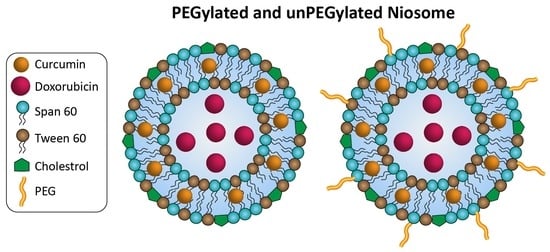
Empowering Cancer Therapy: Comparing PEGylated and Non-PEGylated Niosomes Loaded with Curcumin and Doxorubicin on MCF-7 Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Construction of Niosomes without Coating and with PEG Polymer Coating
2.3. Characterization
2.4. Bio-Activity Assessments
2.4.1. Evaluation of Encapsulation Efficiency (EE) in Niosomes with Two Drugs In Vitro
2.4.2. Stability Evaluation of the Particles
2.4.3. Drug Release Determination
2.4.4. Cytotoxicity Investigations
2.5. Statical Analysis
3. Results
3.1. Characterizations
3.1.1. Size and Surface Charge Determining of Niosomes
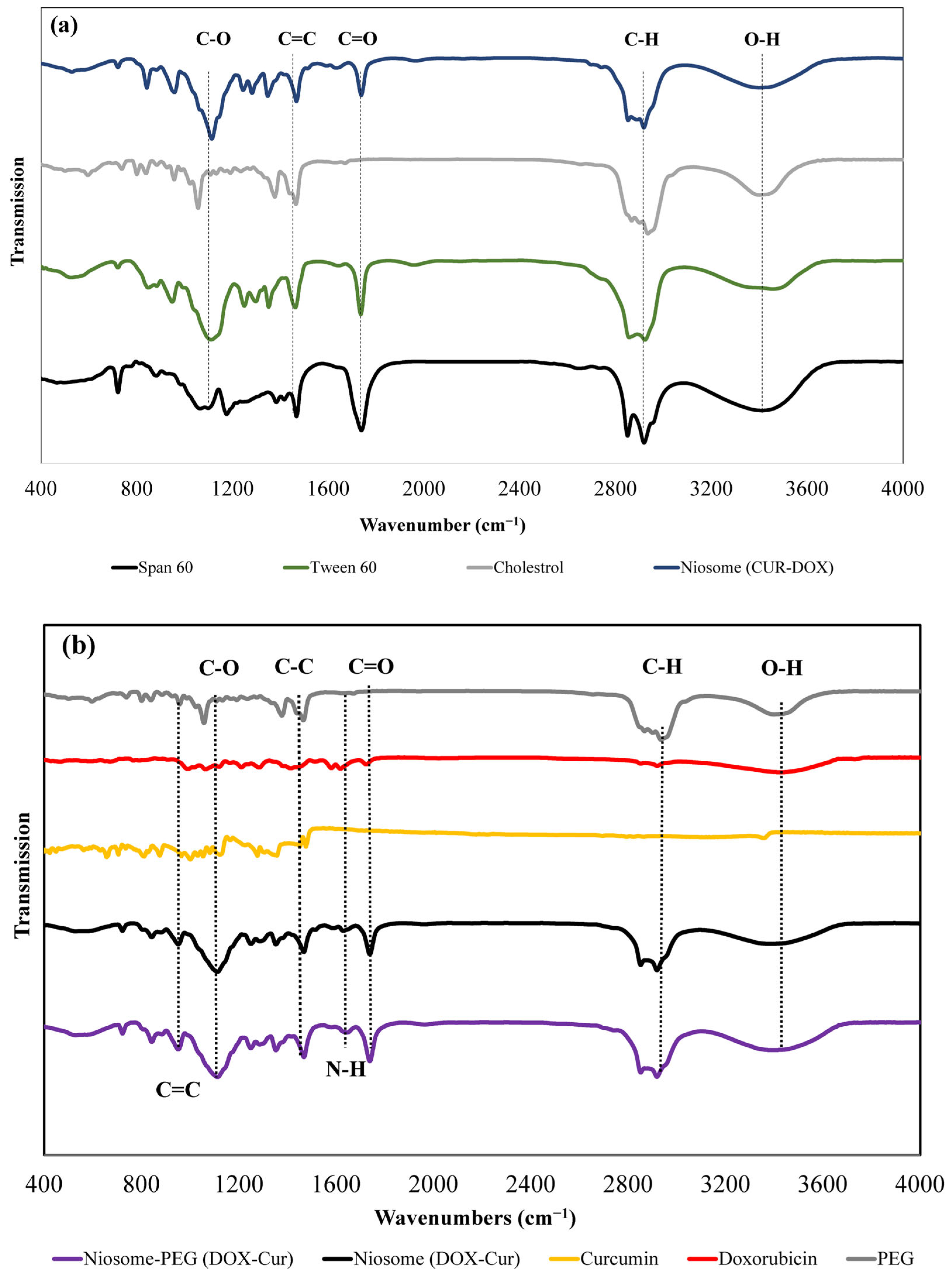
3.1.2. Fourier-Transform Infrared Spectroscopy Analysis
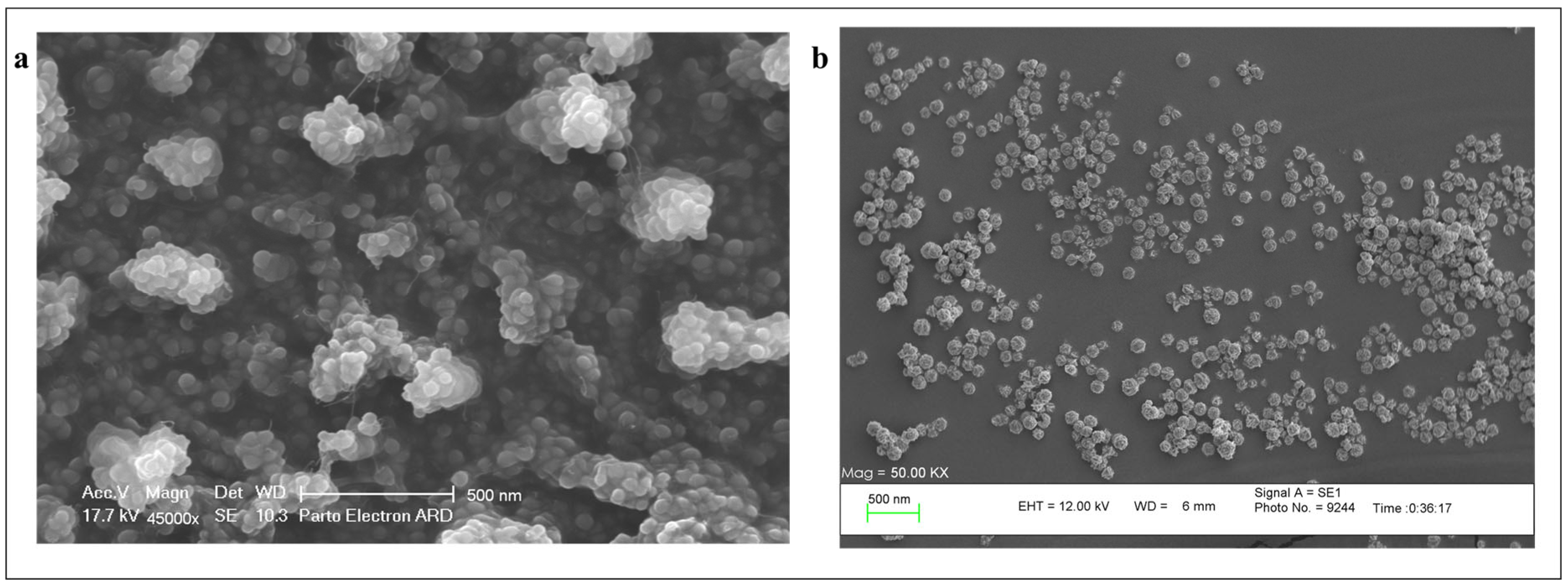
3.1.3. Scanning Electron Microscopy (SEM)
3.2. Bioactivity Assessments
3.2.1. Encapsulation Efficiency (EE%) Measurement
3.2.2. Stability Evaluation of the Particles
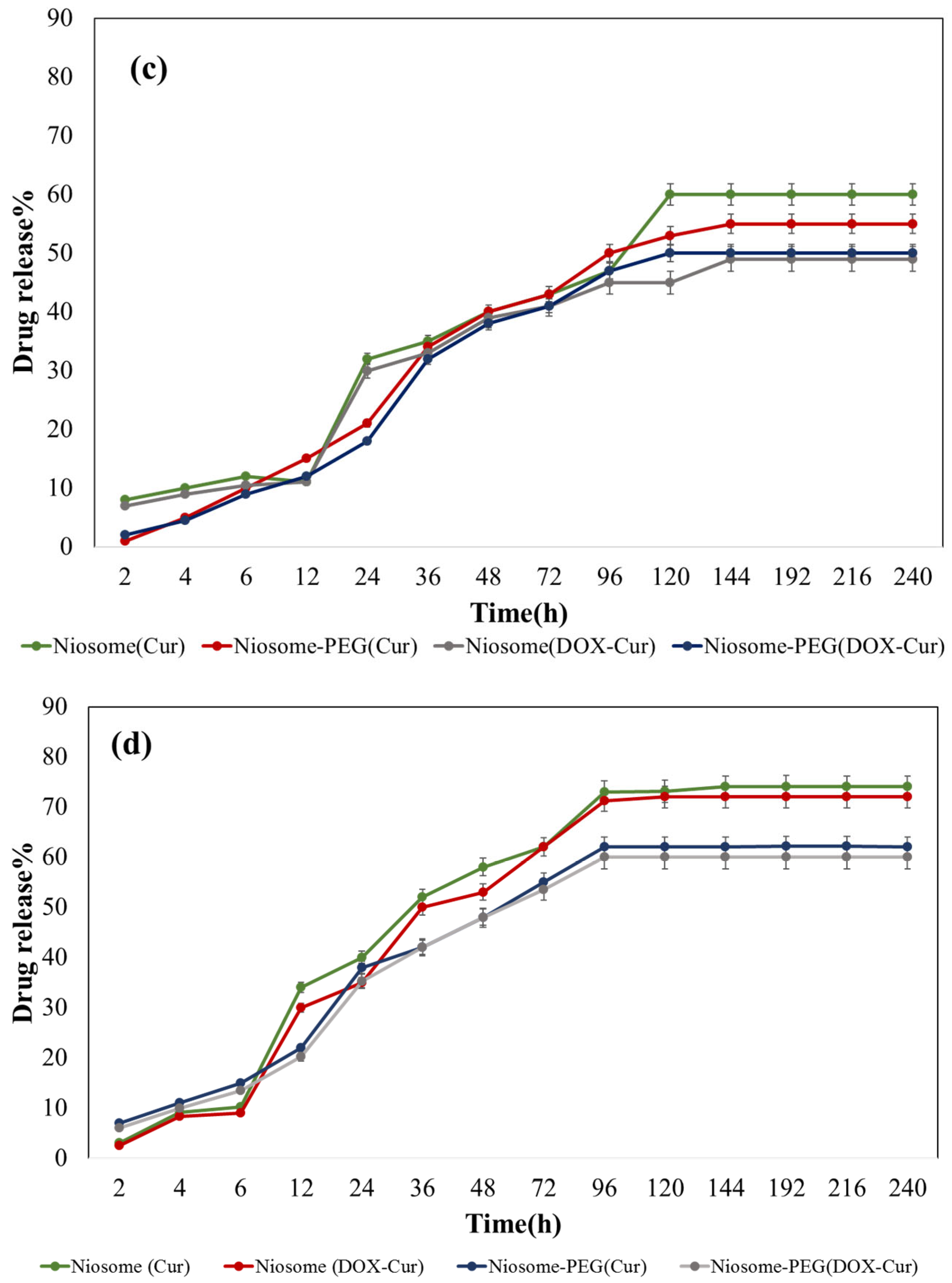
3.2.3. Drug Release Profile of the Particles
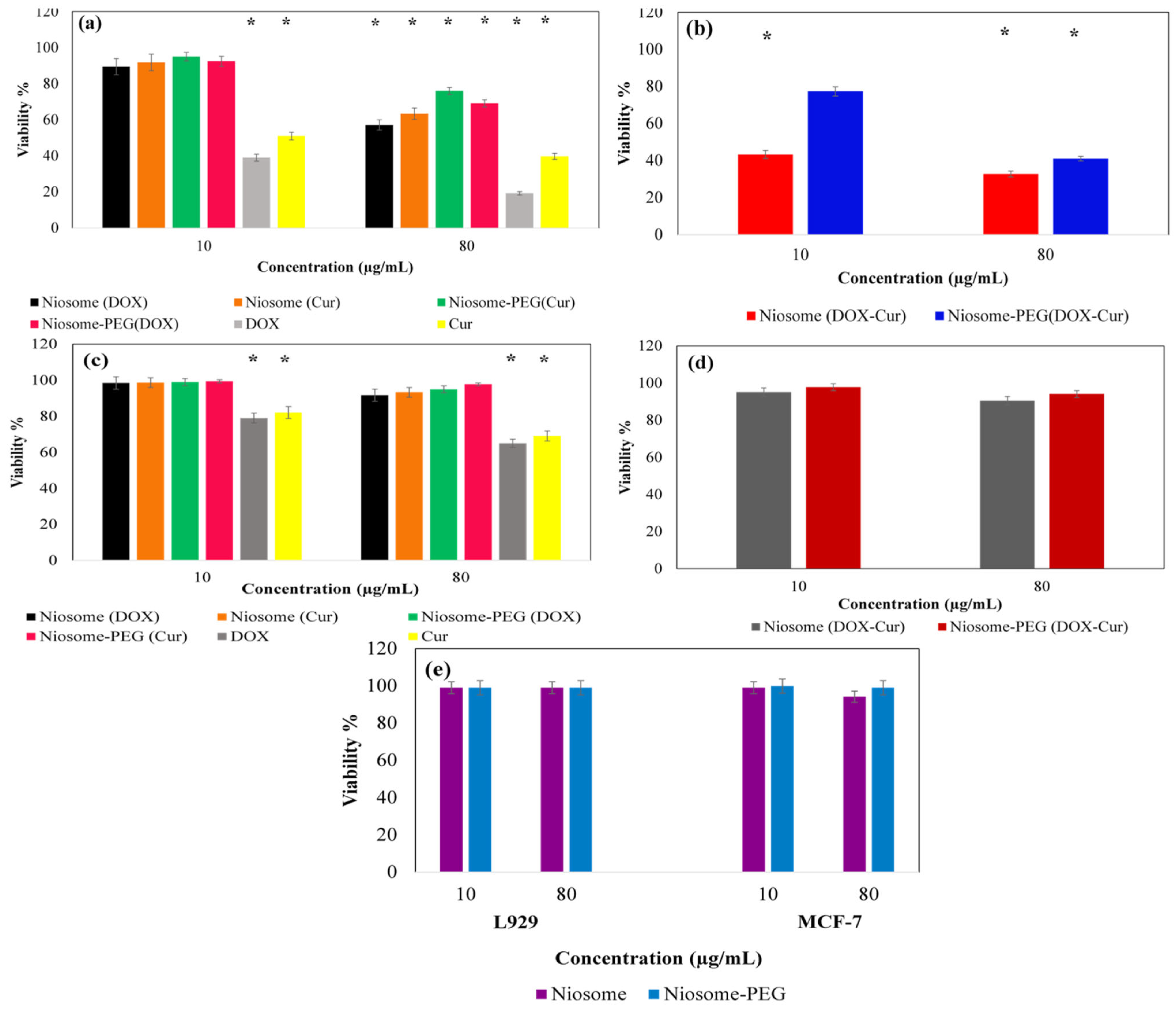
3.2.4. In Vitro MTT Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bidram, E.; Esmaeili, Y.; Ranji-Burachaloo, H.; Al-Zaubai, N.; Zarrabi, A.; Stewart, A.; Dunstan, D.E. A concise review on cancer treatment methods and delivery systems. J. Drug Deliv. Sci. Technol. 2019, 54, 101350. [Google Scholar] [CrossRef]
- Thallinger, C.; Füreder, T.; Preusser, M.; Heller, G.; Müllauer, L.; Höller, C.; Prosch, H.; Frank, N.; Swierzewski, R.; Berger, W. Review of cancer treatment with immune checkpoint inhibitors. Wien. Klin. Wochenschr. 2018, 130, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Peter, S.; Alven, S.; Maseko, R.B.; Aderibigbe, B.A. Doxorubicin-Based Hybrid Compounds as Potential Anticancer Agents: A Review. Molecules 2022, 27, 4478. [Google Scholar] [CrossRef]
- Paknia, F.; Parchami, N.; Nasry, N.; Mohabatkar, H. Therapeutic activities and biological effects of curcumin, as a natural multi-target compound, on human health: A minireview. J. Shahrekord Univ. Med. Sci. 2022, 24, 145–152. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Alipour, M.; Dalir Abdolahinia, E.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, H.; Rahbar Saadat, Y.; Sharifi, S.; Zununi Vahed, S. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytother. Res. 2022, 36, 1156–1181. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Rajera, R.; Nagpal, K.; Singh, S.K.; Mishra, D.N. Niosomes: A controlled and novel drug delivery system. Biol. Pharm. Bull. 2011, 34, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Moghtaderi, M.; Sedaghatnia, K.; Bourbour, M.; Fatemizadeh, M.; Salehi Moghaddam, Z.; Hejabi, F.; Heidari, F.; Quazi, S.; Farasati Far, B. Niosomes: A novel targeted drug delivery system for cancer. Med. Oncol. 2022, 39, 240. [Google Scholar] [CrossRef]
- Kianinejad, N.; Kwon, Y.M. Preparation and Size Characterization of Niosomal Nanocarriers for Targeted Drug Delivery. Master’s Thesis, College of Pharmacy, Ft. Lauderdale, FL, USA, 2022. [Google Scholar]
- Durak, S.; Esmaeili Rad, M.; Alp Yetisgin, A.; Eda Sutova, H.; Kutlu, O.; Cetinel, S.; Zarrabi, A. Niosomal drug delivery systems for ocular disease—Recent advances and future prospects. Nanomaterials 2020, 10, 1191. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Thabet, Y.; Elsabahy, M.; Eissa, N.G. Methods for preparation of niosomes: A focus on thin-film hydration method. Methods 2022, 199, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cosco, D.; Paolino, D.; Muzzalupo, R.; Celia, C.; Citraro, R.; Caponio, D.; Picci, N.; Fresta, M. Novel PEG-coated niosomes based on bola-surfactant as drug carriers for 5-fluorouracil. Biomed. Microdevices 2009, 11, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Abouzeid, A.H.; Patel, N.R.; Rachman, I.M.; Senn, S.; Torchilin, V.P. Anti-cancer activity of anti-GLUT1 antibody-targeted polymeric micelles co-loaded with curcumin and doxorubicin. J. Drug Target. 2013, 21, 994–1000. [Google Scholar] [CrossRef]
- Chou, T.-C.; Motzer, R.J.; Tong, Y.; Bosl, G.J. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: A rational approach to clinical protocol design. JNCI J. Natl. Cancer Inst. 1994, 86, 1517–1524. [Google Scholar] [CrossRef]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Guo, Y.; Terazzi, E.; Seemann, R.; Fleury, J.B.; Baulin, V.A. Direct proof of spontaneous translocation of lipid-covered hydrophobic nanoparticles through a phospholipid bilayer. Sci. Adv. 2016, 2, e1600261. [Google Scholar] [CrossRef]
- Fu, X.; Kong, W.; Zhang, Y.; Jiang, L.; Wang, J.; Lei, J. Novel solid–solid phase change materials with biodegradable trihydroxy surfactants for thermal energy storage. RSC Adv. 2015, 5, 68881–68889. [Google Scholar] [CrossRef]
- Mokale, V.; Patil, H.; Patil, A.; Shirude, P.; Naik, J.; Naik, B. Formulation and optimisation of famotidine proniosomes: An in vitro and ex vivo study. J. Exp. Nanosci. 2015, 11, 97–110. [Google Scholar] [CrossRef]
- Motevalli, S.M.; Eltahan, A.S.; Liu, L.; Magrini, A.; Rosato, N.; Guo, W.; Bottini, M.; Liang, X.-J. Co-encapsulation of curcumin and doxorubicin in albumin nanoparticles blocks the adaptive treatment tolerance of cancer cells. Biophys. Rep. 2019, 5, 19–30. [Google Scholar] [CrossRef]
- Hsieh, Y.F.; Chen, T.L.; Wang, Y.T.; Chang, J.H.; Chang, H.M. Properties of liposomes prepared with various lipids. J. Food Sci. 2002, 67, 2808–2813. [Google Scholar] [CrossRef]
- Rehman, A.U.; Omran, Z.; Anton, H.; Mély, Y.; Akram, S.; Vandamme, T.F.; Anton, N. Development of doxorubicin hydrochloride loaded pH-sensitive liposomes: Investigation on the impact of chemical nature of lipids and liposome composition on pH-sensitivity. Eur. J. Pharm. Biopharm. 2018, 133, 331–338. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Choy, K.L.; Lai, S.K.; Suk, J.S.; Tang, B.C.; Prabhu, S.; Hanes, J. PEGylation of nanoparticles improves their cytoplasmic transport. Int. J. Nanomed. 2007, 2, 735–741. [Google Scholar]
- Zmerli, I.; Michel, J.-P.; Makky, A. Bioinspired polydopamine nanoparticles: Synthesis, nanomechanical properties, and efficient PEGylation strategy. J. Mater. Chem. B 2020, 8, 4489–4504. [Google Scholar] [CrossRef] [PubMed]
- Tully, M.; Dimde, M.; Weise, C.; Pouyan, P.; Licha, K.; Schirner, M.; Haag, R. Polyglycerol for half-life extension of proteins—Alternative to PEGylation? Biomacromolecules 2021, 22, 1406–1416. [Google Scholar] [CrossRef]
- Feng, H.; Kang, J.-H.; Qi, S.; Kishimura, A.; Mori, T.; Katayama, Y. Preparation of a PEGylated liposome that co-encapsulates l-arginine and doxorubicin to achieve a synergistic anticancer effect. RSC Adv. 2021, 11, 34101–34106. [Google Scholar] [CrossRef]
- Najlah, M.; Said Suliman, A.; Tolaymat, I.; Kurusamy, S.; Kannappan, V.; Elhissi, A.M.; Wang, W. Development of injectable PEGylated liposome encapsulating disulfiram for colorectal cancer treatment. Pharmaceutics 2019, 11, 610. [Google Scholar] [CrossRef]
- Mohammed, I.; Mahmoud, M.; Al Shehri, D.; Kamal, M.S.; Alade, O.S. Effects of the Reservoir Environment and Oilfield Operations on the Iron Mineral Surface Charge Development: An Insight into Their Role in Wettability Alteration. Energy Fuels 2022, 36, 1676–1687. [Google Scholar] [CrossRef]
- Talebi, V.; Ghanbarzadeh, B.; Hamishehkar, H.; Pezeshki, A.; Ostadrahimi, A. Effects of different stabilizers on colloidal properties and encapsulation efficiency of vitamin D3 loaded nano-niosomes. J. Drug Deliv. Sci. Technol. 2021, 61, 101284. [Google Scholar] [CrossRef]
- Ghaferi, M.; Raza, A.; Koohi, M.; Zahra, W.; Akbarzadeh, A.; Ebrahimi Shahmabadi, H.; Alavi, S.E. Impact of PEGylated Liposomal Doxorubicin and Carboplatin Combination on Glioblastoma. Pharmaceutics 2022, 14, 2183. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, F.; Marverti, G.; D’Arca, D.; Severi, L.; Maretti, E.; Iannuccelli, V.; Pacifico, S.; Ponterini, G.; Costi, M.P.; Leo, E. pH-promoted release of a novel anti-tumour peptide by “stealth” liposomes: Effect of nanocarriers on the drug activity in cis-platinum resistant cancer cells. Pharm. Res. 2018, 35, 206. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Z.; Luo, R.; Fu, J. Travoprost-loaded PEGylated solid lipid nanoparticle-laden silicone contact lens for managing glaucoma. J. Drug Deliv. Sci. Technol. 2021, 66, 102731. [Google Scholar] [CrossRef]
- Seleci, D.A.; Seleci, M.; Jochums, A.; Walter, J.-G.; Stahl, F.; Scheper, T. Aptamer mediated niosomal drug delivery. RSC Adv. 2016, 6, 87910–87918. [Google Scholar] [CrossRef]
- Zhou, M.; Huang, H.; Wang, D.; Lu, H.; Chen, J.; Chai, Z.; Yao, S.Q.; Hu, Y. Light-triggered PEGylation/dePEGylation of the nanocarriers for enhanced tumor penetration. Nano Lett. 2019, 19, 3671–3675. [Google Scholar] [CrossRef] [PubMed]
- Firouzi Amoodizaj, F.; Baghaeifar, S.; Taheri, E.; Farhoudi Sefidan Jadid, M.; Safi, M.; Seyyed Sani, N.; Hajazimian, S.; Isazadeh, A.; Shanehbandi, D. Enhanced anticancer potency of doxorubicin in combination with curcumin in gastric adenocarcinoma. J. Biochem. Mol. Toxicol. 2020, 34, e22486. [Google Scholar] [CrossRef]
- Khan, M.M.; Madni, A.; Tahir, N.; Parveen, F.; Khan, S.; Jan, N.; Ali, A.; Abdurrahim, M.; Farooq, U.; Khan, M.I. Co-Delivery of Curcumin and Cisplatin to Enhance Cytotoxicity of Cisplatin Using Lipid-Chitosan Hybrid Nanoparticles. Int. J. Nanomed. 2020, 15, 2207–2217. [Google Scholar] [CrossRef]
- Hemati, M.; Haghiralsadat, F.; Jafary, F.; Moosavizadeh, S.; Moradi, A. Targeting cell cycle protein in gastric cancer with CDC20siRNA and anticancer drugs (doxorubicin and quercetin) co-loaded cationic PEGylated nanoniosomes. Int. J. Nanomed. 2019, 14, 6575–6585. [Google Scholar] [CrossRef]


| Time (min) | Acetonitrile (% v/v) | Water with 0.2% TFA (% v/v) | Methanol (% v/v) |
|---|---|---|---|
| 0 | 25 | 50 | 25 |
| 7 | 25 | 50 | 25 |
| 8.5 | 40 | 40 | 20 |
| 10 | 60 | 30 | 10 |
| 11.5 | 80 | 20 | 0 |
| 13 | 100 | 0 | 0 |
| 16 | 100 | 0 | 0 |
| 17 | 25 | 50 | 25 |
| 20 | 25 | 50 | 25 |
| Sample Name | Size (nm ± SD) | PDI ± SD | Surface Charge (mV ± SD) |
|---|---|---|---|
| DOX-loaded niosome | 147.4 ± 1.5 | 0.34 ± 0.02 | −19.1 ± 1.5 |
| CUR-loaded niosome | 185.9 ± 0.9 | 0.28 ± 0.05 | −4.9 ± 0.4 |
| Co-loaded niosome | 188.9 ± 1.1 | 0.37 ± 0.11 | −18.3 ± 1.2 |
| DOX-loaded PEGylated niosome | 196.6 ± 2.2 | 0.33 ± 0.09 | −46.4 ± 2.3 |
| CUR-loaded PEGylated niosome | 236.8 ± 1.9 | 0.30 ± 0.06 | −46.7 ± 1.9 |
| Co-loaded PEGylated niosome | 273.1 ± 3.2 | 0.39 ± 0.08 | −43.2 ± 1.0 |
| Sample Name | DOX EE% ± SD | CUR EE% ± SD |
|---|---|---|
| DOX-loaded niosome (non-coated) | 19.31 ± 1.3 | - |
| CUR-loaded niosome (non-coated) | - | 83.10 ± 3.1 |
| DOX and CUR co-loaded niosome (non-coated) | 23.31 ± 2.2 | 86.50 ± 4.02 |
| DOX-loaded niosome (coated with PEG) | 84.00 ± 3.1 | - |
| CUR-loaded niosome (coated with PEG) | - | 95.02 ± 2.4 |
| DOX and CUR co-loaded niosome (coated with PEG) | 62.90 ± 1.1 | 96.50 ± 3.7 |
| Sample Name | EE% of DOX ± SD | EE% of CUR ± SD |
|---|---|---|
| DOX and CUR co-loaded niosome (non-PEGylated) | 13.1 ± 1.5 | 61.4 ± 2.3 |
| DOX and CUR co-loaded niosome (PEGylated) | 54.3 ± 0.9 | 76.2 ± 3.3 |
| Treatment Name | IC50 Value (µg/mL) |
|---|---|
| Niosome (DOX) | 26.4 ± 2.4 |
| Niosome (Cur) | 57.1 ± 0.99 |
| Niosome (DOX-Cur) | 15.8 ± 2.1 |
| Niosome-PEG (DOX) | 36.4 ± 1.6 |
| Niosome-PEG (Cur) | 64.6 ± 1.4 |
| Niosome-PEG (DOX-Cur) | 20.7 ± 2.3 |
| Sample | CI Value | Interaction Type |
|---|---|---|
| Pure DOX + Pure Cur | 0.41 | Synergic |
| Niosome (DOX-Cur) | 0.45 | Synergic |
| Niosome-PEG (DOX-Cur) | 0.70 | synergic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saharkhiz, S.; Zarepour, A.; Zarrabi, A. Empowering Cancer Therapy: Comparing PEGylated and Non-PEGylated Niosomes Loaded with Curcumin and Doxorubicin on MCF-7 Cell Line. Bioengineering 2023, 10, 1159. https://doi.org/10.3390/bioengineering10101159
Saharkhiz S, Zarepour A, Zarrabi A. Empowering Cancer Therapy: Comparing PEGylated and Non-PEGylated Niosomes Loaded with Curcumin and Doxorubicin on MCF-7 Cell Line. Bioengineering. 2023; 10(10):1159. https://doi.org/10.3390/bioengineering10101159
Chicago/Turabian StyleSaharkhiz, Shaghayegh, Atefeh Zarepour, and Ali Zarrabi. 2023. "Empowering Cancer Therapy: Comparing PEGylated and Non-PEGylated Niosomes Loaded with Curcumin and Doxorubicin on MCF-7 Cell Line" Bioengineering 10, no. 10: 1159. https://doi.org/10.3390/bioengineering10101159