Biofuels from Renewable Sources, a Potential Option for Biodiesel Production
Abstract
:1. Introduction
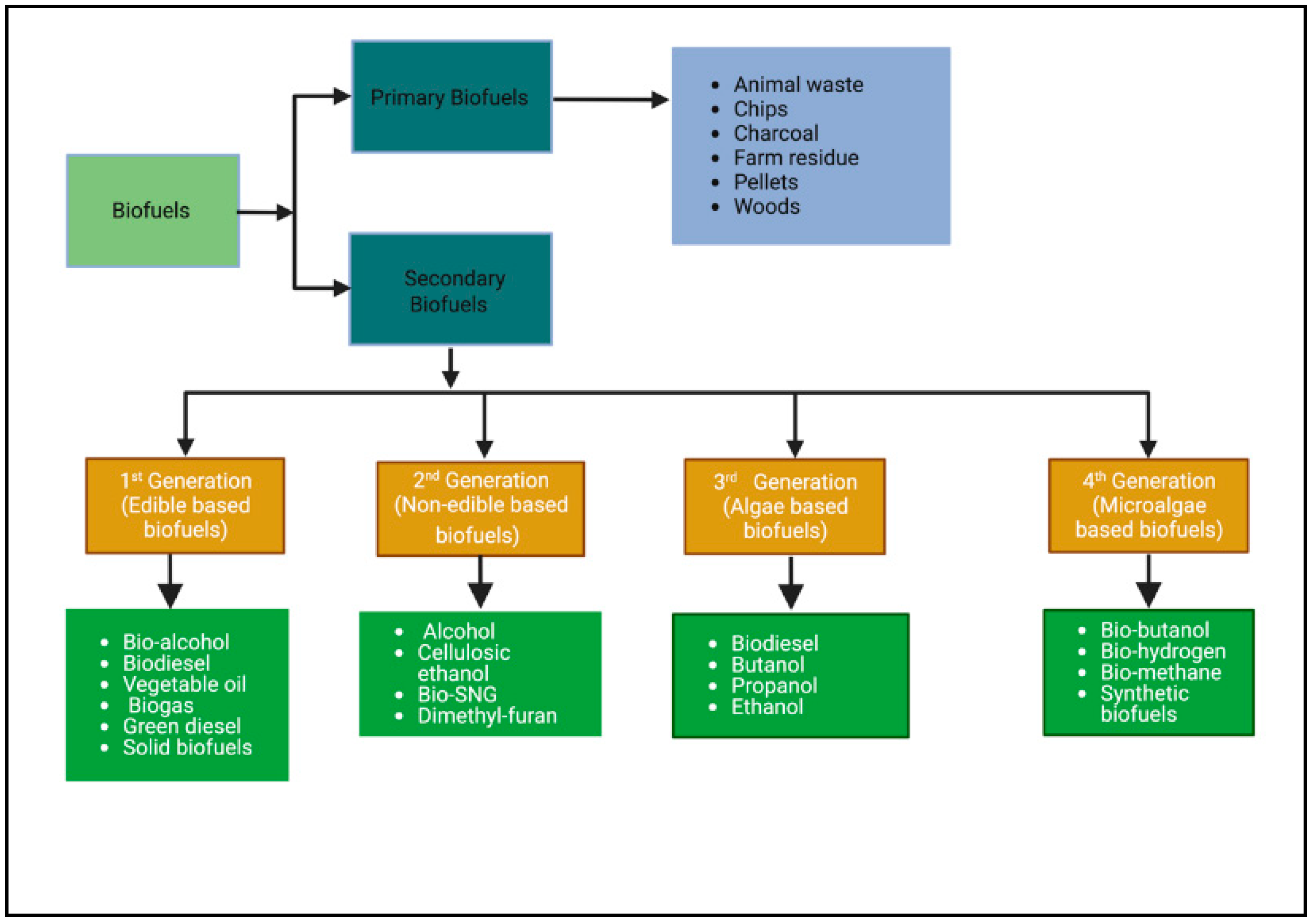
2. Types and Generation of Biofuels
2.1. First-Generation Biofuels
2.1.1. Bio Alcohols
2.1.2. Biodiesels
2.1.3. Vegetable Oil
2.1.4. Green Diesel
2.1.5. Biogas
2.1.6. Solid Biofuels
2.2. Second-Generation Biofuels
2.2.1. Cellulosic Ethanol
2.2.2. Algae-Based Biofuels
2.2.3. Alcohol
2.2.4. Dimethylfuran
2.2.5. Biosynthetic Natural Gas (Bio-SNG)
2.3. Third-Generation Biofuels
2.4. Fourth-Generation Biofuels
3. Biomass Sources for Biodiesel Production
4. Biodiesel and Its Properties
4.1. Cloud Point
4.2. Cetane Number
4.3. Oxidative Stability
4.4. Saponification Value
4.5. Iodine Number
4.6. Acid Value
5. Procedures for Biodiesel Production
5.1. Micro-Emulsion
5.2. Pyrolysis
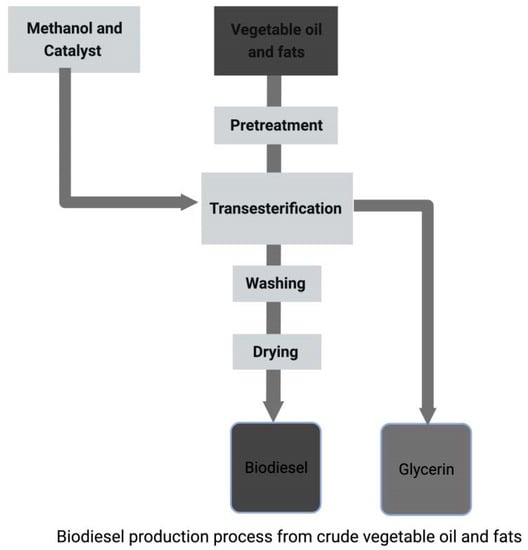
5.3. Transesterification
6. Factors Affecting Biodiesel Production
6.1. Free Fatty Acids
6.2. Water Content
6.3. Types of Alcohol
6.4. Alcohol to Oil Ratio
6.5. Reaction Time
6.6. Reaction Temperature
6.7. pH
6.8. Catalyst Concentration
6.9. Agitation Speed
7. Catalyst Use for Biodiesel Production
7.1. Acidic Catalysts
7.2. Alkaline Catalysts
7.3. Enzyme Catalysts
7.4. Homogeneous Catalysts
7.5. Heterogeneous Catalysts
8. Evaluation of Greenhouse Gas Emissions from Biodiesel Production
9. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Neupane, D.; Adhikari, P.; Bhattarai, D.; Rana, B.; Ahmed, Z.; Sharma, U.; Adhikari, D. Does Climate Change Affect the Yield of the Top Three Cereals and Food Security in the World? Earth 2022, 3, 45–71. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Ibrahim, M.; Rashid, U.; Nawaz, M.; Ali, S.; Hussain, A.; Gull, M. Biomass production for bioenergy using marginal lands. Sustain. Prod. Consum. 2017, 9, 3–21. [Google Scholar] [CrossRef]
- Maheshwari, P.; BelalHaider, M.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 335, 131588. [Google Scholar] [CrossRef]
- Singh, A.R.; Singh, S.K.; Jain, S. A review on bioenergy and biofuel production. Mater. Today Proc. 2022, 49, 510–516. [Google Scholar]
- Alengebawy, A.; Mohamed, B.A.; Ghimire, N.; Jin, K.; Liu, T.; Samer, M.; Ai, P. Understanding the environmental impacts of biogas utilization for energy production through life cycle assessment: An action towards reducing emissions. Environ. Res. 2022, 213, 113632. [Google Scholar] [CrossRef]
- Edrisi, S.A.; Abhilash, P. Exploring marginal and degraded lands for biomass and bioenergy production: An Indian scenario. Renew. Sustain. Energy Rev. 2016, 54, 1537–1551. [Google Scholar] [CrossRef]
- BP. BP, 2022. Statistical Review of World Energy-August 2022. Available online: https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy/primary-energy.html (accessed on 21 July 2022).
- Yusuf, M.; Beg, M.; Ubaidullah, M.; Shaikh, S.F.; Keong, L.K.; Hellgardt, K.; Abdullah, B. Kinetic studies for DRM over high-performance Ni–W/Al2O3–MgO catalyst. Int. J. Hydrog. Energy 2021, 47, 42150–42159. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
- Bacenetti, J.; Negri, M.; Fiala, M.; González-García, S. Anaerobic digestion of different feedstocks: Impact on energetic and environmental balances of biogas process. Sci. Total Environ. 2013, 463, 541–551. [Google Scholar] [CrossRef]
- Fatima, Z.; Ahmed, M.; Hussain, M.; Abbas, G.; Ul-Allah, S.; Ahmad, S.; Ahmed, N.; Ali, M.A.; Sarwar, G.; Iqbal, P. The fingerprints of climate warming on cereal crops phenology and adaptation options. Sci. Rep. 2020, 10, 1–21. [Google Scholar] [CrossRef]
- Sharma, R.K.; Kumar, S.; Vatta, K.; Bheemanahalli, R.; Dhillon, J.; Reddy, K.N. Impact of recent climate change on corn, rice, and wheat in southeastern USA. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, F.; Abdullah, B.; Oladipo, H.; Yusuf, M.; Alenazey, F.; Nguyen, T.D.; Ayoub, M. Chapter 18—Recent Developments in Photocatalytic Irradiation from CO2 to Methanol. In Nanostructured Photocatalysts; Nguyen, V.-H., Vo, D.-V.N., Nanda, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 519–540. [Google Scholar] [CrossRef]
- Rosdin, R.D.b.; Yusuf, M.; Abdullah, B. Dry reforming of methane over Ni-based catalysts: Effect of ZrO2 and MgO addition as support. Mater. Lett. X 2021, 12, 100095. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. R. Soc. A 2020, 476, 20200351. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, F.; Strømman, A.H. Life cycle assessment of bioenergy systems: State of the art and future challenges. Bioresour. Technol. 2011, 102, 437–451. [Google Scholar] [CrossRef]
- Tayari, S.; Abedi, R.; Rahi, A. Comparative assessment of engine performance and emissions fueled with three different biodiesel generations. Renew. Energy 2020, 147, 1058–1069. [Google Scholar] [CrossRef]
- Okoye, P.U.; Longoria, A.; Sebastian, P.J.; Wang, S.; Li, S.; Hameed, B.H. A review on recent trends in reactor systems and azeotrope separation strategies for catalytic conversion of biodiesel-derived glycerol. Sci. Total Environ. 2020, 719, 134595. [Google Scholar] [CrossRef]
- Garcia, R.; Figueiredo, F.; Brandão, M.; Hegg, M.; Castanheira, É.; Malça, J.; Nilsson, A.; Freire, F. A meta-analysis of the life cycle greenhouse gas balances of microalgae biodiesel. Int. J. Life Cycle Assess. 2020, 25, 1737–1748. [Google Scholar] [CrossRef]
- Sun, S.; Li, K. Biodiesel production from phoenix tree seed oil catalyzed by liquid lipozyme TL100L. Renew. Energy 2020, 151, 152–160. [Google Scholar] [CrossRef]
- Esmaeili, H. A critical review on the economic aspects and life cycle assessment of biodiesel production using heterogeneous nanocatalysts. Fuel Process. Technol. 2022, 230, 107224. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Kumar, M. Impact of n-butanol as an additive with eucalyptus biodiesel-diesel blends on the performance and emission parameters of the diesel engine. Fuel 2020, 277, 118178. [Google Scholar] [CrossRef]
- Deora, P.S.; Verma, Y.; Muhal, R.A.; Goswami, C.; Singh, T. Biofuels: An alternative to conventional fuel and energy source. Mater. Today Proc. 2022, 48, 1178–1184. [Google Scholar]
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.H.; Nguyen, T.H.P. Sustainability of the four generations of biofuels–a review. Int. J. Energy Res. 2020, 44, 9266–9282. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.; Madzimbamuto, T.; Ikhu-Omoregbe, D. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Obergruber, M.; Hönig, V.; Procházka, P.; Kučerová, V.; Kotek, M.; Bouček, J.; Mařík, J. Physicochemical properties of biobutanol as an advanced biofuel. Materials 2021, 14, 914. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A review on the prospects of sustainable biodiesel production: A global scenario with an emphasis on waste-oil biodiesel utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Salehi Jouzani, G.; Aghbashlo, M.; Tabatabaei, M. Biofuels: Types, Promises, Challenges, and Role of Fungi. In Fungi in Fuel Biotechnology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–14. [Google Scholar]
- Corsini, A.; Marchegiani, A.; Rispoli, F.; Sciulli, F.; Venturini, P. Vegetable Oils as Fuels in Diesel Engine. Engine Performance and Emissions. Energy Procedia 2015, 81, 942–949. [Google Scholar] [CrossRef] [Green Version]
- Faungnawakij, K.; Suriye, K. New and Future Developments in Catalysis: Chapter 4. Current Catalytic Processes with Hybrid Materials and Composites for Heterogeneous Catalysis; Elsevier Inc. Chapters: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Ai, P.; Jin, K.; Alengebawy, A.; Elsayed, M.; Meng, L.; Chen, M.; Ran, Y. Effect of application of different biogas fertilizer on eggplant production: Analysis of fertilizer value and risk assessment. Environ. Technol. Innov. 2020, 19, 101019. [Google Scholar] [CrossRef]
- Aryal, N.; Ghimire, N.; Bajracharya, S. Coupling of microbial electrosynthesis with anaerobic digestion for waste valorization. In Advances in Bioenergy; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 101–127. [Google Scholar]
- do Nascimento Santos, T.; Dutra, E.D.; do Prado, A.G.; Leite, F.C.B.; de Souza, R.d.F.R.; dos Santos, D.C.; de Abreu, C.A.M.; Simões, D.A.; de Morais Jr, M.A.; Menezes, R.S.C. Potential for biofuels from the biomass of prickly pear cladodes: Challenges for bioethanol and biogas production in dry areas. Biomass Bioenergy 2016, 85, 215–222. [Google Scholar] [CrossRef]
- Wasajja, H.; Lindeboom, R.E.; van Lier, J.B.; Aravind, P. Techno-economic review of biogas cleaning technologies for small scale off-grid solid oxide fuel cell applications. Fuel Process. Technol. 2020, 197, 106215. [Google Scholar] [CrossRef]
- Bhuiya, M.; Rasul, M.; Khan, M.M.K.; Ashwath, N.; Azad, A.K.; Hazrat, M. Second generation biodiesel: Potential alternative to-edible oil-derived biodiesel. Energy Procedia 2014, 61, 1969–1972. [Google Scholar] [CrossRef] [Green Version]
- Perlack, R.D. Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply; Oak Ridge National Laboratory: Oak Ridge, Tennessee, 2005. [Google Scholar]
- Werling, B.P.; Dickson, T.L.; Isaacs, R.; Gaines, H.; Gratton, C.; Gross, K.L.; Liere, H.; Malmstrom, C.M.; Meehan, T.D.; Ruan, L. Perennial grasslands enhance biodiversity and multiple ecosystem services in bioenergy landscapes. Proc. Natl. Acad. Sci. USA 2014, 111, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Lynd, L.R.; Sow, M.; Chimphango, A.F.; Cortez, L.A.; Brito Cruz, C.H.; Elmissiry, M.; Laser, M.; Mayaki, I.A.; Moraes, M.A.; Nogueira, L.A. Bioenergy and African transformation. Biotechnol. Biofuels 2015, 8, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirbas, A. Use of algae as biofuel sources. Energy Convers. Manag. 2010, 51, 2738–2749. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, C.-Y.; Lee, D.-J.; Chang, J.-S. Perspectives on microalgal CO2-emission mitigation systems—A review. Biotechnol. Adv. 2011, 29, 189–198. [Google Scholar] [CrossRef]
- Posten, C.; Schaub, G. Microalgae and terrestrial biomass as source for fuels—A process view. J. Biotechnol. 2009, 142, 64–69. [Google Scholar] [CrossRef]
- Hong, M.; Zhukareva, V.; Vogelsberg-Ragaglia, V.; Wszolek, Z.; Reed, L.; Miller, B.I.; Geschwind, D.H.; Bird, T.D.; McKeel, D.; Goate, A. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 1998, 282, 1914–1917. [Google Scholar] [CrossRef]
- Xu, H.; Wang, C. A Comprehensive Review of 2,5-Dimethylfuran as a Biofuel Candidate. Biofuels Lignocellul. Biomass Innov. Beyond Bioethanol. 2016, 105–129. [Google Scholar]
- Zhang, W.; He, J.; Engstrand, P.; Björkqvist, O. Economic evaluation on bio-synthetic natural gas production integrated in a thermomechanical pulp mill. Energies 2015, 8, 12795–12809. [Google Scholar] [CrossRef] [Green Version]
- Alaswad, A.; Dassisti, M.; Prescott, T.; Olabi, A.G. Technologies and developments of third generation biofuel production. Renew. Sustain. Energy Rev. 2015, 51, 1446–1460. [Google Scholar] [CrossRef]
- Wirth, R.; Lakatos, G.; Böjti, T.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Anaerobic gaseous biofuel production using microalgal biomass–a review. Anaerobe 2018, 52, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, R.; Manigandan, S.; Samuel, M.S.; Shanmuganathan, R.; Brindhadevi, K.; Chi, N.T.L.; Duc, P.A.; Pugazhendhi, A. A review on prospective production of biofuel from microalgae. Biotechnol. Rep. 2020, 27, e00509. [Google Scholar] [CrossRef] [PubMed]
- Subramani, L.; Venu, H. Evaluation of methyl ester derived from novel Chlorella emersonii as an alternative feedstock for DI diesel engine & its combustion, performance and tailpipe emissions. Heat Mass Transf. 2019, 55, 1513–1534. [Google Scholar]
- Popovich, C.A.; Pistonesi, M.; Hegel, P.; Constenla, D.; Bielsa, G.B.; Martin, L.A.; Damiani, M.C.; Leonardi, P.I. Unconventional alternative biofuels: Quality assessment of biodiesel and its blends from marine diatom Navicula cincta. Algal Res. 2019, 39, 101438. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Wang, X.; Zhang, B.; Tian, W.; Zhang, J. Hydrothermal liquefaction of microalgae over transition metal supported TiO2 catalyst. Bioresour. Technol. 2018, 250, 474–480. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Aka, R.J.N. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Process. Technol. 2022, 227, 107120. [Google Scholar] [CrossRef]
- Godbole, V.; Pal, M.K.; Gautam, P. A critical perspective on the scope of interdisciplinary approaches used in fourth-generation biofuel production. Algal Res. 2021, 58, 102436. [Google Scholar] [CrossRef]
- Abdullah, B.; Muhammad, S.A.F.a.S.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.M.A. Fourth generation biofuel: A review on risks and mitigation strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Hu, G.; Dauvillee, D.; Sommerfeld, M.; Ball, S.; Hu, Q. Chlamydomonas starchless mutant defective in ADP-glucose pyrophosphorylase hyper-accumulates triacylglycerol. Metab. Eng. 2010, 12, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Lan, E.I.; Liao, J.C. Metabolic engineering of cyanobacteria for 1-butanol production from carbon dioxide. Metab. Eng. 2011, 13, 353–363. [Google Scholar] [CrossRef] [PubMed]
- USDA. Agricultural Projections to 2025. Available online: https://www.usda.gov/oce/commodity/projections/USDA_Agricultural_Projections_to_2025.pdf (accessed on 2 June 2018).
- OECD-FAO. Agricultural Outlook. OECD Agriculture Statistics (Database). Available online: http://www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Oilcrops/Documents/ECD_Reports/OECD_biofuels2015_2024.pdf (accessed on 21 June 2022).
- EIA. Monthly Energy Review. In U.S. Energy Information Administration (EIA), Monthly Energy Review. Available online: https://www.eia.gov/totalenergy/data/monthly/pdf/mer.pdf (accessed on 21 June 2022).
- Jaganmohan, M. US Biomass Energy Production Forecast from 2021 to 2050. In Energy Information Administration (EIA). Annual Energy Outlook 2022; Available online: https://www.statista.com/statistics/264029/us-biomass-energy-production/ (accessed on 3 March 2022).
- Kaur, M.; Ali, A. Lithium ion impregnated calcium oxide as nano catalyst for the biodiesel production from karanja and jatropha oils. Renew. Energy 2011, 36, 2866–2871. [Google Scholar] [CrossRef]
- Mahdavi, M.; Abedini, E.; hosein Darabi, A. Biodiesel synthesis from oleic acid by nano-catalyst (ZrO2/Al2O3) under high voltage conditions. RSC Adv. 2015, 5, 55027–55032. [Google Scholar] [CrossRef]
- Demirbas, A. Production of biodiesel fuels from linseed oil using methanol and ethanol in non-catalytic SCF conditions. Biomass Bioenergy 2009, 33, 113–118. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Umana, U.S.; Ebong, M.S.; Godwin, E.O. Biomass production from oil palm and its value chain. J. Hum. Earth Future 2020, 1, 30–38. [Google Scholar] [CrossRef]
- Kim, D.S.; Hanifzadeh, M.; Kumar, A. Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ. Prog. Sustain. Energy 2018, 37, 7–19. [Google Scholar] [CrossRef]
- Gülşen, E.; Olivetti, E.; Freire, F.; Dias, L.; Kirchain, R. Impact of feedstock diversification on the cost-effectiveness of biodiesel. Appl. Energy 2014, 126, 281–296. [Google Scholar] [CrossRef] [Green Version]
- Thurmond, W. Global Biodiesel Market Trends, Outlook and Opportunities. Emerg. Mark. Online Glob. Energy Biofuels Intell. 2008, 2. [Google Scholar]
- Schumacher, L.G.; Borgelt, S.C.; Fosseen, D.; Goetz, W.; Hires, W. Heavy-duty engine exhaust emission tests using methyl ester soybean oil/diesel fuel blends. Bioresour. Technol. 1996, 57, 31–36. [Google Scholar] [CrossRef]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.; Haywood, J.; Lean, J.; Lowe, D.; Myhre, G. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tingor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 129–234. [Google Scholar]
- Carter, M.S.; Hauggaard-Nielsen, H.; Heiske, S.; Jensen, M.; Thomsen, S.T.; Schmidt, J.E.; Johansen, A.; Ambus, P. Consequences of field N2O emissions for the environmental sustainability of plant-based biofuels produced within an organic farming system. GCB Bioenergy 2012, 4, 435–452. [Google Scholar] [CrossRef] [Green Version]
- Smeets, E.M.; Bouwman, L.F.; Stehfest, E.; Van Vuuren, D.P.; Posthuma, A. Contribution of N2O to the greenhouse gas balance of first-generation biofuels. Glob. Change Biol. 2009, 15, 1–23. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Gashaw, A.; Lakachew, A. Production of biodiesel from non edible oil and its properties. Int. J. Sci. Environ. Technol. 2014, 3, 1544–1562. [Google Scholar]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef]
- Paul Abishek, M.; Patel, J.; Prem Rajan, A. Algae oil: A sustainable renewable fuel of future. Biotechnol. Res. Int. 2014, 2014, 272814. [Google Scholar] [CrossRef] [Green Version]
- Adewale, P.; Dumont, M.-J.; Ngadi, M. Recent trends of biodiesel production from animal fat wastes and associated production techniques. Renew. Sustain. Energy Rev. 2015, 45, 574–588. [Google Scholar] [CrossRef]
- Sangkharak, K.; Mhaisawat, S.; Rakkan, T.; Paichid, N.; Yunu, T. Utilization of mixed chicken waste for biodiesel production using single and combination of immobilized lipase as a catalyst. Biomass Convers. Biorefinery 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Zhao, C.; Brück, T.; Lercher, J.A. Catalytic deoxygenation of microalgae oil to green hydrocarbons. Green Chem. 2013, 15, 1720–1739. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, J. Influence of feed/inoculum ratios and waste cooking oil content on the mesophilic anaerobic digestion of food waste. Waste Manag. 2018, 73, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, Z.; Wang, Q.; Liu, Y. Biodiesels from microbial oils: Opportunity and challenges. Bioresour. Technol. 2018, 263, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Arruda, L.F.d.; Borghesi, R.; Oetterer, M. Use of fish waste as silage: A review. Braz. Arch. Biol. Technol. 2007, 50, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Samuchia, D.; Sharma, P.; Mandeewal, R.L.; Nitharwal, P.K.; Meena, M. Effect of Nitrogen and Sulphur in the Production of the Mustard Crop [Brassica juncea (L.)]. Int. J. Environ. Clim. Change 2022, 12, 132–137. [Google Scholar] [CrossRef]
- Koh, M.Y.; Mohd; Ghazi, T.I. A review of biodiesel production from Jatropha curcas L. oil. Renew. Sustain. Energy Rev. 2011, 15, 2240–2251. [Google Scholar] [CrossRef]
- Ho, D.P.; Ngo, H.H.; Guo, W. A mini review on renewable sources for biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neupane, D.; Bhattarai, D.; Ahmed, Z.; Das, B.; Pandey, S.; Solomon, J.K.; Qin, R.; Adhikari, P. Growing Jatropha (Jatropha curcas L.) as a potential second-generation biodiesel feedstock. Inventions 2021, 6, 60. [Google Scholar] [CrossRef]
- Li, Y.; Khanal, S.K. Bioenergy: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Cecrle, E.; Depcik, C.; Duncan, A.; Guo, J.; Mangus, M.; Peltier, E.; Stagg-Williams, S.; Zhong, Y. Investigation of the effects of biodiesel feedstock on the performance and emissions of a single-cylinder diesel engine. Energy Fuels 2012, 26, 2331–2341. [Google Scholar] [CrossRef]
- Behcet, R.; Aydın, H.; Ilkılıc, C.; İşcan, B.; Aydın, S. Diesel engine applications for evaluation of performance and emission behavior of biodiesel from different oil stocks. Environ. Prog. Sustain. Energy 2015, 34, 890–896. [Google Scholar] [CrossRef]
- Akbar, E.; Yaakob, Z.; Kamarudin, S.K.; Ismail, M.; Salimon, J. Characteristic and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock feedstock. Eur. J. Sci. Res. 2009, 29, 396–403. [Google Scholar]
- Leung, D.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Canoira, L.; Rodriguez-Gamero, M.; Querol, E.; Alcántara, R.n.; Lapuerta, M.n.; Oliva, F.n. Biodiesel from low-grade animal fat: Production process assessment and biodiesel properties characterization. Ind. Eng. Chem. Res. 2008, 47, 7997–8004. [Google Scholar] [CrossRef]
- Islam, M.A.; Magnusson, M.; Brown, R.J.; Ayoko, G.A.; Nabi, M.N.; Heimann, K. Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 2013, 6, 5676–5702. [Google Scholar] [CrossRef] [Green Version]
- Wan Ghazali, W.N.M.; Mamat, R.; Masjuki, H.H.; Najafi, G. Effects of biodiesel from different feedstocks on engine performance and emissions: A review. Renew. Sustain. Energy Rev. 2015, 51, 585–602. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Sharma, M.P. Review of process parameters for biodiesel production from different feedstocks. Renew. Sustain. Energy Rev. 2016, 62, 1063–1071. [Google Scholar] [CrossRef]
- Sakthivel, R.; Ramesh, K.; Purnachandran, R.; Mohamed Shameer, P. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; Rodríguez-Fernández, J. Effect of biodiesel fuels on diesel engine emissions. Prog. Energy Combust. Sci. 2008, 34, 198–223. [Google Scholar] [CrossRef]
- Mostafa, S.S.M.; El-Gendy, N.S. Evaluation of fuel properties for microalgae Spirulina platensis bio-diesel and its blends with Egyptian petro-diesel. Arab. J. Chem. 2017, 10, S2040–S2050. [Google Scholar] [CrossRef] [Green Version]
- Dixit, S.; kanakraj, S.; Rehman, A. Linseed oil as a potential resource for bio-diesel: A review. Renew. Sustain. Energy Rev. 2012, 16, 4415–4421. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, P.; Garg, S. Alkali transesterification of linseed oil for biodiesel production. Fuel 2013, 104, 553–560. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Masjuki, H.H.; Kalam, M.A.; Wakil, M.A.; Rashedul, H.K.; Abedin, M.J. Performance and emission characteristics of a CI engine fueled with Cocos nucifera and Jatropha curcas B20 blends accompanying antioxidants. Ind. Crops Prod. 2014, 57, 132–140. [Google Scholar] [CrossRef]
- Kumar, K.; Sharma, M. Performance and emission characteristics of a diesel engine fuelled with biodiesel blends. Int. J. Renew. Energy Res. 2016, 6, 658–662. [Google Scholar]
- Neupane, D.; Lohaus, R.H.; Solomon, J.K.; Cushman, J.C. Realizing the Potential of Camelina sativa as a Bioenergy Crop for a Changing Global Climate. Plants 2022, 11, 772. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Bamishaiye, E.; Bamishaiye, O.; Usman, L.; Salawu, M.; Nafiu, M.; Oloyede, O. Physicochemical properties and fatty acid composition of cyperus esculentus (Tiger Nut) Tuber Oil. Biores Bull 2011, 5, 51–54. [Google Scholar]
- Standard Organization of Nigeria. Standards for Edible Refined Palm Oil and Its Processed Form; Scientific Research Publishing Inc.: Irvine, CA, USA, 2000; pp. 2–5. [Google Scholar]
- NIS. Nigerian Industrial Standards. Standard for Edible Vegetable Oil; Scientific Research Publishing Inc.: Irvine, CA, USA, 1992; pp. 5–12. [Google Scholar]
- Nduka, J.K.C.; Omozuwa, P.O.; Imanah, O.E. Effect of heating time on the physicochemical properties of selected vegetable oils. Arab. J. Chem. 2021, 14, 103063. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Kyriakidis, N.B.; Katsiloulis, T. Calculation of iodine value from measurements of fatty acid methyl esters of some oils: Comparison with the relevant American oil chemists society method. J. Am. Oil Chem. Soc. 2000, 77, 1235–1238. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Rasouli, H.; Esmaeili, H. Characterization of MgO nanocatalyst to produce biodiesel from goat fat using transesterification process. 3 Biotech. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process. Technol. 2019, 191, 232–247. [Google Scholar] [CrossRef]
- Attaphong, C.; Do, L.; Sabatini, D.A. Vegetable oil-based microemulsions using carboxylate-based extended surfactants and their potential as an alternative renewable biofuel. Fuel 2012, 94, 606–613. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Lee, W.-J.; Lin, S.-L.; Wang, L.-C. Green energy: Water-containing acetone–butanol–ethanol diesel blends fueled in diesel engines. Appl. Energy 2013, 109, 182–191. [Google Scholar] [CrossRef]
- Attaphong, C.; Sabatini, D.A. Phase behaviors of vegetable oil-based microemulsion fuels: The effects of temperatures, surfactants, oils, and water in ethanol. Energy Fuels 2013, 27, 6773–6780. [Google Scholar] [CrossRef]
- Lif, A.; Holmberg, K. Water-in-diesel emulsions and related systems. Adv. Colloid Interface Sci. 2006, 123, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Grebemariam, S.; Marchetti, J.M. Biodiesel Production Technologies: Review. AIMS Energy 2017, 5, 425–457. [Google Scholar]
- Baskar, G.; Aiswarya, R. Trends in catalytic production of biodiesel from various feedstocks. Renew. Sustain. Energy Rev. 2016, 57, 496–504. [Google Scholar] [CrossRef]
- Agarwal, A.K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 2007, 33, 233–271. [Google Scholar] [CrossRef]
- Akram, F.; ul Haq, I.; Raja, S.I.; Mir, A.S.; Qureshi, S.S.; Aqeel, A.; Shah, F.I. Current trends in biodiesel production technologies and future progressions: A possible displacement of the petro-diesel. J. Clean. Prod. 2022, 133479. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Karmakar, B.; Halder, G. Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies. Energy Convers. Manag. 2019, 182, 307–339. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Avhad, M.; Marchetti, J. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Sani, Y.; Daud, W.; Aziz, A.A. Biodiesel feedstock and production technologies: Successes, challenges and prospects. Biodiesel-Feedstocks Prod. Appl. 2012, 10, 52790. [Google Scholar]
- Vyas, A.P.; Verma, J.L.; Subrahmanyam, N. A review on FAME production processes. Fuel 2010, 89, 1–9. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels securing the planet’s future energy needs. Energy Convers. Manag. 2009, 50, 2239–2249. [Google Scholar] [CrossRef]
- Hashmi, S.; Gohar, S.; Mahmood, T.; Nawaz, U.; Farooqi, H. Biodiesel production by using CaO-Al2O3 Nano catalyst. Int. J. Eng. Res. Sci. 2016, 2, 43–49. [Google Scholar]
- Rodrigues, R.C.; Volpato, G.; Wada, K.; Ayub, M.A.Z. Enzymatic synthesis of biodiesel from transesterification reactions of vegetable oils and short chain alcohols. J. Am. Oil Chem. Soc. 2008, 85, 925–930. [Google Scholar] [CrossRef]
- Iso, M.; Chen, B.; Eguchi, M.; Kudo, T.; Shrestha, S. Production of biodiesel fuel from triglycerides and alcohol using immobilized lipase. J. Mol. Catal. B Enzym. 2001, 16, 53–58. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Gude, V.G.; Patil, P.; Martinez-Guerra, E.; Deng, S.; Nirmalakhandan, N. Microwave energy potential for biodiesel production. Sustain. Chem. Process. 2013, 1. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Bonini, M.; Fratini, E.; Tondi, G.; Gartner, K.; Bridgwater, A.; Grimm, H.; Soldaini, I.; Webster, A.; Baglioni, P. Development of emulsions from biomass pyrolysis liquid and diesel and their use in engines—Part 1: Emulsion production. Biomass Bioenergy 2003, 25, 85–99. [Google Scholar] [CrossRef]
- Shaah, M.A.H.; Hossain, M.S.; Allafi, F.A.S.; Alsaedi, A.; Ismail, N.; Ab Kadir, M.O.; Ahmad, M.I. A review on non-edible oil as a potential feedstock for biodiesel: Physicochemical properties and production technologies. RSC Adv. 2021, 11, 25018–25037. [Google Scholar] [CrossRef]
- Mahanta, P.; Shrivastava, A. Technology development of bio-diesel as an energy alternative. Dep. Mech. Eng. Indian Inst. Technol. 2004, 1, 1–19. [Google Scholar]
- Yan, B.; Zhang, S.; Chen, W.; Cai, Q. Pyrolysis of tobacco wastes for bio-oil with aroma compounds. J. Anal. Appl. Pyrolysis 2018, 136, 248–254. [Google Scholar] [CrossRef]
- Shadangi, K.P.; Mohanty, K. Production and characterization of pyrolytic oil by catalytic pyrolysis of Niger seed. Fuel 2014, 126, 109–115. [Google Scholar] [CrossRef]
- Naik, M.; Meher, L.; Naik, S.; Das, L. Production of biodiesel from high free fatty acid Karanja (Pongamia pinnata) oil. Biomass Bioenergy 2008, 32, 354–357. [Google Scholar] [CrossRef]
- Gaurav, A.; Ng, F.T.; Rempel, G.L. A new green process for biodiesel production from waste oils via catalytic distillation using a solid acid catalyst–Modeling, economic and environmental analysis. Green Energy Environ. 2016, 1, 62–74. [Google Scholar] [CrossRef] [Green Version]
- Bilgin, A.; Durgun, O.; Sahin, Z. The effects of diesel-ethanol blends on diesel engine performance. Energy Sources 2002, 24, 431–440. [Google Scholar] [CrossRef]
- Kiss, A.A.; Bildea, C.S. A review of biodiesel production by integrated reactive separation technologies. J. Chem. Technol. Biotechnol. 2012, 87, 861–879. [Google Scholar] [CrossRef]
- Adenuga, A.A.; Oyekunle, J.A.O.; Idowu, O.O. Pathway to reduce free fatty acid formation in Calophyllum inophyllum kernel oil: A renewable feedstock for biodiesel production. J. Clean. Prod. 2021, 316, 128222. [Google Scholar] [CrossRef]
- Elgharbawy, A.S.; Sadik, W.A.; Sadek, O.M.; Kasaby, M.A. Maximizing biodiesel production from high free fatty acids feedstocks through glycerolysis treatment. Biomass Bioenergy 2021, 146, 105997. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Chang, M.Y.; Chan, E.-S.; Song, C.P. Biodiesel production catalysed by low-cost liquid enzyme Eversa® Transform 2.0: Effect of free fatty acid content on lipase methanol tolerance and kinetic model. Fuel 2021, 283, 119266. [Google Scholar] [CrossRef]
- Felizardo, P.; Baptista, P.; Menezes, J.C.; Correia, M.J.N. Multivariate near infrared spectroscopy models for predicting methanol and water content in biodiesel. Anal. Chim. Acta 2007, 595, 107–113. [Google Scholar] [CrossRef]
- Fregolente, P.B.L.; Fregolente, L.V.; Wolf Maciel, M.R. Water Content in Biodiesel, Diesel, and Biodiesel–Diesel Blends. J. Chem. Eng. Data 2012, 57, 1817–1821. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Possible methods for biodiesel production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Parthiban, K.S.; Pandian, S.; Subramanian, D. Conventional and in-situ transesterification of Annona squamosa seed oil for biodiesel production: Performance and emission analysis. Environ. Technol. Innov. 2021, 23, 101593. [Google Scholar] [CrossRef]
- Musa, I.A. The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt. J. Pet. 2016, 25, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Norjannah, B.; Ong, H.C.; Masjuki, H.; Juan, J.; Chong, W. Enzymatic transesterification for biodiesel production: A comprehensive review. RSC Adv. 2016, 6, 60034–60055. [Google Scholar] [CrossRef]
- IŞIk, M.Z. Comparative experimental investigation on the effects of heavy alcohols- safflower biodiesel blends on combustion, performance and emissions in a power generator diesel engine. Appl. Therm. Eng. 2021, 184, 116142. [Google Scholar] [CrossRef]
- Razak, N.H.; Hashim, H.; Yunus, N.A.; Klemeš, J.J. Reducing diesel exhaust emissions by optimisation of alcohol oxygenates blend with diesel/biodiesel. J. Clean. Prod. 2021, 316, 128090. [Google Scholar] [CrossRef]
- Binhweel, F.; Bahadi, M.; Pyar, H.; Alsaedi, A.; Hossain, S.; Ahmad, M.I. A comparative review of some physicochemical properties of biodiesels synthesized from different generations of vegetative oils. J. Phys. Conf. Ser. 2021, 1900, 012009. [Google Scholar] [CrossRef]
- Hossain, A.; Boyce, A.; Salleh, A.; Chandran, S. Impacts of alcohol type, ratio and stirring time on the biodiesel production from waste canola oil. Afr. J. Agric. Res. 2010, 5, 1851–1859. [Google Scholar]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification kinetics of soybean oil 1. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Mahlia, T.M.I.; Ong, H.C.; Riayatsyah, T.M.I.; Kusumo, F.; Ibrahim, H.; Dharma, S.; Gumilang, D. A comparative study of biodiesel production methods for Reutealis trisperma biodiesel. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 2006–2014. [Google Scholar] [CrossRef]
- Refaat, A.; Attia, N.; Sibak, H.A.; El Sheltawy, S.; ElDiwani, G. Production optimization and quality assessment of Biodiesel from waste vegetable oil. Int. J. Environ. Sci. Technol. 2008, 5, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Kusdiana, D.; Saka, S. Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 2001, 80, 693–698. [Google Scholar] [CrossRef]
- Daramola, M.; Mtshali, K.; Senokoane, L.; Fayemiwo, O. Influence of operating variables on the transesterification of waste cooking oil to biodiesel over sodium silicate catalyst: A statistical approach. J. Taibah Univ. Sci. 2016, 10, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Mathiyazhagan, M.; Ganapathi, A. Factors affecting biodiesel production. Res. Plant Biol. 2011, 1. [Google Scholar]
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gupta, M.N. Lipase catalyzed preparation of biodiesel from Jatropha oil in a solvent free system. Process Biochem. 2007, 42, 409–414. [Google Scholar] [CrossRef]
- Ganesan, D.; Rajendran, A.; Thangavelu, V. An overview on the recent advances in the transesterification of vegetable oils for biodiesel production using chemical and biocatalysts. Rev. Environ. Sci. Bio/Technol. 2009, 8, 367. [Google Scholar] [CrossRef]
- Vicente, G.; Martínez, M.; Aracil, J. Optimisation of integrated biodiesel production. Part I. A study of the biodiesel purity and yield. Bioresour. Technol. 2007, 98, 1724–1733. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Jia, L. Variables Affecting Biodiesel Production from Zanthoxylum bungeanum Seed Oil with High Free Fatty Acids. Ind. Eng. Chem. Res. 2012, 51, 3525–3530. [Google Scholar] [CrossRef]
- Chen, L.; Yin, P.; Liu, X.; Yang, L.; Yu, Z.; Guo, X.; Xin, X. Biodiesel production over copper vanadium phosphate. Energy 2011, 36, 175–180. [Google Scholar] [CrossRef]
- Kumari, A.; Mahapatra, P.; Garlapati, V.K.; Banerjee, R. Enzymatic transesterification of Jatropha oil. Biotechnol. Biofuels 2009, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Diasakou, M.; Louloudi, A.; Papayannakos, N. Kinetics of the non-catalytic transesterification of soybean oil. Fuel 1998, 77, 1297–1302. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2018, 3, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, M.G.; Gopinath, R.; Meher, L.C.; Dalai, A.K. Solid acid catalyzed biodiesel production by simultaneous esterification and transesterification. Green Chem. 2006, 8, 1056–1062. [Google Scholar] [CrossRef]
- Ranganathan, S.V.; Narasimhan, S.L.; Muthukumar, K. An overview of enzymatic production of biodiesel. Bioresour. Technol. 2008, 99, 3975–3981. [Google Scholar] [CrossRef]
- Aarthy, M.; Saravanan, P.; Gowthaman, M.K.; Rose, C.; Kamini, N.R. Enzymatic transesterification for production of biodiesel using yeast lipases: An overview. Chem. Eng. Res. Des. 2014, 92, 1591–1601. [Google Scholar] [CrossRef]
- Hama, S.; Noda, H.; Kondo, A. How lipase technology contributes to evolution of biodiesel production using multiple feedstocks. Curr. Opin. Biotechnol. 2018, 50, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Refaat, A.A. Different techniques for the production of biodiesel from waste vegetable oil. Int. J. Environ. Sci. Technol. 2010, 7, 183–213. [Google Scholar] [CrossRef] [Green Version]
- Schuchardt, U.; Sercheli, R.; Vargas, R.M. Transesterification of vegetable oils: A review. J. Braz. Chem. Soc. 1998, 9, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Inorganic heterogeneous catalysts for biodiesel production from vegetable oils. Biomass Bioenergy 2011, 35, 3787–3809. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, X.; Chen, G. 99% yield biodiesel production from rapeseed oil using benzyl bromide–CaO catalyst. Environ. Chem. Lett. 2013, 11, 203–208. [Google Scholar] [CrossRef]
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Heterogeneous solid base nanocatalyst: Preparation, characterization and application in biodiesel production. Bioresour. Technol. 2011, 102, 4150–4156. [Google Scholar] [CrossRef]
- Ruhul, A.; Kalam, M.; Masjuki, H.; Fattah, I.R.; Reham, S.; Rashed, M. State of the art of biodiesel production processes: A review of the heterogeneous catalyst. RSC Adv. 2015, 5, 101023–101044. [Google Scholar] [CrossRef]
- Liu, X.; Piao, X.; Wang, Y.; Zhu, S.; He, H. Calcium methoxide as a solid base catalyst for the transesterification of soybean oil to biodiesel with methanol. Fuel 2008, 87, 1076–1082. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Piraman, S. Biodiesel synthesis by TiO2–ZnO mixed oxide nanocatalyst catalyzed palm oil transesterification process. Bioresour. Technol. 2013, 150, 55–59. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Qi, D.; Geng, L.; Chen, H.; Bian, Y.Z.; Liu, J.; Ren, X.C. Combustion and performance evaluation of a diesel engine fueled with biodiesel produced from soybean crude oil. Renew. Energy 2009, 34, 2706–2713. [Google Scholar] [CrossRef]
- Ganapathy, T.; Gakkhar, R.; Murugesan, K. Influence of injection timing on performance, combustion and emission characteristics of Jatropha biodiesel engine. Appl. Energy 2011, 88, 4376–4386. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Kumar, N.; Cho, H.M. A study on the performance and emission of a diesel engine fueled with Jatropha biodiesel oil and its blends. Energy 2012, 37, 616–622. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Jayaprabakar, J.; Williams, R.D. Experimental Investigations on Diesel engine using Methyl esters of Jatropha oil and fish oil. IOP Conf. Ser. Mater. Sci. Eng. 2017, 197, 012020. [Google Scholar] [CrossRef] [Green Version]
- Canakci, M.; Van Gerpen, J.H. Comparison of engine performance and emissions for petroleum diesel fuel, yellow grease biodiesel, and soybean oil biodiesel. Trans. ASAE 2003, 46, 937. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Morgan, M.R.; McCurdy, A.T.; Willis, R.M.; Morgan, M.D.; Dye, D.J.; Bugbee, B.; Wood, B.D.; Seefeldt, L.C. Biodiesel from microalgae, yeast, and bacteria: Engine performance and exhaust emissions. Energy Fuels 2013, 27, 220–228. [Google Scholar] [CrossRef]
- Tüccar, G.; Aydın, K. Evaluation of methyl ester of microalgae oil as fuel in a diesel engine. Fuel 2013, 112, 203–207. [Google Scholar] [CrossRef]
- Fisher, B.C.; Marchese, A.J.; Volckens, J.; Lee, T.; Collett, J.L. Measurement of gaseous and particulate emissions from algae-based fatty acid methyl esters. SAE Int. J. Fuels Lubr. 2010, 3, 292–321. [Google Scholar] [CrossRef] [Green Version]
- Alptekin, E.; Canakci, M.; Ozsezen, A.N.; Turkcan, A.; Sanli, H. Using waste animal fat based biodiesels–bioethanol–diesel fuel blends in a DI diesel engine. Fuel 2015, 157, 245–254. [Google Scholar] [CrossRef]
- McCormick, R.L.; Graboski, M.S.; Alleman, T.L.; Herring, A.M.; Tyson, K.S. Impact of biodiesel source material and chemical structure on emissions of criteria pollutants from a heavy-duty engine. Environ. Sci. Technol. 2001, 35, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Lira, T.A.M.; Santos, A.P.; Moreti, T.C.F.; Lopes, A.; de Oliveira, M.C.J.; Neves, M.C.T.; Iamaguti, P.S.; de Lima, L.P.; Koike, G.H.A.; de Abreu Silva, R. Performance of agricultural tractor consuming diesel and biodiesel derived from babassu (‘Orbinya martiana’). Aust. J. Crop Sci. 2019, 13, 1037–1044. [Google Scholar] [CrossRef]
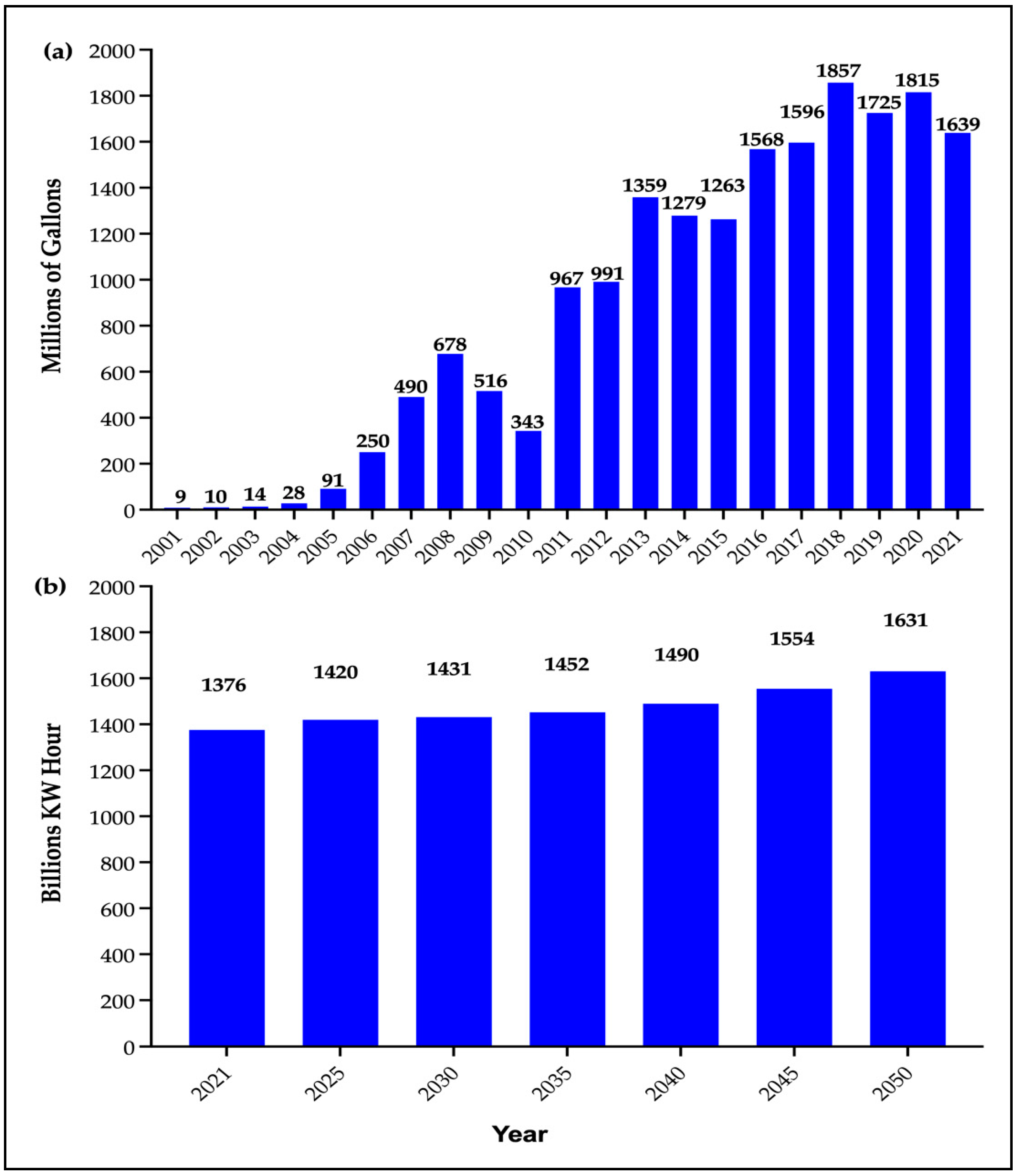
| Period | Vegetable Oil (Million kg) | Animal Fats (Million kg) | |||||
|---|---|---|---|---|---|---|---|
| Canola Oil | Corn Oil | Cottonseed Oil | Soybean Oil | Other | Poultry | Tallow | |
| January | 49.4 | 80.3 | - | 236.3 | W | 5.0 | W |
| February | 42.4 | 60.7 | - | 260.7 | W | 5.4 | 9.4 |
| March | 59.6 | 65.7 | - | 297.6 | W | 10.7 | W |
| April | 62.8 | 38.0 | - | 304.7 | S | W | 10.9 |
| May | 58.9 | 38.3 | - | 365.3 | W | 3.9 | 5.3 |
| June | 50.0 | 42.7 | W | 338.9 | 5.9 | W | 9.7 |
| July | W | 60.5 | W | 351.5 | W | W | 24.6 |
| August | W | 67.3 | W | 338.0 | W | W | 20.0 |
| September | W | 61.7 | - | 334.0 | W | 10.4 | 12.4 |
| October | W | 45.8 | - | 328.0 | W | 9.5 | 23.6 |
| November | W | 60.3 | - | 309.8 | - | 6.4 | 15.0 |
| December | W | 66.7 | - | 337.5 | - | 3.2 | 17.2 |
| Total | 565.2 | 687.6 | 0.3 | 3802.5 | W | 78.5 | 166.9 |
| % of total | 10.7 | 13.0 | 0.0 | 71.7 | 0.1 | 1.5 | 3.1 |
| Feedstocks | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|---|---|---|---|
| Rapeseed oil | 6500 | 5710 | 6200 | 6400 | 6060 | 6300 | 5200 | 5000 |
| Used cooking oil (UCO) | 800 | 1150 | 1890 | 2400 | 2620 | 2770 | 2860 | 2750 |
| Palm oil | 1535 | 2340 | 2240 | 2340 | 2315 | 2650 | 2570 | 2640 |
| Soybean oil | 720 | 870 | 840 | 540 | 610 | 930 | 1000 | 1100 |
| Animal fats | 360 | 420 | 920 | 1030 | 795 | 795 | 800 | 800 |
| Sunflower oil | 300 | 290 | 310 | 210 | 250 | 180 | 185 | 190 |
| other, pine/tall oils, fatty acid | 220 | 335 | 370 | 560 | 615 | 635 | 680 | 700 |
| Share of rapeseed oil (%) | 62.3 | 51.4 | 48.6 | 47.5 | 45.7 | 44.2 | 39.1 | 37.9 |
| Edible Oils | Oil Content (%) | Non-Edible Oils | Oil Content (%) | Animal Fats and Other Sources | Oil Contents (%) |
|---|---|---|---|---|---|
| Sunflower oil | 25–35 | 1 Jatropha oil | 30–60 | Mutton fat | - |
| Soybean oil | 15–20 | Stillingia oil | 44.15 | Broiler chicken waste | 41 [80] |
| Rapeseed oil | 38–46 | 1 Karanja oil | 27–40 | Algae oil | 20–60 [81] |
| Peanut oil | 45–55 | Neem oil | 20–30 | Waste cooking oil | 33–53 [82] |
| Palm oil | 30–60 | 1 Castor oil | 45–60 | Microbial oil | 23–70 [83] |
| Olive oil | 45–70 | Rubber seed oil | 53.7–68.4 | Waste fish oil | 40–65 [84] |
| Mustard oil | 40–42 [85] | 1 Mahua | 35–40 | Microalgae | 30–70, 15–77 |
| 1 Linseed oil | 35–45 | - | - | Pine and Kapok oil | - |
| Coconut oil | 63–65 | - | - | - | - |
| Canola oil | 40–45 | - | - | - | - |
| Fatty Acid | Octanoic C8:0 | Decanoic C10:0 | Lauric C12:0 | Myristic C14:0 | Palmitic C16:0 | Palmitoleic C16:1 | Stearic C18:0 | Oleic C18:1 | Linoleic C18:2 | Linolenic C18:3 | Arachidic C20:0 | Eicosenoic C20:1 | Eicosapentaenoic C20:5 | Behenaic C22:0 | Erucic C22:1 | others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Edible | ||||||||||||||||
| Soybean | 0.1 a | 6–11 abc | 11 a | 2–5 abc | 20–30 abc | 50–60 abc | 5–11 abc | |||||||||
| Rapeseed | 1–3.5 bc | 9.1 c | 0–1 bc | 10–15 b, 64.1 c | 12–15 b, 22.3 c | 8–12 b, 0.1 c | 7–10 b | 45–60 b | ||||||||
| Sunflower | 5–8 b | 2–6 ab | 15–40 ab | 30–70 ab | 3–5 b | 0.3 a | ||||||||||
| Peanut | 8–9 b | 2–3 b | 50–65 b | 20–30 b | ||||||||||||
| Olive | 9–10 b | 2–3 b | 72–85 b | 10–12 b | 0–1 b | |||||||||||
| Palm | 16.3 a, 0.5–2 b | 8.4 a, 39–48 bc | 2.4–6 abc | 15.4 a, 36–44 bc | 2.4 a, 9–12 bc | 0.1 a | ||||||||||
| Mustard | 1–2 b | 8–23 b | 10–24 b | 8–18 b | 5–13 b | 20–50 b | ||||||||||
| Coconut | 45–53 b | 16–21 b | 7–10 b | 2–4 b | 5–10 b | 1–2.5 b | ||||||||||
| Almond kernel | 6.5 e | 1.4 e | 70.7 e | 20 e | 0.9 e | |||||||||||
| Walnut kernel | 7.2 e | 1.9 e | 18.5 e | 56 e | 16.2 e | |||||||||||
| Sesame | 13 e | 4 e | 53 e | 30 e | ||||||||||||
| Non-edible | ||||||||||||||||
| Linseed | 4–7 b | 2–4 b | 25–40 b | 35–40 b | 25–60 b | |||||||||||
| Neem | 13.6–16.2 b | 49.1–61.9 b | ||||||||||||||
| Jatropha | 0–0.1 a, 14.1–15.3 b | 14.1–15.3 ac, 0–13 b | 0–1.3 a | 3.7–9.8 ac | 34.3–45.8 abc | 14.1–15.3 b, 29–44.2 ac | 0–0.3 ab | 0–0.3 a | 0–0.2 a | 1.4 | ||||||
| Cotton seed | 23–28.3 b | 0.8–0.9 b | 13.3–18.3 b | 0.2 b | ||||||||||||
| Rubber | 2.2 f | 10.2 f | 8.7 f | 24.6 f | 39.6 f | 16.3 f | ||||||||||
| Karanja | 9.8 a, 3.7–7.9 f | 2.4–8.6 af | 44.5–72.2 af | 10.8–18.3 af | ||||||||||||
| Pongamia | 11.65 f | 51.5 f | 11.65 f | |||||||||||||
| Stillingia | 0.4 f | 0.1 f | 7.5 f | 2.3 f | 16.7 f | 31.5 f | 41.5 f | |||||||||
| Animal fat and other sources | ||||||||||||||||
| Animal fats | 2.52 c | 28.4 c | 15.7 c | 42.2 c | 9.4 c | 0.6 c | 0.16 c | 0.86 c | 0.01 c | 0.01 c | ||||||
| Chicken fats | 3.1 g | 19.82 g | 3.06 g | 37.62 g | ||||||||||||
| Used/waste cooking oil | 0.9 c | 20.4 c, 8.5 g | 4.6 c | 4.8 c, 3.1 g | 52.9 c, 21.2 g | 13.5 c, 55.2 g | 0.8 c, 5.9 g | 0.12 c | 0.84 c | 0.03 c | 0.07 c | 0.04 c | ||||
| Tallow | 23.3 f | 19.3 f | 42.4 f | 2.9 f | 0.9 f | 2.9 f | ||||||||||
| Brown grease | 1.66 f | 22.83 f | 12.54 f | 42.36 f | 12.09 f | 0.82 f | ||||||||||
| Microalgal | 0.2 d | 12–15 g | 34.8 d, 10–20 g | 32 d | 1.1 d | 21.7 d | 1.4 d | 8.9 d | ||||||||
| Yellow grease | 2.43 fh | 23.24 fh | 12.96 fh | 44.32 fh | 6.97 fh | 0.67 fh | ||||||||||
| Sources | CP (°C) | CN | OS (mg/100 mL) | SV | IN | AV (mg KOH/g oil) |
|---|---|---|---|---|---|---|
| Soybean oil | 0.9 | 47 | 16.0 | 189–195 | 117–143 | 0.1–0.2 |
| Canola oil | −3.3 | 55 | 44.9 | 188–193 | 109–126 | 0.6–0.8 |
| Olive | - | - | - | 184–196 | 75–94 | 0.94–2.11 |
| Corn | - | - | - | 187–198 | 103–140 | 0.1–5.75 |
| Jatropha curcas | 5.66 | 55.43 | - | 177–189 | 92–112 | 15.6–43 |
| Palm oil | 14.24 | 60.21 | - | 186–209 | 35–61 | 6.9–50.8 |
| Rapeseed | - | 168–187 | 94–129 | 0.2 | ||
| Sunflower | - | 186–194 | 110–143 | 0.2–0.5 | ||
| Camelina | 2.5 | 48.91 | - | 146.5 | 0.2 | |
| Poultry fat | - | - | - | - | 78.8 | 0.55 |
| Choice white grease | 7.0 | 64 | 72.0 | - | - | - |
| Inedible tallow | 16.0 | 62 | 6.2 | - | - | - |
| Yellow grease | 6.0 | 58 | 2.3 | - | - | - |
| Ultra-low sulfur diesel (ULSD) | −45 to −7 | 47 | - | - | - | - |
| Production Technologies | Merits | Demerits |
|---|---|---|
| Micro-emulsion | Micro-emulsion is a simple process, a potential solution for solving the problem of vegetable oil viscosity [136]. It is the dispersion of water, oil, and surfactant. Alcohols such as methanol and ethanol are used to lower viscosity, higher alcohols are used as surfactants, and alkyl nitrates are used as cetane improvers [137]. Micro-emulsion is an alternative method that produces biofuel with suitable properties with low energy consumption [138]. | Some of the disadvantages of micro-emulsion include high viscosity, poor stability, and volatility. Therefore, pre-treatment technology such as cracking, blending, and hydrodeoxygenation is required to minimize the viscosity and FFAs content before producing biodiesel [138]. |
| Pyrolysis | Pyrolysis is a simple and pollution-free process. The product from pyrolysis has a lower viscosity, flash point, and pour point than petroleum diesel; however, it has equivalent calorific values and a lower value of cetane number. Thus, pyrolyzed vegetable oil has an acceptable amount of sulfur, water, sediment, and copper corrosion values [139]. A study suggested that pyrolytic oil, also known as bio-oil, derived from non-edible feedstock such as Jatropha, Castor, Kusum, Mahua, Neem, and Polanga, has drawn interest to be used as an alternative biofuel. The advantages of using pyrolytic bio-oil are that it is easy to handle, store, and transport and has a high cetane number, low viscosity, and low sulfur quantities [138,140]. | The bio-oils derived from edible and non-edible plant seeds are acidic. They are denser than petroleum diesel fuel and thus require a pre-treatment process to remove moisture and neutralize prior to use as an alternative biofuel [138,141]. The disadvantages of pyrolysis include high temperature, expensive apparatus, and low purity due to intolerable amounts of carbon residue and clinker [138,141]. |
| Transesterification | The transesterification process has several advantages over the biodiesel synthesis methods, which include eco-friendly, mild chemical reactions, and are suitable for biodiesel feedstock. It effectively reduces moisture, FFAs, and viscosity during producing biodiesel from non-edible oil [138,142]. | The type of catalyst used will determine the conversion efficiency, reusability, cost, and applicability of feedstocks with water and high fatty acid content. The enzymes used during the process are costly, and the reaction is time-consuming [4]. |
| Catalytic distillation | Catalytic distillation is a green reactor technology that integrates chemical reactions and product separation into a single operation. This method simultaneously carries out the chemical reaction and product separation within a single-stage operation. The continuous removal of the product from the reactive section via distillation action can lead to increased product yield and enhanced productivity. Catalytic distillation has several advantages, such as mitigating catalyst hot spots, better temperature control, and improved energy integration due to the conduction of an exothermic chemical reaction in a boiling medium. Recent studies show that catalytic distillation is a novel approach to biodiesel production, which is more efficient and cost-effective [143]. | The conversion process and solvent usage for post-treatment depend on catalyst recovery. |
| Dilution | Dilution is a simple process that results in a reduction in the viscosity and density of vegetable oils. A study revealed that adding 4% ethanol to diesel fuel increases the brake thermal efficiency, brake torque, and power [144]. Another study reported that blending non-edible oil with diesel fuel increases the storability, potential improvement of physical properties, and engine performance. Additionally, dilution reduces poor atomization and difficulty handling by conventional fuel injection systems of compression ignition engines [55]. | The issues with blending include the formation of carbon in the engine and incomplete combustion. |
| Microwave technology | The electromagnetic waves generated in the microwave through electric energy transfer energy directly at the molecular level, allowing quick reaction activity and better energy transfer [135]. The catalyst (homogeneous or heterogeneous) in microwave radiation lowers microwave power usage while keeping the reaction equilibrium and achieving transesterification at very low input power with a very fast conversion rate [53]. The high input power can directly degrade oils into different byproducts. Thus, controlling the radiation level is vital to achieving a complete transesterification reaction. | Removal of the catalyst after the process is needed, and process conversion depends on catalyst activity and is not appropriate for solid feedstocks. |
| Reactive distillation | Reactive distillation offers new and exciting opportunities for manufacturing fatty acid alkyl esters in the industrial production of biodiesel and specialty chemicals. The processes can be enhanced by heat integration and powered by heterogeneous catalysts to eliminate all conventional catalyst-related operations by efficiently using raw materials and reaction volume. At the same time, reactive distillation offers higher conversion, selectivity, and high energy savings [145]. This method combines the reaction and separation stages in a single unit, thereby reducing the capital cost and increasing heat integration [25]. Overall, this method is applicable with feedstock with high FFAs content, simple process, less use of methanol, and easy to separate product. | However, it requires high energy, and process conversion depends on catalyst efficiency. |
| Supercritical fluid method | In the supercritical fluid method, the reaction is carried out at supercritical conditions. The mixture becomes homogeneous, where both the esterification of free fatty acids and the transesterification of triglycerides occur without needing a catalyst, making the process suitable for all types of raw materials. The combination of two stages has attracted research interest recently, where simultaneous extraction and reaction from solid matrices are carried out using methanol with supercritical CO2 as a co-solvent [25]. This method involves less reaction time, high conversion, and no catalyst required. | This method demands a high cost of apparatus and energy consumption. |
| Transesterification Process | Merits | Demerits |
|---|---|---|
| Acid-based catalyzed reaction | Suitable in the presence of high levels of FFA and water. No need for pretreatment. Fewer environmental problems and less toxic effect. Few main processing units. | Slow reaction. High temperature, pressure, and alcohol/oil ratio. Environmental contamination. Required costly equipment. |
| Alkali-based catalyzed reaction | Low temperature, pressure, and alcohol/oil ratio. High reaction rate. Smaller equipment, good corrosion resistance properties. Low cost of catalyst. | Need of pretreatment. Low ester yields and byproducts without pretreatment. Saponification occurs. |
| Factors | Homogeneous Catalysis | Heterogenous Catalysis |
|---|---|---|
| Reaction rate | Fast and high conversion | Moderate conversion |
| Post-treatment | No recovery of catalyst | Catalysts can be recovered |
| Processing methodology | Mild reaction and less energy consumption | Continuous operation possible |
| Process of water and FFA | Sensitive and not suitable | Not sensitive and suitable |
| Reuse of catalyst | Not possible | Possible |
| Cost | Comparatively cost-effective than the currently available heterogeneous catalyzed transesterification | Potentially cheaper, high conversion efficiency, and technologically available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neupane, D. Biofuels from Renewable Sources, a Potential Option for Biodiesel Production. Bioengineering 2023, 10, 29. https://doi.org/10.3390/bioengineering10010029
Neupane D. Biofuels from Renewable Sources, a Potential Option for Biodiesel Production. Bioengineering. 2023; 10(1):29. https://doi.org/10.3390/bioengineering10010029
Chicago/Turabian StyleNeupane, Dhurba. 2023. "Biofuels from Renewable Sources, a Potential Option for Biodiesel Production" Bioengineering 10, no. 1: 29. https://doi.org/10.3390/bioengineering10010029
APA StyleNeupane, D. (2023). Biofuels from Renewable Sources, a Potential Option for Biodiesel Production. Bioengineering, 10(1), 29. https://doi.org/10.3390/bioengineering10010029