A Nanostructured Protein Filtration Device for Possible Use in the Treatment of Alzheimer’s Disease—Concept and Feasibility after In Vivo Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Development of the Nanoporous Ceramic Filter
2.2. Development of the CSF Filtration Device
2.3. Device Implantation on Mice
2.4. Nanoporous Ceramic Filter Assessment after a 4-Week Trial Period
3. Results
3.1. Development and Characterization of the Functionalized Nanoporous Ceramic Filter
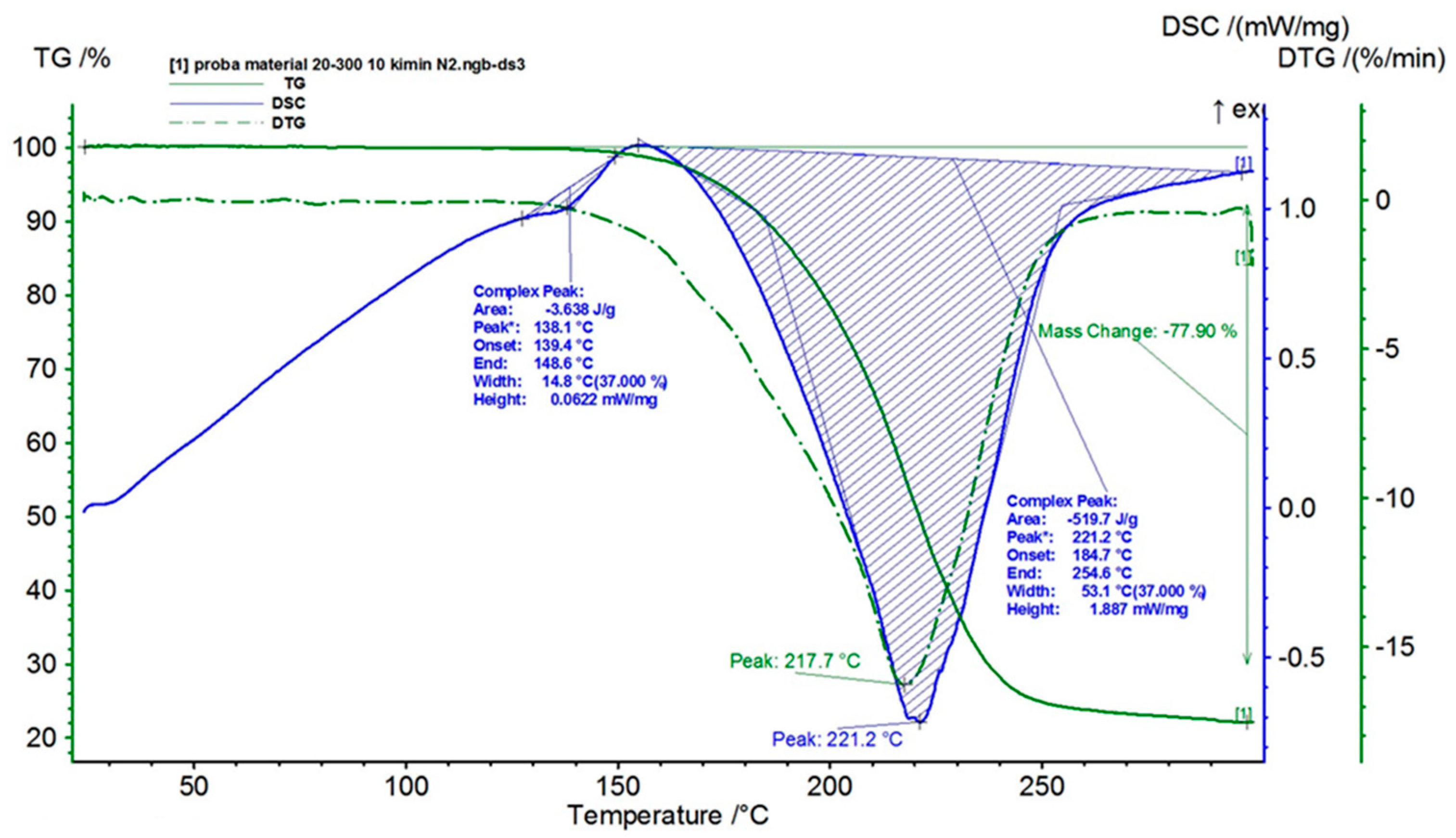
3.2. Analysis of the Nanoporous Ceramic Filter after the 4-Week In Vivo Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Counts, N.; Chen, S.; Seligman, B.; Tortorice, D.; Vigo, D.; Bloom, D.E. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: A value of statistical life approach. EClinicalMedicine 2022, 51, 101580. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.E.Z.N.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimer’s Dement. (New York N.Y.) 2022, 8, e12295. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Hinz, R.; Gentleman, S.; Hampshire, A.; Dani, M.; Brooks, D.J.; Edison, P. Neuroinflammation is independently associated with brain network dysfunction in Alzheimer’s disease. Mol. Psychiatry 2023, 28, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative Stress and Beta Amyloid in Alzheimer’s Disease. Which Comes First: The Chicken or the Egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Zhang, L.; Meng, R.N.; Gao, J.; Jin, M.; Li, M.; Wang, X.P. Effect of metal ions on Alzheimer’s disease. Brain Behav. 2022, 12, e2527. [Google Scholar] [CrossRef]
- Cai, W.; Wu, T.; Chen, N. The Amyloid-Beta Clearance: From Molecular Targets to Glial and Neural Cells. Biomolecules 2023, 13, 313. [Google Scholar] [CrossRef]
- Beshir, S.A.; Aadithsoorya, A.M.; Parveen, A.; Goh, S.S.L.; Hussain, N.; Menon, V.B. Aducanumab Therapy to Treat Alzheimer’s Disease: A Narrative Review. Int. J. Alzheimers Dis. 2022, 2022, 9343514. [Google Scholar] [CrossRef]
- Qiao, Y.; Chi, Y.; Zhang, Q.; Ma, Y. Safety and efficacy of lecanemab for Alzheimer’s disease: A systematic review and meta-analysis of randomized clinical trials. Front. Aging Neurosci. 2023, 15, 1169499. [Google Scholar] [CrossRef]
- Achar, A.; Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood-Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef]
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef]
- Zeiadeh, I.; Najjar, A.; Karaman, R. Strategies for Enhancing the Permeation of CNS-Active Drugs through the Blood-Brain Barrier: A Review. Molecules 2018, 23, 1289. [Google Scholar] [CrossRef]
- De Andres, J.; Hayek, S.; Perruchoud, C.; Lawrence, M.M.; Reina, M.A.; De Andres-Serrano, C.; Rubio-Haro, R.; Hunt, M.; Yaksh, T.L. Intrathecal Drug Delivery: Advances and Applications in the Management of Chronic Pain Patient. Front. Pain Res. (Lausanne) 2022, 3, 900566, Erratum in Front. Pain Res. (Lausanne) 2023, 4, 1190014. [Google Scholar] [CrossRef]
- Menendez-Gonzalez, M.; Padilla-Zambrano, H.S.; Alvarez, G.; Capetillo-Zarate, E.; Tomas-Zapico, C.; Costa, A. Targeting Beta-Amyloid at the CSF: A New Therapeutic Strategy in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.G.; Menéndez-González, M.; Popescu, B.O. The “Cerebrospinal Fluid Sink Therapeutic Strategy” in Alzheimer’s Disease—From Theory to Design of Applied Systems. Biomedicines 2022, 10, 1509. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.G.; Tamba, B.I.; Mihai, C.T.; L˝orinczi, A.; Baibarac, M.; Ciobanu, R.C.; Popescu, B.O. Nanoporous Ceramic filters for the Filtration of Proteins from Biological Fluids: Biocompatibility Tests on Cell Cultures and Suggested Applications for the Treatment of Alzheimer’s Disease. J. Clin. Med. 2022, 11, 5846. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Victorio, G.; Peto-Gutiérrez, C.; Díaz-Bello, B.; Cano-Jorge, M.; Pérez-Calixto, D.; Jiménez-Escobar, A.; Espinosa-Matías, S.; Martínez, R.L.; Courson, R.; Malaquin, L.; et al. Building a microfluidic cell culture platform with stiffness control using Loctite 3525 glue. Lab Chip. 2019, 19, 3512–3525. [Google Scholar] [CrossRef] [PubMed]
- Lok, K.; Zhao, H.; Shen, H.; Wang, Z.; Gao, X.; Zhao, W.; Yin, M. Characterization of the APP/PS1 mouse model of Alzheimer’s disease in senescence accelerated background. Neurosci. Lett. 2013, 557 Pt B, 84–89. [Google Scholar] [CrossRef]
- DeVos, S.L.; Miller, T.M. Direct intraventricular delivery of drugs to the rodent central nervous system. J. Vis. Exp. 2013, 75, e50326. [Google Scholar]
- Coto-Vilcapoma, M.A.; Castilla-Silgado, J.; Fernández-García, B.; Pinto-Hernández, P.; Cipriani, R.; Capetillo-Zarate, E.; Menéndez-González, M.; Álvarez-Vega, M.; Tomás-Zapico, C. New, Fully Implantable Device for Selective Clearance of CSF-Target Molecules: Proof of Concept in a Murine Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 9256. [Google Scholar] [CrossRef]
- Akhtar, K.; Khan, S.A.; Khan, S.B.; Asiri, A.M. Scanning Electron Microscopy: Principle and Applications in Nanomaterials Characterization. In Handbook of Materials Characterization; Sharma, S., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Egerton, R.F.; Zhu, Y. Spatial resolution in secondary-electron microscopy [published correction appears in Microscopy (Oxf). 2023;72(1):64]. Microscopy 2023, 72, 66–77. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Rayung, M.; Norrrahim, M.N.F.; Shazleen, S.S.; Rani, M.S.A.; Shafi, A.R.; Aisyah, H.A.; Radzi, M.H.M.; Sabaruddin, F.A.; et al. Thermogravimetric Analysis Properties of Cellulosic Natural Fiber Polymer Composites: A Review on Influence of Chemical Treatments. Polymers 2021, 13, 2710. [Google Scholar] [CrossRef]
- Wesolowski, M.; Leyk, E. Coupled and Simultaneous Thermal Analysis Techniques in the Study of Pharmaceuticals. Pharmaceutics. 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, N.S.; Omanwar, S.K. Low-Temperature Stearic Acid Sol–Gel Synthesis of α-Al2O3 Quantum Dots and Its Optical Properties. J. Sol.-Gel. Sci. Technol. 2015, 75, 1–5. [Google Scholar] [CrossRef]
- Naayi, S.; Hassan, A.; Salim, E. FTIR and X-ray Diffraction Analysis of Al2O3 Nanostructured Thin Film Prepared at Low Temperature Using Spray Pyrolysis Method. Int. J. Nanoelectron. Mater. 2018, 11, 1–6. [Google Scholar]
- Kim, Y.; Park, J.H.; Lee, H.; Nam, J.M. How Do the Size, Charge and Shape of Nanoparticles Affect Amyloid β Aggregation on Brain Lipid Bilayer? Sci. Rep. 2016, 6, 19548. [Google Scholar] [CrossRef]
- Liu, X.; He, D. Atomic layer deposited aluminium oxide ceramic filters for selective hydrogen separation through molecular sieving. J. Membr. Sci. 2022, 662, 121011. [Google Scholar] [CrossRef]
- Yanagishita, T.; Itoh, H.; Masuda, H. Efficient fabrication of ordered alumina through-hole ceramic filters using a TiO2 protective layer prepared by atomic layer deposition. RSC Adv. 2022, 12, 3662–3671. [Google Scholar] [CrossRef]
- Xiong, S.; Qian, X.; Zhong, Z.; Wang, Y. Atomic layer deposition for ceramic filter modification, functionalization and preparation: A review. J. Membr. Sci. 2022, 658, 120740. [Google Scholar] [CrossRef]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Soler, G.J.; Bao, M.; Jaiswal, D.; Zaveri, H.P.; DiLuna, M.L.; Grant, R.A.; Hoshino, K. A Review of Cerebral Shunts, Current Technologies, and Future Endeavors. Yale J. Biol. Med. 2018, 91, 313–321. [Google Scholar]
- Tag, S.H.; Kim, B.; Bae, J.; Chang, K.A.; Im, H.I. Neuropathological and behavioral features of an APP/PS1/MAPT (6xTg) transgenic model of Alzheimer’s disease. Mol. Brain 2022, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.L.R.; Hauglund, N.L.; von Holstein-Rathlou, S.; Li, Q.; Sanggaard, S.; Lou, N.; Lundgaard, I.; Nedergaard, M. Cannula Implantation into the Cisterna Magna of Rodents. J. Vis. Exp. 2018, 135, 57378. [Google Scholar] [CrossRef]
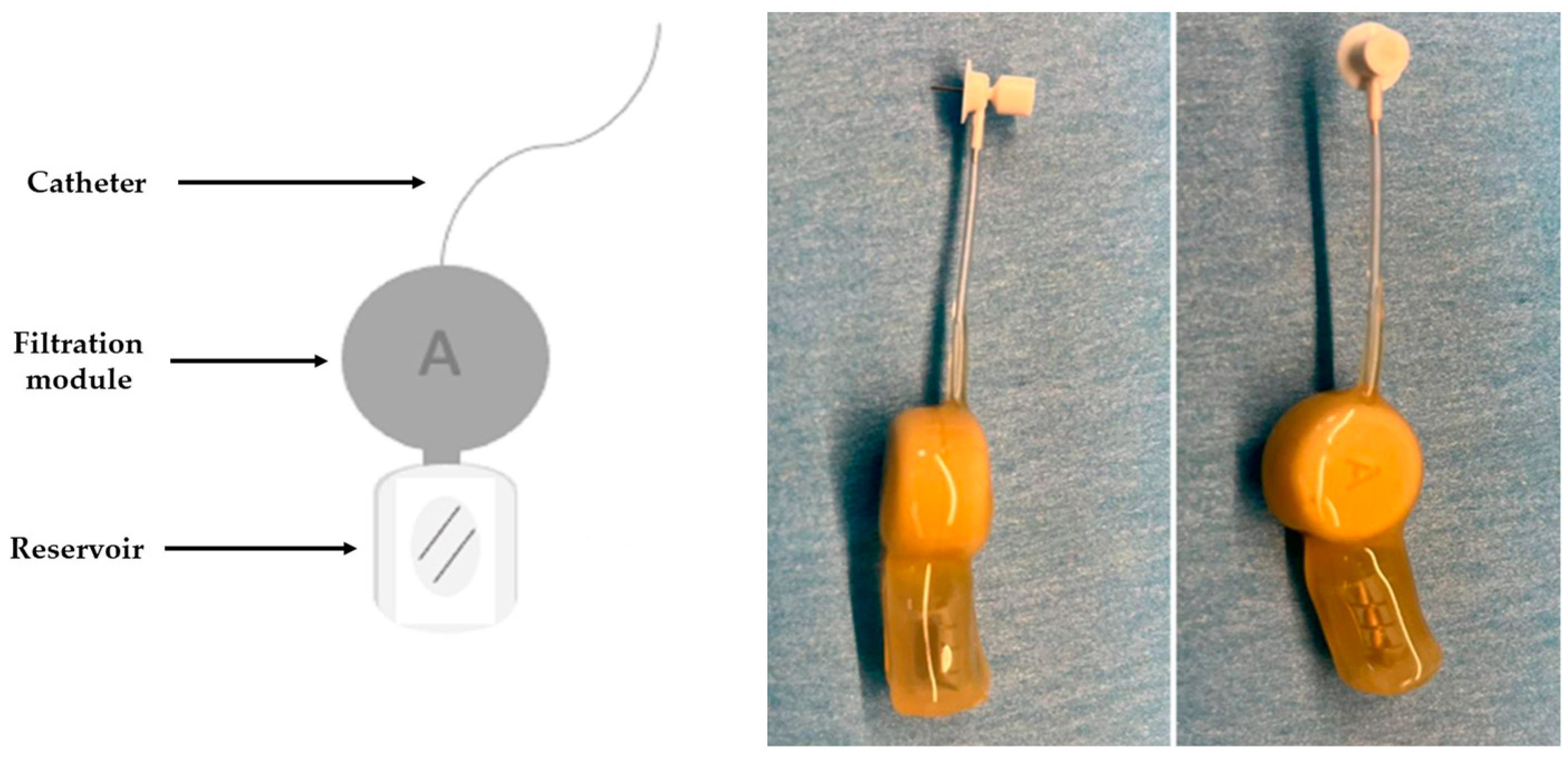

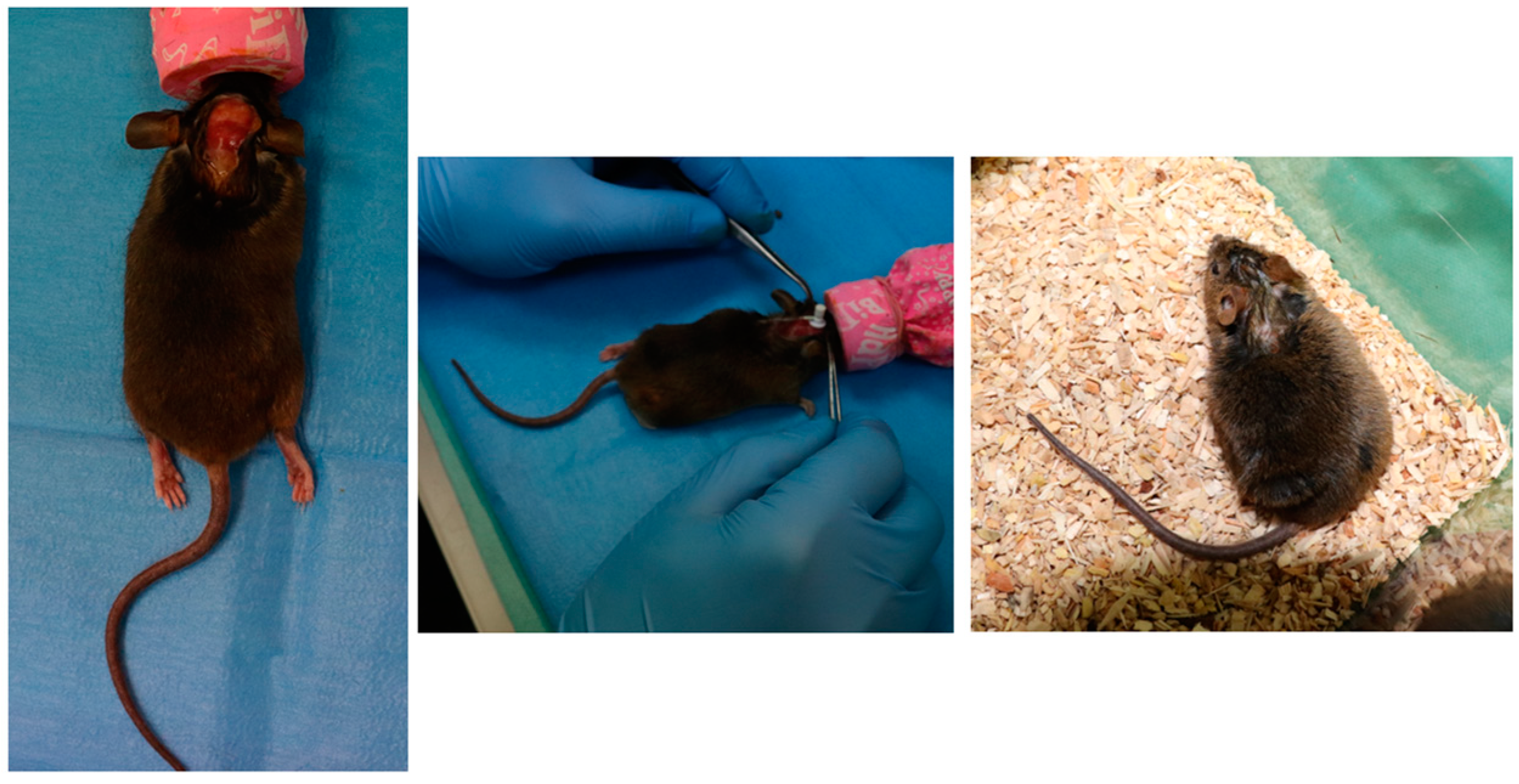

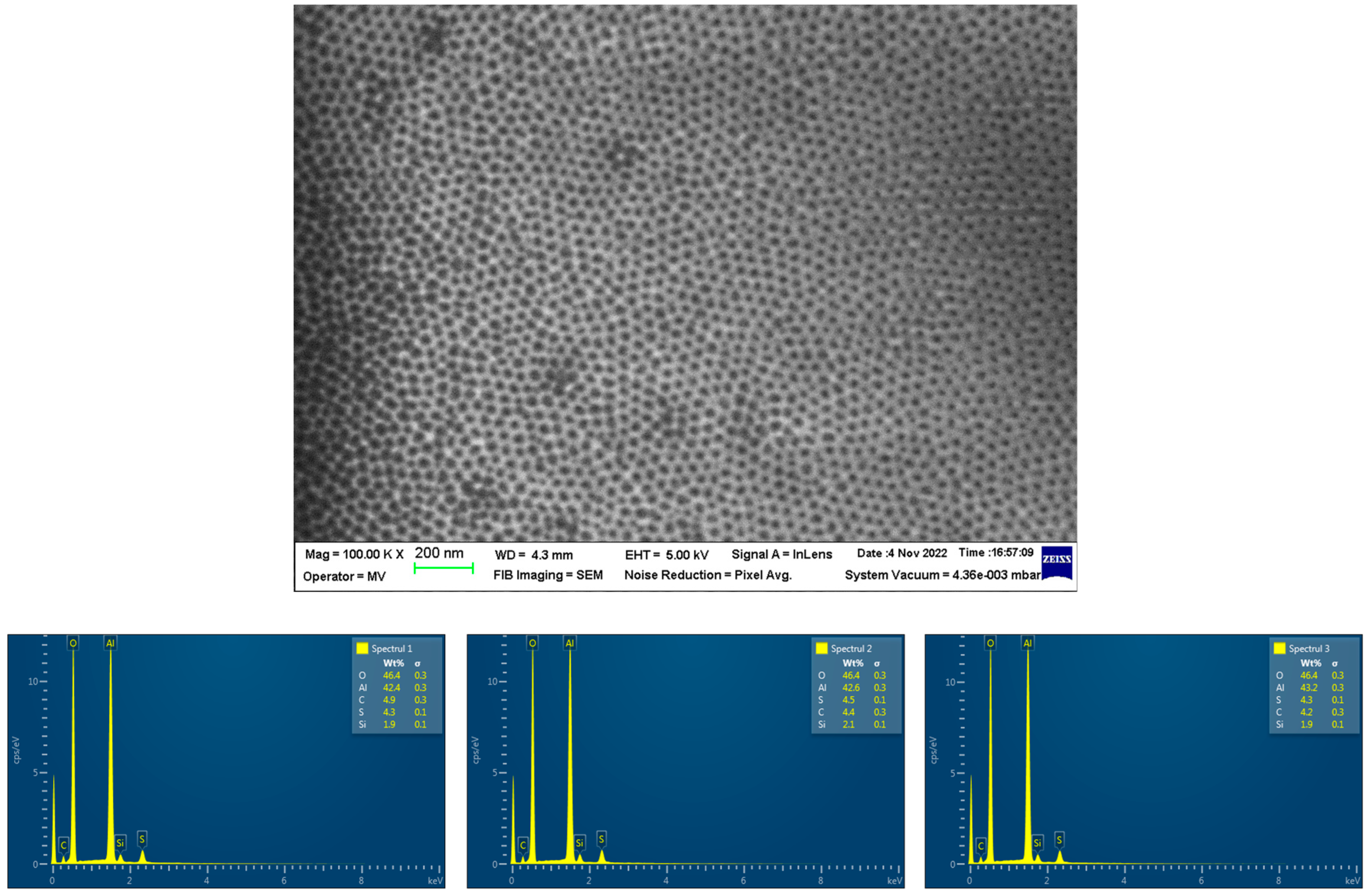

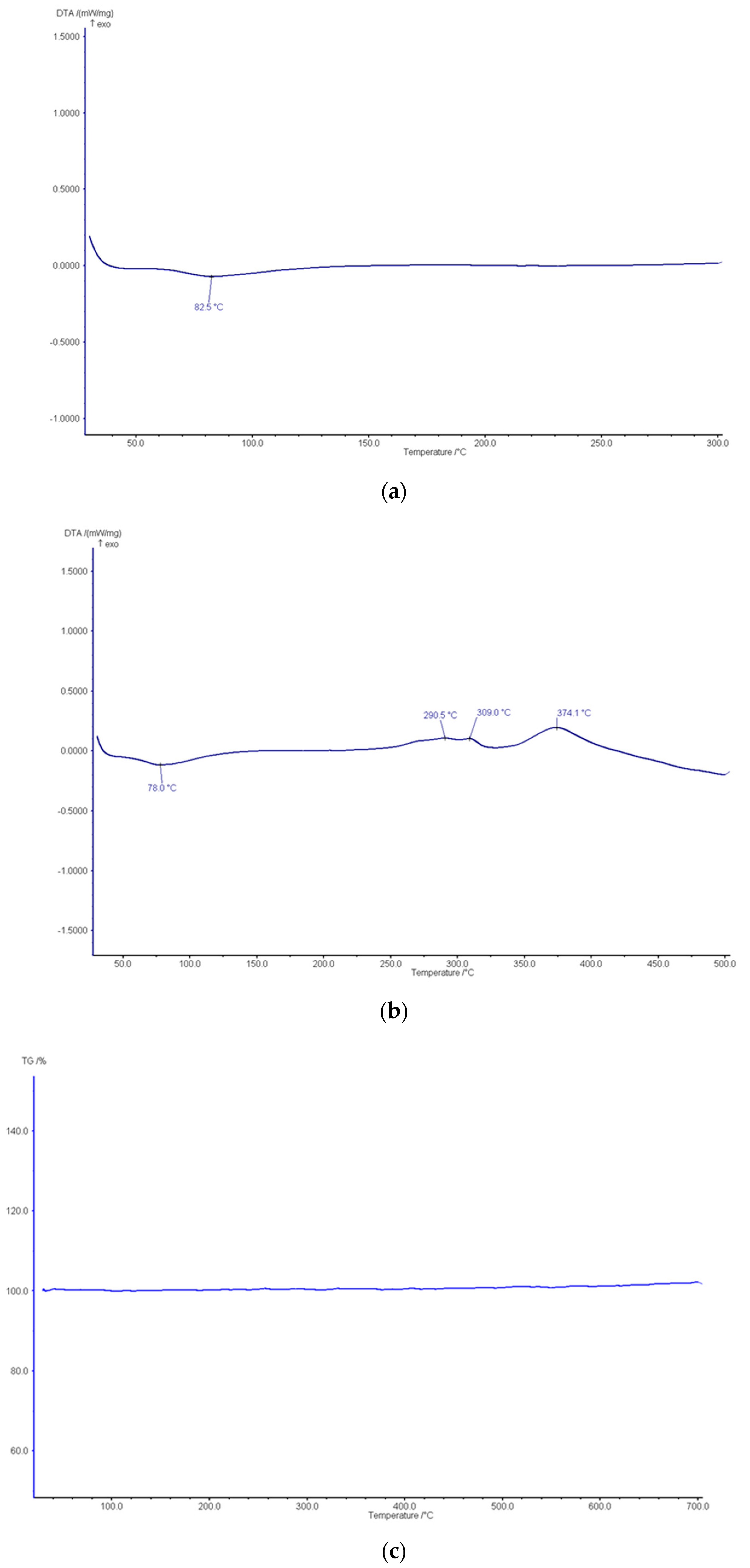
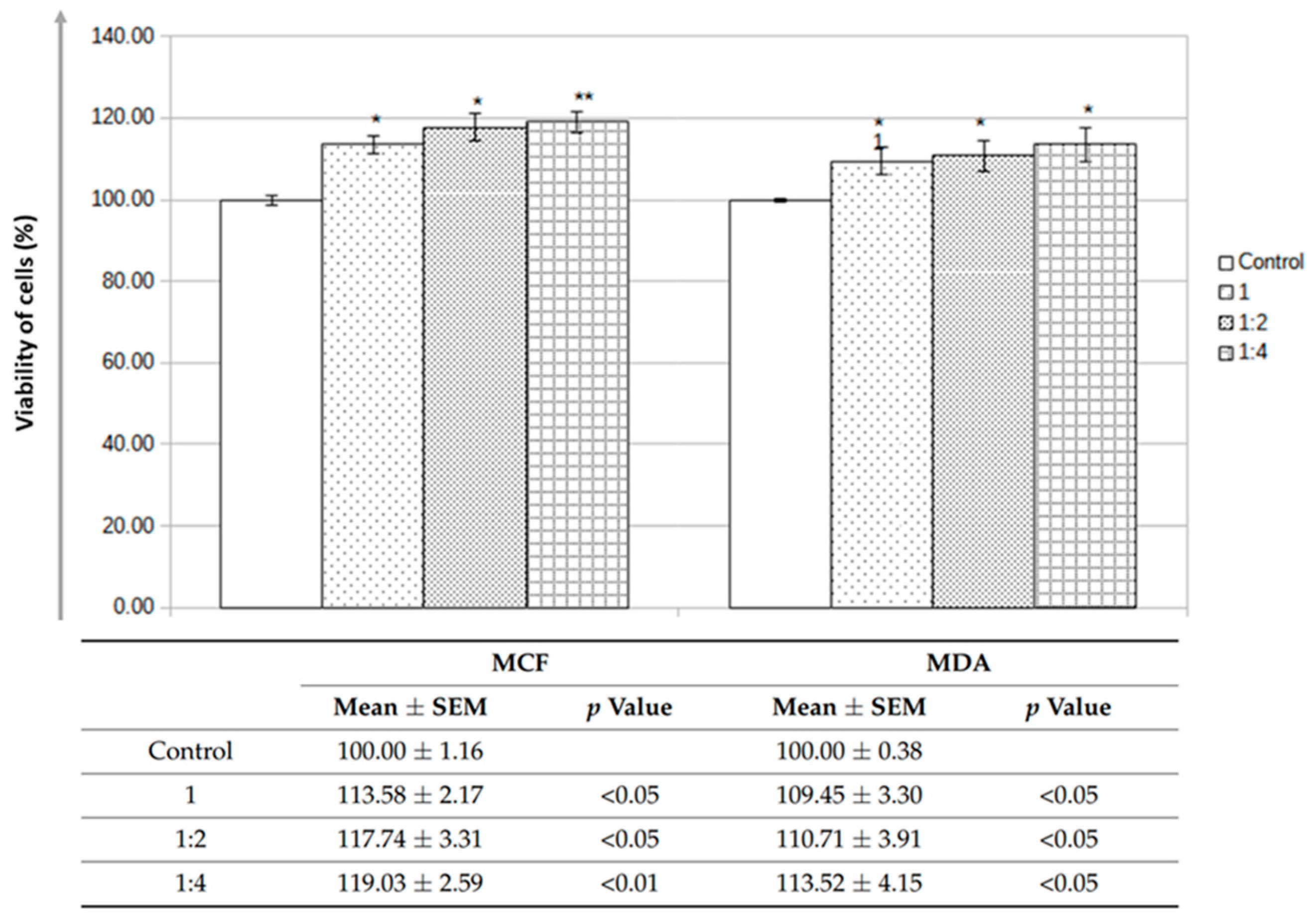
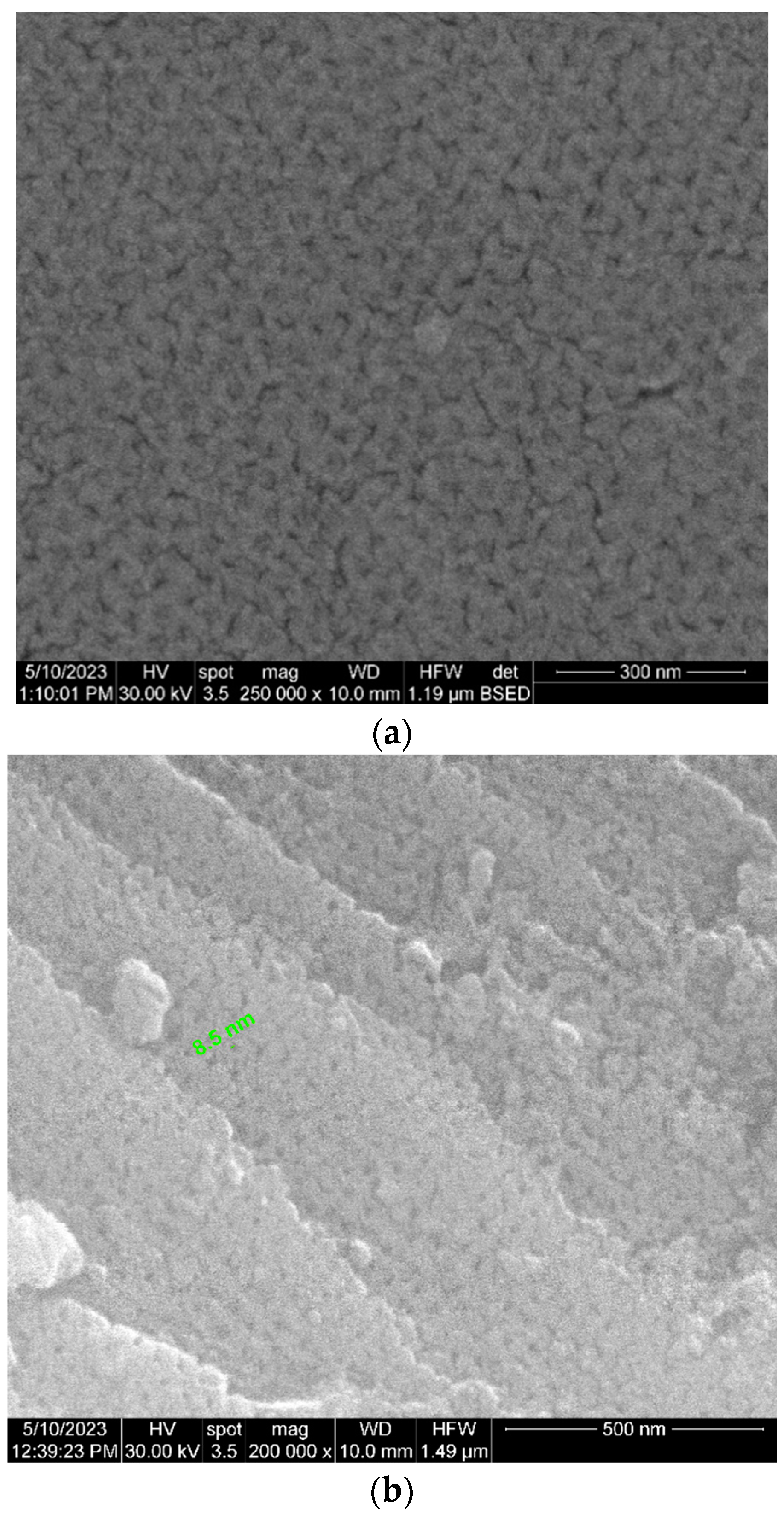
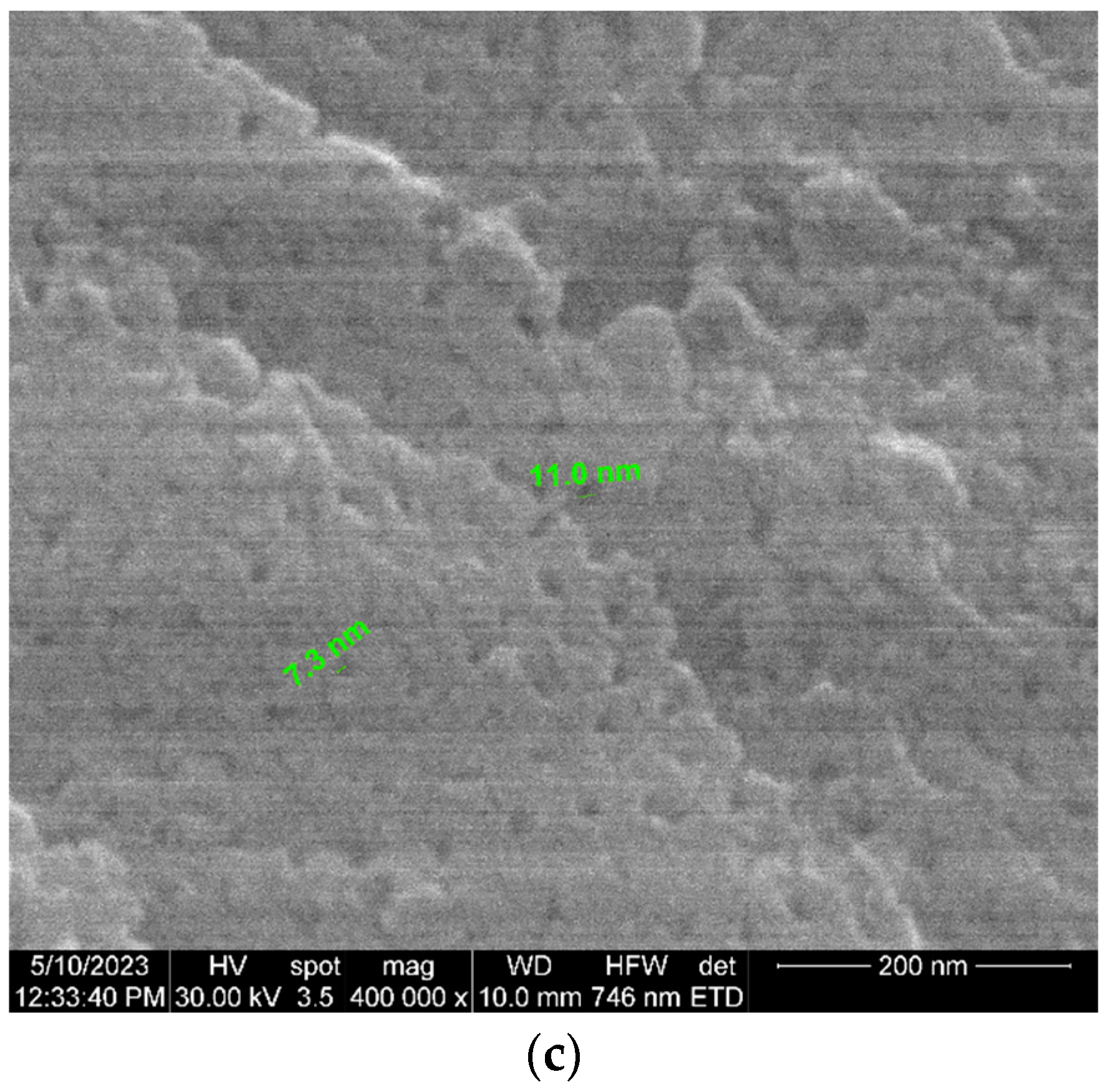
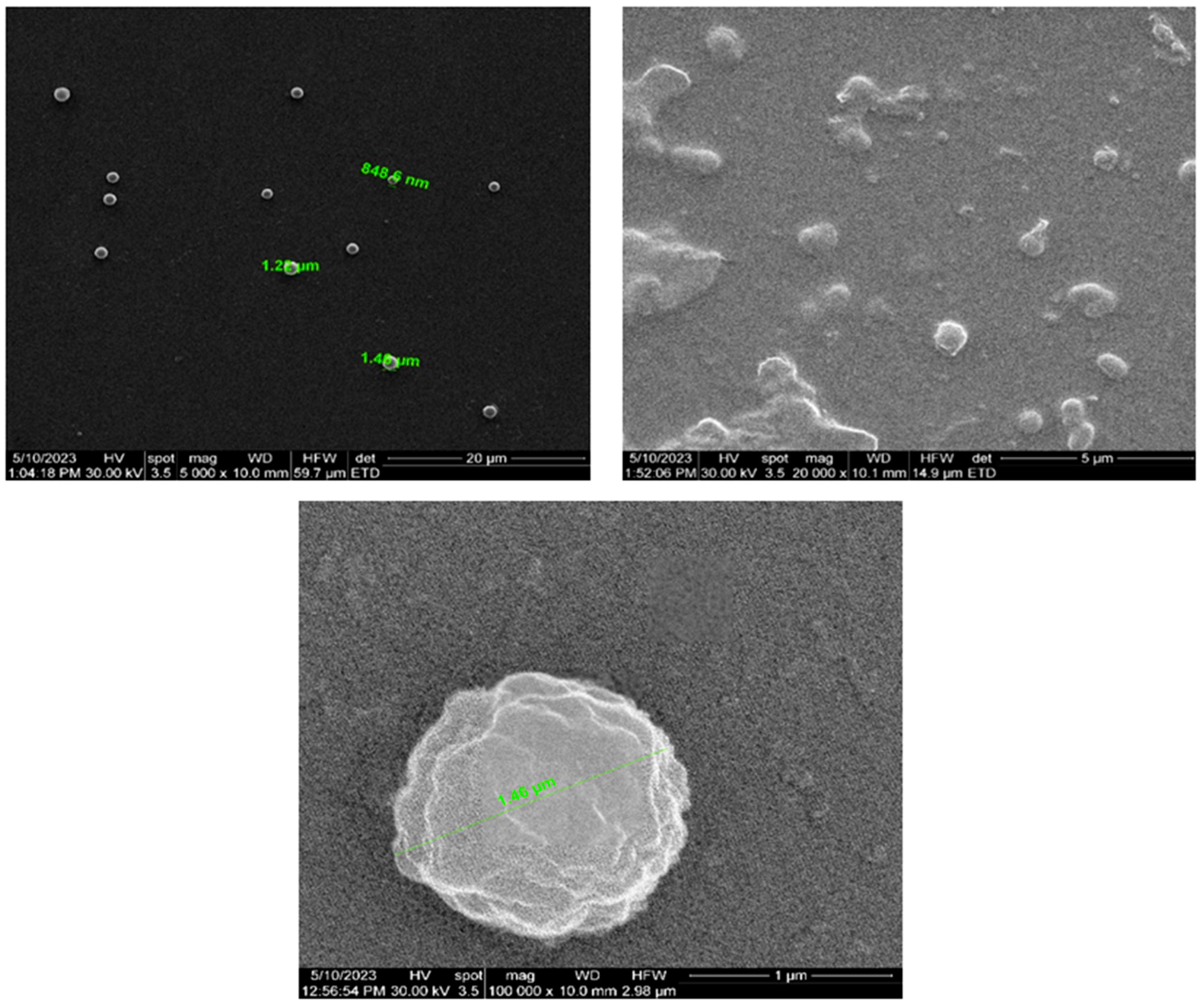
| Parameter | Units | Average Values |
|---|---|---|
| Ceramic filter thickness | µm | 48 |
| Pore diameter | nm | 17 (±1.7) |
| Pore period | nm | 42 (±4.2) |
| Pore density | 1/cm2 | 3.2 × 1010 (±20%) |
| Porosity | % | 12 |
| Molecular Weight Cut-Off (MWCO) | kDa | 140 |
| (Aβ) Diffusivity | % | 100 |
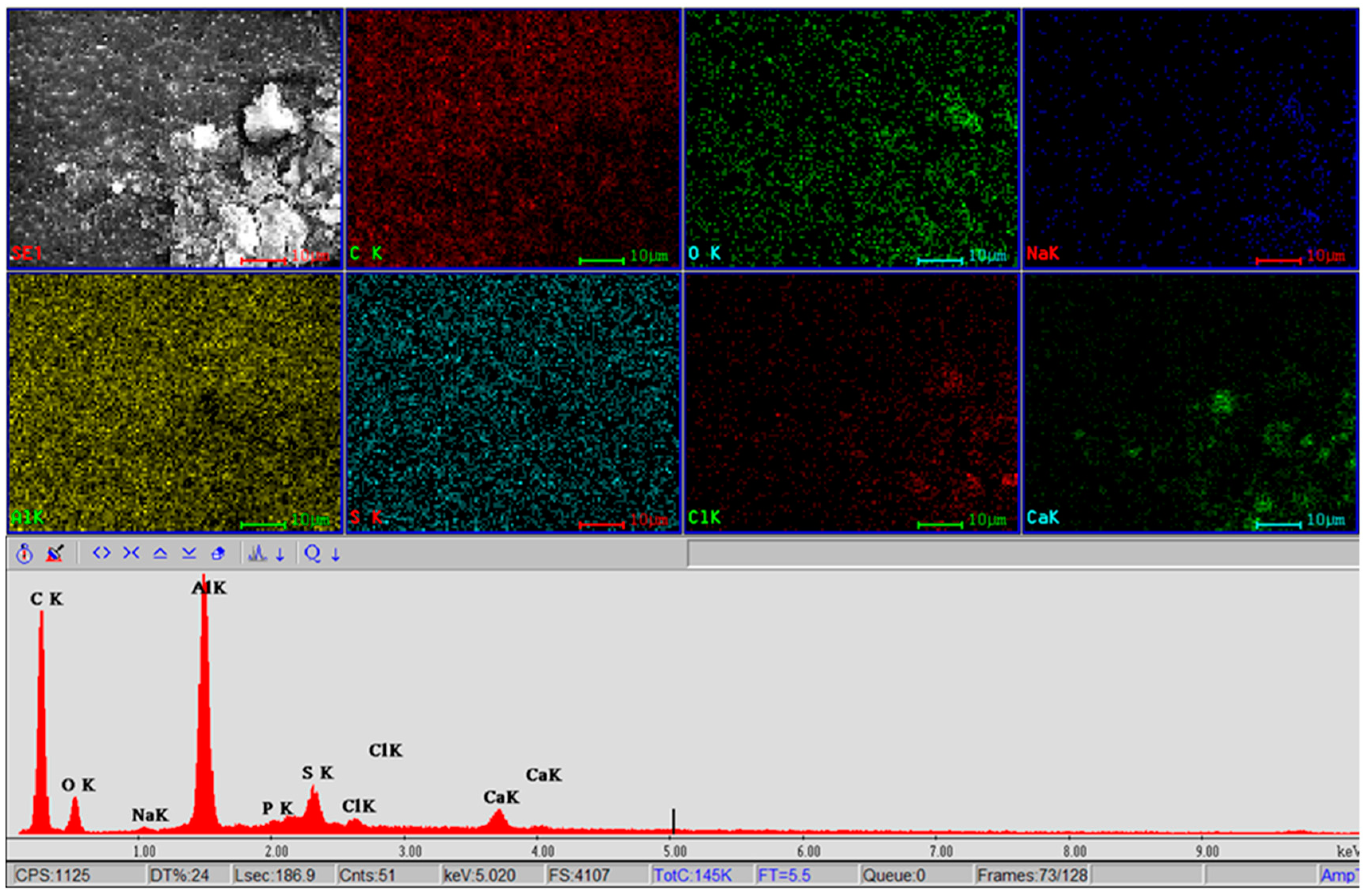
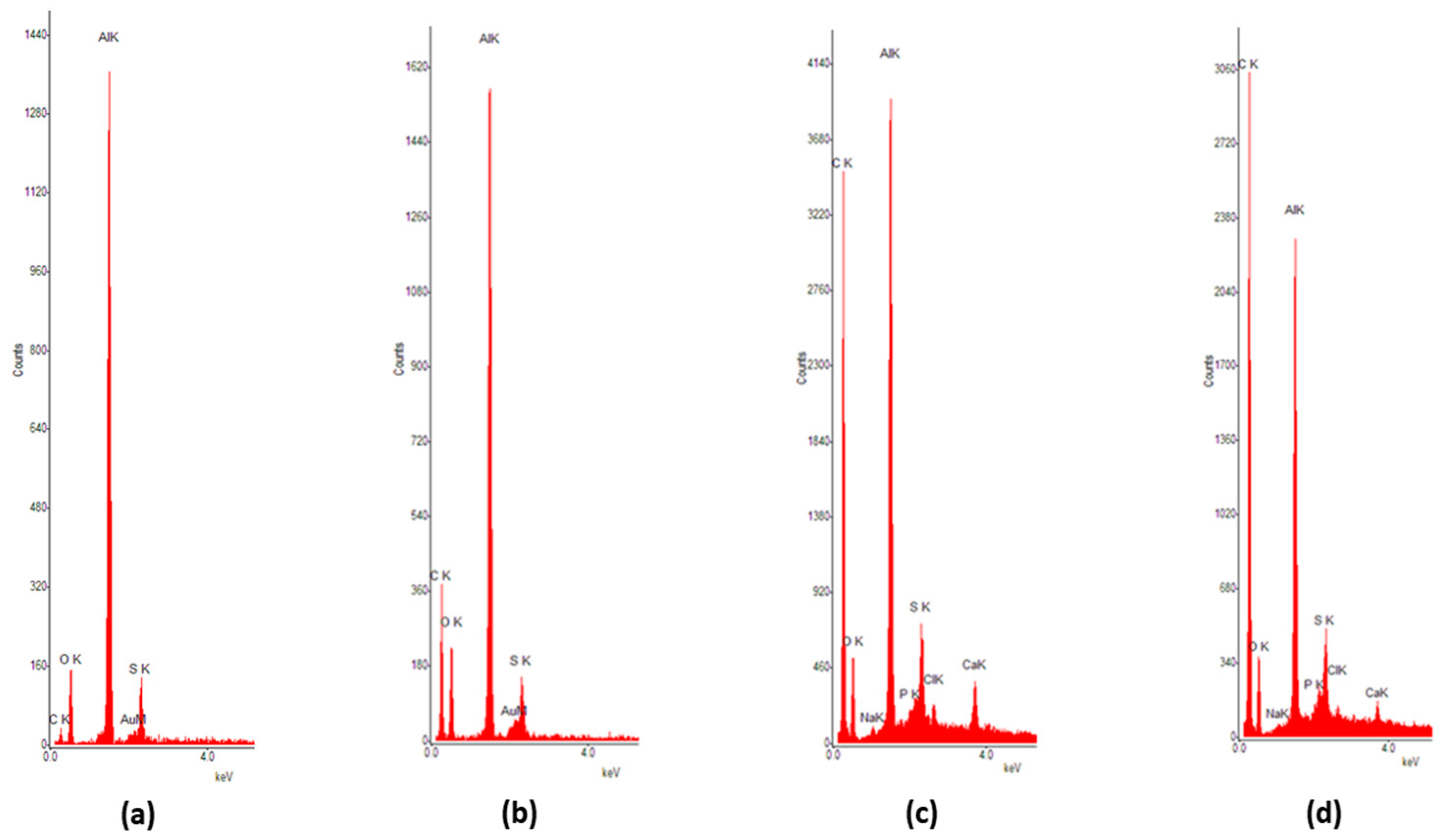
| Element | Figure 12a | Figure 12b | Figure 12c | Figure 12d |
|---|---|---|---|---|
| C | 6.62 | 41.25 | 70.9 | 77.68 |
| O | 36.82 | 23.13 | 9.25 | 7.23 |
| Na | 0 | 0.04 | 0.42 | 0.46 |
| Al | 51.43 | 31.89 | 14.49 | 10.22 |
| Si | 0.17 | 0.08 | 0.46 | 0.42 |
| P | 0.08 | 0.03 | 0.61 | 0.67 |
| S | 3.7 | 2.61 | 1.85 | 1.28 |
| Cl | 0 | 0.06 | 0.72 | 0.81 |
| Ca | 0.04 | 0.02 | 0.55 | 0.36 |
| Au | 0.96 | 0.88 | 0.77 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiner, T.G.; Menéndez-González, M.; Adam, M.; Popescu, B.O.; Szilagyi, A.; Stanciu, G.D.; Tamba, B.I.; Ciobanu, R.C. A Nanostructured Protein Filtration Device for Possible Use in the Treatment of Alzheimer’s Disease—Concept and Feasibility after In Vivo Tests. Bioengineering 2023, 10, 1303. https://doi.org/10.3390/bioengineering10111303
Schreiner TG, Menéndez-González M, Adam M, Popescu BO, Szilagyi A, Stanciu GD, Tamba BI, Ciobanu RC. A Nanostructured Protein Filtration Device for Possible Use in the Treatment of Alzheimer’s Disease—Concept and Feasibility after In Vivo Tests. Bioengineering. 2023; 10(11):1303. https://doi.org/10.3390/bioengineering10111303
Chicago/Turabian StyleSchreiner, Thomas Gabriel, Manuel Menéndez-González, Maricel Adam, Bogdan Ovidiu Popescu, Andrei Szilagyi, Gabriela Dumitrita Stanciu, Bogdan Ionel Tamba, and Romeo Cristian Ciobanu. 2023. "A Nanostructured Protein Filtration Device for Possible Use in the Treatment of Alzheimer’s Disease—Concept and Feasibility after In Vivo Tests" Bioengineering 10, no. 11: 1303. https://doi.org/10.3390/bioengineering10111303







