Performance Assessment of a Permeable Reactive Barrier on Reducing Groundwater Transport of Nitrate from an Onsite Wastewater Treatment System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Groundwater Sampling
2.3. Statistical Analyses
3. Results
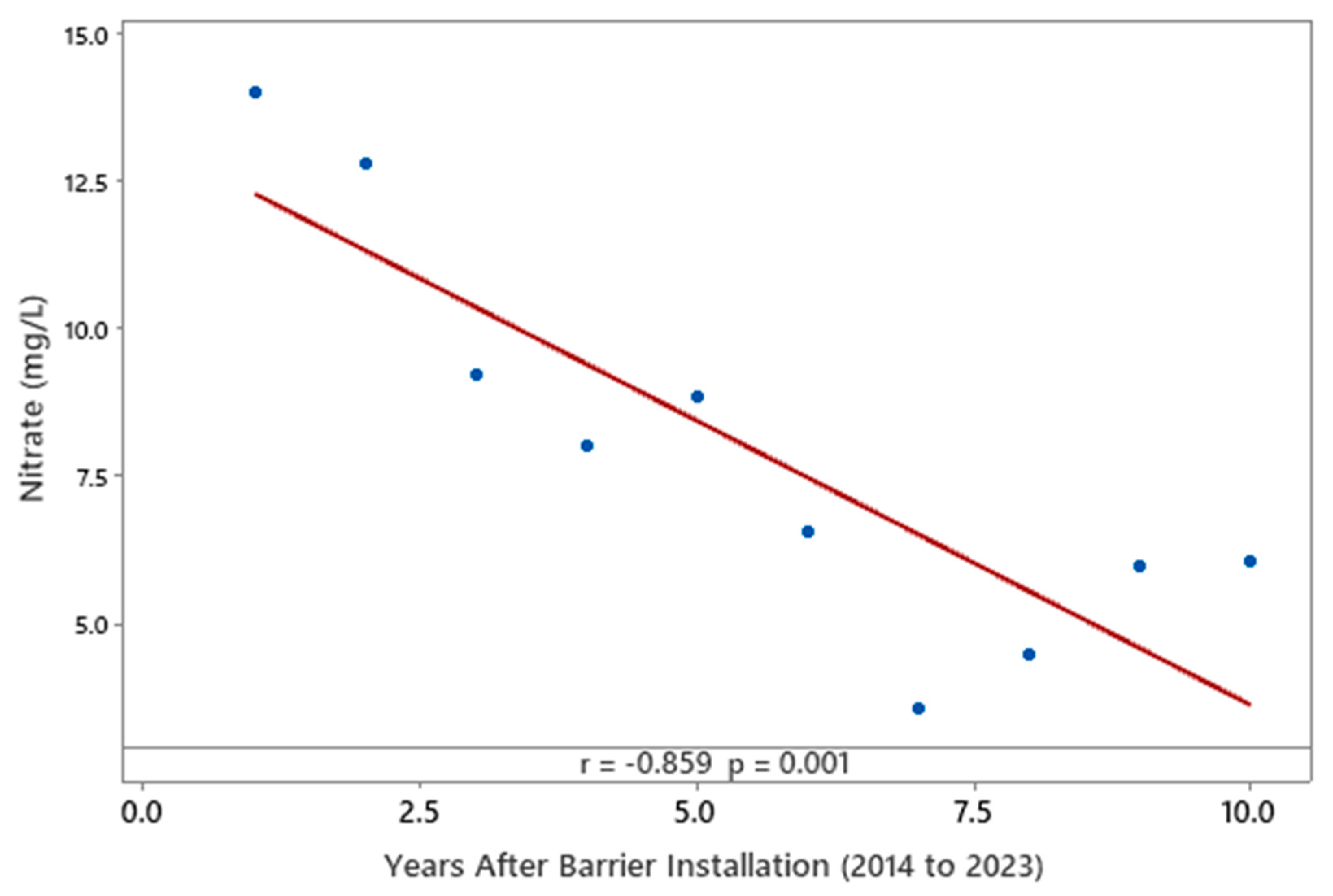
3.1. Nitrate Concentrations
3.2. Physicochemical Characteristics of Groundwater
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gill, L.; O’Luanaigh, N.; Johnston, P.; Misstear, B.; O’Suilleabhain, C. Nutrient loading on subsoils from on-site wastewatereffluent, comparing septic tank and secondary treatment systems. Water Res. 2009, 43, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Aravena, R.; Evans, M.L.; Cherry, J.A. Stable istopes of oxygen and nitrogen in source identification of nitrate from septic systems. Groundwater 1993, 31, 180–186. [Google Scholar] [CrossRef]
- Geary, P.; Lucas, S. Contamination of estuaries from failing septic tank systems: Difficulties in scaling up from monitored individual systems to cumulative impact. Environ. Sci. Pollut. Res. 2018, 26, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Onsite Wastewater Treatment Systems Manual. 2002. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/2004_07_07_septics_septic_2002_osdm_all.pdf (accessed on 6 November 2024).
- Richards, S.; Paterson, E.; Withers, P.J.A.; Stutter, M. Septic tank discharges as multi-pollutant hotspots in catchments. Sci. Total Environ. 2016, 542, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Nokes, C.; Simunek, J.; Kikkert, H.; Hector, R. Modeling the impact of clustered septic tanks systems on groundwater quality. Vadose Zone J. 2006, 5, 599–609. [Google Scholar] [CrossRef]
- Lusk, M.; Toor, G.; Yang, Y.Y.; Mechtensimer, S.; De, M.; Obreza, T. A review of the fate and transport of nitrogen, phosphorus, pathogens, and trace organic chemicals in septic systems. Crit. Rev. Environ. Sci. Technol. 2017, 47, 455–541. [Google Scholar] [CrossRef]
- Nasr, F.A.; Mkhaeil, B. Treatment of domestic wastewater using conventional and baffled septic tanks. Environ. Technol. 2013, 34, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Hoover, M.T.; Clark, G.H.; Gumpertz, M.; Wollum, A.G.; Cobb, C.; Strock, J. Septic tank additive impacts on microbial populations. J. Environ. Health 2008, 70, 22–27. [Google Scholar]
- Lin, H.; Wang, Y.; Lierop, L.; Zamalloa, C.; Furlong, C.; Keleman, M.; Hu, B. Study of food waste degredation in a simulated septic tank. Waste Manag. Res. 2019, 37, 1199–1206. [Google Scholar] [CrossRef]
- Patel, T.; O’Luanaigh, N.; Gill, L.W. A comparison of gravity distribution devices used in on-site domestic wastewater treatment systems. Water Air Soil Pollut. 2008, 191, 55–69. [Google Scholar] [CrossRef]
- Christopherson, S.; Wheeler, D.; Wittwer, J.; Haeg, T. Field comparison of rock-filled and chambered trench systems. J. Hydrol. Eng. 2008, 13, 671–680. [Google Scholar] [CrossRef]
- Carr, M.E.; Jumper, D.L.; Yelderman, J.C., Jr. A comparison of disposal methods for on-site sewage facilties within the state of Texas, USA. Environmentalist 2009, 29, 381–387. [Google Scholar] [CrossRef]
- Radcliffe, D.E.; West, L.T.; Singer, J. Gravel effect on wastewater infiltration from septic system trenches. Soil Sci. Soc. Am. J. 2005, 69, 1217–1224. [Google Scholar] [CrossRef]
- Humphrey, C.P., Jr.; Iverson, G.; Underwood, W.J.; Cary, S.S.; Skibiel, C.; O’Driscoll, M. Nitrogen treatment in soil beneath high-flow and low-flow onsite wastewater systems. J. Sustain. Water Built Environ. 2019, 5, 4. [Google Scholar] [CrossRef]
- Howarth, R.W.; Marino, R. Nitrogen as a lmiting nutrient for eutrophication in coastal marine ecosystems: Evolving views over three decades. Limnol. Oceanogr. 2006, 51, 364–376. [Google Scholar] [CrossRef]
- Brush, G.S. Historical land use, nitrogen, and coastals eutrophication: A paleoecological perspective. Estuaries Coasts 2009, 32, 18–28. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Dodds, W.K.; Smith, V.H. Nitrogen, phosphorus, and eutrophication in streams. Inland Waters 2016, 6, 155–164. [Google Scholar] [CrossRef]
- Bunnell, J.; Zampella, R.; Morgan, M.; Gray, D. A comparison of nitrogen removal by subsurface pressure dosing and standard septic systems in sandy soils. J. Environ. Manag. 1999, 56, 209–219. [Google Scholar] [CrossRef]
- Humphrey, C.P., Jr.; Iverson, G.; Hvastkovs, E.; Pradhan, S. Occurrence and concentrations of traditional and emerging contaminants in onsite wastewater systems and water supply wells in eastern North Carolina, USA. J. Water Health 2024, 22, 550–564. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Toor, G.S. Nitrogen transformations in the mounded drainfields of drip dispersal and gravel trench septic systems. Ecol. Eng. 2017, 102, 352–360. [Google Scholar] [CrossRef]
- Cooper, J.A.; Morales, I.; Amador, J.A. Nitrogen transformations in different types of soil treatment areas receiving domestic wastewater. Ecol. Eng. 2016, 94, 22–29. [Google Scholar] [CrossRef]
- Robertson, W.D.; Cherry, J.A.; Sudicky, E.A. Ground-water contamination from two small septic systems on sand aquifers. Groundwater 1991, 29, 82–92. [Google Scholar] [CrossRef]
- Humphrey, C.P.; O’Driscoll, M.A.; Zarate, M.A. Controls on groundwater nitrogen contributions from on-site wastewater systems in coastal North Carolina. Water Sci. Technol. 2010, 62, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Humprey, C.P., Jr.; O’Driscoll, M.; Iverson, G. Comparison of nitrogen treatment by four onsite wastewater systems in nutrient sensitive watersheds of the North Carolina Coastal Plain. Nitrogen 2021, 2, 268–286. [Google Scholar] [CrossRef]
- Mari, A.; Taro, M.; Takayuki, M. Recent water quality incidents and a methemoglobinemia outbreak in infants due to inadequate plumbing of a university hospital’s private water supply. J. Natl. Inst. Public Health 2023, 72, 31–42. [Google Scholar]
- Ward, M.H.; Mark, S.D.; Cantor, K.P.; Weisenburger, D.D.; Corrrea-Villasenor, A.; ZAhm, S.H. Drinking water nitrate and the risk of non-hodgkin’s lymphoma. Epidemiology 1996, 7, 465–471. [Google Scholar] [CrossRef]
- Weyer, P.J.; Cerhan, J.R.; Kross, B.C.; Hallberg, G.R.; Kantamneni, J.; Breuer, G.; Jones, M.P.; Zheng, W.; Lynch, C.F. Municipal drinking water nitrate level and cancer risk in oder women: The Iowa women’s health study. Epidemiology 2001, 12, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Schullehner, J.; Hansen, B.; Thygesen, M.; Pedersen, C.B.; Sigsgaard, T. Nitrate in drinking water and colorectal cancer risk: A nationwide population-based cohort study. Int. J. Cancer 2018, 143, 73–79. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.A.; Humphrey, C.P.; Deal, N.E.; Lindbo, D.L.; Zarate-Bermudez, M.A. Meteorological influences on nitrogen dynamics of a coastal onsite wastewater treatment system. J. Environ. Qual. 2014, 43, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Harden, H.S.; Roeder, E.; Hooks, M.; Chanton, J.P. Evaluation of onsite sewage treatment and disposal systems in shallow karst terrain. Water Res. 2008, 42, 2585–2597. [Google Scholar] [CrossRef] [PubMed]
- Hoghooghi, N.; Radcliffe, D.E.; Habteselassie, M.Y.; Clarke, J.S. Confirmation of the impact of onsite wastewater treatment systems on stream base-flow nitrogen concentrations in urban watersheds of metropolitan Atlanta, GA. J. Environ. Qual. 2016, 45, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Iverson, G.; O’Driscoll, M.; Humphrey Jr, C.; Manda, A.; Anderson-Evans, E. Wastewater nitrogen contributions to coastal plain watersheds, NC, USA. Water Air Soil Pollut. 2015, 226, 325. [Google Scholar] [CrossRef]
- Aravena, R.; Robertson, W.D. Use of multiple isotope tracers to evaluate denitrification in ground water: Study of nitrate from a large-flux septic system plume. Groundwater 1998, 36, 975–982. [Google Scholar] [CrossRef]
- Del Rosario, K.L.; Humphrey, C.P.; Mitra, S.; O’Driscoll, M.A. Nitrogen and carbon dynamics beneath on-site wastewater treatment systems in Pitt County, North Carolina. Water Sci. Technol. 2013, 69, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Robertson, W.D.; Cherry, J.A. In situ denitrification of septic-system derived nitrate using reactive porous media barriers: Field trials. Ground Water 1995, 33, 99–111. [Google Scholar] [CrossRef]
- Mayer, P.M.; Reynolds, S.K.; McCutchen, M.D.; Canfield, T.J. Meta-Analysis of Nitrogen Removal in Riparian Buffers. J. Environ. Qual. 2007, 36, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, M.; Humphrey, C.P.; DeWalle, D.; Iverson, G. Groundwater seeps: Portholes to evaluate groundwater’s influence on stream water quality. J. Contemp. Water Resour. Educ. 2019, 166, 57–78. [Google Scholar] [CrossRef]
- Schipper, L.A.; Vojvodi’c-Vukovi’c, M. Five years of nitrate removal, denitrification and carbon dynamics in a denitrification wall. Water Res. 2001, 35, 3473–3477. [Google Scholar] [CrossRef]
- Long, L.M.; Schipper, L.A.; Bruesewitz, D.A. Long-term nitrate removal in a denitrification wall. Agric. Ecosyst. Environ. 2011, 140, 514–520. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Clark, M.W. Efficacy of a denitrification wall to treat continuously high nitrate loads. Ecol. Eng. 2012, 42, 203–211. [Google Scholar] [CrossRef]
- Humphrey, C.; Pradhan, S.; Bean, E.; O’Driscoll, M.; Iverson, G. Preliminary evaluation of a permeable reactive barrier for reducing groundwater nitrate transport from a large onsite wastewater system. Am. J. Environ. Sci. 2015, 11, 216–226. [Google Scholar] [CrossRef]
- Gibert, O.; Assal, A.; Devlin, H.; Elliot, T.; Kalin, R.M. Performance of a field-scale biological permeable reactive barrier for in-situ remediation of nitrate- contaminated groundwater. Sci. Total Environ. 2019, 659, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ponnada, E.V.; Lynn, T.J.; Peterson, M.; Ergas, S.J.; Mihelcic, J.R. Application of denitrifying wood chip bioreactors for management of residential non-point sources of nitrogen. J. Biol. Eng. 2017, 11, 16. [Google Scholar] [CrossRef]
- United States Climate Data. Williamston, NC Climate Weather Averages. Available online: https://www.usclimatedata.com/climate/williamston/north-carolina/united-states/usnc0757 (accessed on 14 December 2024).
- De, M.; Toor, G.S. Fate of effluent-borne nitrogen in the mounded drainfield of an onsite wastewater treatment system. Vadose Zone J. 2015, 14, 1–12. [Google Scholar] [CrossRef]
- Reay, W.G. Septic tank impacts on ground water quality and nearshore sediment nutrient flux. Ground Water 2004, 42, 1079–1089. [Google Scholar] [CrossRef]
- Lie, E.; Welander, T. Influence of dissolved oxygen and oxidation-reduction potential on the denitrification rate of activated sludge. Water Sci. Technol. 1994, 30, 91–100. [Google Scholar] [CrossRef]
- Robertson, W.D.; Vogan, J.L.; Lombardo, P.S. Nitrate removal rates in a 15-year-old permeable reactive barrier treating septic system nitrate. Ground Water Monit. Remediat. 2008, 28, 65–72. [Google Scholar] [CrossRef]
- Cameron, S.G.; Schipper, L.A. Nitrate removal and hydraulic performance of organic carbon for use in denitrification beds. Ecol. Eng. 2010, 36, 1588–1595. [Google Scholar] [CrossRef]
- Peterson, I.J.; Igielski, S.; Davis, A.P. Enhanced denitrification in bioretention using woodchips as an organic carbon source. J. Sustain. Water Built Environ. 2015, 1, 04015004. [Google Scholar] [CrossRef]
- Moorman, T.B.; Parkin, T.B.; Kaspar, T.C.; Jaynes, D.B. Denitrification activity, wood loss, and N2O emissions over 9 years from a wood chip bioreactor. Ecol. Eng. 2010, 36, 1567–1574. [Google Scholar] [CrossRef]
- O’Driscoll, M.; Bean, E.; Mahoney, R.N.; Humphrey, C.P., Jr. Coastal tourism and its influence on wastewater nitrogen loading: A barrier island case study. Environ. Manag. 2019, 64, 436–455. [Google Scholar] [CrossRef]
- Lancellotti, B.; Loomis, G.; Hoyt, K.; Avizinis, E.; Amador, J. Evaluation of nitrogen concentration in final effluent of advanced nitrogen-removal onsite wastewater treatment systems (OWTS). Water Air Soil Pollut. 2017, 228, 383. [Google Scholar] [CrossRef]
- Ross, B.N.; Hoyt, K.P.; Loomis, G.W.; Amador, J.A. Effectiveness of advanced nitrogen-removal onsite wastewater treatment systems in a New England Coastal Community. Water Air Soil Pollut. 2020, 251, 543. [Google Scholar] [CrossRef]
- Diaz-Elsayed, N.; Xu, X.; Balaguer-Barbosa, M.; Zhang, Q. An evaluation of the sustainability of onsite wastewater treatment systems for nutrient management. Water Res. 2017, 121, 186–196. [Google Scholar] [CrossRef]








| Location | pH | Temp (°C) | ORP (mV) | SC (uS/cm) | DOC (mg/L) | Cl (mg/L) |
|---|---|---|---|---|---|---|
| Well 1 | 4.8 (0.3) | 17.5 (5.6) | 52.8 (36.7) | 72.0 (2.9) | 0.8 (0.2) | 4.3 (0.1) |
| Well 2 | 4.8 (0.2) | 17.2 (7.0) | −40.8 (86.9) | 259.2 (27.4) | 16.9 (9.3) | 14.7 (1.0) |
| Well 3 | 4.7 (0.2) | 19.5 (6.6) | 15.2 (28.9) | 219.0 (22.0) | 3.3 (4.0) | 26.2 (1.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humphrey Jr., C.P.; Iverson, G.; O’Driscoll, M. Performance Assessment of a Permeable Reactive Barrier on Reducing Groundwater Transport of Nitrate from an Onsite Wastewater Treatment System. Hydrology 2025, 12, 18. https://doi.org/10.3390/hydrology12010018
Humphrey Jr. CP, Iverson G, O’Driscoll M. Performance Assessment of a Permeable Reactive Barrier on Reducing Groundwater Transport of Nitrate from an Onsite Wastewater Treatment System. Hydrology. 2025; 12(1):18. https://doi.org/10.3390/hydrology12010018
Chicago/Turabian StyleHumphrey Jr., Charles P., Guy Iverson, and Mike O’Driscoll. 2025. "Performance Assessment of a Permeable Reactive Barrier on Reducing Groundwater Transport of Nitrate from an Onsite Wastewater Treatment System" Hydrology 12, no. 1: 18. https://doi.org/10.3390/hydrology12010018
APA StyleHumphrey Jr., C. P., Iverson, G., & O’Driscoll, M. (2025). Performance Assessment of a Permeable Reactive Barrier on Reducing Groundwater Transport of Nitrate from an Onsite Wastewater Treatment System. Hydrology, 12(1), 18. https://doi.org/10.3390/hydrology12010018










