Exploring the Antimicrobial, Anticancer, and Apoptosis Inducing Ability of Biofabricated Silver Nanoparticles Using Lagerstroemia speciosa Flower Buds against the Human Osteosarcoma (MG-63) Cell Line via Flow Cytometry
Abstract
:1. Introduction
- (i)
- Bio-inspired synthesis of AgNPs utilizing the flower bud extract of the L. speciosa plant;
- (ii)
- Characterization of biosynthesized AgNPs by using various analytical techniques;
- (iii)
- Evaluating the antimicrobial activity of biosynthesized AgNPs against human clinical pathogens;
- (iv)
- Determination of in vitro anticancer potential by MTT assay using biosynthesized AgNPs against MG-63 cell line and exploring the apoptotic inducing ability of biosynthesized AgNPs through flow cytometry.
2. Materials and Methods
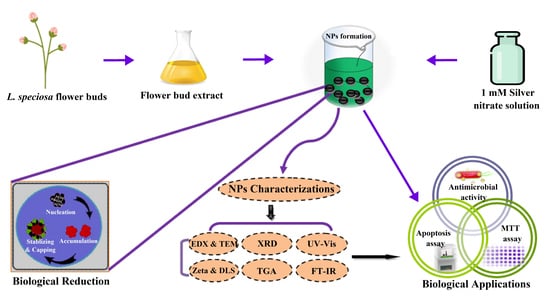
2.1. Collection and Preparation of Flower Bud Extract
2.2. Synthesis of Silver Nanoparticles
2.3. Characterisation of Bio-Fabricated Ls-AgNPs
2.3.1. UV-Visible Spectrophotometric Analysis
2.3.2. Fourier Transform Infrared Spectroscopic Analysis
2.3.3. X-ray Diffraction Analysis
2.3.4. Energy Dispersive X-ray Analysis
2.3.5. Transmission Electron Microscopy
2.3.6. Zeta Potential and Dynamic Light Scattering Analysis
2.3.7. Thermo Gravimetric Analysis
2.4. Antimicrobial Activity of Ls-AgNPs
2.5. In-Vitro Anticancer Activity of Ls-AgNPs
2.5.1. MTT Assay
2.5.2. Annexin-V/Propidium Iodide Apoptosis Detection Assay
2.6. Statistical Analysis
3. Results
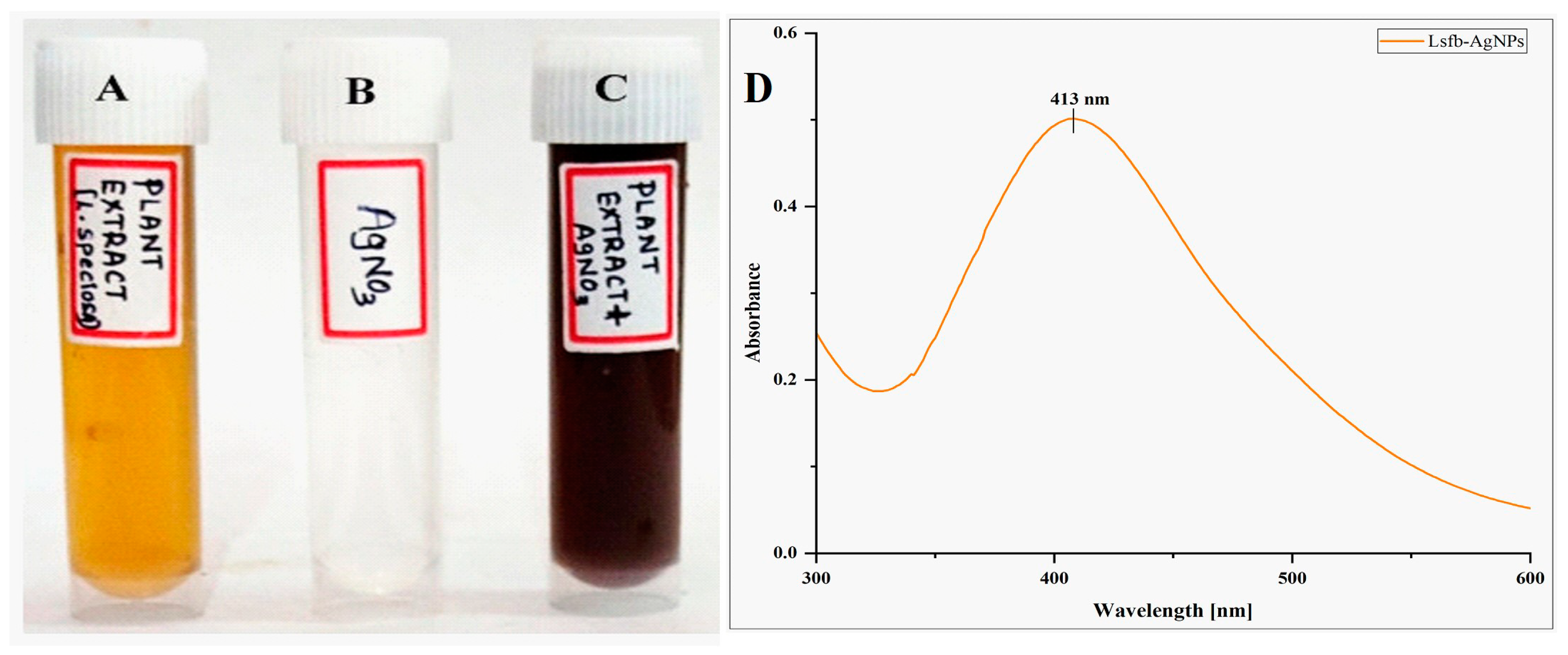
3.1. Synthesis of Silver Nanoparticles and UV-Visible Spectrophotometric Analysis
3.2. FTIR Analysis
3.3. XRD Analysis
3.4. EDX Analysis
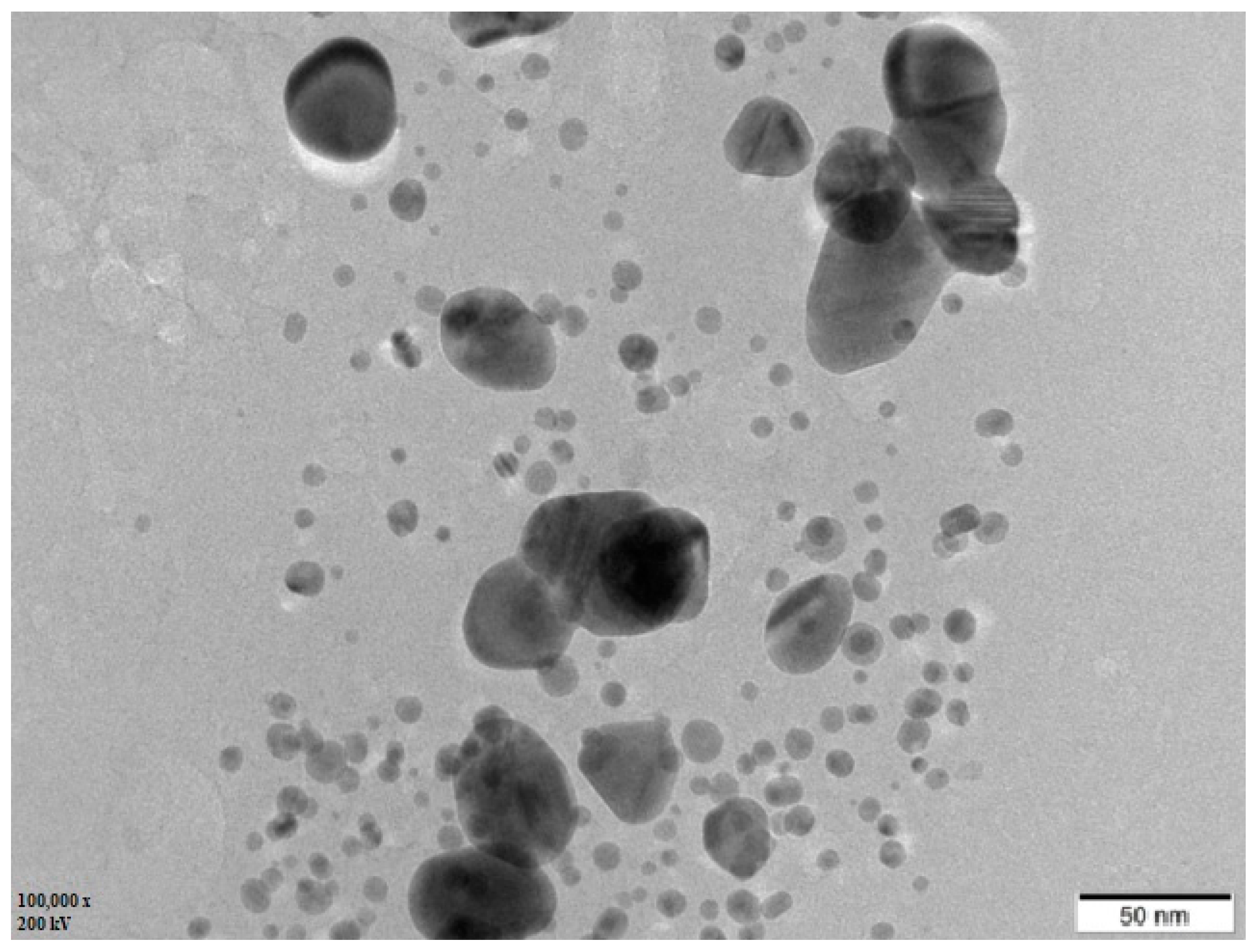
3.5. TEM Analysis
3.6. Zeta Potential and DLS Analysis
3.7. TGA Analysis
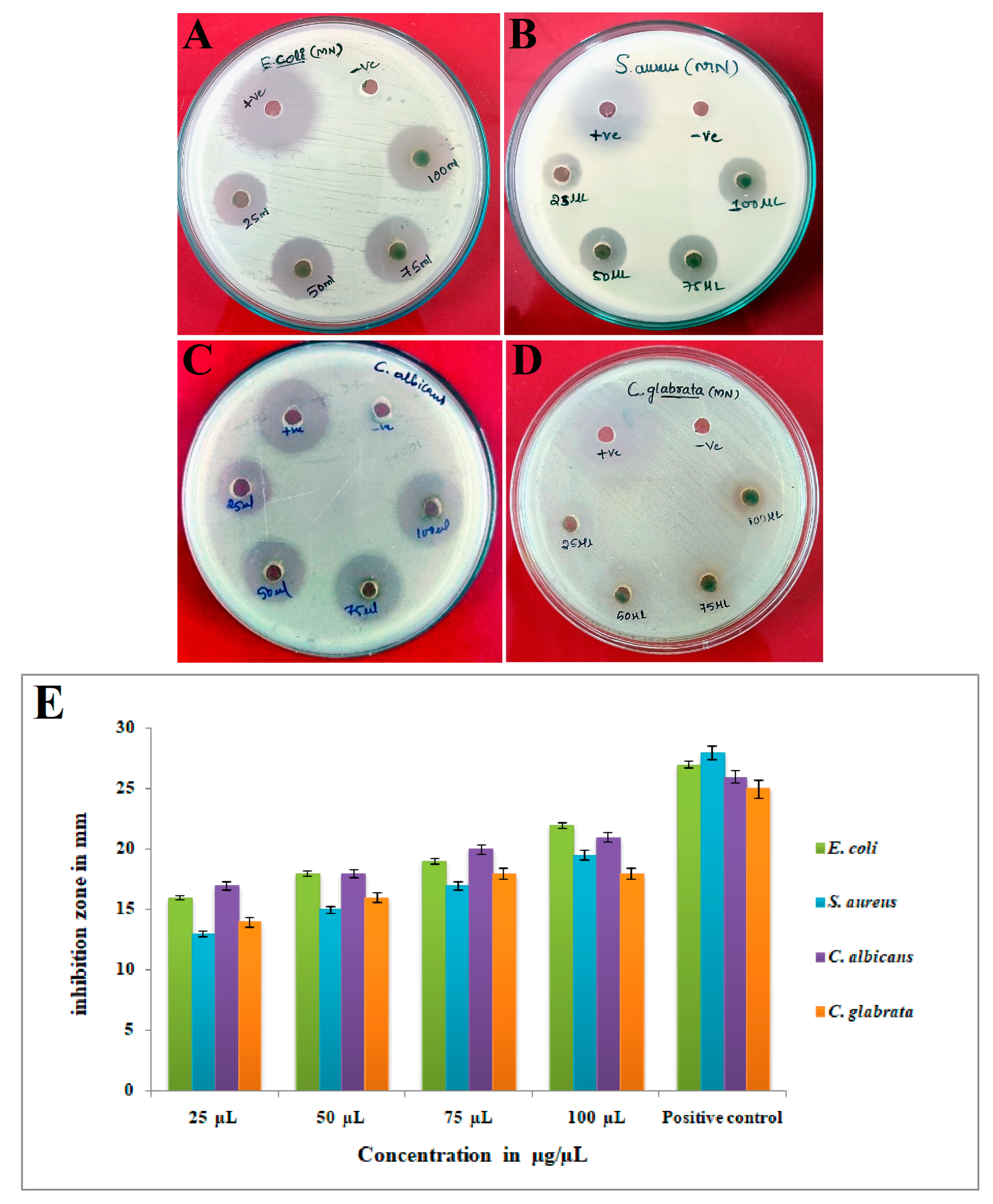
3.8. Antimicrobial Activity of Synthesized Ls-AgNPs
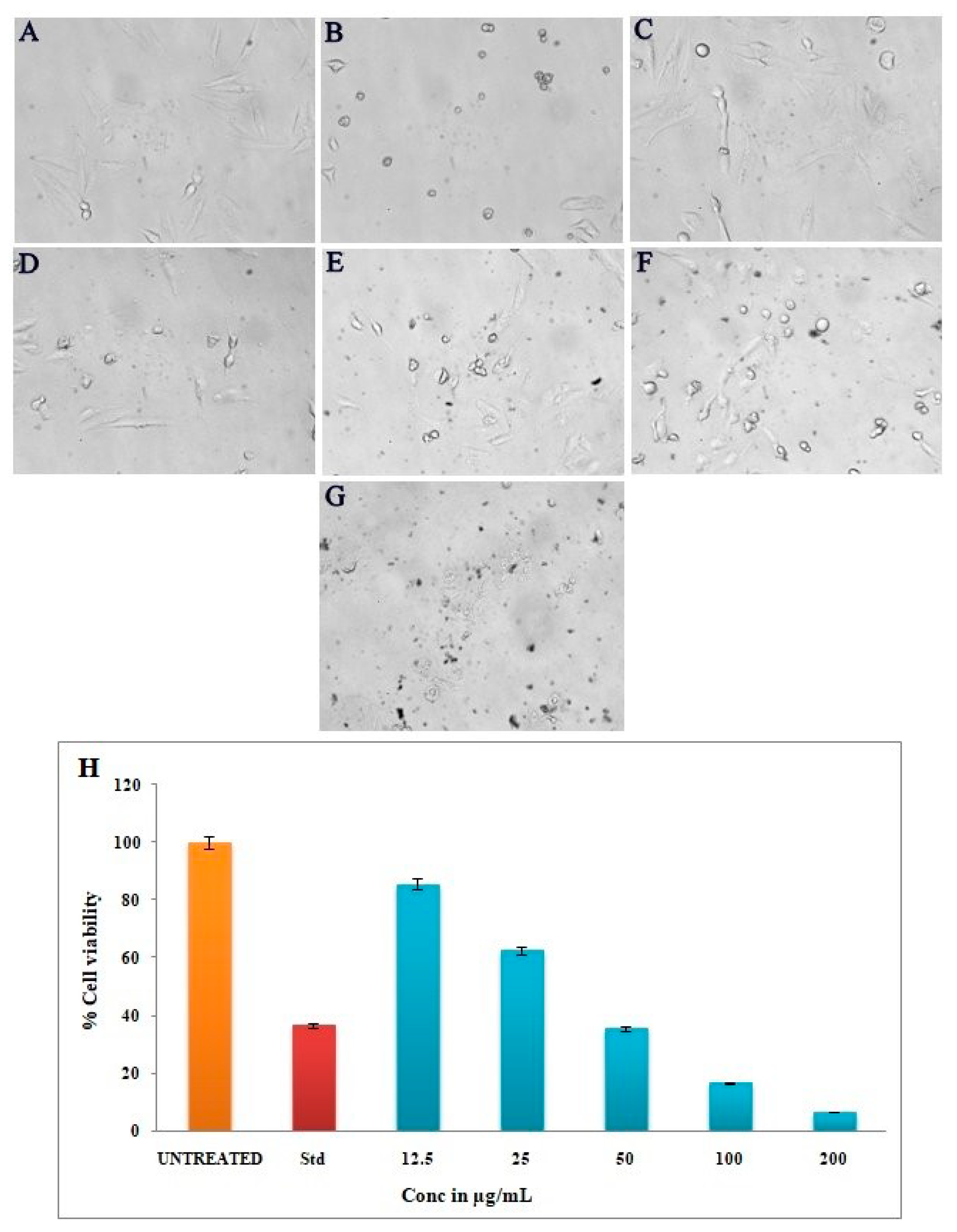
3.9. Anticancer Activity of Silver Nanoparticles by MTT Assay
3.10. Apoptosis/Necrosis Assay by Flow Cytometry
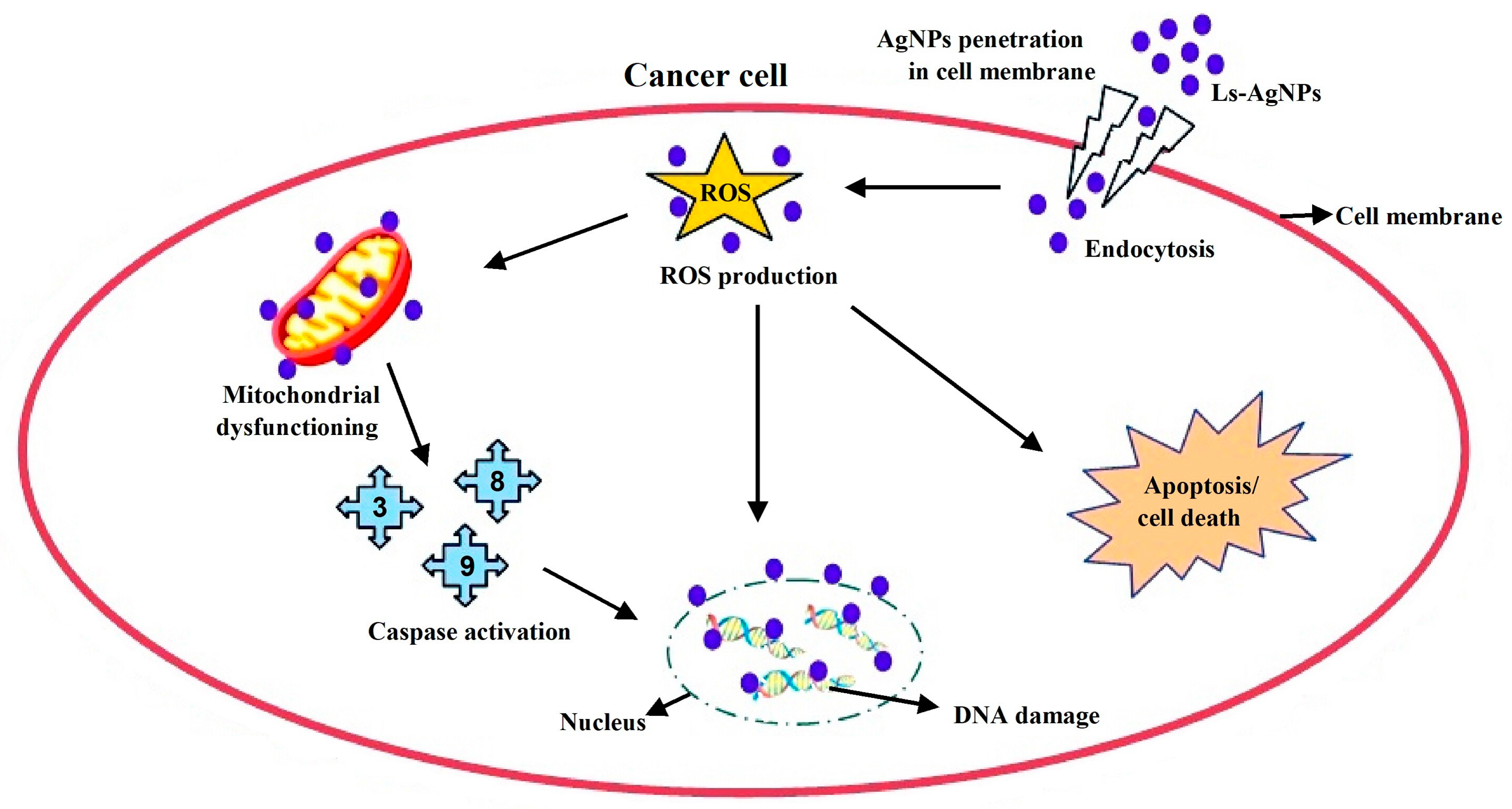
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moodley, J.S.; Krishna, S.B.N.; Pillay, K.; Sershen; Govender, P. Green Synthesis of Silver Nanoparticles from Moringa oleifera Leaf Extracts and Its Antimicrobial Potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015011. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jebril, S.; Fdhila, A.; Dridi, C. Nanoengineering of Eco-Friendly Silver Nanoparticles Using Five Different Plant Extracts and Development of Cost-Effective Phenol Nanosensor. Sci. Rep. 2021, 11, 22060. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of metal nanoparticles and their pharmacological applications: Present status and application prospects. J. Nanostruct. Chem. 2018, 8, 217–254. [Google Scholar] [CrossRef] [Green Version]
- Govindarajan, M.; Rajeswary, M.; Veerakumar, K.; Muthukumaran, U.; Hoti, S.L.; Benelli, G. Green Synthesis and Characterization of Silver Nanoparticles Fabricated Using Anisomeles indica: Mosquitocidal Potential against Malaria, Dengue and Japanese Encephalitis Vectors. Exp. Parasitol. 2016, 161, 40–47. [Google Scholar] [CrossRef]
- Chung, I.-M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Nagaraja, S.K.; Niazi, S.K.; Bepari, A.; Assiri, R.A.; Nayaka, S. Leonotis nepetifolia Flower Bud Extract Mediated Green Synthesis of Silver Nanoparticles, their Characterization, and in vitro Evaluation of Biological Applications. Materials 2022, 15, 8990. [Google Scholar] [CrossRef]
- Muthukumaran, U.; Govindarajan, M.; Rajeswary, M.; Hoti, S.L. Synthesis and Characterization of Silver Nanoparticles Using Gmelina asiatica Leaf Extract against Filariasis, Dengue, and Malaria Vector Mosquitoes. Parasitol. Res. 2015, 114, 1817–1827. [Google Scholar] [CrossRef]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf Extract Mediated Green Synthesis of Silver Nanoparticles from Widely Available Indian Plants: Synthesis, Characterization, Antimicrobial Property and Toxicity Analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Kolakul, P.; Sripanidkulchai, B. Phytochemicals and Anti-Aging Potentials of the Extracts from Lagerstroemia speciosa and Lagerstroemia floribunda. Ind. Crops Prod. 2017, 109, 707–716. [Google Scholar] [CrossRef]
- Mousa, A.M.; El-Sammad, N.M.; Abdel-Halim, A.H.; Anwar, N.; Khalil, W.K.B.; Nawwar, M.; Hashim, A.N.; Elsayed, E.A.; Hassan, S.K. Lagerstroemia speciosa (L.) Pers Leaf Extract Attenuates Lung Tumorigenesis via Alleviating Oxidative Stress, Inflammation and Apoptosis. Biomolecules 2019, 9, 871. [Google Scholar] [CrossRef] [Green Version]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma Incidence and Survival Rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Vasco, V.R.; Leopizzi, M.; Di Maio, V.; Della Rocca, C. U-73122 Reduces the Cell Growth in Cultured MG-63 Ostesarcoma Cell Line Involving Phosphoinositide-Specific Phospholipases C. SpringerPlus 2016, 5, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiller, C.A.; Craft, A.W.; Corazziari, I. Survival of Children with Bone Sarcoma in Europe since 1978. Eur. J. Cancer 2001, 37, 760–766. [Google Scholar] [CrossRef]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of Osteosarcoma Cell Lines MG-63, Saos-2 and U-2 OS in Comparison to Human Osteoblasts. Anticancer. Res. 2004, 24, 3743–3748. [Google Scholar]
- Nithya, P.; Raghunathan, S.; Prabakaran, M.; Antony, S.A.; MubarakAli, D. Synthesis and Characterization of Tween-20 Capped Biosynthesized Silver Nanoparticles for Anticancer and Antimicrobial Property. Appl. Biochem. Biotechnol. 2023, 195, 2282–2293. [Google Scholar] [CrossRef]
- De Giglio, E.; Cafagna, D.; Cometa, S.; Allegretta, A.; Pedico, A.; Giannossa, L.C.; Sabbatini, L.; Mattioli-Belmonte, M.; Iatta, R. An Innovative, Easily Fabricated, Silver Nanoparticle-Based Titanium Implant Coating: Development and Analytical Characterization. Anal. Bioanal. Chem. 2013, 405, 805–816. [Google Scholar] [CrossRef]
- Ud Din, Q.M.; Kangaraj, R. Anti-Metastatic Properties of Bio Synthesized AgNPs on Osteosarcoma (MG-63) Cancer Cell Lines. BJSTR 2017, 1, 1120–1123. [Google Scholar] [CrossRef] [Green Version]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Kumari, M.; Nayak, B. Bark Extract Mediated Green Synthesis of Silver Nanoparticles: Evaluation of Antimicrobial Activity and Antiproliferative Response against Osteosarcoma. Mater. Sci. Eng. C 2016, 58, 44–52. [Google Scholar] [CrossRef]
- Singh, N.; Chatterjee, A.; Chakraborty, K.; Chatterjee, S.; Abraham, J. Cytotoxic Effect on MG-63 Cell Line and Antimicrobial and Antioxidant Properties of Silver Nanoparticles Synthesized with Seed Extracts of Capsicum sp. Rec. Nat. Prod. 2016, 10, 47–57. [Google Scholar]
- Nagaraja, S.K.; Nayaka, S.; Kumar, R.S. Phytochemical Analysis, GC-MS profiling, and In vitro Evaluation of Biological Applications of Different Solvent Extracts of Leonotis nepetifolia (L.) R.Br. Flower Buds. Appl. Biochem. Biotechnol. 2023, 195, 1197–1215. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Chakraborty, B.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Kotresha, D.; Pallavi, S.S.; Dhanyakumara, S.B.; Shashiraj, K.N.; Nayaka, S. Biogenic Synthesis, Characterization and Antimicrobial Activity of Ixora brachypoda (DC) Leaf Extract Mediated Silver Nanoparticles. J. King Saud Univ. Sci. 2021, 33, 101296. [Google Scholar] [CrossRef]
- Gopinath, K.; Gowri, S.; Arumugam, A. Phytosynthesis of Silver Nanoparticles using Pterocarpus santalinus Leaf Extract and Their Antibacterial Properties. J. Nanostr. Chem. 2013, 3, 68. [Google Scholar] [CrossRef]
- Wang, D.; Markus, J.; Wang, C.; Kim, Y.-J.; Mathiyalagan, R.; Aceituno, V.C.; Ahn, S.; Yang, D.C. Green Synthesis of Gold and Silver Nanoparticles using Aqueous Extract of Cibotium barometz Root. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasa, N.; Bidhayak, C.; Pallavi, S.S.; Bhat, M.P.; Shashiraj, K.N.; Ghasti, B. Synthesis of Biogenic Silver Nanoparticles using Zanthoxylum rhetsa (Roxb.) DC Seed Coat Extract as Reducing agent and In-Vitro Assessment of Anticancer Effect on A549 Lung Cancer Cell Line. Int. J. Pharm. Res. 2020, 12, 302–314. [Google Scholar]
- Basavarajappa, D.S.; Kumar, R.S.; Almansour, A.I.; Chakraborty, B.; Bhat, M.P.; Nagaraja, S.K.; Hiremath, H.; Perumal, K.; Nayaka, S. Biofunctionalized Silver Nanoparticles Synthesized from Passiflora vitifolia Leaf Extract and Evaluation of Its Antimicrobial, Antioxidant and Anticancer Activities. Biochem. Eng. J. 2022, 187, 108517. [Google Scholar] [CrossRef]
- Kambale, E.K.; Nkanga, C.I.; Mutonkole, B.-P.I.; Bapolisi, A.M.; Tassa, D.O.; Liesse, J.-M.I.; Krause, R.W.M.; Memvanga, P.B. Green Synthesis of Antimicrobial Silver Nanoparticles Using Aqueous Leaf Extracts from Three Congolese Plant Species (Brillantaisia Patula, Crossopteryx Febrifuga and Senna Siamea). Heliyon 2020, 6, e04493. [Google Scholar] [CrossRef]
- Dongargaonkar, A.A.; Clogston, J.D. Quantisation of Surface Coating on Nanoparticles using Thermo Gravimetric Analysis. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1682, pp. 57–63. ISBN 978-1-4939-7350-7. [Google Scholar]
- Chand, K.; Cao, D.; Eldin Fouad, D.; Hussain Shah, A.; Qadeer Dayo, A.; Zhu, K.; Nazim Lakhan, M.; Mehdi, G.; Dong, S. Green Synthesis, Characterization and Photocatalytic Application of Silver Nanoparticles Synthesized by Various Plant Extracts. Arab. J. Chem. 2020, 13, 8248–8261. [Google Scholar] [CrossRef]
- Aritonang, H.F.; Koleangan, H.; Wuntu, A.D. Synthesis of Silver Nanoparticles Using Aqueous Extract of Medicinal Plants’ (Impatiens balsamina and Lantana camara) Fresh Leaves and Analysis of Antimicrobial Activity. Int. J. Microbiol. 2019, 2019, 8642303. [Google Scholar] [CrossRef] [Green Version]
- Mussin, J.; Robles-Botero, V.; Casañas-Pimentel, R.; Rojas, F.; Angiolella, L.; San Martín-Martínez, E.; Giusiano, G. Antimicrobial and Cytotoxic Activity of Green Synthesis Silver Nanoparticles Targeting Skin and Soft Tissue Infectious Agents. Sci. Rep. 2021, 11, 14566. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, M.; Rudayni, H.A.; Assiri, R.A.; Bepari, A.; Basavarajappa, D.S.; Nagaraja, S.K.; Chakraborty, B.; Swamy, P.S.; Agadi, S.N.; Niazi, S.K.; et al. Plumeria alba-Mediated Green Synthesis of Silver Nanoparticles Exhibits Antimicrobial Effect and Anti-Oncogenic Activity against Glioblastoma U118 MG Cancer Cell Line. Nanomaterials 2022, 12, 493. [Google Scholar] [CrossRef]
- O’Brien, M.C.; Bolton, W.E. Comparison of Cell Viability Probes Compatible with Fixation and Permeabilization for Combined Surface and Intracellular Staining in Flow Cytometry. Cytometry 1995, 19, 243–255. [Google Scholar] [CrossRef]
- Pereira, T.M.; Polez, V.L.P.; Sousa, M.H.; Silva, L.P. Modulating Physical, Chemical, and Biological Properties of Silver Nanoparticles Obtained by Green Synthesis Using Different Parts of the Tree Handroanthus heptaphyllus (Vell.) Mattos. Colloid Interface Sci. Commun. 2020, 34, 100224. [Google Scholar] [CrossRef]
- Shashiraj, K.N.; Nayaka, S.; Kumar, R.S.; Kantli, G.B.; Basavarajappa, D.S.; Gunagambhire, P.V.; Almansour, A.I.; Perumal, K. Rotheca serrata Flower Bud Extract Mediated Bio-Friendly Preparation of Silver Nanoparticles: Their Characterizations, Anticancer, and Apoptosis Inducing Ability against Pancreatic Ductal Adenocarcinoma Cell Line. Processes 2023, 11, 893. [Google Scholar] [CrossRef]
- Gnanakani, P.E.; Santhanam, P.; Premkumar, K.; Eswar Kumar, K.; Dhanaraju, M.D. Nannochloropsis Extract–Mediated Synthesis of Biogenic Silver Nanoparticles, Characterization and In Vitro Assessment of Antimicrobial, Antioxidant and Cytotoxic Activities. Asian. Pac. J. Cancer Prev. 2019, 20, 2353–2364. [Google Scholar] [CrossRef]
- Rajesh Kumar, T.V.; Murthy, J.S.R.; Narayana Rao, M.; Bhargava, Y. Evaluation of Silver Nanoparticles Synthetic Potential of Couroupita guianensis Aubl., Flower Buds Extract and Their Synergistic Antibacterial Activity. 3 Biotech 2016, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Nagaraja, S.K.; Kumar, R.S.; Chakraborty, B.; Hiremath, H.; Almansour, A.I.; Perumal, K.; Gunagambhire, P.V.; Nayaka, S. Biomimetic Synthesis of Silver Nanoparticles Using Cucumis sativus var. hardwickii Fruit Extract and Their Characterizations, Anticancer Potential and Apoptosis Studies against Pa-1 (Human Ovarian Teratocarcinoma) Cell Line via Flow Cytometry. Appl. Nanosci. 2023, 13, 3073–3084. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Lee, Y.; Kim, M.J.; Ahn, C.W. Biomimetic Synthesis of Silver Nanoparticles Using Syzygium aromaticum (Clove) Extract: Catalytic and Antimicrobial Effects. Appl. Organometal. Chem. 2019, 33, e4867. [Google Scholar] [CrossRef]
- Lakhan, M.N.; Chen, R.; Shar, A.H.; Chand, K.; Shah, A.H.; Ahmed, M.; Ali, I.; Ahmed, R.; Liu, J.; Takahashi, K.; et al. Eco-Friendly Green Synthesis of Clove Buds Extract Functionalized Silver Nanoparticles and Evaluation of Antibacterial and Antidiatom Activity. J. Microbiol. Methods 2020, 173, 105934. [Google Scholar] [CrossRef] [PubMed]
- El-Aswar, E.I.; Zahran, M.M.; El-Kemary, M. Optical and Electrochemical Studies of Silver Nanoparticles Biosynthesized by Haplophyllum tuberculatum Extract and Their Antibacterial Activity in Waste water Treatment. Mater. Res. Express 2019, 6, 105016. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of Silver Nanoparticles by Green Synthesis Method using Pedalium murex Leaf Extract and Their Antibacterial Activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Moteriya, P.; Chanda, S. Synthesis and Characterization of Silver Nanoparticles Using Caesalpinia pulcherrima Flower Extract and Assessment of Their in Vitro Antimicrobial, Antioxidant, Cytotoxic, and Genotoxic Activities. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1556–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K. Antibacterial Activity of Synthesized Silver Nanoparticles from Tinospora cordifolia against Multi Drug Resistant Strains of Pseudomonas aeruginosa Isolated from Burn Patients. J. Nanomed. Nanotechnol. 2014, 5, 1000192. [Google Scholar] [CrossRef]
- Lee, Y.J.; Song, K.; Cha, S.-H.; Cho, S.; Kim, Y.S.; Park, Y. Sesquiterpenoids from Tussilago farfara Flower Bud Extract for the Eco-Friendly Synthesis of Silver and Gold Nanoparticles Possessing Antibacterial and Anticancer Activities. Nanomaterials 2019, 9, 819. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Mohan, S.; Venkatesh, K.S.; Esakkirajan, M.; Kaleeswarran, P.; Alharbi, N.S.; Kadaikunnan, S.; Govindarajan, M.; et al. Green Synthesis of Silver, Gold and Silver/Gold Bimetallic Nanoparticles Using the Gloriosa superba Leaf Extract and Their Antibacterial and Antibiofilm Activities. Microb. Pathog. 2016, 101, 1–11. [Google Scholar] [CrossRef]
- Donga, S.; Chanda, S. Facile Green Synthesis of Silver Nanoparticles using Mangifera indica seed Aqueous Extract and its Antimicrobial, Antioxidant and Cytotoxic Potential (3-in-1 system). Artif. Cells Nanomed. Biotechnol. 2021, 49, 292–302. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Govindarajan, M.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Alyahya, S.A.; Al-anbr, M.N.; Gopinath, K.; Sudha, A. Nanosilver Crystals Capped with Bauhinia acuminata Phytochemicals as New Antimicrobials and Mosquito Larvicides. J. Trace Elem. Med. Biol. 2018, 50, 146–153. [Google Scholar] [CrossRef]
- Riaz, M.; Mutreja, V.; Sareen, S.; Ahmad, B.; Faheem, M.; Zahid, N.; Jabbour, G.; Park, J. Exceptional Antibacterial and Cytotoxic Potency of Monodisperse Areener AgNPs Prepared under Optimized pH and Temperature. Sci. Rep. 2021, 11, 2866. [Google Scholar] [CrossRef]
- Niu, G.; Yousefi, B.; Qujeq, D.; Marjani, A.; Asadi, J.; Wang, Z.; Mir, S.M. Melatonin and Doxorubicin Co-Delivered via a Functionalized Graphene-Dendrimeric System Enhances Apoptosis of Osteosarcoma Cells. Mater. Sci. Eng. C 2021, 119, 111554. [Google Scholar] [CrossRef]
- Iram, S.; Khan, S.; Ansary, A.A.; Arshad, M.; Siddiqui, S.; Ahmad, E.; Khan, R.H.; Khan, M.S. Biogenic Terbium Oxide Nanoparticles as the Vanguard against Osteosarcoma. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.; Ali, R.; Abd El-Monem, D.; El-Magd, M. Graviola Leaves Extract Enhances the Anticancer Effect of Cisplatin on Various Cancer Cell Lines. Mol. Cell. Toxicol. 2020, 16, 385–399. [Google Scholar] [CrossRef]
- Lekshmi, A.; Varadarajan, S.N.; Lupitha, S.S.; Indira, D.; Mathew, K.A.; Chandrasekharan, N.A.; Nair, M.; Prasad, T.; Sekar, H.; Gopalakrishnan, A.K.; et al. A Quantitative Real-time Approach for Discriminating Apoptosis and Necrosis. Cell Death Discov. 2017, 3, 16101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayromlou, A.; Masoudi, S.; Mirzaie, A. Scorzonera calyculata Aerial Part Extract Mediated Synthesis of Silver Nanoparticles: Evaluation of Their Antibacterial, Antioxidant and Anticancer Activities. J. Clust. Sci. 2019, 30, 1037–1050. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shashiraj, K.N.; Hugar, A.; Kumar, R.S.; Rudrappa, M.; Bhat, M.P.; Almansour, A.I.; Perumal, K.; Nayaka, S. Exploring the Antimicrobial, Anticancer, and Apoptosis Inducing Ability of Biofabricated Silver Nanoparticles Using Lagerstroemia speciosa Flower Buds against the Human Osteosarcoma (MG-63) Cell Line via Flow Cytometry. Bioengineering 2023, 10, 821. https://doi.org/10.3390/bioengineering10070821
Shashiraj KN, Hugar A, Kumar RS, Rudrappa M, Bhat MP, Almansour AI, Perumal K, Nayaka S. Exploring the Antimicrobial, Anticancer, and Apoptosis Inducing Ability of Biofabricated Silver Nanoparticles Using Lagerstroemia speciosa Flower Buds against the Human Osteosarcoma (MG-63) Cell Line via Flow Cytometry. Bioengineering. 2023; 10(7):821. https://doi.org/10.3390/bioengineering10070821
Chicago/Turabian StyleShashiraj, Kariyellappa Nagaraja, Anil Hugar, Raju Suresh Kumar, Muthuraj Rudrappa, Meghashyama Prabhakara Bhat, Abdulrahman I. Almansour, Karthikeyan Perumal, and Sreenivasa Nayaka. 2023. "Exploring the Antimicrobial, Anticancer, and Apoptosis Inducing Ability of Biofabricated Silver Nanoparticles Using Lagerstroemia speciosa Flower Buds against the Human Osteosarcoma (MG-63) Cell Line via Flow Cytometry" Bioengineering 10, no. 7: 821. https://doi.org/10.3390/bioengineering10070821
APA StyleShashiraj, K. N., Hugar, A., Kumar, R. S., Rudrappa, M., Bhat, M. P., Almansour, A. I., Perumal, K., & Nayaka, S. (2023). Exploring the Antimicrobial, Anticancer, and Apoptosis Inducing Ability of Biofabricated Silver Nanoparticles Using Lagerstroemia speciosa Flower Buds against the Human Osteosarcoma (MG-63) Cell Line via Flow Cytometry. Bioengineering, 10(7), 821. https://doi.org/10.3390/bioengineering10070821