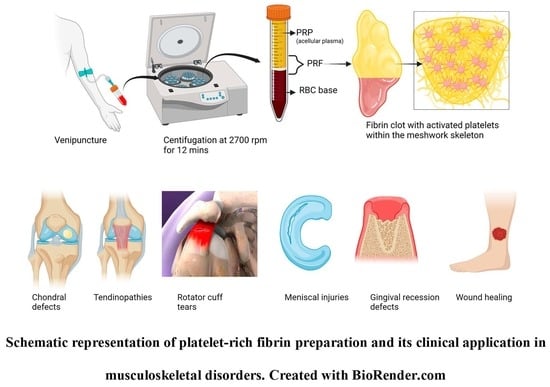
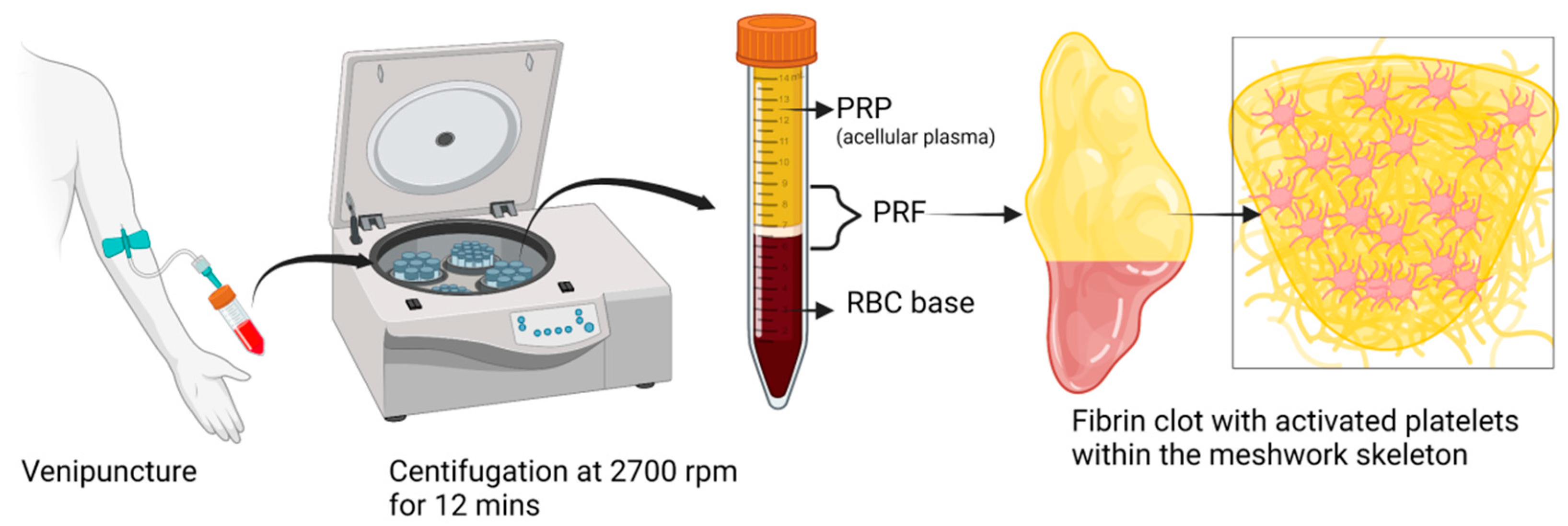
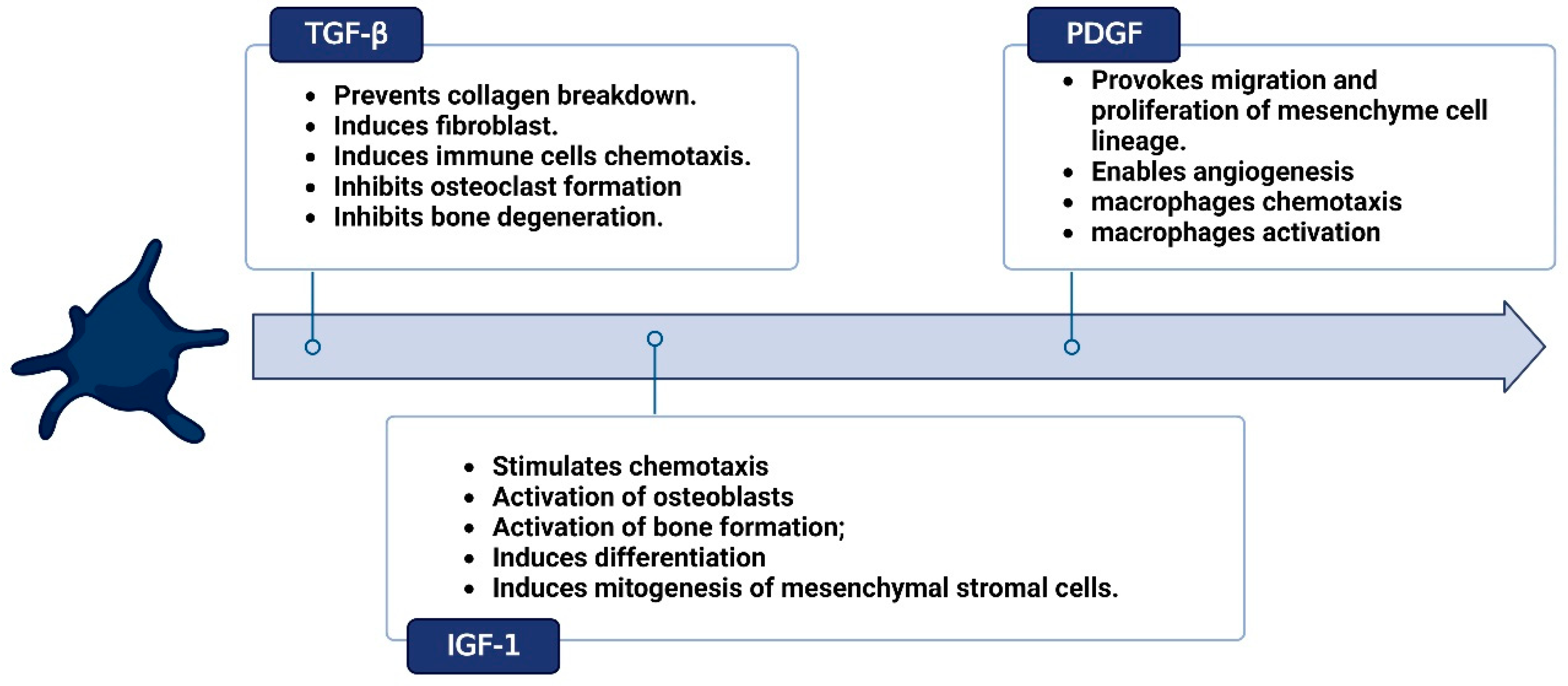
Evolution and Clinical Advances of Platelet-Rich Fibrin in Musculoskeletal Regeneration
Abstract
1. Introduction
2. Evolution of Platelet Concentrates
2.1. Fibrin Glue
2.2. Platelet-Rich Plasma (PRP)
2.3. Platelet-Rich Fibrin (PRF)
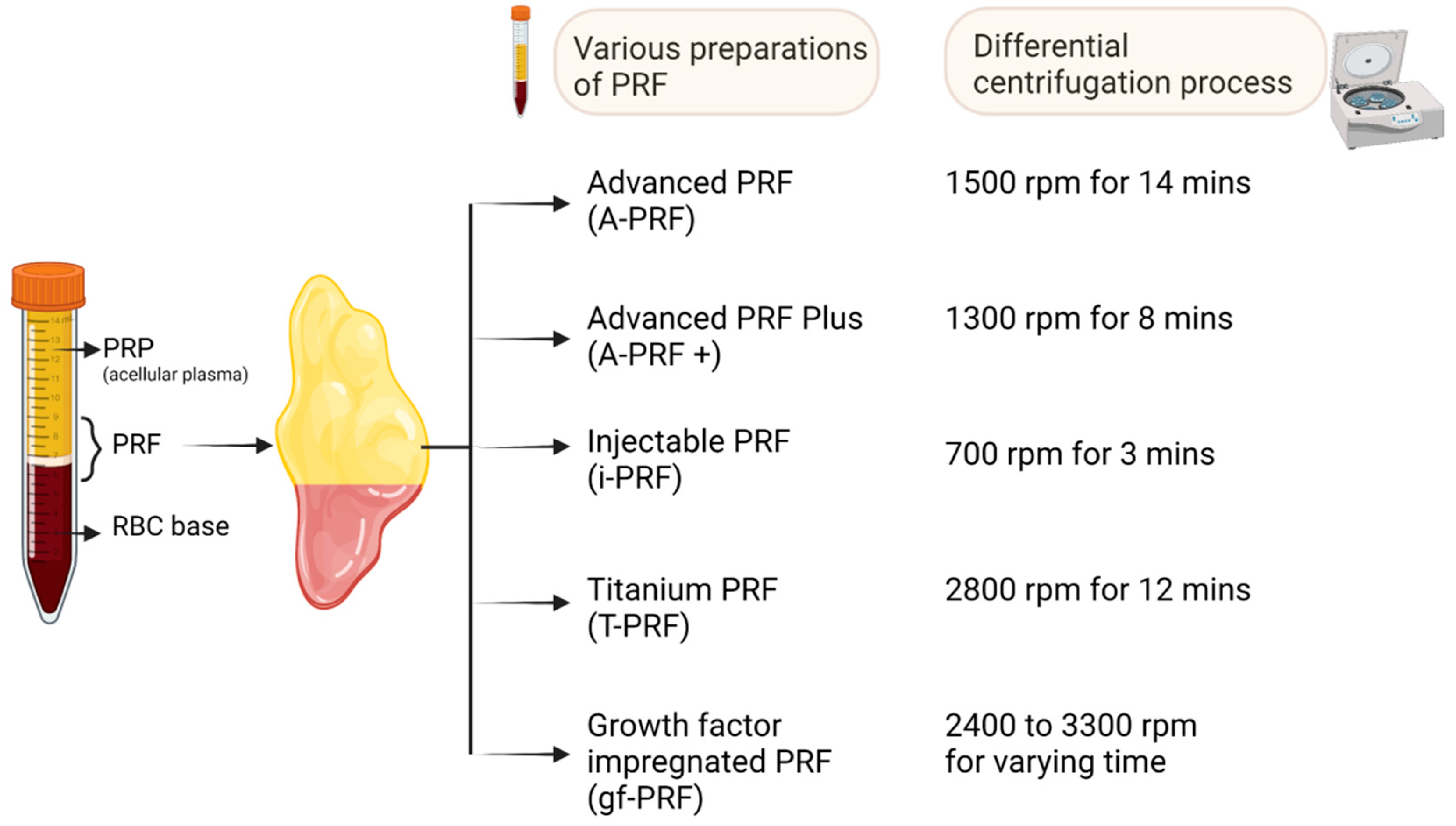
3. Other PRF Formulations
3.1. Advanced PRF (A-PRF)
3.2. Advanced PRF plus (A-PRF+)
3.3. Injectable PRF (i-PRF)
3.4. Titanium-PRF (T-PRF)
3.5. Growth-Factor-Impregnated PRF (gf-PRF)
4. Clinical Applications of PEF in Musculoskeletal Conditions
4.1. Cartilage Regeneration
4.2. Tendon Repair, Augmentation, and Regeneration
4.3. Sports and Over-Use Related Injuries
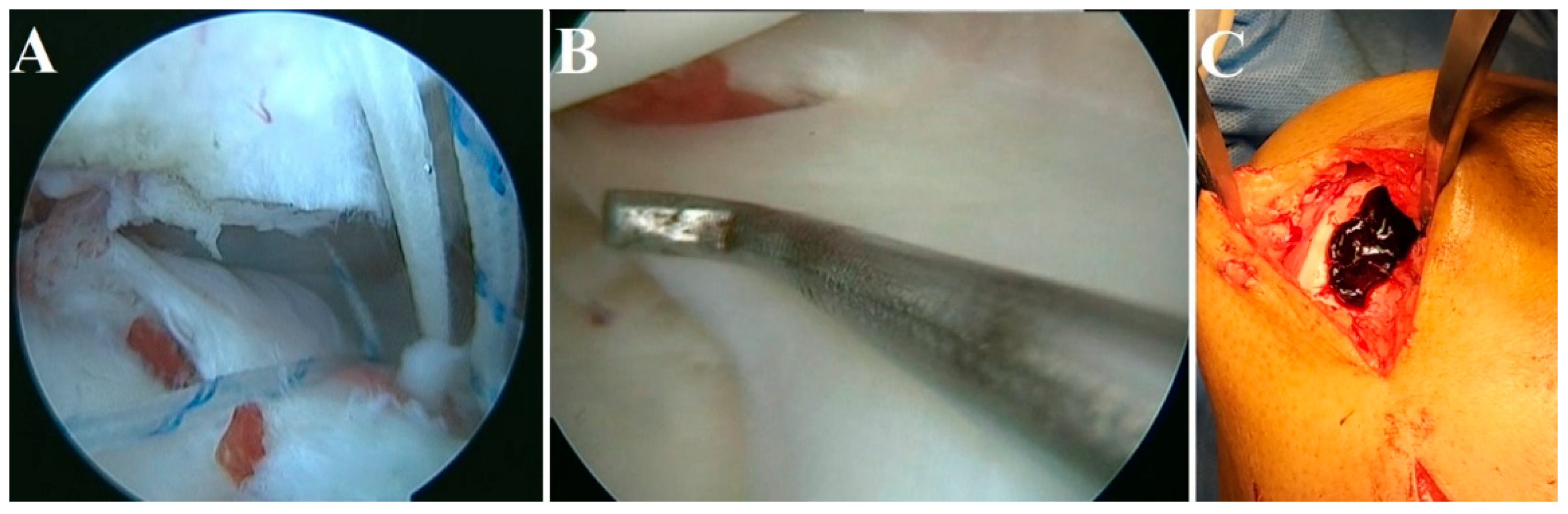
4.4. Meniscal Injuries
5. Author’s Perspectives
6. Limitations
7. Future Prospects
- (a)
- PRF Lysate (PRF-Ly)
- (b)
- Lyophilized PRF (Ly-PRF)
- (c)
- Albumin PRF (Alb-PRF)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariani, E.; Pulsatelli, L. Platelet Concentrates in Musculoskeletal Medicine. Int. J. Mol. Sci. 2020, 21, 1328. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A. Evolution, Current Status and Advances in Application of Platelet Concentrate in Periodontics and Implantology. World J. Clin. Cases 2017, 5, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Saluja, H.; Dehane, V.; Mahindra, U. Platelet-Rich Fibrin: A Second Generation Platelet Concentrate and a New Friend of Oral and Maxillofacial Surgeons. Ann. Maxillofac. Surg. 2011, 1, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.; Chopra, P.; Sheokand, V. Journey of Platelet Concentrates: A Review. Biomed. Pharmacol. J. 2020, 13, 185–191. [Google Scholar] [CrossRef]
- Egle, K.; Salma, I.; Dubnika, A. From Blood to Regenerative Tissue: How Autologous Platelet-Rich Fibrin Can Be Combined with Other Materials to Ensure Controlled Drug and Growth Factor Release. Int. J. Mol. Sci. 2021, 22, 11553. [Google Scholar] [CrossRef]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How It All Starts: Initiation of the Clotting Cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 319–327. [Google Scholar]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-Rich Fibrin: Basics of Biological Actions and Protocol Modifications. Open Med. Wars 2021, 16, 446–454. [Google Scholar] [CrossRef]
- Miron, R.J.; Chai, J.; Fujioka-Kobayashi, M.; Sculean, A.; Zhang, Y. Evaluation of 24 Protocols for the Production of Platelet-Rich Fibrin. BMC Oral Health 2020, 20, 310. [Google Scholar] [CrossRef]
- Crisci, A.; Lombardi, D.; Serra, E.; Lombardi, G.; Cardillo, F.; Crisci, M. Standardized Protocol Proposed for Clinical Use of L-PRF and the Use of L-PRF Wound Box®. J. Unexplored Med. Data 2017, 2, 77–87. [Google Scholar] [CrossRef]
- Reksodiputro, M.H.; Harahap, A.R.; Setiawan, L.; Yosia, M. A Modified Preparation Method of Ideal Platelet-Rich Fibrin Matrix From Whole Blood. Front. Med. 2021, 8, 724488. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced Platelet-Rich Fibrin: A New Concept for Cell-Based Tissue Engineering by Means of Inflammatory Cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.; Tehreem, F.; Ahmed, F.; Marya, A.; Karobari, M.I. Platelet-Rich Fibrin Used in Regenerative Endodontics and Dentistry: Current Uses, Limitations, and Future Recommendations for Application. Int. J. Dent. 2021, 2021, e4514598. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.R.; Genmorgan, K.; Abdul Rahman, S.M.; Rajan, M.A.; Kumar, T.A.; Prasad, V.S. Role of Plasma-Rich Fibrin in Oral Surgery. J. Pharm. Bioallied Sci. 2016, 8, S36–S38. [Google Scholar] [CrossRef] [PubMed]
- Borie, E.; Oliví, D.G.; Orsi, I.A.; Garlet, K.; Weber, B.; Beltrán, V.; Fuentes, R. Platelet-Rich Fibrin Application in Dentistry: A Literature Review. Int. J. Clin. Exp. Med. 2015, 8, 7922–7929. [Google Scholar]
- Yu, P.; Zhai, Z.; Jin, X.; Yang, X.; Qi, Z. Clinical Application of Platelet-Rich Fibrin in Plastic and Reconstructive Surgery: A Systematic Review. Aesthetic Plast. Surg. 2018, 42, 511–519. [Google Scholar] [CrossRef]
- Sclafani, A.P.; Saman, M. Platelet-Rich Fibrin Matrix for Facial Plastic Surgery. Facial Plast. Surg. Clin. 2012, 20, 177–186. [Google Scholar] [CrossRef]
- Surucu, Y.; Saadoun, R.; Loder, S.; Guneren, E. Platelet Rich Fibrin (PRF) Enhances Scar Resolution of High-Tension Wounds in Rats. Plast. Reconstr. Surg. Glob. Open 2022, 10, 54. [Google Scholar] [CrossRef]
- Xiong, S.; Qiu, L.; Su, Y.; Zheng, H.; Yi, C. Platelet-Rich Plasma and Platelet-Rich Fibrin Enhance the Outcomes of Fat Grafting: A Comparative Study. Plast. Reconstr. Surg. 2019, 143, 1201e. [Google Scholar] [CrossRef]
- Grecu, A.F.; Reclaru, L.; Ardelean, L.C.; Nica, O.; Ciucă, E.M.; Ciurea, M.E. Platelet-Rich Fibrin and Its Emerging Therapeutic Benefits for Musculoskeletal Injury Treatment. Medicina 2019, 55, E141. [Google Scholar] [CrossRef]
- Chenna, D.; Shastry, S.; Das, S. Cocktail Protocol for Preparation of Platelet-Rich Fibrin Glue for Autologous Use. Malays. J. Med. Sci. 2021, 28, 35–40. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Mechanisms of Fibrin Polymerization and Clinical Implications. Blood 2013, 121, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Su, C.-Y.; Radosevich, M.; Goubran, H.; El-Ekiaby, M. Blood-Derived Biomaterials: Fibrin Sealant, Platelet Gel and Platelet Fibrin Glue. ISBT Sci. Ser. 2009, 4, 136–142. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. SAD 2018, 4, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Alsousou, J.; Thompson, M.; Hulley, P.; Noble, A.; Willett, K. The Biology of Platelet-Rich Plasma and Its Application in Trauma and Orthopaedic Surgery. J. Bone Jt. Surg. Br. Vol. 2009, 91, 987–996. [Google Scholar] [CrossRef]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef]
- Ramaswamy Reddy, S.H.; Reddy, R.; Babu, N.C.; Ashok, G.N. Stem-Cell Therapy and Platelet-Rich Plasma in Regenerative Medicines: A Review on Pros and Cons of the Technologies. J. Oral Maxillofac. Pathol. 2018, 22, 367–374. [Google Scholar] [CrossRef]
- Redler, L.H.; Thompson, S.A.; Hsu, S.H.; Ahmad, C.S.; Levine, W.N. Platelet-Rich Plasma Therapy: A Systematic Literature Review and Evidence for Clinical Use. Phys. Sportsmed. 2011, 39, 42–51. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part I: Technological Concepts and Evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef]
- Barbon, S.; Stocco, E.; Macchi, V.; Contran, M.; Grandi, F.; Borean, A.; Parnigotto, P.P.; Porzionato, A.; De Caro, R. Platelet-Rich Fibrin Scaffolds for Cartilage and Tendon Regenerative Medicine: From Bench to Bedside. Int. J. Mol. Sci. 2019, 20, 1701. [Google Scholar] [CrossRef]
- Miron, R.J.; Chai, J.; Zhang, P.; Li, Y.; Wang, Y.; de Almeida Barros Mourão, C.F.; Sculean, A.; Fujioka Kobayashi, M.; Zhang, Y. A Novel Method for Harvesting Concentrated Platelet-Rich Fibrin (C-PRF) with a 10-Fold Increase in Platelet and Leukocyte Yields. Clin. Oral Investig. 2020, 24, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part II: Platelet-Related Biologic Features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part III: Leucocyte Activation: A New Feature for Platelet Concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e51–e55. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, S.M. Safety Issues Associated With Platelet-Rich Fibrin Method. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 587. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; Corso, M.D.; Kang, B.-S.; Nally, M.; Lanata, N.; Wang, H.-L.; Quirynen, M. The Impact of the Centrifuge Characteristics and Centrifugation Protocols on the Cells, Growth Factors, and Fibrin Architecture of a Leukocyte- and Platelet-Rich Fibrin (L-PRF) Clot and Membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef]
- Everts, P.A.M.; Knape, J.T.A.; Weibrich, G.; Schönberger, J.P.A.M.; Hoffmann, J.; Overdevest, E.P.; Box, H.A.M.; van Zundert, A. Platelet-Rich Plasma and Platelet Gel: A Review. J. Extra Corpor. Technol. 2006, 38, 174–187. [Google Scholar]
- Ghanaati, S.; Mourão, C.F.; Adam, E.H.; Sader, R.; Zadeh, H.H.; Al-Maawi, S. The Role of Centrifugation Process in the Preparation of Therapeutic Blood Concentrates: Standardization of the Protocols to Improve Reproducibility. Int. J. Growth Factors Stem Cells Dent. 2019, 2, 41. [Google Scholar] [CrossRef]
- El Bagdadi, K.; Kubesch, A.; Yu, X.; Al-Maawi, S.; Orlowska, A.; Dias, A.; Booms, P.; Dohle, E.; Sader, R.; Kirkpatrick, C.J.; et al. Reduction of Relative Centrifugal Forces Increases Growth Factor Release within Solid Platelet-Rich-Fibrin (PRF)-Based Matrices: A Proof of Concept of LSCC (Low Speed Centrifugation Concept). Eur. J. Trauma Emerg. Surg. 2019, 45, 467–479. [Google Scholar] [CrossRef]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative Release of Growth Factors from PRP, PRF, and Advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Qiao, J.; An, N.; Ouyang, X. Quantification of Growth Factors in Different Platelet Concentrates. Platelets 2017, 28, 774–778. [Google Scholar] [CrossRef]
- The Opportunity in Perio-Implantology: The PRF—ScienceOpen. Available online: https://www.scienceopen.com/document?vid=38d30c61-808c-4355-bda4-1eb440085011 (accessed on 23 October 2022).
- Ravi, S.; Santhanakrishnan, M. Mechanical, Chemical, Structural Analysis and Comparative Release of PDGF-AA from L-PRF, A-PRF and T-PRF—An in Vitro Study. Biomater. Res. 2020, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Watanebe, T.; Kawabata, H.; Kitamura, Y.; Okudera, T.; Okudera, H.; Uematsu, K.; Okuda, K.; Nakata, K.; Tanaka, T.; et al. Mechanical and Degradation Properties of Advanced Platelet-Rich Fibrin (A-PRF), Concentrated Growth Factors (CGF), and Platelet-Poor Plasma-Derived Fibrin (PPTF). Int. J. Implant Dent. 2017, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, H.; Raoofi, S.; Bagheri, R.; Banihashemi, H. Comparison of the Mechanical Properties of Early Leukocyte- and Platelet-Rich Fibrin versus PRGF/Endoret Membranes. Int. J. Dent. 2016, 2016, 1849207. [Google Scholar] [CrossRef]
- Kubesch, A.; Barbeck, M.; Al-Maawi, S.; Orlowska, A.; Booms, P.F.; Sader, R.A.; Miron, R.J.; Kirkpatrick, C.J.; Choukroun, J.; Ghanaati, S. A Low-Speed Centrifugation Concept Leads to Cell Accumulation and Vascularization of Solid Platelet-Rich Fibrin: An Experimental Study in Vivo. Platelets 2019, 30, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Simões-Pedro, M.; Tróia, P.M.B.P.S.; dos Santos, N.B.M.; Completo, A.M.G.; Castilho, R.M.; de Oliveira Fernandes, G.V. Tensile Strength Essay Comparing Three Different Platelet-Rich Fibrin Membranes (L-PRF, A-PRF, and A-PRF+): A Mechanical and Structural In Vitro Evaluation. Polymers 2022, 14, 1392. [Google Scholar] [CrossRef]
- Li, W.; Sigley, J.; Pieters, M.; Helms, C.C.; Nagaswami, C.; Weisel, J.W.; Guthold, M. Fibrin Fiber Stiffness Is Strongly Affected by Fiber Diameter, but Not by Fibrinogen Glycation. Biophys. J. 2016, 110, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef]
- Farshidfar, N.; Jafarpour, D.; Firoozi, P.; Sahmeddini, S.; Hamedani, S.; de Souza, R.F.; Tayebi, L. The Application of Injectable Platelet-Rich Fibrin in Regenerative Dentistry: A Systematic Scoping Review of In Vitro and In Vivo Studies. Jpn. Dent. Sci. Rev. 2022, 58, 89–123. [Google Scholar] [CrossRef]
- Castro, A.B.; Andrade, C.; Li, X.; Pinto, N.; Teughels, W.; Quirynen, M. Impact of g Force and Timing on the Characteristics of Platelet-Rich Fibrin Matrices. Sci. Rep. 2021, 11, 6038. [Google Scholar] [CrossRef]
- Ragunanthan, N.; Devi, C.A.; Hemavani, V. A Novel Approach of Harvesting Concentrated Plasma-Rich Fibrin (PRF) with Increased Platelet Count. JMRI 2022, 6, 11–18. [Google Scholar] [CrossRef]
- Shao, Z.; Lyu, C.; Teng, L.; Xie, X.; Sun, J.; Zou, D.; Lu, J. An Injectable Fibrin Scaffold Rich in Growth Factors for Skin Repair. BioMed Res. Int. 2021, 2021, e8094932. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable Platelet Rich Fibrin (i-PRF): Opportunities in Regenerative Dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Tunalı, M.; Özdemir, H.; Küçükodacı, Z.; Akman, S.; Yaprak, E.; Toker, H.; Fıratlı, E. A Novel Platelet Concentrate: Titanium-Prepared Platelet-Rich Fibrin. BioMed Res. Int. 2014, 2014, 209548. [Google Scholar] [CrossRef] [PubMed]
- Tunali, M.; Özdemir, H.; Küçükodacı, Z.; Akman, S.; Öncü, E.; Aydınbelge, M.; Akman, M.; Firatli, E. A New Centrifugation Method for the Improvement of Platelet-Rich Fibrin Products: A Preliminary Study. J. Adv. Med. Med. Res. 2016, 13, 1–10. [Google Scholar] [CrossRef]
- Shirbhate, U.; Bajaj, P. Third-Generation Platelet Concentrates in Periodontal Regeneration: Gaining Ground in the Field of Regeneration. Cureus 2022, 14, e28072. [Google Scholar] [CrossRef]
- Tunalı, M.; Özdemir, H.; Küçükodacı, Z.; Akman, S.; Fıratlı, E. In Vivo Evaluation of Titanium-Prepared Platelet-Rich Fibrin (T-PRF): A New Platelet Concentrate. Br. J. Oral Maxillofac. Surg. 2013, 51, 438–443. [Google Scholar] [CrossRef]
- Gummaluri, S.S.; Bhattacharya, H.S.; Astekar, M.; Cheruvu, S. Evaluation of Titanium-Prepared Platelet-Rich Fibrin and Leucocyte Platelet-Rich Fibrin in the Treatment of Intra-Bony Defects: A Randomized Clinical Trial. J. Dent. Res. Dent. Clin. Dent. Prospect. 2020, 14, 83–91. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, Y.; Ma, Y.; Ao, Y.; Hu, X.; Meng, Q. Engineering of MSC-Derived Exosomes: A Promising Cell-Free Therapy for Osteoarthritis. Membranes 2022, 12, 739. [Google Scholar] [CrossRef]
- Chien, C.-S.; Ho, H.-O.; Liang, Y.-C.; Ko, P.-H.; Sheu, M.-T.; Chen, C.-H. Incorporation of Exudates of Human Platelet-Rich Fibrin Gel in Biodegradable Fibrin Scaffolds for Tissue Engineering of Cartilage. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 948–955. [Google Scholar] [CrossRef]
- Abd El Raouf, M.; Wang, X.; Miusi, S.; Chai, J.; Mohamed AbdEl-Aal, A.B.; Nefissa Helmy, M.M.; Ghanaati, S.; Choukroun, J.; Choukroun, E.; Zhang, Y.; et al. Injectable-Platelet Rich Fibrin Using the Low Speed Centrifugation Concept Improves Cartilage Regeneration When Compared to Platelet-Rich Plasma. Platelets 2019, 30, 213–221. [Google Scholar] [CrossRef]
- Wong, C.-C.; Chen, C.-H.; Chan, W.P.; Chiu, L.-H.; Ho, W.-P.; Hsieh, F.-J.; Chen, Y.-T.; Yang, T.-L. Single-Stage Cartilage Repair Using Platelet-Rich Fibrin Scaffolds With Autologous Cartilaginous Grafts. Am. J. Sport. Med. 2017, 45, 3128–3142. [Google Scholar] [CrossRef] [PubMed]
- de Souza, F.G.; Fernandes, B.L.; Rebelatto, C.L.K.; de Aguiar, A.M.; Fracaro, L.; Brofman, P.R.S. Proliferation and Differentiation of Stem Cells in Contact with Eluate from Fibrin-Rich Plasma Membrane. Rev. Bras. Ortop. 2017, 53, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Metineren, H.; Dülgeroğlu, T.C. Regenerative Effect of Platelet-Rich Fibrin on Articular Cartilage Defects in an Experimental Rat Model. Eur. Res. J. 2019, 5, 299–305. [Google Scholar] [CrossRef]
- Wang, M.; Gao, W. Fixation of Platelet-Rich Plasma and Fibrin Gels on Knee Cartilage Defects after Microfracture with Arthroscopy. Int. Orthop. 2022, 46, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, D.; Fakhrjou, A.; Dizaji, V.M.; Alishahi, M.K. Effect of Autologous Platelet Rich Fibrin on the Healing of Experimental Articular Cartilage Defects of the Knee in an Animal Model. BioMed Res. Int. 2014, 2014, 486436. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Sheu, S.-Y.; Hsu, L.-H.; Yang, K.-C.; Tseng, C.-C.; Kuo, T.-F. Intra-Articular Injection of Platelet-Rich Fibrin Releasates in Combination with Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Articular Cartilage Defects: An in Vivo Study in Rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, F.; L Duré, G.; P Klein, C.; F Bampi, V.; V Padoin, A.; D Silva, V.; Braga-Silva, J. Platelet-Rich Fibrin Promotes an Accelerated Healing of Achilles Tendon When Compared to Platelet-Rich Plasma in Rat. World J. Plast. Surg. 2015, 4, 101–109. [Google Scholar]
- Anitua, E.; Sanchez, M.; Nurden, A.T.; Zalduendo, M.; de la Fuente, M.; Orive, G.; Azofra, J.; Andia, I. Autologous Fibrin Matrices: A Potential Source of Biological Mediators That Modulate Tendon Cell Activities. J. Biomed. Mater. Res. A 2006, 77, 285–293. [Google Scholar] [CrossRef]
- Visser, L.C.; Arnoczky, S.P.; Caballero, O.; Egerbacher, M. Platelet-Rich Fibrin Constructs Elute Higher Concentrations of Transforming Growth Factor-Β1 and Increase Tendon Cell Proliferation over Time When Compared to Blood Clots: A Comparative in Vitro Analysis. Vet. Surg. 2010, 39, 811–817. [Google Scholar] [CrossRef]
- Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Russell, R.P.; Apostolakos, J.; Ramos, D.M.; Kumbar, S.G.; Imhoff, A.B.; Arciero, R.A.; Mazzocca, A.D. Properties of Biologic Scaffolds and Their Response to Mesenchymal Stem Cells. Arthroscopy 2014, 30, 289–298. [Google Scholar] [CrossRef]
- Zumstein, M.A.; Berger, S.; Schober, M.; Boileau, P.; Nyffeler, R.W.; Horn, M.; Dahinden, C.A. Leukocyte- and Platelet-Rich Fibrin (L-PRF) for Long-Term Delivery of Growth Factor in Rotator Cuff Repair: Review, Preliminary Results and Future Directions. Curr. Pharm. Biotechnol. 2012, 13, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Castricini, R.; Longo, U.G.; De Benedetto, M.; Panfoli, N.; Pirani, P.; Zini, R.; Maffulli, N.; Denaro, V. Platelet-Rich Plasma Augmentation for Arthroscopic Rotator Cuff Repair: A Randomized Controlled Trial. Am. J. Sport. Med. 2011, 39, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Barber, F.A. Triple-Loaded Single-Row Versus Suture-Bridge Double-Row Rotator Cuff Tendon Repair With Platelet-Rich Plasma Fibrin Membrane: A Randomized Controlled Trial. Arthroscopy 2016, 32, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Bergeson, A.G.; Tashjian, R.Z.; Greis, P.E.; Crim, J.; Stoddard, G.J.; Burks, R.T. Effects of Platelet-Rich Fibrin Matrix on Repair Integrity of at-Risk Rotator Cuff Tears. Am. J. Sport. Med. 2012, 40, 286–293. [Google Scholar] [CrossRef]
- Hasan, S.; Weinberg, M.; Khatib, O.; Jazrawi, L.; Strauss, E.J. The Effect of Platelet-Rich Fibrin Matrix on Rotator Cuff Healing in a Rat Model. Int. J. Sport. Med. 2016, 37, 36–42. [Google Scholar] [CrossRef]
- Weber, S.C.; Kauffman, J.I.; Parise, C.; Weber, S.J.; Katz, S.D. Platelet-Rich Fibrin Matrix in the Management of Arthroscopic Repair of the Rotator Cuff: A Prospective, Randomized, Double-Blinded Study. Am. J. Sport. Med. 2013, 41, 263–270. [Google Scholar] [CrossRef]
- Alviti, F.; Gurzì, M.; Santilli, V.; Paoloni, M.; Padua, R.; Bernetti, A.; Bernardi, M.; Mangone, M. Achilles Tendon Open Surgical Treatment With Platelet-Rich Fibrin Matrix Augmentation: Biomechanical Evaluation. J. Foot Ankle Surg. 2017, 56, 581–585. [Google Scholar] [CrossRef]
- Saltzman, B.M.; Ukwuani, G.; Makhni, E.C.; Stephens, J.P.; Nho, S.J. The Effect of Platelet-Rich Fibrin Matrix at the Time of Gluteus Medius Repair: A Retrospective Comparative Study. Arthroscopy 2018, 34, 832–841. [Google Scholar] [CrossRef]
- Beyzadeoglu, T.; Pehlivanoglu, T.; Yildirim, K.; Buldu, H.; Tandogan, R.; Tuzun, U. Does the Application of Platelet-Rich Fibrin in Anterior Cruciate Ligament Reconstruction Enhance Graft Healing and Maturation? A Comparative MRI Study of 44 Cases. Orthop. J. Sport. Med. 2020, 8, 2325967120902013. [Google Scholar] [CrossRef]
- Matsunaga, D.; Akizuki, S.; Takizawa, T.; Omae, S.; Kato, H. Compact Platelet-Rich Fibrin Scaffold to Improve Healing of Patellar Tendon Defects and for Medial Collateral Ligament Reconstruction. Knee 2013, 20, 545–550. [Google Scholar] [CrossRef]
- Andersson-Molina, H.; Karlsson, H.; Rockborn, P. Arthroscopic Partial and Total Meniscectomy: A Long-Term Follow-up Study with Matched Controls. Arthroscopy 2002, 18, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lindhorst, E.; Vail, T.P.; Guilak, F.; Wang, H.; Setton, L.A.; Vilim, V.; Kraus, V.B. Longitudinal Characterization of Synovial Fluid Biomarkers in the Canine Meniscectomy Model of Osteoarthritis. J. Orthop. Res. 2000, 18, 269–280. [Google Scholar] [CrossRef] [PubMed]
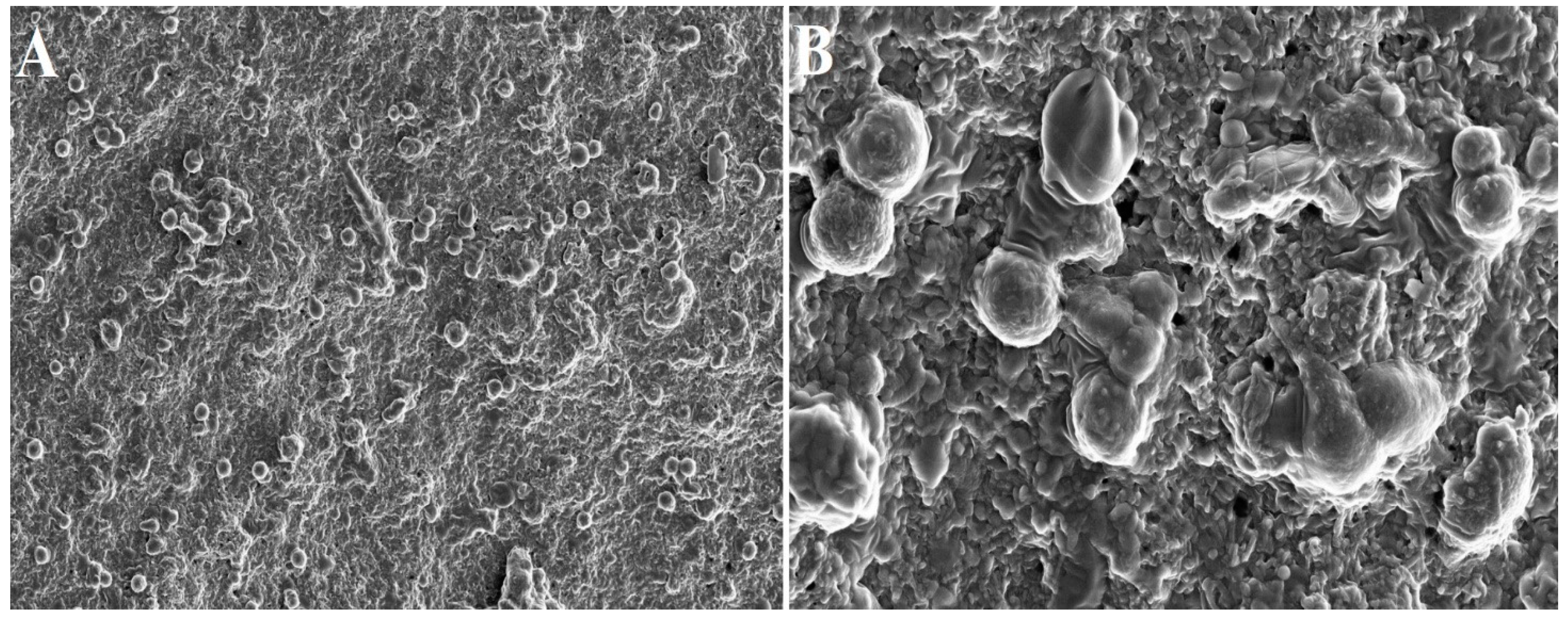
- Bai, M.-Y.; Wang, C.-W.; Wang, J.-Y.; Lin, M.-F.; Chan, W.P. Three-Dimensional Structure and Cytokine Distribution of Platelet-Rich Fibrin. Clinics 2017, 72, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Tatullo, M. Influence of PRF in the Healing of Bone and Gingival Tissues. Clinical and Histological Evaluations. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1958–1962. [Google Scholar] [PubMed]
- Narayanaswamy, R.; Sha, I. Arthroscopic Meniscal Repair With Second-Generation Platelet-Rich Fibrin Clot Augmentation. Arthrosc. Tech. 2022, 11, e1569–e1575. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-C.; Kuo, T.-F.; Yang, T.-L.; Tsuang, Y.-H.; Lin, M.-F.; Chang, C.-H.; Lin, Y.-H.; Chan, W.P. Platelet-Rich Fibrin Facilitates Rabbit Meniscal Repair by Promoting Meniscocytes Proliferation, Migration, and Extracellular Matrix Synthesis. Int. J. Mol. Sci. 2017, 18, 1722. [Google Scholar] [CrossRef]
- Kurnaz, R.; Balta, O. Effect of Platelet-Rich Plasma and Platelet-Rich Fibrin Matrix on Healing of Vertical Meniscal Tears in a Rabbit Model. Acta Orthop. Traumatol. Turc. 2020, 54, 186–195. [Google Scholar] [CrossRef]
- Meidyawati, R.; Suprastiwi, E. The Ability of Lysate-PRF Induces Proliferation of Fibroblast Cells in Endodontic Regenerative Therapy. Open J. Stomatol. 2018, 8, 182–187. [Google Scholar] [CrossRef]
- Potential Ability of PRF Lysate and A-PRF in HDPSCs Proliferation IADR Abstract Archives. Available online: https://iadr.abstractarchives.com/abstract/18iags-2957986/potential-ability-of-prf-lysate-and-a-prf-in-hdpscs-proliferation (accessed on 25 October 2022).
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and Cells for Tissue Regeneration: Different Scaffold Pore Sizes-Different Cell Effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Ngah, N.A.; Dias, G.J.; Tong, D.C.; Mohd Noor, S.N.F.; Ratnayake, J.; Cooper, P.R.; Hussaini, H.M. Lyophilised Platelet-Rich Fibrin: Physical and Biological Characterisation. Molecules 2021, 26, 7131. [Google Scholar] [CrossRef]
- Li, Q.; Reed, D.A.; Min, L.; Gopinathan, G.; Li, S.; Dangaria, S.J.; Li, L.; Geng, Y.; Galang, M.-T.; Gajendrareddy, P.; et al. Lyophilized Platelet-Rich Fibrin (PRF) Promotes Craniofacial Bone Regeneration through Runx2. Int. J. Mol. Sci. 2014, 15, 8509–8525. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, L.; Sun, T.; Wang, W.; Li, X.; Wu, B. Preparation and Effect of Lyophilized Platelet-Rich Fibrin on the Osteogenic Potential of Bone Marrow Mesenchymal Stem Cells in Vitro and in Vivo. Heliyon 2019, 5, e02739. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Schaller, B.; Mourão, C.F.D.A.B.; Zhang, Y.; Sculean, A.; Miron, R.J. Biological Characterization of an Injectable Platelet-Rich Fibrin Mixture Consisting of Autologous Albumin Gel and Liquid Platelet-Rich Fibrin (Alb-PRF). Platelets 2021, 32, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Giancola, C.; De Sena, C.; Fessas, D.; Graziano, G.; Barone, G. DSC Studies on Bovine Serum Albumin Denaturation Effects of Ionic Strength and SDS Concentration. Int. J. Biol. Macromol. 1997, 20, 193–204. [Google Scholar] [CrossRef]
- Gheno, E.; de Almeida Barros Mourão, C.F.; de Mello-Machado, R.C.; Stellet Lourenço, E.; Miron, R.J.; Catarino, K.F.F.; Alves, A.T.; Alves, G.G.; Calasans-Maia, M.D. In Vivo Evaluation of the Biocompatibility and Biodegradation of a New Denatured Plasma Membrane Combined with Liquid PRF (Alb-PRF). Platelets 2021, 32, 542–554. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanaswamy, R.; Patro, B.P.; Jeyaraman, N.; Gangadaran, P.; Rajendran, R.L.; Nallakumarasamy, A.; Jeyaraman, M.; Ramani, P.; Ahn, B.-C. Evolution and Clinical Advances of Platelet-Rich Fibrin in Musculoskeletal Regeneration. Bioengineering 2023, 10, 58. https://doi.org/10.3390/bioengineering10010058
Narayanaswamy R, Patro BP, Jeyaraman N, Gangadaran P, Rajendran RL, Nallakumarasamy A, Jeyaraman M, Ramani P, Ahn B-C. Evolution and Clinical Advances of Platelet-Rich Fibrin in Musculoskeletal Regeneration. Bioengineering. 2023; 10(1):58. https://doi.org/10.3390/bioengineering10010058
Chicago/Turabian StyleNarayanaswamy, Ragunanthan, Bishnu Prasad Patro, Naveen Jeyaraman, Prakash Gangadaran, Ramya Lakshmi Rajendran, Arulkumar Nallakumarasamy, Madhan Jeyaraman, Prasanna Ramani, and Byeong-Cheol Ahn. 2023. "Evolution and Clinical Advances of Platelet-Rich Fibrin in Musculoskeletal Regeneration" Bioengineering 10, no. 1: 58. https://doi.org/10.3390/bioengineering10010058
APA StyleNarayanaswamy, R., Patro, B. P., Jeyaraman, N., Gangadaran, P., Rajendran, R. L., Nallakumarasamy, A., Jeyaraman, M., Ramani, P., & Ahn, B.-C. (2023). Evolution and Clinical Advances of Platelet-Rich Fibrin in Musculoskeletal Regeneration. Bioengineering, 10(1), 58. https://doi.org/10.3390/bioengineering10010058