Enhanced Enzymatic Sugar Recovery of Dilute-Acid-Pretreated Corn Stover by Sodium Carbonate Deacetylation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Chemicals
2.2. The Deacetylation of Corn Stover with Sodium Carbonate
2.3. Dilute Acid Treatment of Deacetylated Corn Stover
2.4. Enzymatic Hydrolysis of Treated Corn Stover
2.5. Composition Analysis of Treated Samples
2.6. Scanning Electron Microscopy (SEM) Analysis
2.7. FT-IR Analysis
2.8. Statistical Analysis
3. Results and Discussion
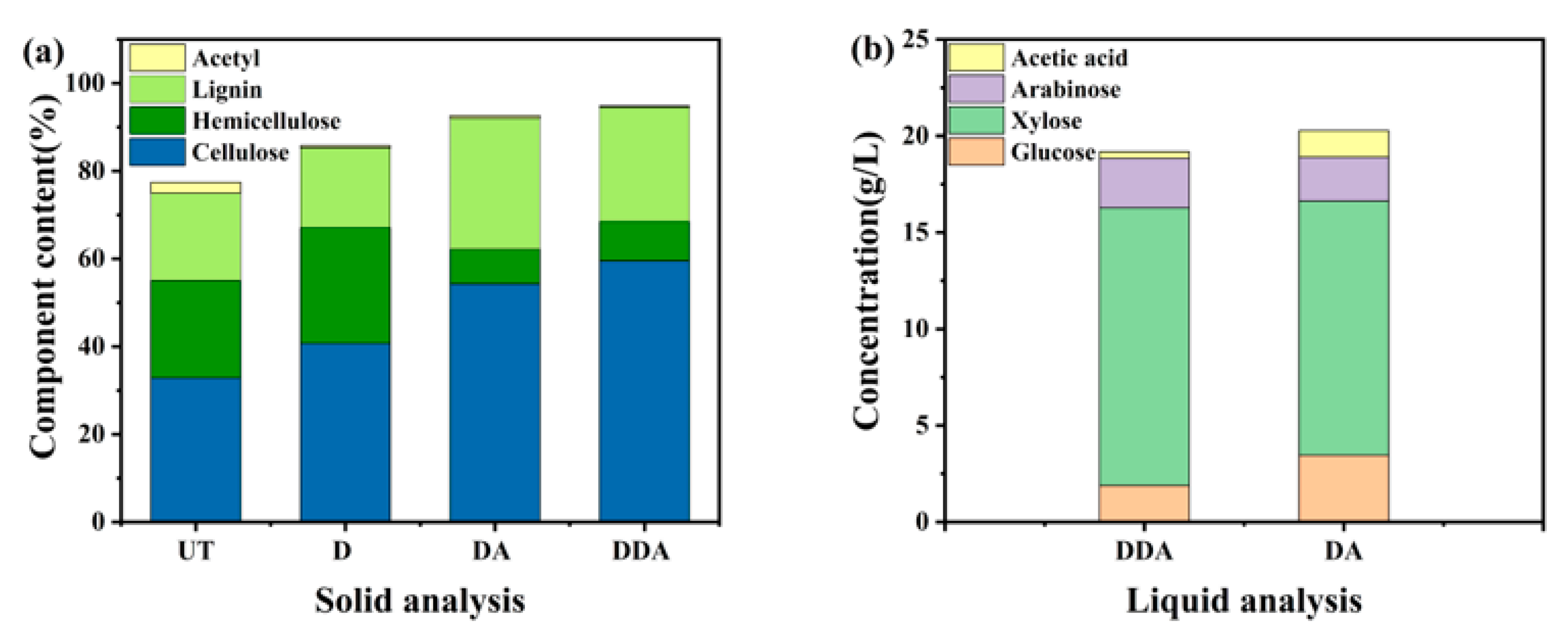
3.1. The Compositional Change of Corn Stover after Sodium Carbonate Deacetylation
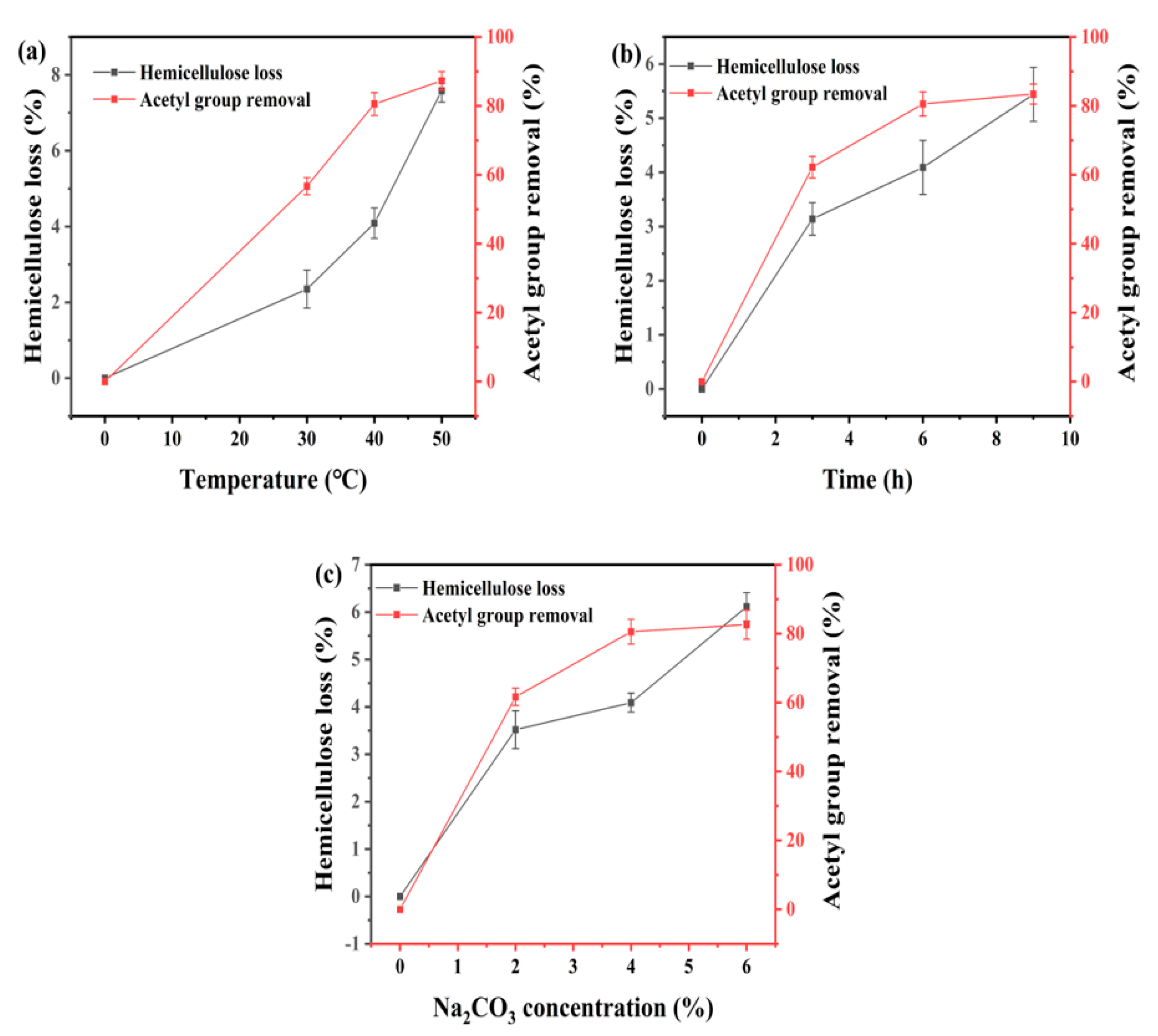
3.2. Effect of Temperature on Na2CO3 Deacetylation of Corn Stover
3.3. Effect of Reaction Time on Deacetylation of Corn Stover
3.4. Effect of Na2CO3 Concentration on Deacetylation of Corn Stover
3.5. Effect of Sodium Carbonate Deacetylation on Dilute Acid Pretreatment
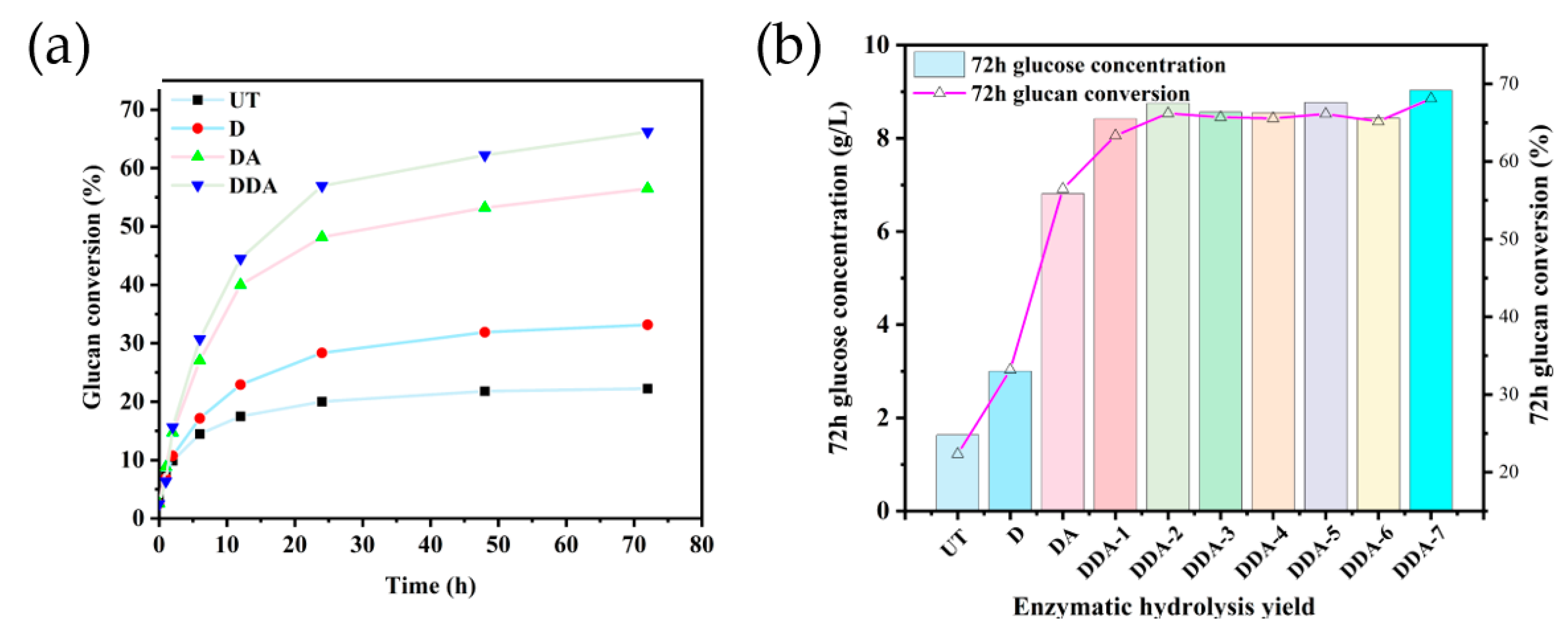
3.6. Effect of Na2CO3 Deacetylation on Enzymatic Hydrolysis
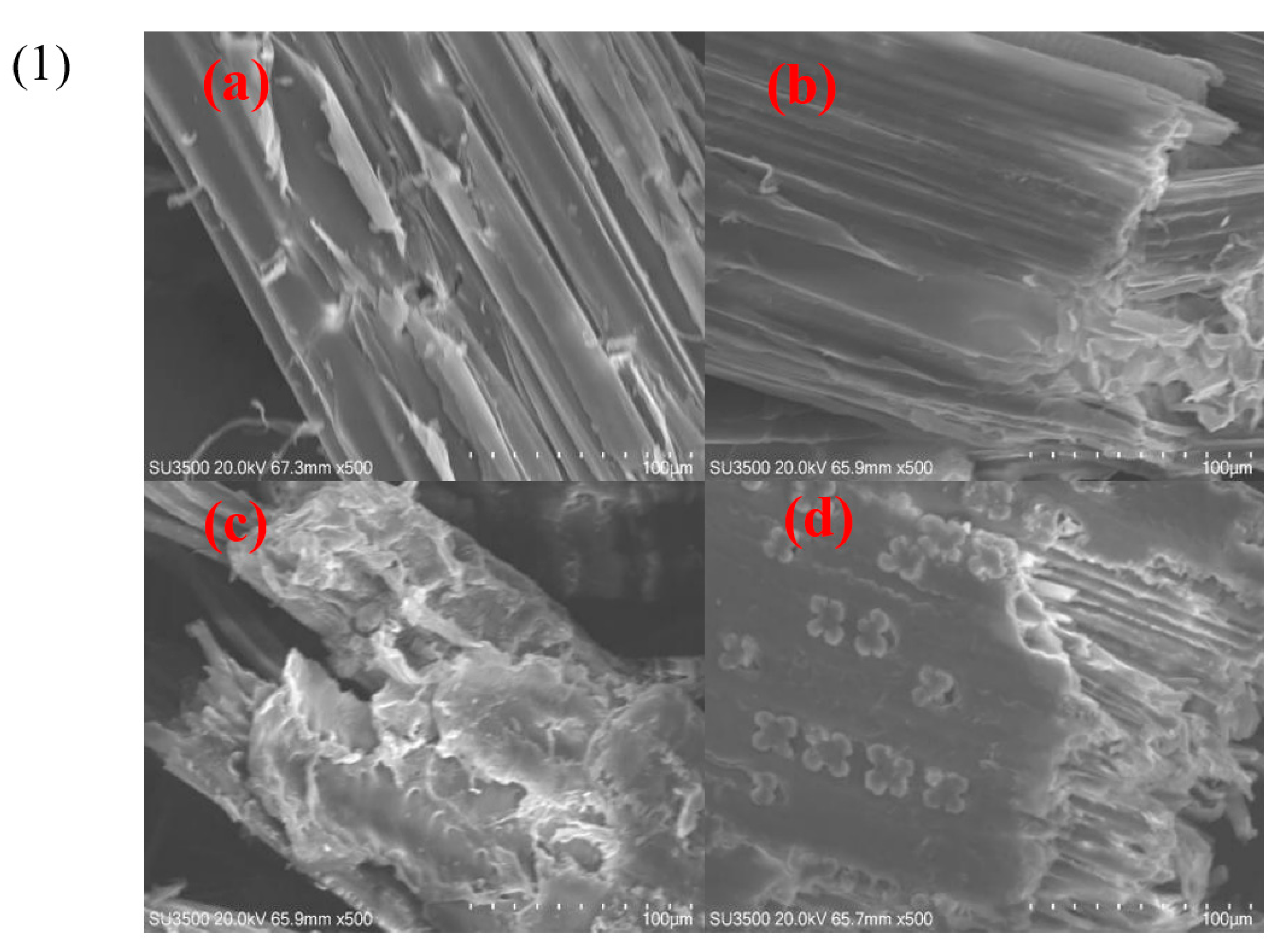
3.7. SEM Analysis and FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.B.; Liu, Y.Q.; Hao, L. Contributions of open crop straw burning emissions to PM2.5 concentrations in China. Environ. Res. Lett. 2016, 11, 014014. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. R. Soc. a-Math. Phys. Eng. Sci. 2020, 476, 20200351. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, S.K.; Kumar, J.; Ahluwalia, V. A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresour. Technol. 2020, 299, 122633. [Google Scholar] [CrossRef]
- Peng, F.; Ren, J.L.; Xu, F.; Sun, R.C. Chemicals from Hemicelluloses: A Review. In Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass; American Chemical Society: Washington, DC, USA, 2011; Chapter 9; pp. 219–259. [Google Scholar] [CrossRef]
- Grohmann, K.; Mitchell, D.J.; Himmel, M.E.; Dale, B.E.; Schroeder, H.A. The role of ester groups in resistance of plant cell wall polysaccharides to enzymatic hydrolysis. Appl. Biochem. Biotechnol. 1989, 20–21, 45–61. [Google Scholar] [CrossRef]
- Kothari, U.D.; Lee, Y.Y. Inhibition effects of dilute-acid prehydrolysate of corn stover on enzymatic hydrolysis of Solka Floc. Appl. Biochem. Biotechnol. 2011, 165, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shekiro, J.; Elander, R.; Tucker, M. Improved Xylan Hydrolysis of Corn Stover by Deacetylation with High Solids Dilute Acid Pretreatment. Ind. Eng. Chem. Res. 2011, 51, 70–76. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Kim, S.R.; Cate, J.H.; Jin, Y.-S. Enhanced biofuel production through coupled acetic acid and xylose consumption by engineered yeast. Nat. Commun. 2013, 4, 2580. [Google Scholar] [CrossRef]
- Wang, Z.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Improving ethanol yields with deacetylated and two-stage pretreated corn stover and sugarcane bagasse by blending commercial xylose-fermenting and wild type Saccharomyces yeast. Bioresour. Technol. 2019, 282, 103–109. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef]
- Madadi, M.; Wang, Y.M.; Xu, C.B.; Liu, P.; Wang, Y.T.; Xia, T.; Tu, Y.Y.; Lin, X.C.; Song, B.; Yang, X.; et al. Using Amaranthus green proteins as universal biosurfactant and biosorbent for effective enzymatic degradation of diverse lignocellulose residues and efficient multiple trace metals remediation of farming lands. J. Hazard. Mater. 2021, 406, 124727. [Google Scholar] [CrossRef]
- Freitas, J.V.; Nogueira, F.G.; Farinas, C.S. Coconut shell activated carbon as an alternative adsorbent of inhibitors from lignocellulosic biomass pretreatment. Ind. Crops Prod. 2019, 137, 16–23. [Google Scholar] [CrossRef]
- Persson, P.; Andersson, J.; Gorton, L.; Larsson, S.; Nilvebrant, N.O.; Jonsson, L.J. Effect of different forms of alkali treatment on specific fermentation inhibitors and on the fermentability of lignocellulose hydrolysates for production of fuel ethanol. J. Agric. Food Chem. 2002, 50, 5318–5325. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cavka, A.; Jonsson, L.J.; Hong, F. Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb. Cell Factories 2013, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.J.; Peinado, S.; Mateo, S.; Fonseca, B.G.; Sanchez, S. Improving bioethanol production from olive pruning biomass by deacetylation step prior acid hydrolysis and fermentation processes. Bioresour. Technol. 2016, 220, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, W.; Ciesielski, P.; Trass, O.; Park, S.; Tao, L.; Tucker, M.P. Improving Sugar Yields and Reducing Enzyme Loadings in the Deacetylation and Mechanical Refining (DMR) Process through Multistage Disk and Szego Refining and Corresponding Techno-Economic Analysis. ACS Sustain. Chem. Eng. 2016, 4, 324–333. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.O.; Scarlata, C.; Templeton, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Chen, L.J.; Li, J.B.; Lu, M.S.; Guo, X.M.; Zhang, H.Y.; Han, L.J. Integrated chemical and multi-scale structural analyses for the processes of acid pretreatment and enzymatic hydrolysis of corn stover. Carbohydr. Polym. 2016, 141, 1–9. [Google Scholar] [CrossRef]
- Yang, J.; Gao, C.; Yang, X.; Su, Y.; Shi, S.; Han, L. Effect of combined wet alkaline mechanical pretreatment on enzymatic hydrolysis of corn stover and its mechanism. Biotechnol. Biofuels Bioprod. 2022, 15, 31. [Google Scholar] [CrossRef]
- Goshadrou, A. Bioethanol production from Cogongrass by sequential recycling of black liquor and wastewater in a mild-alkali pretreatment. Fuel 2019, 258, 116141. [Google Scholar] [CrossRef]
- Prajapati, B.P.; Kango, N. Evaluation of alkali black liquor recycling for rice straw delignification and its effect on enzymatic saccharification. Ind. Crops Prod. 2022, 180, 114709. [Google Scholar] [CrossRef]
- Hendriks, A.T.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Shekiro, J.; Chen, X.; Smith, H.; Tucker, M.P. Development and characterization of a high-solids deacetylation process. Sustain. Chem. Process. 2016, 4, 6. [Google Scholar] [CrossRef]
- Kong, F.; Engler, C.R.; Soltes, E.J. Effects of cell-wall acetate, xylan backbone, and lignin on enzymatic hydrolysis of aspen wood. Appl. Biochem. Biotechnol. 1992, 34–35, 23–35. [Google Scholar] [CrossRef]
- Martins, J.R.; Schmatz, A.A.; Salazar-Bryan, A.M.; Brienzo, M. Effect of Dilute Acid Pretreatment on the Sugarcane Leaf for Fermentable Sugars Production. Sugar Tech 2022, 24, 1540–1550. [Google Scholar] [CrossRef]
- Alvira, P.; Tomas-Pejo, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Selig, M.J.; Adney, W.S.; Himmel, M.E.; Decker, S.R. The impact of cell wall acetylation on corn stover hydrolysis by cellulolytic and xylanolytic enzymes. Cellulose 2009, 16, 711–722. [Google Scholar] [CrossRef]
- Pan, X.J.; Gilkes, N.; Saddler, J.N. Effect of acetyl groups on enzymatic hydrolysis of cellulosic substrates. Holzforschung 2006, 60, 398–401. [Google Scholar] [CrossRef]
- Ying, W.J.; Sun, F.B.; Li, X.; Zhang, J.H. Efficient high solid loading enzymatic hydrolysis of hydrogen peroxide/acetic acid-pretreated bamboo for monosaccharides production. Ind. Crops Prod. 2023, 197, 116588. [Google Scholar] [CrossRef]
- Wang, W.; Kang, L.; Wei, H.; Arora, R.; Lee, Y.Y. Study on the Decreased Sugar Yield in Enzymatic Hydrolysis of Cellulosic Substrate at High Solid Loading. Appl. Biochem. Biotechnol. 2011, 164, 1139–1149. [Google Scholar] [CrossRef]
- Jiang, W.; Peng, H.D.; Li, H.Q.; Xu, J. Effect of acetylation/deacetylation on enzymatic hydrolysis of corn stalk. Biomass Bioenergy 2014, 71, 294–298. [Google Scholar] [CrossRef]
- Pandey, K.K. A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 1999, 71, 1969–1975. [Google Scholar] [CrossRef]
- Zhao, X.B.; Wang, L.; Liu, D.H. Effect of several factors on peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis. J. Chem. Technol. Biotechnol. 2007, 82, 1115–1121. [Google Scholar] [CrossRef]


| Deacetylation Condition | Cellulose | Cellulose | Hemicellulose | Hemicellulose | Lignin | Lignin | Acetyl | Acetyl | Solid | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp | Time | Na2CO3 | (%) | Loss (%) | (%) | Loss (%) | (%) | Remove (%) | (%) | Remove (%) | Recovery (%) |
| Raw (control) | 32.83 ± 0.23 | - | 22.19 ± 0.31 | - | 19.93 ± 0.62 | - | 2.47 ± 0.23 | - | - | ||
| 40 °C | 6 h | 2% | 40.85 ± 0.11 | 0.70 ± 0.14 | 26.40 ± 0.28 | 3.52 ± 0.39 | 18.38 ± 0.51 | 26.40 ± 0.83 | 1.13 ± 0.11 | 61.66 ± 2.48 | 79.80 ± 0.92 |
| 40 °C | 6 h | 4% | 40.69 ± 0.16 | 1.62 ± 0.23 | 26.39 ± 0.36 | 4.09 ± 0.35 | 18.21 ± 0.37 | 27.47 ± 0.42 | 0.44 ± 0.04 | 80.55 ± 3.36 | 79.38 ± 1.23 |
| 40 °C | 6 h | 6% | 40.92 ± 0.34 | 2.38 ± 0.28 | 26.18 ± 0.52 | 6.11 ± 0.32 | 18.29 ± 0.42 | 28.15 ± 0.66 | 0.40 ± 0.02 | 82.61 ± 4.23 | 78.31 ± 0.80 |
| 40 °C | 3 h | 4% | 40.45 ± 0.27 | 1.40 ± 0.31 | 26.44 ± 0.13 | 3.14 ± 0.28 | 19.21 ± 0.36 | 22.89 ± 0.71 | 0.85 ± 0.05 | 62.21 ± 3.12 | 80.02 ± 1.35 |
| 40 °C | 9 h | 4% | 41.32 ± 0.42 | 2.24 ± 0.37 | 26.59 ± 0.60 | 5.44 ± 0.47 | 18.22 ± 0.64 | 28.97 ± 0.41 | 0.39 ± 0.02 | 83.43 ± 2.95 | 77.68 ± 0.69 |
| 30 °C | 6 h | 4% | 39.53 ± 0.26 | 1.60 ± 0.29 | 26.10 ± 0.44 | 2.35 ± 0.51 | 19.26 ± 0.58 | 21.04 ± 0.67 | 0.96 ± 0.04 | 56.70 ± 2.50 | 81.72 ± 1.04 |
| 50 °C | 6 h | 4% | 42.91 ± 0.50 | 2.36 ± 0.33 | 27.02 ± 0.67 | 7.58 ± 0.31 | 18.02 ± 0.71 | 32.48 ± 0.92 | 0.31 ± 0.06 | 87.24 ± 2.71 | 74.70 ± 1.62 |
| Sample | Deacetylation Condition | Dilute Sulfuric Acid Pretreatment | Cellulose | Hemicellulose | Lignin | Acetic Acid | ||
|---|---|---|---|---|---|---|---|---|
| Temp | Time | Na2CO3 | (%) | (%) | (%) | Concentrate (g/L) | ||
| Raw | 1.5 wt% H2SO4, 121 °C, 1 h | |||||||
| DDA-1 | 40 °C | 6 h | 2% | 59.88 ± 0.26 | 9.01 ± 0.25 | 26.03 ± 0.60 | 0.77 ± 0.08 | |
| DDA-2 | 40 °C | 6 h | 4% | 59.50 ± 0.43 | 9.28 ± 0.22 | 25.62 ± 0.50 | 0.34 ± 0.04 | |
| DDA-3 | 40 °C | 6 h | 6% | 58.60 ± 0.15 | 9.41 ± 0.34 | 26.04 ± 0.28 | 0.21 ± 0.03 | |
| DDA-4 | 40 °C | 3 h | 4% | 58.69 ± 0.36 | 8.91 ± 0.20 | 26.48 ± 1.02 | 0.50 ± 0.05 | |
| DDA-5 | 40 °C | 9 h | 4% | 60.21 ± 0.28 | 9.74 ± 0.14 | 24.68 ± 0.79 | 0.17 ± 0.03 | |
| DDA-6 | 30 °C | 6 h | 4% | 58.25 ± 0.42 | 8.75 ± 0.11 | 27.11 ± 0.43 | 0.67 ± 0.05 | |
| DDA-7 | 50 °C | 6 h | 4% | 59.67 ± 0.23 | 9.80 ± 0.30 | 24.22 ± 0.56 | 0.14 ± 0.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Wu, S.; Wang, C.; Thangalazhy-Gopakumar, S.; Kothari, U.; Shi, S.; Han, L. Enhanced Enzymatic Sugar Recovery of Dilute-Acid-Pretreated Corn Stover by Sodium Carbonate Deacetylation. Bioengineering 2023, 10, 1197. https://doi.org/10.3390/bioengineering10101197
Fu W, Wu S, Wang C, Thangalazhy-Gopakumar S, Kothari U, Shi S, Han L. Enhanced Enzymatic Sugar Recovery of Dilute-Acid-Pretreated Corn Stover by Sodium Carbonate Deacetylation. Bioengineering. 2023; 10(10):1197. https://doi.org/10.3390/bioengineering10101197
Chicago/Turabian StyleFu, Weng, Shengbo Wu, Chun Wang, Suchithra Thangalazhy-Gopakumar, Urvi Kothari, Suan Shi, and Lujia Han. 2023. "Enhanced Enzymatic Sugar Recovery of Dilute-Acid-Pretreated Corn Stover by Sodium Carbonate Deacetylation" Bioengineering 10, no. 10: 1197. https://doi.org/10.3390/bioengineering10101197
APA StyleFu, W., Wu, S., Wang, C., Thangalazhy-Gopakumar, S., Kothari, U., Shi, S., & Han, L. (2023). Enhanced Enzymatic Sugar Recovery of Dilute-Acid-Pretreated Corn Stover by Sodium Carbonate Deacetylation. Bioengineering, 10(10), 1197. https://doi.org/10.3390/bioengineering10101197








