Non-Invasive Wearable Devices for Monitoring Vital Signs in Patients with Type 2 Diabetes Mellitus: A Systematic Review
Abstract
:1. Introduction/Background
2. Methods
2.1. Systematic Literature Search, Information Sources and Article Selection
- (i)
- Studies focusing on patients with type 2 diabetes mellitus;
- (ii)
- Studies that used sensors or wearable devices to measure vital signs, including body temperature (BT), blood pressure (BP), heart rate (HR), or respiratory rate (RR);
- (iii)
- Studies with any control group;
- (iv)
- Studies yielding any outcome;
- (v)
- All evidence from randomized controlled trials (RCTs), systematic reviews (SRs), or meta-analyses (MAs) published since the beginning of the year 2017.
2.2. Data Extraction, Risk of Bias Assessment Tool and Quality Scales
- Brief description of the study design,
- Technical details of the device used,
- Demographic information of the participants and
- Main results—primary and secondary endpoints.
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Wearable Technology
3.2.2. Outcomes
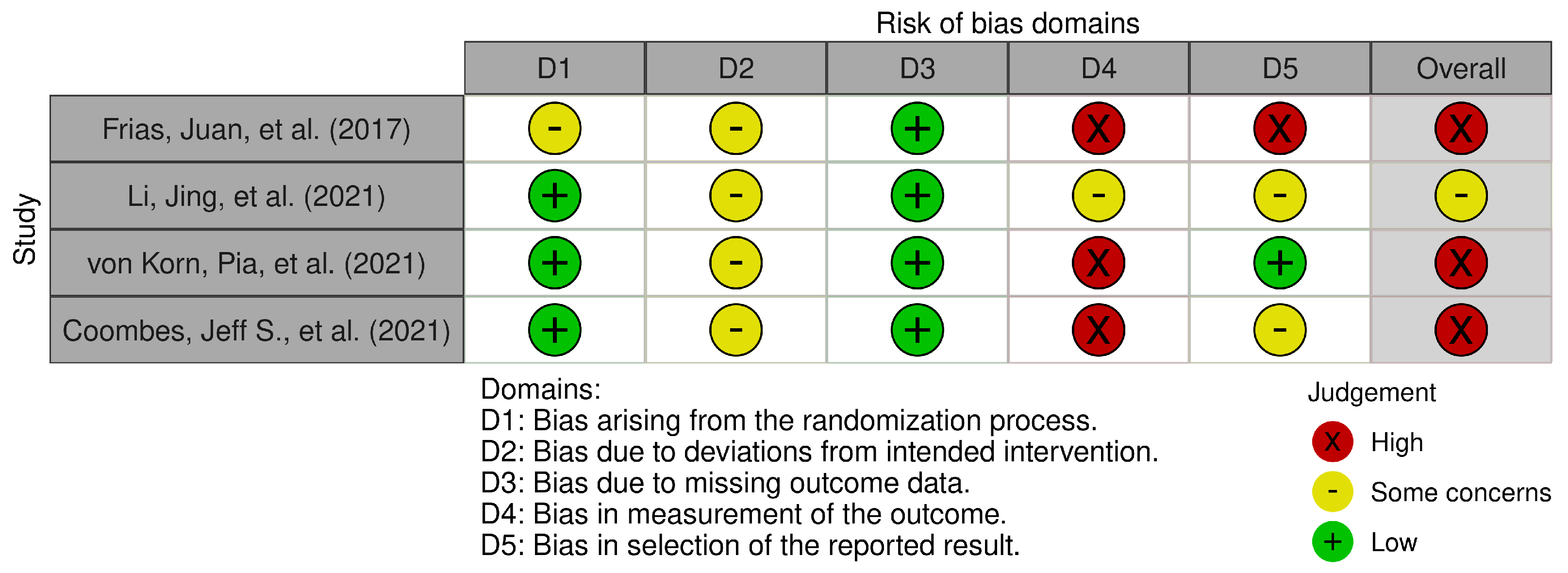
3.3. Risk of Bias Assessment and Quality Appraisal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Risk of Bias Using the Revised Cochrane Tool

References
- Castelyn, G.; Laranjo, L.; Schreier, G.; Gallego, B. Predictive performance and impact of algorithms in remote monitoring of chronic conditions: A systematic review and meta-analysis. Int. J. Med. Inform. 2021, 156, 104620. [Google Scholar] [CrossRef]
- Welch, G.; Balder, A.; Zagarins, S. Telehealth Program for Type 2 Diabetes: Usability, Satisfaction, and Clinical Usefulness in an Urban Community Health Center. Telemed. e-Health 2015, 21, 395–403. [Google Scholar] [CrossRef]
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 26 June 2023).
- Gojka, R. WHO Global report on diabetes: A summary. Int. J. Noncommunicable Dis. 2016, 1, 4. [Google Scholar]
- International Diabetes Federation. Prevalence of Diabetes among People Aged between 20 and 79 Years in Selected Countries Worldwide in 2021. Available online: https://de.statista.com/statistik/daten/studie/182587/umfrage/praevalenz-von-diabetes-in-ausgewaehlten-laendern/ (accessed on 25 May 2023).
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 25 May 2023).
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity management as a primary treatment goal for type 2 diabetes: Time to reframe the conversation. Lancet 2022, 399, 394–405. [Google Scholar] [CrossRef]
- Laha, S.; Rajput, A.; Laha, S.S.; Jadhav, R. A concise and systematic review on non-invasive glucose monitoring for potential diabetes management. Biosensors 2022, 12, 965. [Google Scholar] [CrossRef]
- Leenen, J.P.; Leerentveld, C.; van Dijk, J.D.; van Westreenen, H.L.; Schoonhoven, L.; Patijn, G.A. Current Evidence for Continuous Vital Signs Monitoring by Wearable Wireless Devices in Hospitalized Adults: Systematic Review. J. Med. Internet Res. 2020, 22, e18636. [Google Scholar] [CrossRef]
- Jacobsen, M.; Dembek, T.A.; Kobbe, G.; Gaidzik, P.W.; Heinemann, L. Noninvasive Continuous Monitoring of Vital Signs with Wearables: Fit for Medical Use? J. Diabetes Sci. Technol. 2021, 15, 34–43. [Google Scholar] [CrossRef]
- Soon, S.; Svavarsdottir, H.; Downey, C.; Jayne, D.G. Wearable devices for remote vital signs monitoring in the outpatient setting: An overview of the field. BMJ Innov. 2020, 6, 55–71. [Google Scholar] [CrossRef]
- Prieto-Avalos, G.; Cruz-Ramos, N.A.; Alor-Hernández, G.; Sánchez-Cervantes, J.L.; Rodríguez-Mazahua, L.; Guarneros-Nolasco, L.R. Wearable Devices for Physical Monitoring of Heart: A Review. Biosensors 2022, 12, 292. [Google Scholar] [CrossRef]
- Krakauer, M.; Botero, J.F.; Lavalle-González, F.J.; Proietti, A.; Barbieri, D.E. A review of flash glucose monitoring in type 2 diabetes. Diabetol. Metab. Syndr. 2021, 13, 42. [Google Scholar] [CrossRef]
- Kropff, J.; Choudhary, P.; Neupane, S.; Barnard, K.; Bain, S.C.; Kapitza, C.; Forst, T.; Link, M.; Dehennis, A.; DeVries, J.H. Accuracy and Longevity of an Implantable Continuous Glucose Sensor in the PRECISE Study: A 180-Day, Prospective, Multicenter, Pivotal Trial. Diabetes Care 2017, 40, 63–68. [Google Scholar] [CrossRef]
- Kamusheva, M.; Tachkov, K.; Dimitrova, M.; Mitkova, Z.; García-Sáez, G.; Hernando, M.E.; Goettsch, W.; Petrova, G. A Systematic Review of Collective Evidences Investigating the Effect of Diabetes Monitoring Systems and Their Application in Health Care. Front. Endocrinol. Sec. Clin. Diabetes 2021, 12, 63–68. [Google Scholar] [CrossRef]
- Kamei, T.; Kanamori, T.; Yamamoto, Y.; Edirippulige, S. The use of wearable devices in chronic disease management to enhance adherence and improve telehealth outcomes: A systematic review and meta-analysis. J. Telemed. Telecare 2022, 28. [Google Scholar] [CrossRef]
- Weenk, M.; Bredie, S.J.; Koeneman, M.; Hesselink, G.; van Goor, H.; van de Belt, T.H. Continuous Monitoring of Vital Signs in the General Ward Using Wearable Devices: Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e15471. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Wieseler, B.; McGauran, N. Reporting a systematic review. Chest 2010, 137, 1240–1246. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Chapter 8: Risk of bias in randomized trials. In Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3; John Wiley & Sons: Chichester, UK, 2022; Available online: https://training.cochrane.org/handbook/current (accessed on 15 November 2023).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Chapter 24: Including nonrandomized studies. In Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3; John Wiley & Sons: Chichester, UK, 2022; Available online: https://training.cochrane.org/handbook/current (accessed on 15 November 2023).
- Onoue, T.; Goto, M.; Kobayashi, T.; Tominaga, T.; Ando, M.; Honda, H.; Yoshida, Y.; Tosaki, T.; Yokoi, H.; Kato, S.; et al. Randomized controlled trial for assessment of Internet of Things system to guide intensive glucose control in diabetes outpatients: Nagoya Health Navigator Study protocol. Nagoya J. Med. Sci. 2017, 79, 323–329. [Google Scholar] [CrossRef]
- Rastogi, S.; Verma, D. A comparative study based on real time analysis of physical activity and quality of sleep in early and late night eater patients with type 2 diabetes, using wearable fitness technology. Diabetologia 2020, 63, 348. [Google Scholar] [CrossRef]
- Price, J.C.; Santos, H.O.; Bueno, A.A. The effectiveness of automated digital health solutions at successfully managing obesity and obesity-associated disorders: A PICO-structured investigation. Digit. Health 2022, 5, 8. [Google Scholar] [CrossRef]
- Mattison, G.; Canfell, O.; Forrester, D.; Dobbins, C.; Smith, D.; Töyräs, J.; Sullivan, C. The Influence of Wearables on Health Care Outcomes in Chronic Disease: Systematic Review. J. Med. Internet Res. 2022, 1, 24. [Google Scholar] [CrossRef]
- Frias, J.; Virdi, N.; Raja, P.; Kim, Y.; Savage, G.; Osterberg, L. Effectiveness of Digital Medicines to Improve Clinical Outcomes in Patients with Uncontrolled Hypertension and Type 2 Diabetes: Prospective, Open-Label, Cluster-Randomized Pilot Clinical Trial. J. Med. Internet Res. 2017, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, D.; Liu, S.; Li, M.; Chen, X.; Chen, L.; Wu, Y.; Zhou, W.; Ouyang, L.; Tan, C.; et al. Efficiency of an mHealth App and Chest-Wearable Remote Exercise Monitoring Intervention in Patients with Type 2 Diabetes: A Prospective, Multicenter Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- von Korn, P.; Sydow, H.; Neubauer, S.; Duvinage, A.; Mocek, A.; Dinges, S.; Hackenberg, B.; Weichenberger, M.; Schoenfeld, J.; Amelung, V.; et al. Lifestyle Intervention in Chronic Ischaemic Heart Disease and Type 2 Diabetes (the LeIKD study): Study protocol of a prospective, multicentre, randomised, controlled trial. BMJ Open 2021, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-León, C.; Villalonga, C.; Munoz-Torres, M.; Ruiz, J.R.; Banos, O. Mobile and Wearable Technology for the Monitoring of Diabetes-Related Parameters: Systematic Review. JMIR Mhealth Uhealth 2021, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.S.; Keating, S.E.; Mielke, G.I.; Fassett, R.G.; Coombes, B.K.; O’Leary, K.P.; Cox, E.R.; Burton, N.W. Personal Activity Intelligence e-Health Program in People with Type 2 Diabetes: A Pilot Randomized Controlled Trial. Med. Sci. Sports Exerc. 2022, 54, 18–27. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn. Meth. 2020, 1–7. [Google Scholar] [CrossRef]
- Jablonski, L.; Jensen, T.; Ahlemann, G.M.; Huang, X.; Tetzlaff-Lelleck, V.V.; Piet, A.; Schmelter, F.; Dinkler, V.S.; Sina, C.; Grzegorzek, M. Sensor-Based Detection of Food Hypersensitivity Using Machine Learning. In Proceedings of the ACM, 8th international Workshop on Sensor-Based Activity Recognition and Artificial Intelligence (iWOAR), Lübeck, Germany, 21–22 September 2023. [Google Scholar] [CrossRef]
- Huang, X.; Shirahama, K.; Irshad, M.T.; Nisar, M.A.; Piet, A.; Grzegorzek, M. Sleep Stage Classification in Children Using Self-Attention and Gaussian Noise Data Augmentation. Sensors 2023, 23, 3446. [Google Scholar] [CrossRef]
- Grünewald, A.; Kroenert, D.; Poehler, J.; Brueck, R.; Li, F.; Littau, J.; Schnieber, K.; Piet, A.; Grzegorzek, M.; Kampling, H.; et al. Biomedical Data Acquisition and Processing to Recognize Emotions for Affective Learning. In Proceedings of the 2018 IEEE 18th International Conference on Bioinformatics and Bioengineering (BIBE), Taichung, Taiwan, 29–31 October 2023. [Google Scholar] [CrossRef]
- Irshad, M.T.; Nisar, M.A.; Huang, X.; Hartz, J.; Flak, O.; Li, F.; Gouverneur, P.; Piet, A.; Oltmanns, K.M.; Grzegorzek, M. SenseHunger: Machine Learning Approach to Hunger Detection Using Wearable Sensors. Sensors 2022, 22, 7711. [Google Scholar] [CrossRef]
- Al-Nabulsi, J.; Owida, H.A.; Ma’touq, J.; Matar, S.; Al-Aazeh, E.; Al-Maaiouf, A.; Bleibel, A. Non-invasive Sensing Techniques for Glucose Detection: A Review. Bull. Electr. Eng. Inform. 2022, 11, 1926–1937. [Google Scholar] [CrossRef]
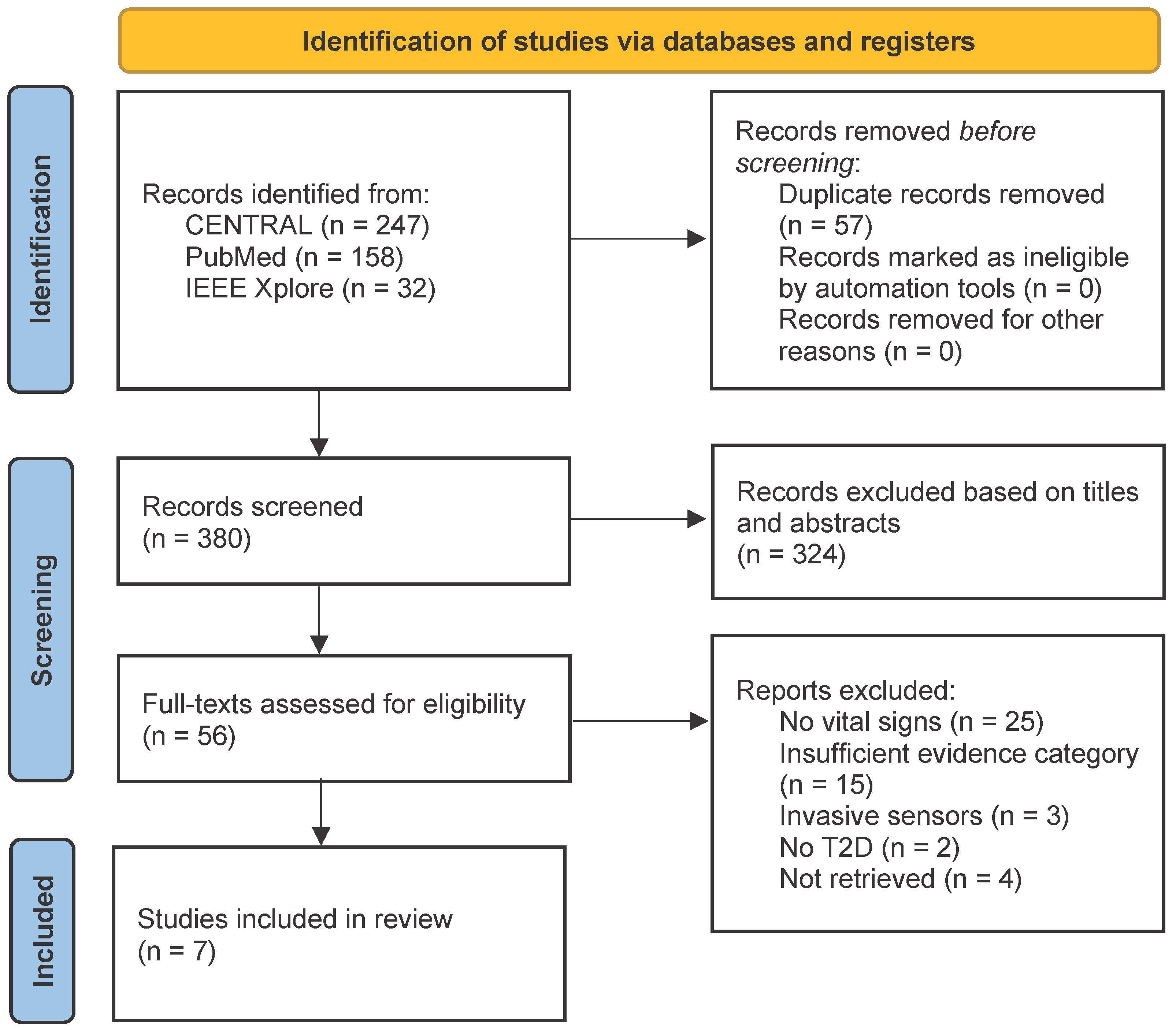

| Study | Study Experiment (Aim, Duration, Intervention) | Study Design | Study Population | Vital Signs Measured | Sensing Technology and Devices | Application of the Sensor | Primary Outcome | Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|
| Frias, Juan, et al. (2017) |
| RCT |
| Heart rate |
|
| Digital med significantly reduced systolic BP at week 4 compared to usual care |
|
| Li, Jing, et al. (2021) |
| RCT |
| Heart rate |
|
|
|
|
| von Korn, Pia, et al. (2021) |
| RCT |
| Heart rate | H7 heart rate sensor, Polar, Kempele, Finland |
|
|
|
| Study | Study Experiment (Aim, Duration, Intervention) | Study Design | Study Population | Vital Signs Measured | Sensing Technology and Devices | Application of the Sensor | Primary Outcome | Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|
| Rodriguez-León, Ciro, et al. (2021) |
| Systematic Review | No information about number of participants and age available | Heart rate |
| Collect data on:
|
|
|
| Coombes, Jeff S., et al. (2021) |
| RCT |
| Heart rate and blood pressure |
|
| Feasibility, acceptability, and efficacy of the PAI e-Health Program |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piet, A.; Jablonski, L.; Daniel Onwuchekwa, J.I.; Unkel, S.; Weber, C.; Grzegorzek, M.; Ehlers, J.P.; Gaus, O.; Neumann, T. Non-Invasive Wearable Devices for Monitoring Vital Signs in Patients with Type 2 Diabetes Mellitus: A Systematic Review. Bioengineering 2023, 10, 1321. https://doi.org/10.3390/bioengineering10111321
Piet A, Jablonski L, Daniel Onwuchekwa JI, Unkel S, Weber C, Grzegorzek M, Ehlers JP, Gaus O, Neumann T. Non-Invasive Wearable Devices for Monitoring Vital Signs in Patients with Type 2 Diabetes Mellitus: A Systematic Review. Bioengineering. 2023; 10(11):1321. https://doi.org/10.3390/bioengineering10111321
Chicago/Turabian StylePiet, Artur, Lennart Jablonski, Jennifer I. Daniel Onwuchekwa, Steffen Unkel, Christian Weber, Marcin Grzegorzek, Jan P. Ehlers, Olaf Gaus, and Thomas Neumann. 2023. "Non-Invasive Wearable Devices for Monitoring Vital Signs in Patients with Type 2 Diabetes Mellitus: A Systematic Review" Bioengineering 10, no. 11: 1321. https://doi.org/10.3390/bioengineering10111321
APA StylePiet, A., Jablonski, L., Daniel Onwuchekwa, J. I., Unkel, S., Weber, C., Grzegorzek, M., Ehlers, J. P., Gaus, O., & Neumann, T. (2023). Non-Invasive Wearable Devices for Monitoring Vital Signs in Patients with Type 2 Diabetes Mellitus: A Systematic Review. Bioengineering, 10(11), 1321. https://doi.org/10.3390/bioengineering10111321









