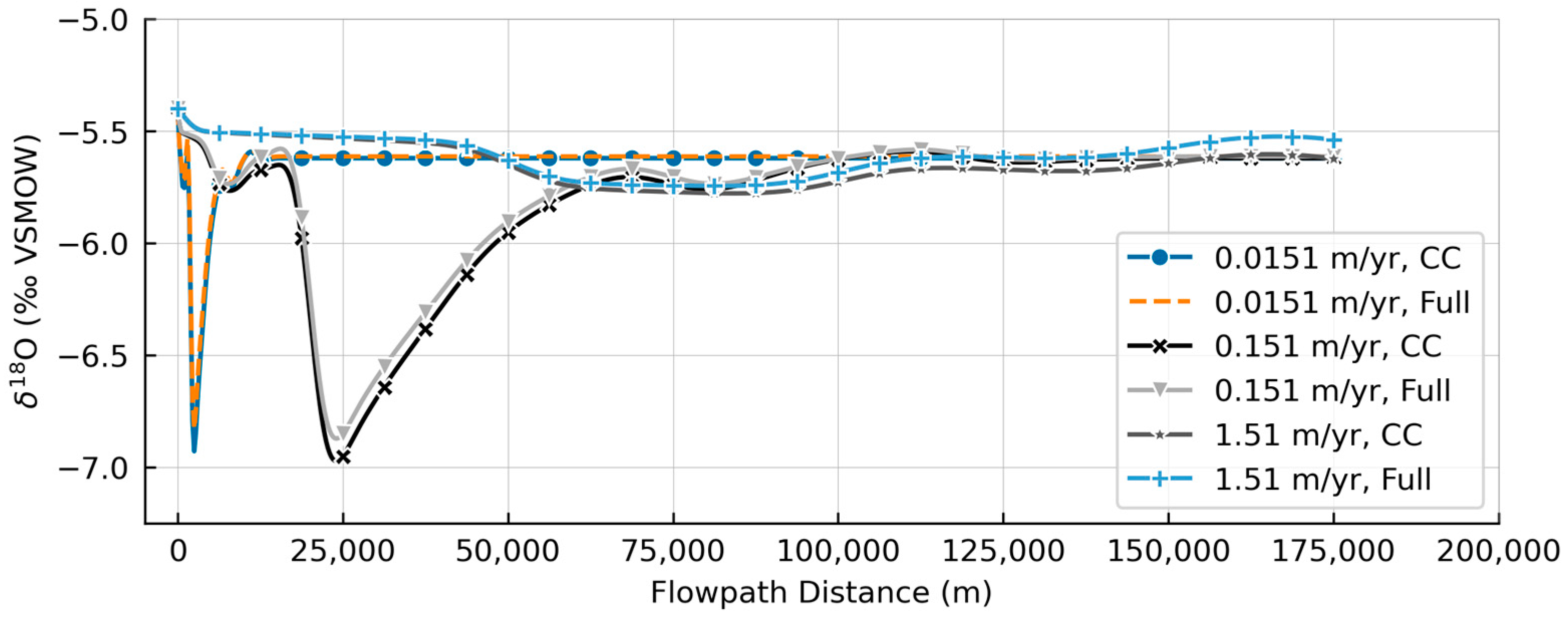
3.2. Model Simulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torgersen, T.; Habermehl, M.A.; Phillips, F.M.; Elmore, D.; Kubik, P.; Jones, G.B.; Hemmick, T.; Gove, H.E. Chlorine 36 dating of very old groundwater: 3. Further studies in the Great Artesian Basin, Australia. Water Resour. Res. 1991, 27, 3201–3213. [Google Scholar] [CrossRef]
- Marty, B.; Torgersen, T.; Meynier, V.; O’Nions, R.K.; de Marsily, G. Helium isotope fluxes and groundwater ages in the Dogger aquifer, Paris Basin. Water Resour. Res. 1993, 29, 1025–1035. [Google Scholar] [CrossRef]
- Sturchio, N.C.; Du, X.; Purtschert, R.; Lehmann, B.E.; Sultan, M.; Patterson, L.J.; Lu, Z.-T.; Müller, P.; Bigler, T.; Bailey, K.; et al. One million year old groundwater in the Sahara revealed by krypton-81 and chlorine-36. Geophys. Res. Lett. 2004, 31, L05503. [Google Scholar] [CrossRef]
- Plummer, L.N.; Eggleston, J.R.; Andreasen, D.C.; Raffensperger, J.P.; Hunt, A.G.; Casile, G.C. Old groundwater in parts of the Upper Patapsco aquifer, Atlantic Coastal Plain, Maryland, USA: Evidence from radiocarbon, chlorine-36 and helium-4. Hydrogeol. J. 2012, 20, 1269–1294. [Google Scholar] [CrossRef]
- Jasechko, S.; Lechler, A.; Pausata, F.S.R.; Fawcett, P.J.; Gleeson, T.; Cendón, D.I.; Galewsky, J.; LeGrande, A.N.; Risi, C.; Sharp, Z.D.; et al. Late-glacial to late-Holocene shifts in global precipitation δ18O. Clim. Past 2015, 11, 1375–1393. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.; Caldwell, E.A. Chapter 2—Fundamentals of isotope geochemistry. In Isotope Tracers in Catchment Hydrology; Kendall, C., McDonnell, J.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 51–86. [Google Scholar]
- Dutton, A.R. Groundwater isotopic evidence for paleorecharge in U.S. High Plains aquifers. Quat. Res. 1995, 43, 221–231. [Google Scholar] [CrossRef]
- Clark, J.F.; Davisson, M.L.; Hudson, G.B.; Macfarlane, P.A. Noble gases, stable isotopes, and radiocarbon as tracers of flow in the Dakota aquifer, Colorado and Kansas. J. Hydrol. 1998, 211, 151–167. [Google Scholar] [CrossRef]
- Stotler, R.; Harvey, F.E.; Gosselin, D.C. A Black Hills-Madison aquifer origin for Dakota aquifer groundwater in northeastern Nebraska. Groundwater 2010, 48, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Jasechko, S. Partitioning young and old groundwater with geochemical tracers. Chem. Geol. 2016, 427, 35–42. [Google Scholar] [CrossRef]
- Jurgens, B.C.; Faulkner, K.; McMahon, P.B.; Hunt, A.G.; Casile, G.; Young, M.B.; Belitz, K. Over a third of groundwater in USA public-supply aquifers is Anthropocene-age and susceptible to surface contamination. Commun. Earth Environ. 2022, 3, 153. [Google Scholar] [CrossRef]
- Hendry, M.J.; Schwartz, F.W. An alternative view on the origin of chemical and isotopic patterns in groundwater from the Milk River aquifer, Canada. Water Resour. Res. 1988, 24, 1747–1763. [Google Scholar] [CrossRef]
- LaBolle, E.M.; Fogg, G.E.; Eweis, J.B.; Gravner, J.; Leaist, D.G. Isotopic fractionation by diffusion in groundwater. Water Resour. Res. 2008, 44, W07405. [Google Scholar] [CrossRef]
- Clark, B.R.; Hart, R.M.; Gurdak, J.J. Groundwater Availability of the Mississippi Embayment; Professional Paper 1785; U.S. Geological Survey: Reston, VA, USA, 2011. [CrossRef]
- Brahana, J.V.; Mesko, T.O.; Busby, J.F.; Kraemer, T.F. Ground-Water Quality Data from the Northern Mississippi Embayment; Arkansas, Missouri, Kentucky, Tennessee, and Mississippi; Open-File Report 85-683; U.S. Geological Survey: Nashville, TN, USA, 1985. [CrossRef]
- Haile, E.; Fryar, A.E. Chemical evolution of groundwater in the Wilcox aquifer of the northern Gulf Coastal Plain, USA. Hydrogeol. J. 2017, 25, 2403–2418. [Google Scholar] [CrossRef]
- Woolery, E.W.; Wang, Z.; Carpenter, N.S.; Street, R.; Brengman, C. The Central United States Seismic Observatory: Site characterization, instrumentation, and recordings. Seismol. Res. Lett. 2016, 87, 215–228. [Google Scholar] [CrossRef]
- Hart, R.M.; Clark, B.R.; Bolyard, S.E. Digital Surfaces and Thicknesses of Selected Hydrogeologic Units within the Mississippi Embayment Regional Aquifer Study (MERAS); Scientific Investigations Report 2008-5098; U.S. Geological Survey: Reston, VA, USA, 2008. [CrossRef]
- Geyh, M. Groundwater: Saturated and unsaturated zone. In Environmental Isotopes in the Hydrological Cycle: Principles and Applications; UNESCO/IAEA: Vienna, Austria, 2001; Volume IV. [Google Scholar]
- Darling, G.W.; Bath, A.H.; Gibson, J.J.; Rozanski, K. Isotopes in water. In Isotopes in Palaeoenvironmental Research; Leng, M.J., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 1–66. [Google Scholar]
- Gat, J.R. Oxygen and hydrogen isotopes in the hydrologic cycle. Annu. Rev. Earth Planet. Sci. 1996, 24, 225–262. [Google Scholar] [CrossRef]
- Plummer, L.N. Stable isotope enrichment in paleowaters of the southeast Atlantic Coastal Plain, United States. Science 1993, 262, 2016–2020. [Google Scholar] [CrossRef]
- Kennedy, C.D.; Genereux, D.P. 14C groundwater age and the importance of chemical fluxes across aquifer boundaries in confined Cretaceous aquifers of North Carolina, USA. Radiocarbon 2007, 49, 1181–1203. [Google Scholar] [CrossRef][Green Version]
- Plummer, L.N.; Busby, J.F.; Lee, R.W.; Hanshaw, B.B. Geochemical modeling of the Madison aquifer in parts of Montana, Wyoming, and South Dakota. Water Resour. Res. 1990, 26, 1981–2014. [Google Scholar] [CrossRef]
- Siegel, D.I. Evidence for dilution of deep, confined ground water by vertical recharge of isotopically heavy Pleistocene water. Geology 1991, 19, 433–436. [Google Scholar] [CrossRef]
- Ma, L.; Castro, M.C.; Hall, C.M. A late Pleistocene–Holocene noble gas paleotemperature record in southern Michigan. Geophys. Res. Lett. 2004, 31, L23204. [Google Scholar] [CrossRef]
- Davis, S.N.; Cecil, D.; Zreda, M.; Sharma, P. Chlorine-36 and the initial value problem. Hydrogeol. J. 1998, 6, 104–114. [Google Scholar] [CrossRef]
- Andrews, J.N.; Fontes, J.-C.; Michelot, J.-L.; Elmore, D. In-situ neutron flux, 36Cl production and groundwater evolution in crystalline rocks at Stripa, Sweden. Earth Planet. Sci. Lett. 1986, 77, 49–58. [Google Scholar] [CrossRef]
- Fontes, J.C.; Brissaud, I.; Michelot, J.L. Hydrological implications of deep production of chlorine-36. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1984, 5, 303–307. [Google Scholar] [CrossRef]
- Bentley, H.W.; Phillips, F.M.; Davis, S.N.; Habermehl, M.A.; Airey, P.L.; Calf, G.E.; Elmore, D.; Gove, H.E.; Torgersen, T. Chlorine 36 dating of very old groundwater: 1. The Great Artesian Basin, Australia. Water Resour. Res. 1986, 22, 1991–2001. [Google Scholar] [CrossRef]
- Purdy, C.B.; Helz, G.R.; Mignerey, A.C.; Kubik, P.W.; Elmore, D.; Sharma, P.; Hemmick, T. Aquia aquifer dissolved Cl− and 36Cl/Cl: Implications for flow velocities. Water Resour. Res. 1996, 32, 1163–1171. [Google Scholar] [CrossRef]
- Lee, M.-K.; Griffin, J.; Saunders, J.; Wang, Y.; Jean, J.-S. Reactive transport of trace elements and isotopes in the Eutaw Coastal Plain aquifer, Alabama. J. Geophys. Res. Biogeosci. 2007, 112, G02026. [Google Scholar] [CrossRef]
- Williamson, A.K.; Grubb, H.F. Ground-Water Flow in the Gulf Coast Aquifer Systems, South-Central United States; Professional Paper 1416-F; U.S. Geological Survey: Reston, VA, USA, 2001. [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Brugger, K. World Map of the Köppen-Geiger Climate Classification Updated Map for the United States of America. Available online: http://koeppen-geiger.vu-wien.ac.at/usa.htm (accessed on 7 June 2024).
- U.S. Climate Data—Monthly Averages. Available online: https://www.usclimatedata.com/ (accessed on 6 June 2024).
- Hosman, R.L. Regional Stratigraphy and Subsurface Geology of Cenozoic Deposits, Gulf Coastal Plain, South-Central United States; Professional Paper 1416-G; U.S. Government Printing Office: Washington, DC, USA, 1996. [CrossRef]
- U.S. Geological Survey Earthquake Hazards Program. Summary of 1811–1812 New Madrid Earthquakes Sequence. Available online: https://www.usgs.gov/programs/earthquake-hazards/science/summary-1811-1812-new-madrid-earthquakes-sequence (accessed on 13 July 2024).
- Brahana, J.V.; Mesko, T.O. Hydrogeology and Preliminary Assessment of Regional Flow in the Upper Cretaceous and Adjacent Aquifers in the Northern Mississippi Embayment; Water-Resources Investigations Report 87-4000; U.S. Geological Survey: Nashville, TN, USA, 1988. [CrossRef]
- Pugh, A.L. Potentiometric Surfaces and Water-Level Trends in the Cockfield (Upper Claiborne) and Wilcox (Lower Wilcox) Aquifers of Southern and Northeastern Arkansas, 2009; Scientific Investigations Report 2010-5014; U.S. Geological Survey: Reston, VA, USA, 2010. [CrossRef]
- Schrader, T.P. Water Levels and Water Quality in the Sparta-Memphis Aquifer (Middle Claiborne Aquifer) in Arkansas, Spring–Summer 2009; Scientific Investigations Report 2013-5100; U.S. Geological Survey: Reston, VA, USA, 2013. [CrossRef][Green Version]
- Arthur, J.K.; Taylor, R.E. Mississippi Embayment aquifer system in Mississippi: Geohydrologic data compilation for flow model simulation. JAWRA J. Am. Water Resour. Assoc. 1986, 22, 1021–1029. [Google Scholar] [CrossRef]
- Larsen, D.; Paul, J.; Cox, R. Geochemical and isotopic evidence for upward flow of saline fluid to the Mississippi River Valley alluvial aquifer, southeastern Arkansas, USA. Hydrogeol. J. 2021, 29, 1421–1444. [Google Scholar] [CrossRef]
- Kresse, T.M.; Hays, P.D.; Merriman, K.R.; Gillip, J.A.; Fugitt, D.T.; Spellman, J.L.; Nottmeier, A.M.; Westerman, D.A.; Blackstock, J.M.; Battreal, J.L. Aquifers of Arkansas: Protection, Management, and Hydrologic and Geochemical Characteristics of Groundwater Resources in Arkansas; Scientific Investigations Report 2014-5149; U.S. Geological Survey: Reston, VA, USA, 2014. [CrossRef]
- Harmon, R.S.; Schwarcz, H.P. Changes of 2H and 18O enrichment of meteoric water and Pleistocene glaciation. Nature 1981, 290, 125–128. [Google Scholar] [CrossRef]
- Rodbell, D.T.; Forman, S.L.; Pierson, J.; Lynn, W.C. Stratigraphy and chronology of Mississippi Valley loess in western Tennessee. GSA Bull. 1997, 109, 1134–1148. [Google Scholar] [CrossRef]
- Markewich, H.W.; Wysocki, D.A.; Pavich, M.J.; Rutledge, E.M.; Millard, H.T.; Rich, F.J.; Maat, P.B.; Rubin, M.; McGeehin, J.P. Paleopedology plus TL, 10Be, and 14C dating as tools in stratigraphic and paleoclimatic investigations, Mississippi River Valley, U.S.A. Quat. Int. 1998, 51–52, 143–167. [Google Scholar] [CrossRef]
- Forman, S.L.; Pierson, J. Late Pleistocene luminescence chronology of loess deposition in the Missouri and Mississippi River valleys, United States. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2002, 186, 25–46. [Google Scholar] [CrossRef]
- Muhs, D.R.; Wehmiller, J.F.; Simmons, K.R.; York, L.L. Quaternary sea-level history of the United States. In Developments in Quaternary Sciences; Elsevier: Amsterdam, The Netherlands, 2003; Volume 1, pp. 147–183. [Google Scholar]
- Williams, J.W.; Shuman, B.N.; Webb III, T. Dissimilarity analyses of late-Quaternary vegetation and climate in eastern North America. Ecology 2001, 82, 3346–3362. [Google Scholar] [CrossRef]
- Rovey, C.W.; Balco, G. Chapter 43—Summary of early and middle Pleistocene glaciations in northern Missouri, USA. In Developments in Quaternary Sciences; Ehlers, J., Gibbard, P.L., Hughes, P.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 15, pp. 553–561. [Google Scholar]
- Bromwich, D.H.; Toracinta, E.R.; Oglesby, R.J.; Fastook, J.L.; Hughes, T.J. LGM summer climate on the southern margin of the Laurentide Ice Sheet: Wet or dry? J. Clim. 2005, 18, 3317–3338. [Google Scholar] [CrossRef]
- USGS Water Data for the Nation. Available online: https://waterdata.usgs.gov/nwis (accessed on 6 June 2024).
- Center for Applied Research and Engagement Systems, University of Missouri. Missouri Source Water Protection and Assessment. Available online: https://drinkingwater.missouri.edu/ (accessed on 13 July 2024).
- Johnston, J. (Arkansas Water Well Construction Commission, Little Rock, AR, USA). Personal communication, 2006.
- Brahana, J.V.; Broshears, R.E. Hydrogeology and Ground-Water Flow in the Memphis and Fort Pillow Aquifers in the Memphis Area, Tennessee; Water-Resources Investigations Report 89-4131; U.S. Geological Survey: Nashville, TN, USA, 2001. [CrossRef]
- Castany, G. Traité Pratique des Eaux Souterraines; Dunod: Paris, France, 1967. [Google Scholar]
- Clark, B.R.; Hart, R.M. The Mississippi Embayment Regional Aquifer Study (MERAS): Documentation of a Groundwater-Flow Model Constructed to Assess Water Availability in the Mississippi Embayment; Scientific Investigations Report 2009-5172; U.S. Geological Survey: Reston, VA, USA, 2009. [CrossRef]
- Haile, E. Chemical Evolution and Residence Time of Groundwater in the Wilcox Aquifer of the Northern Gulf Coastal Plain. Ph.D. Dissertation, University of Kentucky, Lexington, KY, USA, 2011. [Google Scholar]
- Hendry, M.J.; Wassenaar, L.I. Implications of the distribution of δD in pore waters for groundwater flow and the timing of geologic events in a thick aquitard system. Water Resour. Res. 1999, 35, 1751–1760. [Google Scholar] [CrossRef]
- Tanaka, K. Measurements of self-diffusion coefficients of water in pure water and in aqueous electrolyte solutions. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 71, 1127–1131. [Google Scholar] [CrossRef]
- Xu, M.; Eckstein, Y. Use of weighted least-squares method in evaluation of the relationship between dispersivity and field scale. Groundwater 1995, 33, 905–908. [Google Scholar] [CrossRef]
- Davis, S.N.; Moysey, S.; Cecil, D.L.; Zreda, M. Chlorine-36 in groundwater of the United States: Empirical data. Hydrogeol. J. 2003, 11, 217–227. [Google Scholar] [CrossRef]
- Jurgens, B.C.; Musgrove, M.; McAdoo, M.A.; Faulkner, K.E. Data for Distribution of Groundwater Age in Aquifers Used for Public Supply in the Continental United States, 2004–2017 (Version 1.1: June 2022). U.S. Geological Survey Data Release. Available online: https://doi.org/10.5066/P9W7T0DN (accessed on 6 August 2024).
- Dutton, A.; Wilkinson, B.H.; Welker, J.M.; Bowen, G.J.; Lohmann, K.C. Spatial distribution and seasonal variation in 18O/16O of modern precipitation and river water across the conterminous USA. Hydrol. Processes 2005, 19, 4121–4146. [Google Scholar] [CrossRef]
- Arkansas Oil and Gas Commission. Wells, Fields, and Pipelines Maps. Available online: https://www.aogc.state.ar.us/maps/googleEarth.aspx (accessed on 13 July 2024).
- Missouri Department of Natural Resources. Oil and Gas Wells List, March 4, 2024. Available online: https://dnr.mo.gov/media/file/oil-gas-well-list-march-4-2024 (accessed on 15 July 2024).
- Bense, V.F.; Person, M.A. Faults as conduit-barrier systems to fluid flow in siliciclastic sedimentary aquifers. Water Resour. Res. 2006, 42, W05421. [Google Scholar] [CrossRef]
- Clayton, R.N.; Friedman, I.; Graf, D.L.; Mayeda, T.K.; Meents, W.F.; Shimp, N.F. The origin of saline formation waters: 1. Isotopic composition. J. Geophys. Res. 1966, 71, 3869–3882. [Google Scholar] [CrossRef]
- Joyce, J.E.; Tjaisma, L.R.C.; Prutzman, J.M. North American glacial meltwater history for the past 2.3 m.y.: Oxygen isotope evidence from the Gulf of Mexico. Geology 1993, 21, 483–486. [Google Scholar] [CrossRef]
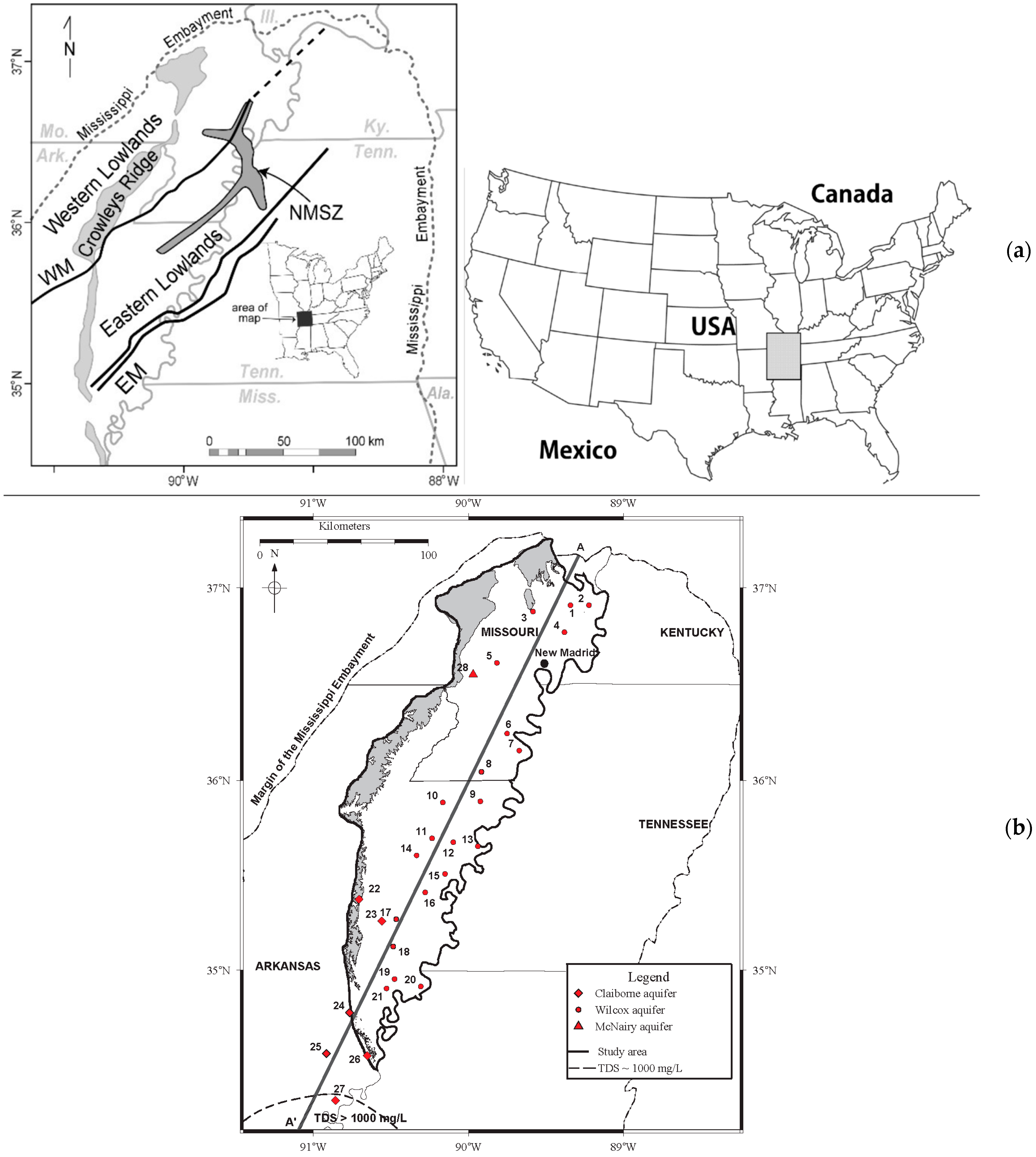
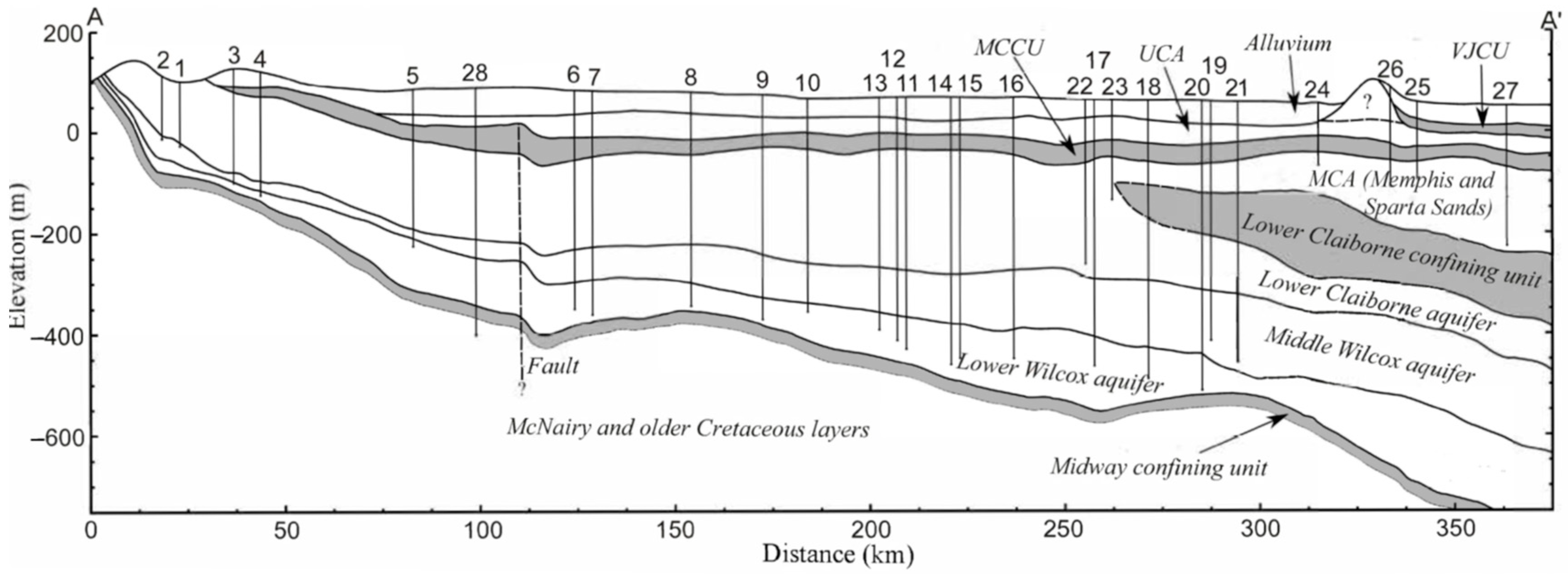
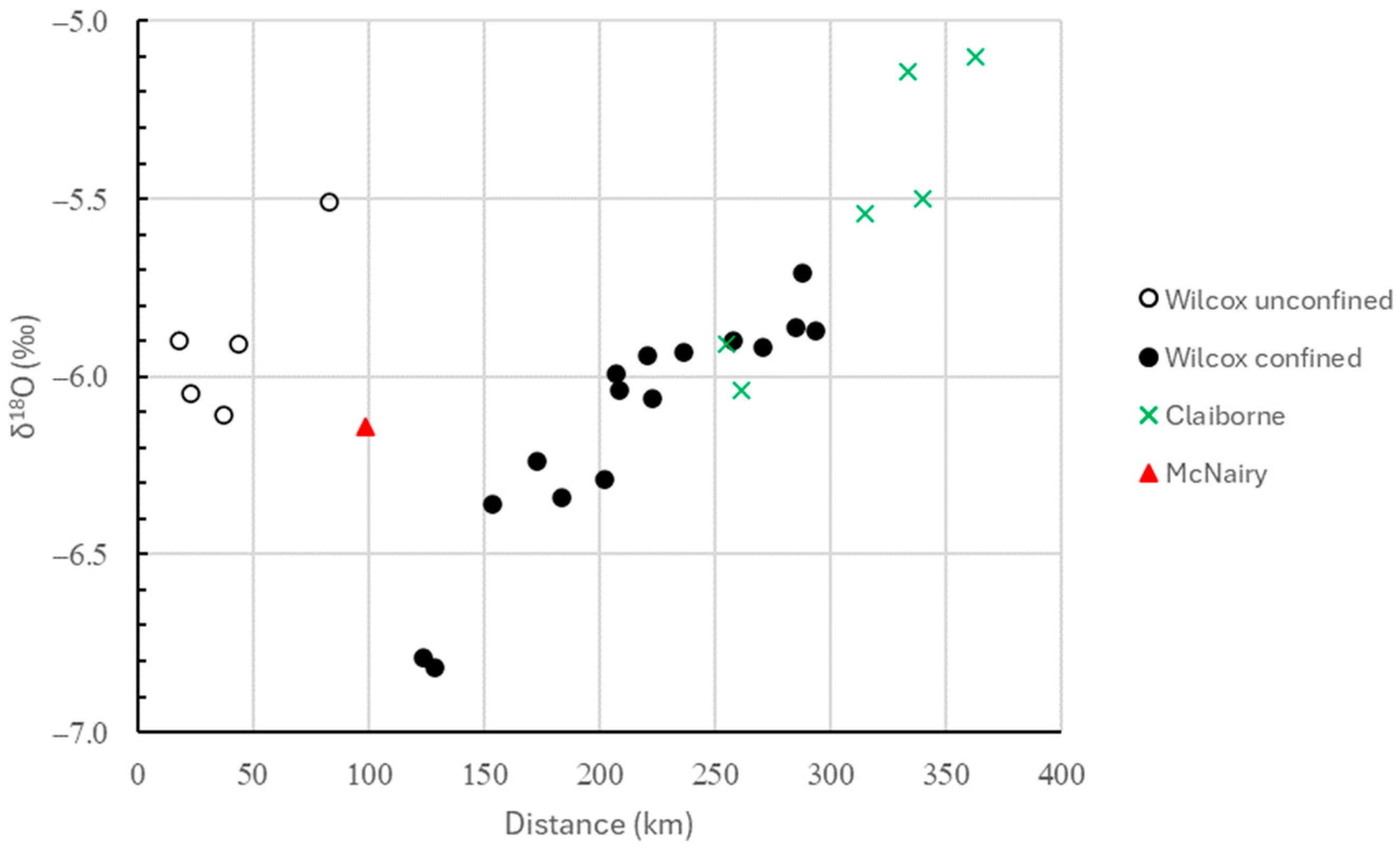

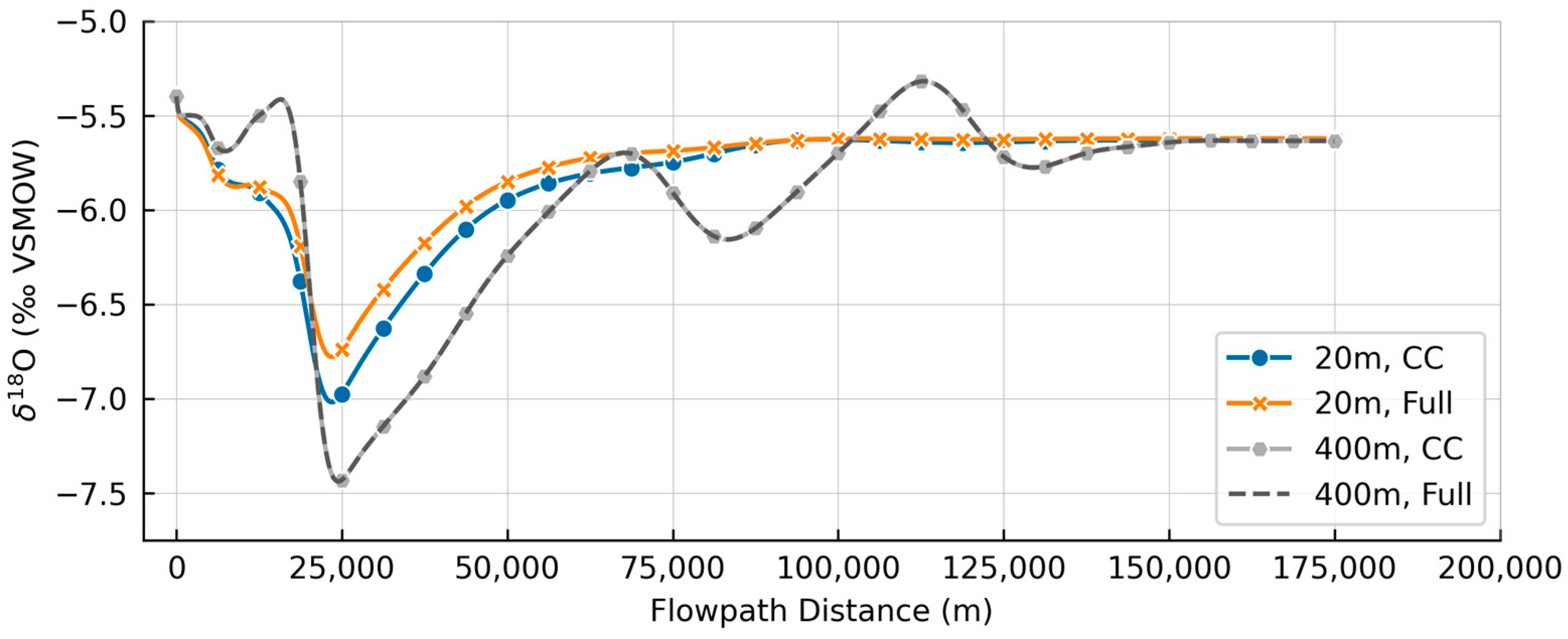
| Well | USGS ID | Location | County/State | Date Sampled | Longitude | Latitude | Altitude | Depth | Distance |
|---|---|---|---|---|---|---|---|---|---|
| (mm/dd/yy) | (masl) | (m) | (km) | ||||||
| Unconfined Wilcox | |||||||||
| 1 | 365444089203001 | Charleston | Mississippi/MO | 7/7/2006 | −89.34156 | 36.91213 | 97.5 | 116.7 | 23 |
| 2 | 365445089132001 | Wyatt | Mississippi/MO | 7/7/2006 | −89.22258 | 36.91185 | 96.6 | 139.3 | 18 |
| 3 | 365242089350601 | Sikeston | Scott/MO | 7/9/2006 | −89.58469 | 36.87838 | 99.7 | 118.0 | 37 |
| 4 | 364618089224901 | East Prairie | Mississippi/MO | 7/8/2006 | −89.38017 | 36.77157 | 93.0 | 179.8 | 44 |
| 5 | 363644089490501 | Parma | New Madrid/MO | 7/9/2006 | −89.81567 | 36.61279 | 85.3 | 141.4 | 83 |
| Confined Wilcox | |||||||||
| 6 | 361415089445801 | Hayti | Pemiscot/MO | 7/10/2006 | −89.74945 | 36.23741 | 82.3 | 395.6 | 124 |
| 7 | 360923089401901 | Caruthersville | Pemiscot/MO | 5/29/2007 | −89.67190 | 36.15632 | 80.8 | 420.6 | 129 |
| 8 | 360239089545501 | Pemiscot Co. | Pemiscot/MO | 7/10/2006 | −89.91523 | 36.04436 | 76.2 | 410.0 | 154 |
| 9 | 355323089552101 | Dogwood | Mississippi/AR | 7/11/2006 | −89.92259 | 35.88980 | 76.2 | 426.7 | 173 |
| 10 | 355252090095701 | Manila | Mississippi/AR | 7/11/2006 | −90.16432 | 35.88488 | 73.2 | 353.0 | 184 |
| 11 | 354133090135001 | Little River | Mississippi/AR | 7/13/2006 | −90.23085 | 35.69225 | 69.2 | 408.4 | 209 |
| 12 | 354033090055201 | Keiser | Mississippi/AR | 7/13/2006 | −90.09675 | 35.67577 | 70.1 | 442.6 | 207 |
| 13 | 353917089561501 | Osceola | Mississippi/AR | 5/30/2007 | −89.93832 | 35.65468 | 74.7 | 457.2 | 202 |
| 14 | 353630090194501 | Lepanto | Poinsett/AR | 7/12/2006 | −90.33063 | 35.60584 | 68.3 | 439.8 | 221 |
| 15 | 353029090085801 | Joiner | Mississippi/AR | 7/15/2006 | −90.15000 | 35.50833 | 70.7 | 461.2 | 223 |
| 16 | 352450090165201 | Gilmore | Crittenden/AR | 7/15/2006 | −90.27889 | 35.41083 | 67.7 | 460.6 | 237 |
| 17 | 351614090275401 | Earle | Crittenden/AR | 7/14/2006 | −90.46458 | 35.27069 | 65.5 | 533.4 | 258 |
| 18 | 350734090290101 | Shell Lake | St Francis/AR | 5/31/2007 | −90.48389 | 35.12528 | 60.7 | 483.1 | 271 |
| 19 | 345710090283002 | Hughes | St Francis/AR | 7/14/2006 | −90.47493 | 34.95326 | 60.7 | 493.5 | 288 |
| 20 | 345448090182701 | Horseshoe Lake | Crittenden/AR | 6/1/2007 | −90.30775 | 34.91349 | 61.3 | 499.3 | 285 |
| 21 | 345416090313801 | Brickeys | Lee/AR | 6/1/2007 | −90.52663 | 34.90369 | 59.7 | 518.8 | 294 |
| Claiborne (Memphis Sand) | |||||||||
| 22 | 352231090421501 | Birdeye | Cross/AR | 5/30/2007 | −90.70514 | 35.37552 | 130.9 | 345.0 | 255 |
| 23 | 351544090334101 | Parkin | Cross/AR | 5/31/2007 | −90.55829 | 35.26059 | 62.5 | 140.8 | 262 |
| Claiborne (Sparta Sand) | |||||||||
| 24 | 344629090455803 | Marianna | Lee/AR | 6/4/2007 | −90.76611 | 34.77472 | 70.4 | 149.0 | 315 |
| 25 | 343324090544601 | Marvell | Phillips/AR | 6/5/2007 | −90.91539 | 34.55676 | 64.0 | 210.0 | 340 |
| 26 | 343242090390201 | West Helena | Phillips/AR | 6/4/2007 | −90.65194 | 34.54524 | 76.2 | 180.1 | 334 |
| 27 | 341822090512401 | Elaine | Phillips/AR | 6/5/2007 | −90.85669 | 34.30724 | 50.6 | 283.5 | 363 |
| McNairy | |||||||||
| 28 | 363442089580901 | Malden | Dunklin/MO | 5/29/2007 | −89.96927 | 36.57825 | 89.6 | 278.9 | 99 |
| Parameter | Value | Units | Source |
|---|---|---|---|
| Model mesh | 250 | m | |
| Time tolerance | 0.1 | ||
| Aquifer hydraulic conductivity | 0.00003 | m/s | Arithmetic mean [41,57] |
| Aquifer thickness | 20–400 | m | Arbitrary |
| Aquifer porosity | 0.15–0.225 | [58] | |
| Confining-unit porosity | 0.35 | [58] | |
| 18O diffusion coefficient | 2.27 × 10−9 | m/s | [62] |
| 18O diffusion coefficient in clay | 1.7 × 10−10 | m/s | [61] |
| Longitudinal dispersion | 0.83 × log(L *)2.414 | m | [63] |
| Well | Cl− | δ18O | δ2H |
|---|---|---|---|
| (mg/L) | ‰ VSMOW | ‰ VSMOW | |
| Unconfined Wilcox | |||
| 1 | 0.9 | −6.05 | −36.18 |
| 2 | 1.5 | −5.90 | −35.94 |
| 3 | 9.0 | −6.11 | −36.97 |
| 4 | 0.43 | −5.91 | −35.15 |
| 5 | 1.3 | −5.51 | −33.13 |
| Confined Wilcox | |||
| 6 | 1.1 | −6.79 | −41.31 |
| 7 | 1.2 | −6.82 | −39.41 |
| 8 | 2.0 | −6.36 | −37.82 |
| 9 | 0.78 | −6.24 | −36.94 |
| 10 | 1.5 | −6.34 | −35.70 |
| 11 | 3.5 | −6.04 | −34.90 |
| 12 | 1.3 | −5.99 | −35.18 |
| 13 | 1.1 | −6.29 | −35.33 |
| 14 | 1.7 | −5.94 | −34.16 |
| 15 | 0.58 | −6.06 | −35.49 |
| 16 | 1.7 | −5.93 | −34.12 |
| 17 | 2.2 | −5.90 | −33.74 |
| 18 | 2.2 | −5.92 | −33.60 |
| 19 | 5.1 | −5.71 | −33.06 |
| 20 | 2.8 | −5.86 | −32.74 |
| 21 | 7.1 | −5.87 | −35.15 |
| Claiborne (Memphis Sand) | |||
| 22 | 2.9 | −5.91 | −33.15 |
| 23 | 2.3 | −6.04 | −34.57 |
| Claiborne (Sparta Sand) | |||
| 24 | 310 | −5.54 | −31.45 |
| 25 | 39 | −5.50 | −31.15 |
| 26 | 83 | −5.14 | −28.07 |
| 27 | 160 | −5.10 | −28.69 |
| McNairy | |||
| 28 | 160 | −6.14 | −32.45 |
| Location | Aquifer | 36Cl/Cl × 10−15 | 14C (pmc) | Low Age (yr) | High Age (yr) | LPM Age (yr) | Distance (km) | Low v (m/s) | High v (m/s) | LPM v (m/s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Osceola, AR | Wilcox | 211.9 | 2.60 × 104 | 1.08 × 105 | 202 | 5.91 × 10−8 | 2.46 × 10−7 | |||
| Shell Lake, AR | Wilcox | 89.6 | 4.00 × 105 | 4.82 × 105 | 271 | 1.78 × 10−8 | 2.15 × 10−8 | |||
| Horseshoe Lake, AR | Wilcox | 59.9 | 5.75 × 105 | 6.57 × 105 | 285 | 1.38 × 10−8 | 1.57 × 10−8 | |||
| Brickeys, AR | Wilcox | 41.0 | 7.39 × 105 | 8.22 × 105 | 294 | 1.13 × 10−8 | 1.26 × 10−8 | |||
| Birdeye, AR * | Claiborne | 205 | 4.04 × 104 | 1.23 × 105 | 255 | 6.58 × 10−8 | 2.00 × 10−7 | |||
| Parkin, AR * | Claiborne | 70 | 5.07 × 105 | 5.89 × 105 | 262 | 1.41 × 10−8 | 1.64 × 10−8 | |||
| Parma, MO | Wilcox | 86.5 | 1090 | 83 | 2.41 × 10−6 | |||||
| Malden, MO | McNairy | 83.8 | 1340 | 99 | 2.34 × 10−6 | |||||
| Caruthersville, MO | Wilcox | 19.2 | 16,000 | 129 | 2.56 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haile, E.; Currens, B.J.; Fryar, A.E. Stable Isotopic Evidence of Paleorecharge in the Northern Gulf Coastal Plain (USA). Hydrology 2024, 11, 118. https://doi.org/10.3390/hydrology11080118
Haile E, Currens BJ, Fryar AE. Stable Isotopic Evidence of Paleorecharge in the Northern Gulf Coastal Plain (USA). Hydrology. 2024; 11(8):118. https://doi.org/10.3390/hydrology11080118
Chicago/Turabian StyleHaile, Estifanos, Benjamin J. Currens, and Alan E. Fryar. 2024. "Stable Isotopic Evidence of Paleorecharge in the Northern Gulf Coastal Plain (USA)" Hydrology 11, no. 8: 118. https://doi.org/10.3390/hydrology11080118
APA StyleHaile, E., Currens, B. J., & Fryar, A. E. (2024). Stable Isotopic Evidence of Paleorecharge in the Northern Gulf Coastal Plain (USA). Hydrology, 11(8), 118. https://doi.org/10.3390/hydrology11080118








