Temporal Statistical Relationship between Regional Cerebral Oxygen Saturation (rSO2) and Brain Tissue Oxygen Tension (PbtO2) in Moderate-to-Severe Traumatic Brain Injury: A Canadian High Resolution-TBI (CAHR-TBI) Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Population
2.3. High-Resolution Physiologic Data Collection
2.4. Physiologic Data Cleaning and Processing
2.5. Physiologic Data Analysis and Statistical Methods
2.5.1. Overview
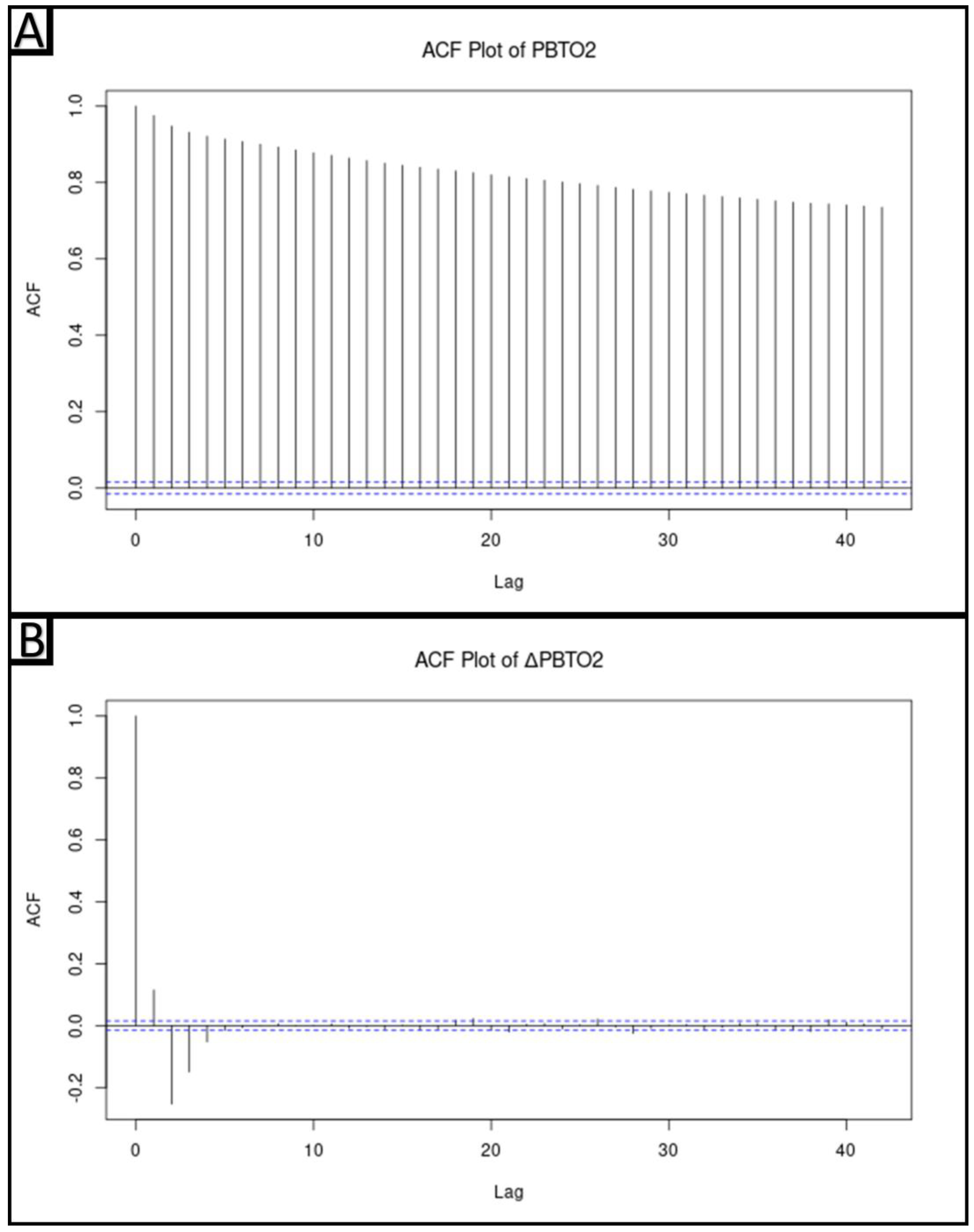
2.5.2. Determination of Stationarity of Physiologic Data
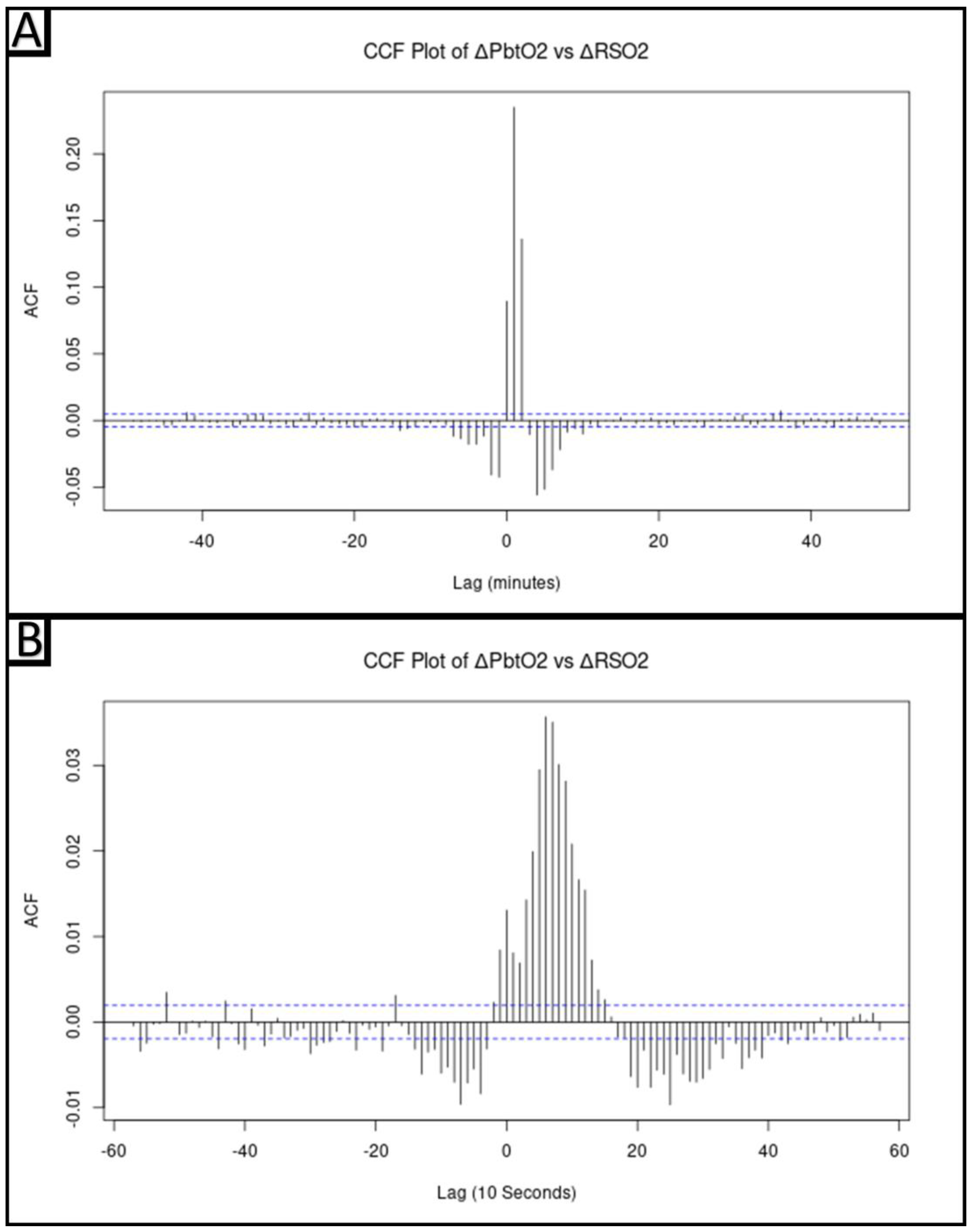
2.5.3. Cross-Correlative Relationship between ΔPbtO2 and ΔrSO2
2.5.4. Vector Autoregressive Modeling and Impulse–Response Function Plots
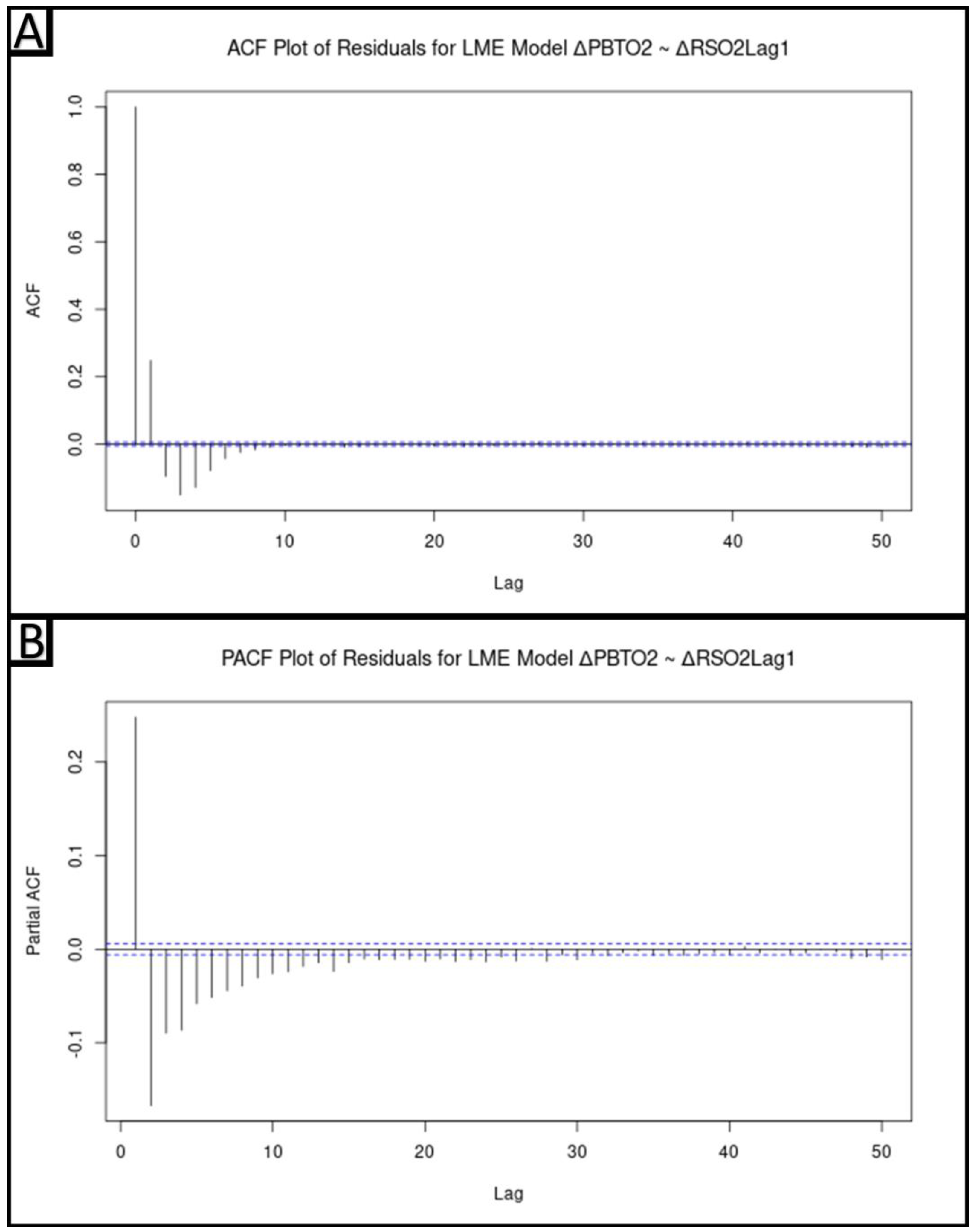
2.5.5. Hierarchical Linear Modeling of ΔPbtO2 from ΔrSO2Lag1
2.5.6. Modeling of ΔPbtO2 from ΔrSO2Lag1 Accounting for Autocorrelative Structure
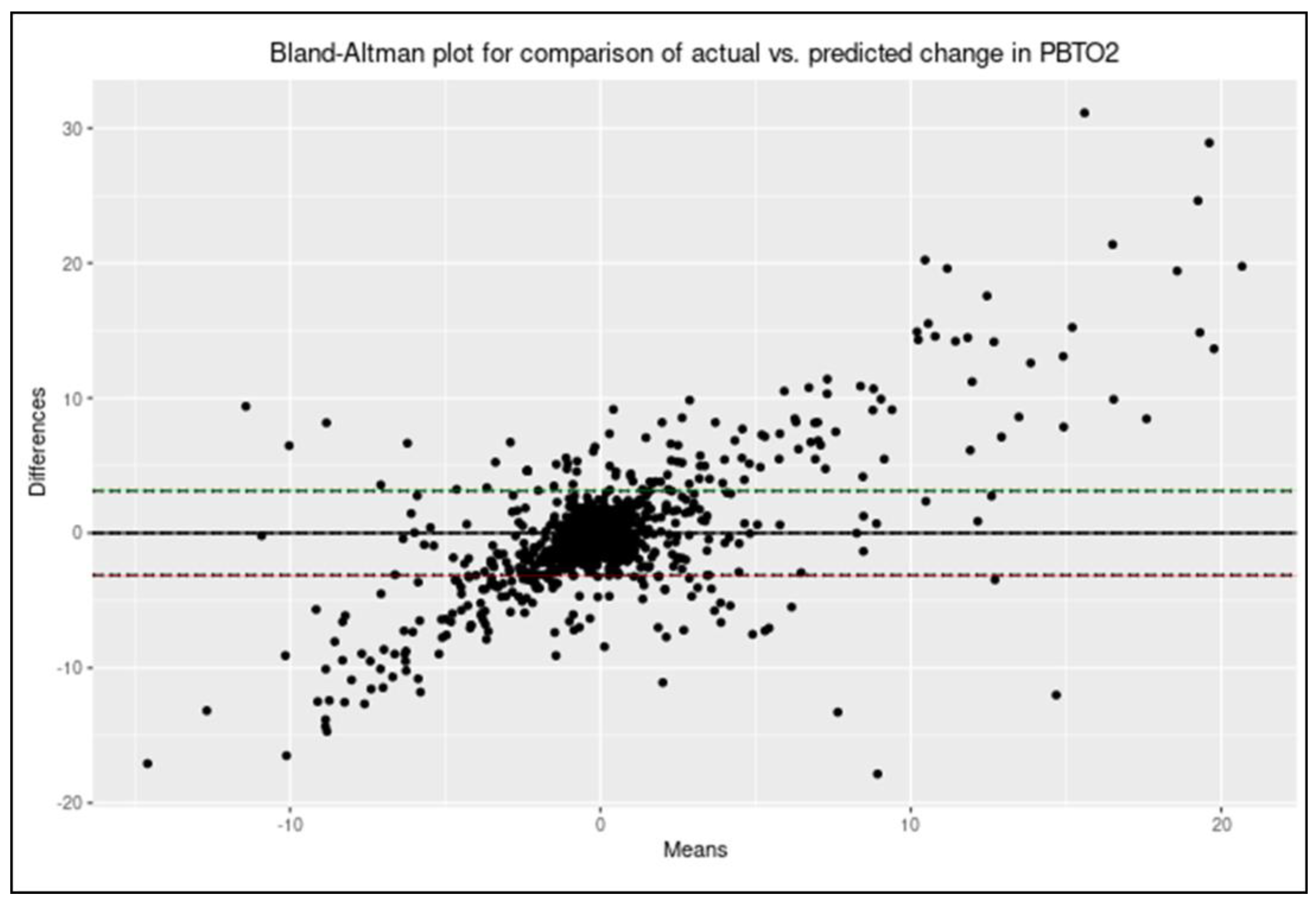
2.5.7. Evaluating Model Correlation and Agreement
3. Results
3.1. Cohort Demographics
3.2. Determination of the Stationarity of the Physiologic Data
3.3. Cross-Correlative Relationship between ΔPbtO2 and ΔrSO2
3.4. Vector Autoregressive Modeling and Impulse–Response Function Plots
3.5. Hierarchical Linear Modeling of ΔPbtO2 from ΔrSO2Lag1
3.6. Modeling of ΔPbtO2 from ΔrSO2Lag1 Accounting for Autocorrelative Structure
3.7. Evaluating Model Correlation and Agreement
4. Discussion
4.1. Limitations
4.2. Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maas, A.I.R.; Marmarou, A.; Murray, G.D.; Teasdale, S.G.M.; Steyerberg, E.W. Prognosis and Clinical Trial Design in Traumatic Brain Injury: The IMPACT Study. J. Neurotrauma 2007, 24, 232–238. [Google Scholar] [CrossRef]
- Maas, A.I.; Stocchetti, N.; Bullock, R. Moderate and Severe Traumatic Brain Injury in Adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.J.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, R.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A Management Algorithm for Adult Patients with Both Brain Oxygen and Intracranial Pressure Monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020, 46, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Czosnyka, M.; Adams, H.; Cardim, D.; Kolias, A.G.; Zeiler, F.A.; Lavinio, A.; Aries, M.; Robba, C.; Smielewski, P.; et al. Twenty-Five Years of Intracranial Pressure Monitoring After Severe Traumatic Brain Injury: A Retrospective, Single-Center Analysis. Neurosurgery 2019, 85, E75–E82. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.; Menon, D.K.; Citerio, G.; Vespa, P.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; Videtta, W.; Armonda, R.; et al. Consensus Summary Statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: A Statement for Healthcare Professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit. Care 2014, 21, 1–26. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic Brain Injury: Integrated Approaches to Improve Prevention, Clinical Care, and Research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Wiegers, E.; Sewalt, C.; Buki, A.; Citerio, G.; De Keyser, V.; Ercole, A.; Kunzmann, K.; Lanyon, L.; Lecky, F.; et al. Case-Mix, Care Pathways, and Outcomes in Patients with Traumatic Brain Injury in CENTER-TBI: A European Prospective, Multicentre, Longitudinal, Cohort Study. Lancet Neurol. 2019, 18, 923–934. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Manley, G.T.; Abrams, M.; Åkerlund, C.; Andelic, N.; Aries, M.; Bashford, T.; Bell, M.J.; Bodien, Y.G.; et al. Traumatic Brain Injury: Progress and Challenges in Prevention, Clinical Care, and Research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef]
- ACTRN12619001328167 The BONANZA Trial—A Randomised Controlled Trial That Is Testing Whether a Management Strategy Guided by Early Brain Tissue Oxygen Monitoring in Patients in with Severe Traumatic Brain Injury Improves Long Term Neurological and Functional Outcomes. 2019. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12619001328167 (accessed on 29 June 2023).
- Payen, J.-F.; Richard, M.; Francony, G.; Audibert, G.; Barbier, E.L.; Bruder, N.; Dahyot-Fizelier, C.; Geeraerts, T.; Gergele, L.; Puybasset, L.; et al. Comparison of Strategies for Monitoring and Treating Patients at the Early Phase of Severe Traumatic Brain Injury: The Multicentre Randomised Controlled OXY-TC Trial Study Protocol. BMJ Open 2020, 10, e040550. [Google Scholar] [CrossRef]
- Bernard, F.; Barsan, W.; Diaz-Arrastia, R.; Merck, L.H.; Yeatts, S.; Shutter, L.A. Brain Oxygen Optimization in Severe Traumatic Brain Injury (BOOST-3): A Multicentre, Randomised, Blinded-Endpoint, Comparative Effectiveness Study of Brain Tissue Oxygen and Intracranial Pressure Monitoring versus Intracranial Pressure Alone. BMJ Open 2022, 12, e060188. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.; Haitsma, I.; Zador, Z.; Hemphill, J.C.; Morabito, D.; Manley, G.; Rosenthal, G. The New Licox Combined Brain Tissue Oxygen and Brain Temperature Monitor: Assessment of in Vitro Accuracy and Clinical Experience in Severe Traumatic Brain Injury. Neurosurgery 2008, 63, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, P.J.; Smielewski, P.; Czosnyka, M.; Menon, D.K.; Pickard, J.D. Near-Infrared Spectroscopy Use in Patients with Head Injury. J. Neurosurg. 1995, 83, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Holzschuh, M.; Woertgen, C.; Metz, C.; Brawanski, A. Dynamic Changes of Cerebral Oxygenation Measured by Brain Tissue Oxygen Pressure and near Infrared Spectroscopy. Neurol. Res. 1997, 19, 246–248. [Google Scholar] [CrossRef]
- Brawanski, A.; Faltermeier, R.; Rothoerl, R.D.; Woertgen, C. Comparison of Near-Infrared Spectroscopy and Tissue p(O2) Time Series in Patients after Severe Head Injury and Aneurysmal Subarachnoid Hemorrhage. J. Cereb. Blood Flow. Metab. 2002, 22, 605–611. [Google Scholar] [CrossRef]
- Rothoerl, R.D.; Faltermeier, R.; Burger, R.; Woertgen, C.; Brawanski, A. Dynamic Correlation between Tissue PO2 and near Infrared Spectroscopy. Acta Neurochir. Suppl. 2002, 81, 311–313. [Google Scholar] [CrossRef]
- McLeod, A.D.; Igielman, F.; Elwell, C.; Cope, M.; Smith, M. Measuring Cerebral Oxygenation during Normobaric Hyperoxia: A Comparison of Tissue Microprobes, near-Infrared Spectroscopy, and Jugular Venous Oximetry in Head Injury. Anesth. Analg. 2003, 97, 851–856. [Google Scholar] [CrossRef]
- Leal-Noval, S.R.; Cayuela, A.; Arellano-Orden, V.; Marín-Caballos, A.; Padilla, V.; Ferrándiz-Millón, C.; Corcia, Y.; García-Alfaro, C.; Amaya-Villar, R.; Murillo-Cabezas, F. Invasive and Noninvasive Assessment of Cerebral Oxygenation in Patients with Severe Traumatic Brain Injury. Intensive Care Med. 2010, 36, 1309–1317. [Google Scholar] [CrossRef]
- Büchner, K.; Meixensberger, J.; Dings, J.; Roosen, K. Near-Infrared Spectroscopy—Not Useful to Monitor Cerebral Oxygenation after Severe Brain Injury. Zentralblatt Neurochir. 2000, 61, 69–73. [Google Scholar] [CrossRef]
- Esnault, P.; Boret, H.; Montcriol, A.; Carre, E.; Prunet, B.; Bordes, J.; Simon, P.; Joubert, C.; Dagain, A.; Kaiser, E.; et al. Assessment of Cerebral Oxygenation in Neurocritical Care Patients: Comparison of a New Four Wavelengths Forehead Regional Saturation in Oxygen Sensor (EQUANOX®) with Brain Tissue Oxygenation. A Prospective Observational Study. Minerva Anestesiol. 2015, 81, 876–884. [Google Scholar]
- de Courson, H.; Proust-Lima, C.; Tuaz, E.; Georges, D.; Verchère, E.; Biais, M. Relationship Between Brain Tissue Oxygen and Near-Infrared Spectroscopy in Patients with Nontraumatic Subarachnoid Hemorrhage. Neurocrit. Care 2022, 37, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Howells, T.; Johnson, U.; McKelvey, T.; Enblad, P. An Optimal Frequency Range for Assessing the Pressure Reactivity Index in Patients with Traumatic Brain Injury. J. Clin. Monit. Comput. 2015, 29, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.P.; Raj, R.; Bellander, B.-M.; Nelson, D.; Piippo-Karjalainen, A.; Siironen, J.; Tanskanen, P.; Hawryluk, G.; Hasen, M.; Unger, B.; et al. Comparison of High versus Low Frequency Cerebral Physiology for Cerebrovascular Reactivity Assessment in Traumatic Brain Injury: A Multi-Center Pilot Study. J. Clin. Monit. Comput. 2020, 34, 971–994. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, J.E.; Askew, S.; Zheng, Y.; Porrill, J.; Westby, G.W.; Redgrave, P.; Rector, D.M.; Harper, R.M. Cerebral Vasomotion: A 0.1-Hz Oscillation in Reflected Light Imaging of Neural Activity. Neuroimage 1996, 4, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Lütkepohl, H. New Introduction to Multiple Time Series Analysis; Springer: New York, NY, USA; Berlin, Germany, 2005; ISBN 978-3-540-40172-8. [Google Scholar]
- Chatfield, C.; Xing, H. The Analysis of Time Series: An Introduction with R, 7th ed.; Chapman & Hall/CRC texts in statistical science series; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Boca Raton, FL, USA, 2019; ISBN 978-1-351-25944-6. [Google Scholar]
- Mills, T.C. Applied Time Series Analysis: A Practical Guide to Modeling and Forecasting; Academic Press: London, UK, 2019; ISBN 978-0-12-813117-6. [Google Scholar]
- Percival, D.B.; Walden, A.T. Spectral Analysis for Univariate Time Series, 1st ed.; Cambridge University Press: Cambridge, UK, 2020; ISBN 978-1-139-23572-3. [Google Scholar]
- Klein, S.P.; Fieuws, S.; Meyfroidt, G.; Depreitere, B. Effects of Norepinephrine, Propofol, and Hemoglobin Concentration on Dynamic Measurements of Cerebrovascular Reactivity in Acute Brain Injury. J. Neurotrauma 2021, 38, 506–512. [Google Scholar] [CrossRef]
- Ponce, L.L.; Pillai, S.; Cruz, J.; Li, X.; Julia, H.; Gopinath, S.; Robertson, C.S. Position of Probe Determines Prognostic Information of Brain Tissue PO2 in Severe Traumatic Brain Injury. Neurosurgery 2012, 70, 1492–1502. [Google Scholar] [CrossRef]
- Gomez, A.; Sainbhi, A.S.; Froese, L.; Batson, C.; Alizadeh, A.; Mendelson, A.A.; Zeiler, F.A. Near Infrared Spectroscopy for High-Temporal Resolution Cerebral Physiome Characterization in TBI: A Narrative Review of Techniques, Applications, and Future Directions. Front. Pharmacol. 2021, 12, 719501. [Google Scholar] [CrossRef]
- Fraser, C.D.; Brady, K.M.; Rhee, C.J.; Easley, R.B.; Kibler, K.; Smielewski, P.; Czosnyka, M.; Kaczka, D.W.; Andropoulos, D.B.; Rusin, C. The Frequency Response of Cerebral Autoregulation. J Appl Physiol 2013, 115, 52–56. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Smielewski, P.; Stevens, A.; Czosnyka, M.; Menon, D.K.; Ercole, A. Non-Invasive Pressure Reactivity Index Using Doppler Systolic Flow Parameters: A Pilot Analysis. J. Neurotrauma 2019, 36, 713–720. [Google Scholar] [CrossRef]
- Budohoski, K.P.; Zweifel, C.; Kasprowicz, M.; Sorrentino, E.; Diedler, J.; Brady, K.M.; Smielewski, P.; Menon, D.K.; Pickard, J.D.; Kirkpatrick, P.J.; et al. What Comes First? The Dynamics of Cerebral Oxygenation and Blood Flow in Response to Changes in Arterial Pressure and Intracranial Pressure after Head Injury. Br. J. Anaesth. 2012, 108, 89–99. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Donnelly, J.; Menon, D.K.; Smielewski, P.; Zweifel, C.; Brady, K.; Czosnyka, M. Continuous Autoregulatory Indices Derived from Multi-Modal Monitoring: Each One Is Not Like the Other. J. Neurotrauma 2017, 34, 3070–3080. [Google Scholar] [CrossRef]
- Lee, J.K.; Kibler, K.K.; Benni, P.B.; Easley, R.B.; Czosnyka, M.; Smielewski, P.; Koehler, R.C.; Shaffner, D.H.; Brady, K.M. Cerebrovascular Reactivity Measured by Near-Infrared Spectroscopy. Stroke 2009, 40, 1820–1826. [Google Scholar] [CrossRef]
- Steiner, L.A.; Pfister, D.; Strebel, S.P.; Radolovich, D.; Smielewski, P.; Czosnyka, M. Near-Infrared Spectroscopy Can Monitor Dynamic Cerebral Autoregulation in Adults. Neurocrit. Care 2009, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Smielewski, P.; Czosnyka, M.; Zweifel, C.; Brady, K.; Hogue, C.; Steiner, L.; Hutchinson, P.; Mennon, D.; Pickard, J. Multicentre Experience of Using ICM+ for Investigations of Cerebrovascular Dynamics with Near-Infrared Spectroscopy. Crit. Care 2010, 14, P348. [Google Scholar] [CrossRef]
- Zweifel, C.; Castellani, G.; Czosnyka, M.; Helmy, A.; Manktelow, A.; Carrera, E.; Brady, K.M.; Hutchinson, P.J.A.; Menon, D.K.; Pickard, J.D.; et al. Noninvasive Monitoring of Cerebrovascular Reactivity with near Infrared Spectroscopy in Head-Injured Patients. J. Neurotrauma 2010, 27, 1951–1958. [Google Scholar] [CrossRef] [PubMed]


| Demographic Parameter | Median or Number of Subjects | |
|---|---|---|
| Age (IQR) | 41 (34.8–49.3) | |
| Gender | Male subjects (%) | 15 (75) |
| Female subjects (%) | 4 (20) | |
| N/A (%) | 1 (5) | |
| Admission GCS | Eye (IQR) | 1 (1–1) |
| Verbal (IQR) | 1 (1–1) | |
| Motor (IQR) | 2 (1–4) | |
| Total (IQR) | 6 (3–7) | |
| Admission Pupils | Bilaterally Reactive (%) | 13 (65) |
| Unilaterally Reactive (%) | 3 (15) | |
| Bilaterally Unreactive (%) | 3 (15) | |
| N/A (%) | 1 (5) | |
| Marshall CT Classification | I (%) | 0 (0) |
| II (%) | 5 (25) | |
| III (%) | 8 (40) | |
| IV (%) | 0 (0) | |
| V (%) | 4 (20) | |
| VI (%) | 0 (0) | |
| N/A, n (%) | 3 (15) | |
| Follow-up GOS | 1 (%) | 4 (20) |
| 2 (%) | 0 (0) | |
| 3 (%) | 1 (5) | |
| 4 (%) | 8 (40) | |
| 5 (%) | 3 (15) | |
| N/A, n (%) | 4 (20) | |
| ABP (IQR) | 87.0 mmHg (78.6–96.70) | |
| ICP (IQR) | 11.0 mmHg (6.7–15.0) | |
| PbtO2 (IQR) | 24.2 mmHg (17.3–32.3) | |
| rSO2 (IQR) | 69.6% (63.6–76.8) | |
| PaO2 (IQR) * | 108 mmHg (88.5–141) | |
| PaCO2 (IQR) * | 38 mmHg (36–41) | |
| Subject ID | Side of rSO2 | Model Autoregressive Order | Model Moving Average Order | Coefficient of ΔrSO2Lag1 as a Regressor (Standard Error) | p-Value of ΔrSO2Lag1 as a Regressor | Pearson Correlation Coefficient of Actual vs. Predicted ΔPbtO2 (95% CI) |
|---|---|---|---|---|---|---|
| 1 | Right | 0 | 3 | −0.012 (0.007) | 0.089 | 0.11 (0.05–0.17) |
| 2 | Right | 5 | 1 | 0.392 (0.017) | <0.001 | 0.28 (0.25–0.30) |
| 3 | Right | 6 | 0 | 0.080 (0.052) | 0.125 | 0.08 (0.02–0.13) |
| 4 | Right | 2 | 2 | 0.246 (0.034) | <0.001 | 0.12 (0.10–0.15) |
| 5 | Right | 3 | 3 | 0.242 (0.019) | <0.001 | 0.19 (0.16–0.21) |
| 6 | Right | 1 | 2 | 1.065 (0.081) | <0.001 | 0.45 (0.40–0.49) |
| 7 | Right | 5 | 1 | −0.101 (0.015) | <0.001 | 0.04 (0.01–0.07) |
| 8 | Right | 4 | 3 | −0.059 (0.031) | 0.055 | 0.10 (0.05–0.16) |
| 9 | Right | 2 | 1 | 0.131 (0.031) | <0.001 | 0.10 (0.7–0.14) |
| 10 | Right | 3 | 2 | −0.059 (0.018) | 0.001 | 0.09 (0.05–0.12) |
| 11 | Right | 0 | 2 | −0.112 (0.032) | <0.001 | 0.03 (−0.03–0.09) |
| 12 | Right | 4 | 3 | 1.072 (0.019) | <0.001 | 0.57 (0.56–0.59) |
| 13 | Right | 1 | 3 | 0.003 (0.002) | 0.159 | 0.14 (0.12–0.15) |
| 14 | Left | 1 | 4 | 0.555 (0.031) | <0.001 | 0.47 (0.43–0.50) |
| 15 | Right | 2 | 2 | 0.055 (0.015) | <0.001 | 0.13 (0.11–0.17) |
| 16 | Right | 3 | 2 | 0.168 (0.017) | <0.001 | 0.13 (0.12–0.16) |
| 17 | Right | 2 | 2 | 0.051 (0.013) | <0.001 | 0.08 (0.06–0.10) |
| 18 | Right | 3 | 1 | 0.134 (0.001) | <0.001 | 0.18 (0.16–0.20) |
| 19 | Right | 1 | 3 | 0.760 (0.028) | <0.001 | 0.40 (0.38–0.41) |
| 20 | Right | 2 | 2 | 0.353 (0.020) | <0.001 | 0.27 (0.25–0.29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, A.; Griesdale, D.; Froese, L.; Yang, E.; Thelin, E.P.; Raj, R.; Aries, M.; Gallagher, C.; Bernard, F.; Kramer, A.H.; et al. Temporal Statistical Relationship between Regional Cerebral Oxygen Saturation (rSO2) and Brain Tissue Oxygen Tension (PbtO2) in Moderate-to-Severe Traumatic Brain Injury: A Canadian High Resolution-TBI (CAHR-TBI) Cohort Study. Bioengineering 2023, 10, 1124. https://doi.org/10.3390/bioengineering10101124
Gomez A, Griesdale D, Froese L, Yang E, Thelin EP, Raj R, Aries M, Gallagher C, Bernard F, Kramer AH, et al. Temporal Statistical Relationship between Regional Cerebral Oxygen Saturation (rSO2) and Brain Tissue Oxygen Tension (PbtO2) in Moderate-to-Severe Traumatic Brain Injury: A Canadian High Resolution-TBI (CAHR-TBI) Cohort Study. Bioengineering. 2023; 10(10):1124. https://doi.org/10.3390/bioengineering10101124
Chicago/Turabian StyleGomez, Alwyn, Donald Griesdale, Logan Froese, Eleen Yang, Eric P. Thelin, Rahul Raj, Marcel Aries, Clare Gallagher, Francis Bernard, Andreas H. Kramer, and et al. 2023. "Temporal Statistical Relationship between Regional Cerebral Oxygen Saturation (rSO2) and Brain Tissue Oxygen Tension (PbtO2) in Moderate-to-Severe Traumatic Brain Injury: A Canadian High Resolution-TBI (CAHR-TBI) Cohort Study" Bioengineering 10, no. 10: 1124. https://doi.org/10.3390/bioengineering10101124






