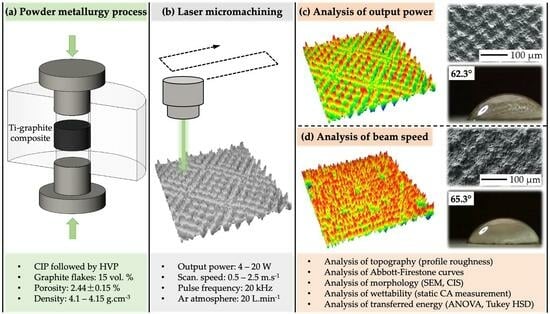
Study on Surface Roughness, Morphology, and Wettability of Laser-Modified Powder Metallurgy-Processed Ti-Graphite Composite Intended for Dental Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Surface Modification Process
2.3. Surface Characterization
2.4. Statistical Analysis
3. Results and Discussion
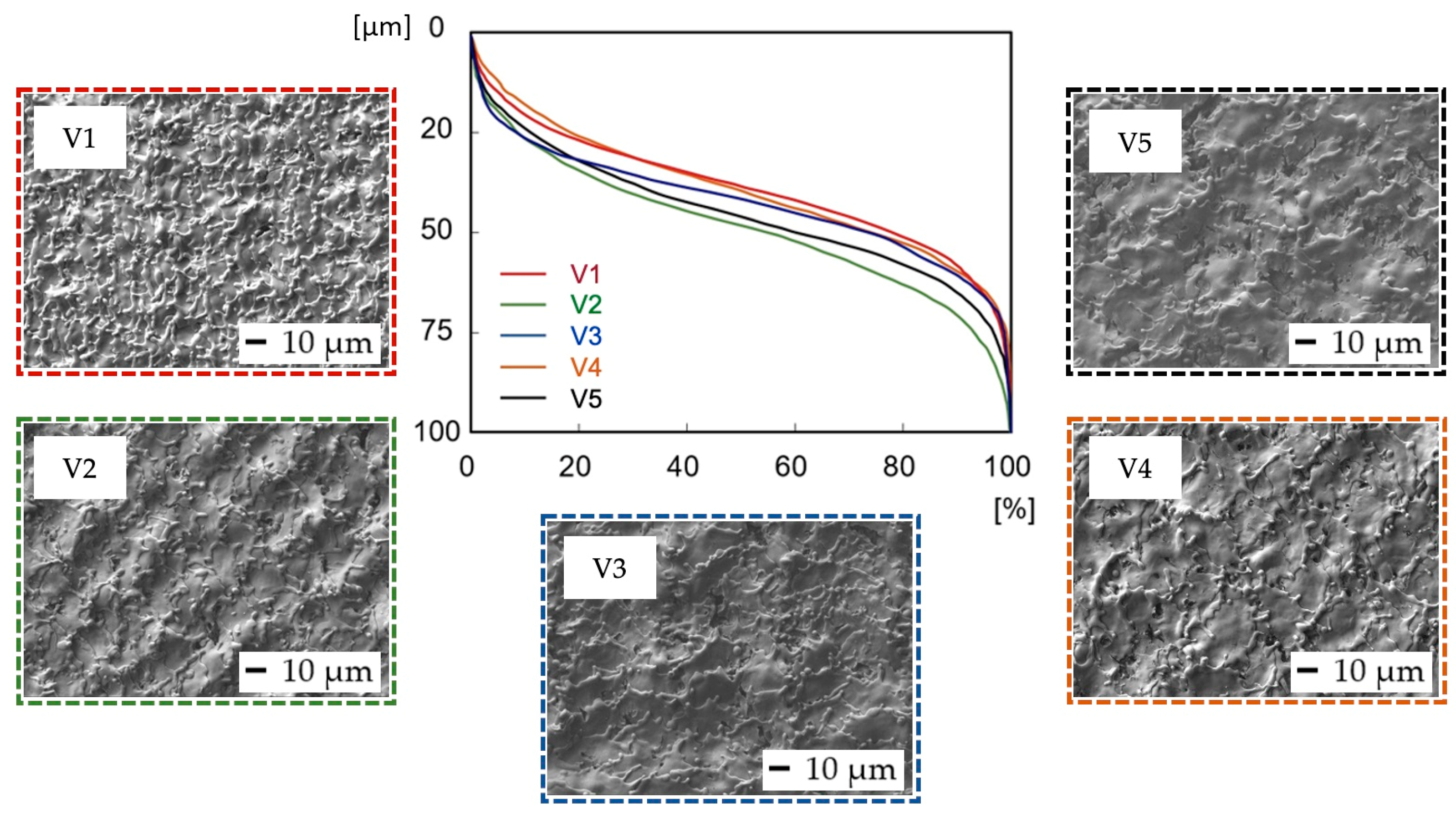
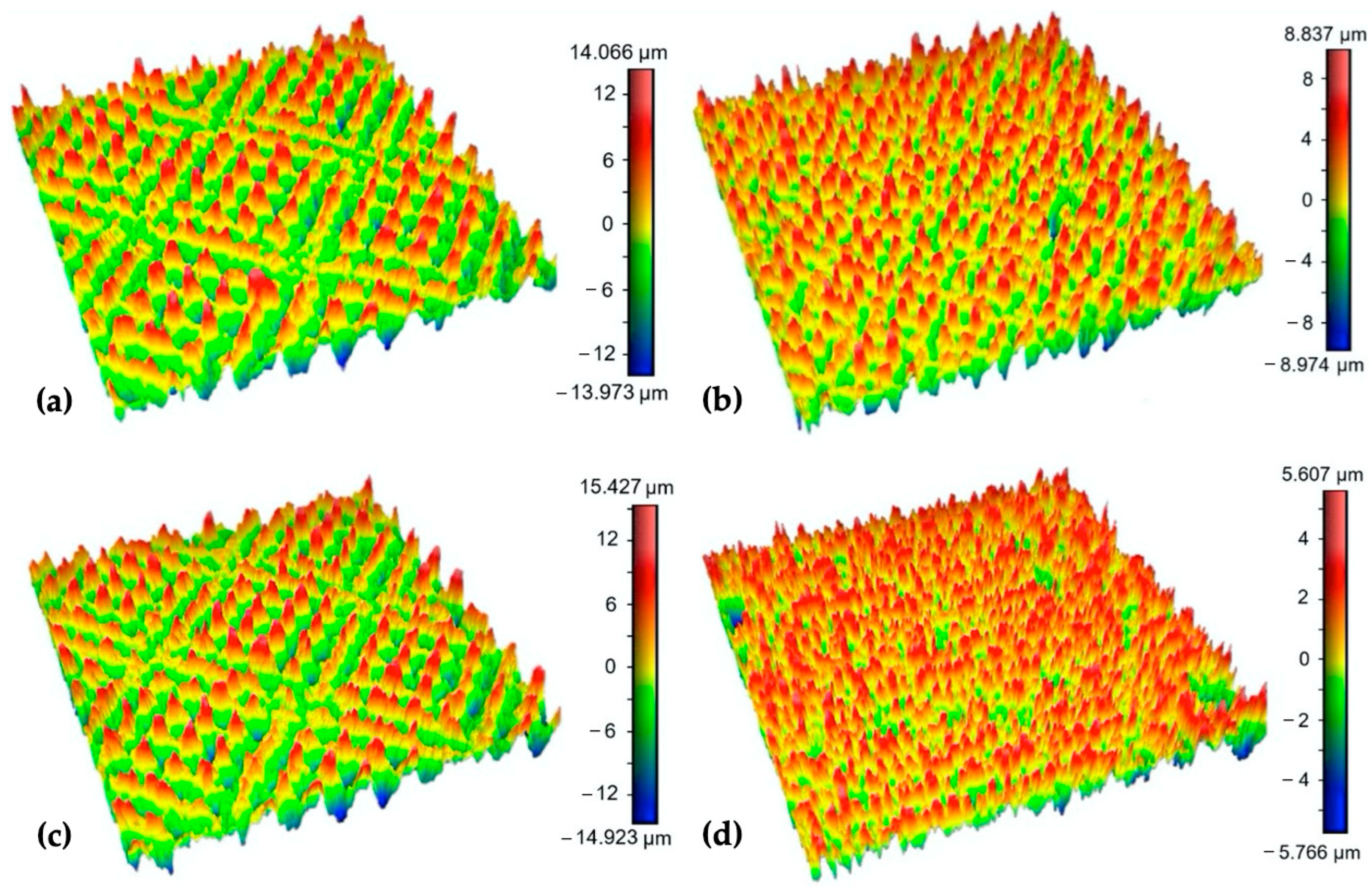
3.1. Surface Roughness Measurement Results
3.2. Scanning Electron Microscopy (SEM) Observation Results (Variation of Output Laser Power)
Scanning Electron Microscopy (SEM) Observation Results (Variation of Scanning Speed)
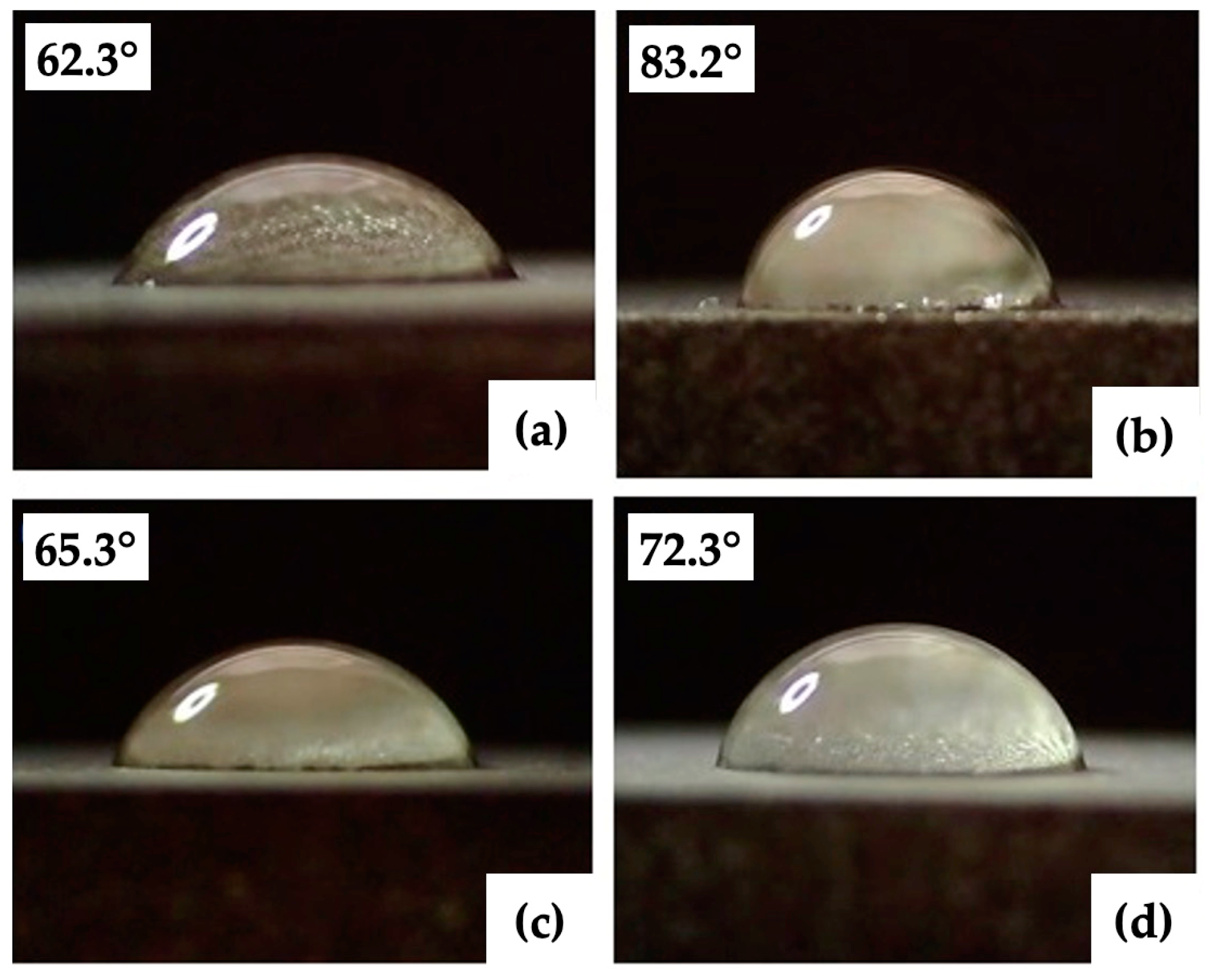
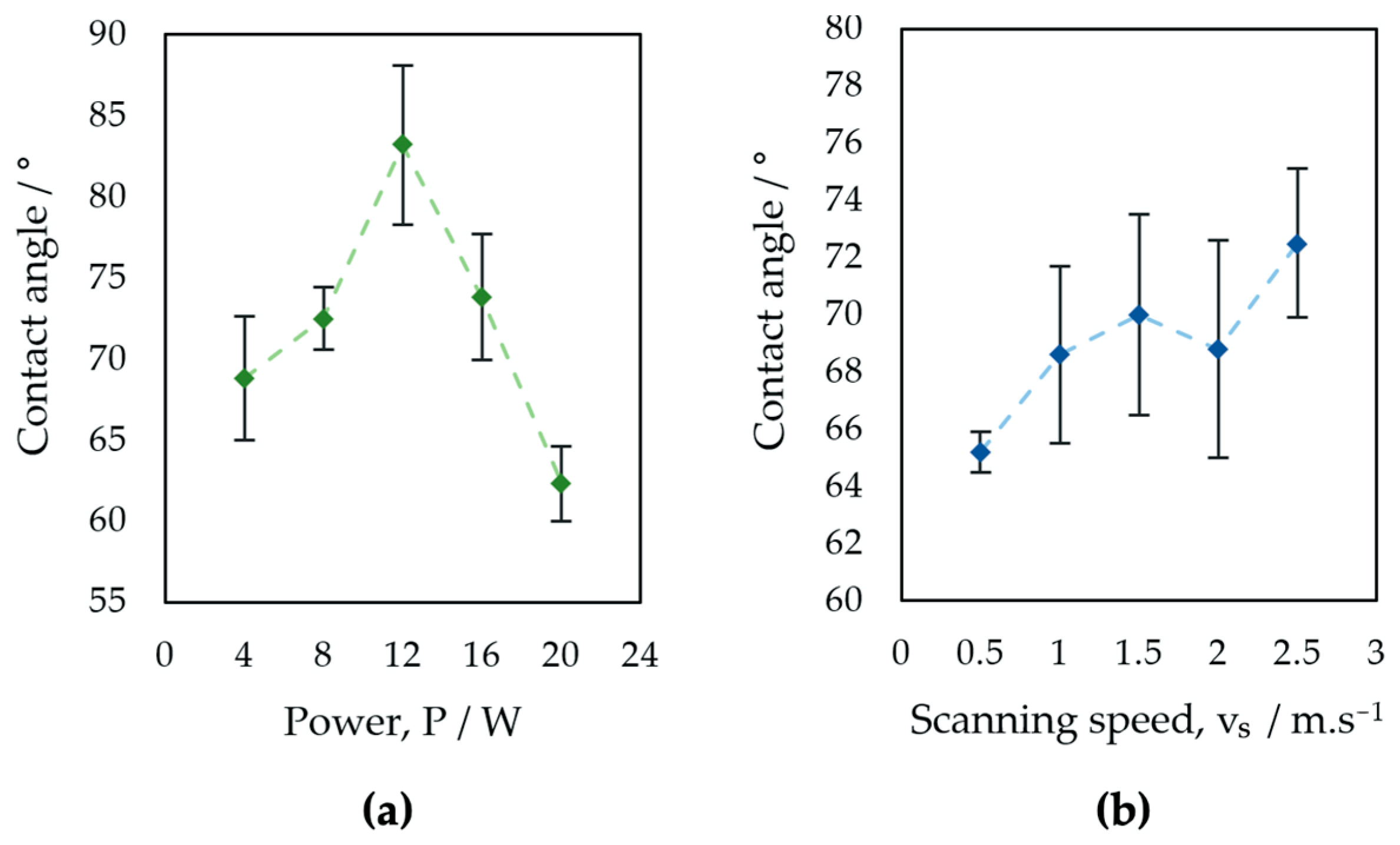
3.3. Wettability Test
4. Conclusions
- (1)
- The moderate level of laser beam energies (0.5 to 1 mJ) helps to induce the presence of regular surface morphology with sufficient spatial distribution, while the higher level of transferred energy (2 to 2.5 mJ) seems to induce irregular surface texture, as well as the formation of nano-geometric features with potential antibacterial effects.
- (2)
- The slot-like morphology observed on the evaluated surfaces might potentially exhibit contact guidance for cultivated cells.
- (3)
- The field surface characteristics that are attributed to high osseointegration relevance (Rsk, Rku, and Rsm) are not statistically different with variation in output power. Interestingly, variation in scanning speed seems to have the most prevalent effect on surface topography when exhibiting statistical differences in all profile roughness parameters except for the Rvk at p-value levels of 0.05 and 0.01.
- (4)
- Applying the same values of the transferred energy while different combinations of laser power and scanning speed were used exhibited different surface morphologies and surface roughness parameters.
- (5)
- An increase in contact angle was documented with increasing laser power up to 12 W, followed by decreasing the contact angle for higher laser power values. An increase in contact angle with increasing laser beam scanning speed was observed, but the changes in contact angle were not statistically significant.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sypniewska, J.; Szkodo, M. Influence of Laser Modification on the Surface Character of Biomaterials: Titanium and Its Alloys—A Review. Coatings 2022, 12, 1371. [Google Scholar] [CrossRef]
- bin Fadzil, A.F.A.; Pramanik, A.; Basak, A.K.; Prakash, C.; Shankar, S. Role of surface quality on biocompatibility of implants—A review. Ann. 3D Print. Med. 2022, 8, 100082. [Google Scholar] [CrossRef]
- Stepanovska, J.; Matejka, R.; Rosina, J.; Bacakova, L.; Kolarova, H. Treatments for enhancing the biocompatibility of titanium implants. Biomed. Pap. 2020, 164, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, E.; Schweitzer, L.; Kieburg, H.; Spielvogel, A.; Huth-Herms, K. The Effects of Laser Microtexturing of Biomedical Grade 5 Ti-6Al-4V Dental Implants (Abutment) on Biofilm Formation. Procedia CIRP 2018, 68, 184–189. [Google Scholar] [CrossRef]
- Medvids, A.; Onufrijevs, P.; Kaupužs, J.; Eglitis, R.; Padgurskas, J.; Zunda, A.; Mimura, H.; Skadins, I.; Varnagiris, S. Anatase or rutile TiO2 nanolayer formation on Ti substrates by laser radiation: Mechanical, photocatalytic and antibacterial properties. Opt. Laser Technol. 2021, 138, 106898. [Google Scholar] [CrossRef]
- Shah, F.A.; Thomsen, P.; Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 2019, 84, 1–15. [Google Scholar] [CrossRef]
- Li, J.; Zhou, P.; Attarilar, S.; Shi, H. Innovative Surface Modification Procedures to Achieve Micro/Nano-Graded Ti-Based Biomedical Alloys and Implants. Coatings 2021, 11, 647. [Google Scholar] [CrossRef]
- Simões, I.G.; dos Reis, A.C.; da Costa Valente, M.L. Analysis of the influence of surface treatment by high-power laser irradiation on the surface properties of titanium dental implants: A systematic review. J. Prosthet. Dent. 2021, 129, 6. [Google Scholar] [CrossRef]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef]
- Günay-Bulutsuz, A.; Berrak, Ö.; Yeprem, H.A.; Arisan, E.D.; Yurci, M.E. Biological responses of ultrafine grained pure titanium and their sand blasted surfaces. Mater. Sci. Eng. C 2018, 91, 382–388. [Google Scholar] [CrossRef]
- Krishna Alla, R.; Ginjupalli, K.; Upadhya, N.; Shammas, M.; Krishna Ravi, R.; Sekhar, A. Surface roughness of implants: A review. Trends Biomater. Artif. Organs 2011, 25, 112–118. [Google Scholar]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral. Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Z.; Li, K.; Hu, J. Effects of surface properties of titanium alloys modified by grinding, sandblasting and acidizing and nanosecond laser on cell proliferation and cytoskeleton. Appl. Surf. Sci. 2020, 501, 144279. [Google Scholar] [CrossRef]
- Wedemeyer, C.; Jablonski, H.; Mumdzic-Zverotic, A.; Fietzek, H.; Mertens, T.; Hilken, G.; Krüger, C.; Wissmann, A.; Heep, H.; Schlepper, R.; et al. Laser-induced nanostructures on titanium surfaces ensure osseointegration of implants in rabbit femora. Materialia 2019, 6, 100266. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2019, 54, 397–425. [Google Scholar] [CrossRef]
- Mukherjee, S.; Dhara, S.; Saha, P. Enhanced corrosion, tribocorrosion resistance and controllable osteogenic potential of stem cells on micro-rippled Ti6Al4V surfaces produced by pulsed laser remelting. J. Manuf. Process. 2021, 65, 119–133. [Google Scholar] [CrossRef]
- Nogués, C.; Blanquer, A.; Barrios, L.; Ibañez, E. Assessment of metallic alloys biocompatibility. In Biomaterials in Clinical Practice: Advances in Clinical Research and Medical Devices; Springer: Cham, Switzerland, 2017; pp. 461–475. [Google Scholar] [CrossRef]
- Solař, P.; Kylián, O.; Marek, A.; Vandrovcová, M.; Bačáková, L.; Hanuš, J.; Vyskočil, J.; Slavínská, D.; Biederman, H. Particles induced surface nanoroughness of titanium surface and its influence on adhesion of osteoblast-like MG-63 cells. Appl. Surf. Sci. 2015, 324, 99–105. [Google Scholar] [CrossRef]
- Hansson, K.N.; Hansson, S. Skewness and Kurtosis: Important Parameters in the Characterization of Dental Implant Surface Roughness—A Computer Simulation. ISRN Mater. Sci. 2011, 2011, 305312. [Google Scholar] [CrossRef]
- Šugár, P.; Ludrovcová, B.; Kalbáčová, M.H.; Šugárová, J.; Sahul, M.; Kováčik, J. Laser Surface Modification of Powder Metallurgy-Processed Ti-Graphite Composite Which Can Enhance Cells’ Osteo-Differentiation. Materials 2021, 14, 6067. [Google Scholar] [CrossRef]
- Starý, V.; Douděrová, M.; Bačáková, L. Influence of surface roughness of carbon materials on human osteoblast-like cell growth. J. Biomed. Mater. Res. A 2014, 102, 1868–1879. [Google Scholar] [CrossRef]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.N. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: A review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Chan, C.W.; Carson, L.; Smith, G.C.; Morelli, A.; Lee, S. Enhancing the antibacterial performance of orthopaedic implant materials by fibre laser surface engineering. Appl. Surf. Sci. 2017, 404, 67–81. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Brambilla, E.; Azzola, F.; Ottobelli, M.; Pellegrini, G.; Francetti, L.A. Laser microtextured titanium implant surfaces reduce in vitro and in situ oral biofilm formation. PLoS ONE 2018, 13, e0202262. [Google Scholar] [CrossRef] [PubMed]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Arshad, K.; Abbasi, M.S.; Maqsood, M.; Shah, R.; Rahim, M. Recent advancements in surface modifications of dental implants. J. Pak. Med. Assoc. 2021, 71, 1655–1661. [Google Scholar] [CrossRef]
- Bonse, J.; Kirner, S.V.; Griepentrog, M.; Spaltmann, D.; Krüger, J. Femtosecond laser texturing of surfaces for tribological applications. Materials 2018, 11, 801. [Google Scholar] [CrossRef]
- Mao, B.; Siddaiah, A.; Liao, Y.; Menezes, P.L. Laser surface texturing and related techniques for enhancing tribological performance of engineering materials: A review. J. Manuf. Process. 2020, 53, 153–173. [Google Scholar] [CrossRef]
- Martínez, J.M.V.; Gómez, J.S.; Ponce, M.B.; Pedemonte, F.J.B. Effects of laser processing parameters on texturized layer development and surface features of Ti6Al4V alloy samples. Coatings 2018, 8, 6. [Google Scholar] [CrossRef]
- Schnell, G.; Staehlke, S.; Duenow, U.; Barbara Nebe, J.; Seitz, H. Femtosecond laser nano/micro textured Ti6Al4V surfaces-effect on wetting and MG-63 cell adhesion. Materials 2019, 12, 2210. [Google Scholar] [CrossRef]
- Shivakoti, I.; Kibria, G.; Cep, R.; Pradhan, B.B.; Sharma, A. Laser surface texturing for biomedical applications: A review. Coatings 2021, 11, 124. [Google Scholar] [CrossRef]
- Böker, K.O.; Kleinwort, F.; Klein-Wiele, J.H.; Simon, P.; Jäckle, K.; Taheri, S.; Lehmann, W.; Schilling, A.F. Laser Ablated Periodic Nanostructures on Titanium and Steel Implants Influence Adhesion and Osteogenic Differentiation of Mesenchymal Stem Cells. Materials 2020, 13, 3526. [Google Scholar] [CrossRef]
- Weng, F.; Chen, C.; Yu, H. Research status of laser cladding on titanium and its alloys: A review. Mater. Des. 2014, 58, 412–425. [Google Scholar] [CrossRef]
- Fedorov, R.; Lederle, F.; Li, M.; Olszok, V.; Wöbbeking, K.; Schade, W.; Hübner, E.G. Formation of Titanium Nitride, Titanium Carbide, and Silicon Carbide Surfaces by High Power Femtosecond Laser Treatment. Chempluschem 2021, 86, 1231–1242. [Google Scholar] [CrossRef]
- Ludrovcová, B.; Šugár, P.; Sahul, M.; Kováčik, J.; Czibor, Z. Oxidation of Biocompatible Graphite–Ti Composite after Laser Ablation in Different Atmospheres. IOP Conf. Ser. Mater. Sci. Eng. 2020, 987, 12032. [Google Scholar] [CrossRef]
- Zeng, C.; Wen, H.; Ettefagh, A.H.; Zhang, B.; Gao, J.; Haghshenas, A.; Raush, J.R.; Guo, S.M. Laser nitriding of titanium surfaces for biomedical applications. Surf. Coat. Technol. 2020, 385, 125397. [Google Scholar] [CrossRef]
- Zeng, C.; Wen, H.; Zhang, B.; Sprunger, P.T.; Guo, S.M. Diffusion of oxygen and nitrogen into titanium under laser irradiation in air. Appl. Surf. Sci. 2020, 505, 144578. [Google Scholar] [CrossRef]
- Chikarakara, E.; Fitzpatrick, P.; Moore, E.; Levingstone, T.; Grehan, L.; Higginbotham, C.; Vázquez, M.; Bagga, K.; Naher, S.; Brabazon, D. In vitro fibroblast and pre-osteoblastic cellular responses on laser surface modified Ti-6Al-4V. Biomed. Mater. 2015, 10, 015007. [Google Scholar] [CrossRef]
- Faeda, R.S.; Tavares, H.S.; Sartori, R.; Guastaldi, A.C.; Marcantonio, E. Evaluation of titanium implants with surface modification by laser beam: Biomechanical study in rabbit tibias. Braz. Oral. Res. 2009, 23, 137–143. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef]
- Laketić, S.; Rakin, M.; Momčilović, M.; Ciganović, J.; Veljović, Đ.; Cvijović-Alagić, I. Surface modifications of biometallic commercially pure Ti and Ti-13Nb-13Zr alloy by picosecond Nd:YAG laser. Int. J. Miner. Metall. Mater. 2021, 28, 285–295. [Google Scholar] [CrossRef]
- Langlade, C.; Vannes, A.B.; Krafft, J.M.; Martin, J.R. Surface modification and tribological behaviour of titanium and titanium alloys after YAG-laser treatments. Surf. Coat. Technol. 1998, 100–101, 383–387. [Google Scholar] [CrossRef]
- Mastrangelo, F.; Quaresima, R.; Abundo, R.; Spagnuolo, G.; Marenzi, G. Esthetic and physical changes of innovative titanium surface properties obtained with laser technology. Materials 2020, 13, 1066. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Tirri, T.; Paldan, H.; Kuntsi-Vaattovaara, H.; Tulamo, R.; Närhi, T. Peri-implant tissue response to TiO2 surface modified implants. Clin. Oral. Implant. Res. 2008, 19, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ao, H.; He, Z.; Wang, Q.; Peng, Z. Surface Modification of Titanium by Femtosecond Laser in Reducing Bacterial Colonization. Coatings 2022, 12, 414. [Google Scholar] [CrossRef]
- Eghbali, N.; Naffakh-Moosavy, H.; Mohammadi, S.S.; Naderi-Manesh, H. The influence of laser frequency and groove distance on cell adhesion, cell viability, and antibacterial characteristics of Ti-6Al-4V dental implants treated by modern fiber engraving laser. Dent. Mater. 2021, 37, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Pfleging, W.; Besser, H.; Majumdar, J.D. Microstructure and corrosion behavior of laser induced periodic patterned titanium based alloy. Opt. Laser Technol. 2019, 116, 196–213. [Google Scholar] [CrossRef]
- Satyanarayana, C.P.; Raju, L.R.; Dumpala, R.; Buradagunta, R.S. Producing high wettable surface on pure titanium sheets by shot peening for bone implant applications. Biointerface Res. Appl. Chem. 2022, 12, 5745–5752. [Google Scholar] [CrossRef]
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2022, 7, e10239. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, J.; Hu, J. Study on surface properties of nanosecond laser textured plasma nitrided titanium alloy. Mater. Today Commun. 2022, 31, 103746. [Google Scholar] [CrossRef]
- Balog, M.; Ibrahim, A.M.H.; Krizik, P.; Bajana, O.; Klimova, A.; Catic, A.; Schauperl, Z. Bioactive Ti + Mg composites fabricated by powder metallurgy: The relation between the microstructure and mechanical properties. J. Mech. Behav. Biomed. Mater. 2019, 90, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, A.; Herrero-Climent, M.; Rios-Carrasco, E.; Rios-Santos, J.V.; Pérez, R.A.; Manero, J.M.; Gil Mur, J. Influence of the Elastic Modulus on the Osseointegration of Dental Implants. Materials 2019, 12, 980. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhao, Y.; Wang, M.; Liu, Z.; Liu, C. Parametric Design of Hip Implant with Gradient Porous Structure. Front. Bioeng. Biotechnol. 2022, 10, 850184. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Huang, L.; Geng, L. Progress on discontinuously reinforced titanium matrix composites. J. Alloys Compd. 2018, 767, 1196–1215. [Google Scholar] [CrossRef]
- Luo, J.P.; Sun, J.F.; Huang, Y.J.; Zhang, J.H.; Zhang, Y.D.; Zhao, D.P.; Yan, M. Low-modulus biomedical Ti–30Nb–5Ta–3Zr additively manufactured by Selective Laser Melting and its biocompatibility. Mater. Sci. Eng. C 2019, 97, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Prakash, C.; Singh, S.; Ramakrishna, S.; Królczyk, G.; Le, C.H. Microwave sintering of porous Ti–Nb-HA composite with high strength and enhanced bioactivity for implant applications. J. Alloys Compd. 2020, 824, 153774. [Google Scholar] [CrossRef]
- Savio, D.; Bagno, A. When the Total Hip Replacement Fails: A Review on the Stress-Shielding Effect. Processes 2022, 10, 612. [Google Scholar] [CrossRef]
- Xu, W.; Tian, J.; Liu, Z.; Lu, X.; Hayat, M.D.; Yan, Y.; Li, Z.; Qu, X.; Wen, C. Novel porous Ti35Zr28Nb scaffolds fabricated by powder metallurgy with excellent osteointegration ability for bone-tissue engineering applications. Mater. Sci. Eng. C 2019, 105, 110015. [Google Scholar] [CrossRef]
- Yılmaz, E.; Kabataş, F.; Gökçe, A.; Fındık, F. Production and Characterization of a Bone-like Porous Ti/Ti-Hydroxyapatite Functionally Graded Material. J. Mater. Eng. Perform. 2020, 29, 6455–6467. [Google Scholar] [CrossRef]
- Yoganandam, K.; Mohanavel, V.; Vairamuthu, J.; Kannadhasan, V. Mechanical properties of titanium matrix composites fabricated via powder metallurgy method. Mater. Today Proc. 2020, 33, 3243–3247. [Google Scholar] [CrossRef]
- ISO 21920-2; Geometrical product specifications (GPS)—Surface texture: Profile—Part 2: Terms, definitions and surface texture parameters. ISO: Geneva, Switzerland, 2021.
- ISO 25178-2; Geometrical product specifications (GPS)—Surface texture: Areal—Part 2: Terms, definitions and surface texture parameters. ISO: Geneva, Switzerland, 2021.
- Zhang, G.; Hua, X.; Li, F.; Zhang, Y.; Shen, C.; Cheng, J. Effect of laser cleaning process parameters on the surface roughness of 5754-grade aluminum alloy. Int. J. Adv. Manuf. Technol. 2019, 105, 2481–2490. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Y.; Wang, R.; Han, Y.; Zhou, H. Effect of Controlled Microtopography on Osteogenic Differentiation of Mesenchymal Stem Cells. J. Healthc. Eng. 2022, 2022, 7179723. [Google Scholar] [CrossRef] [PubMed]
- Veiko, V.; Karlagina, Y.; Zernitckaia, E.; Egorova, E.; Radaev, M.; Yaremenko, A.; Chernenko, G.; Romanov, V.; Shchedrina, N.; Ivanova, E. Laser-Induced µ-Rooms for Osteocytes on Implant Surface: An In Vivo Study. Nanomaterials 2022, 12, 4229. [Google Scholar] [CrossRef]
- Veiko, V.; Karlagina, Y.; Itina, T.; Kuznetsova, D.; Elagin, V.; Zagaynova, E.; Chernenko, G.; Egorova, E.; Zernitskaia, C.; Manokhin, S.; et al. Laser-assisted fabrication and in vitro verification of functionalized surface for cells biointegration. Opt. Laser Technol. 2021, 138, 106871. [Google Scholar] [CrossRef]
- Fan, W.; Yang, Y.; Lou, R.; Yang, Y.; Lou, R.; Chen, X.; Fan, W.; Bai, J.; Cao, W.; Cheng, G.; et al. Influence of energy fluence and overlapping rate of femtosecond laser on surface roughness of Ti-6Al-4V. Opt. Eng. 2019, 58, 106107. [Google Scholar] [CrossRef]
- Frostevarg, J.; Olsson, R.; Powell, J.; Palmquist, A.; Brånemark, R. Formation mechanisms of surfaces for osseointegration on titanium using pulsed laser spattering. Appl. Surf. Sci. 2019, 485, 158–169. [Google Scholar] [CrossRef]
- Klos, A.; Sedao, X.; Itina, T.E.; Helfenstein-Didier, C.; Donnet, C.; Peyroche, S.; Vico, L.; Guignandon, A.; Dumas, V. Ultrafast Laser Processing of Nanostructured Patterns for the Control of Cell Adhesion and Migration on Titanium Alloy. Nanomaterials 2020, 10, 864. [Google Scholar] [CrossRef]
- Tiainen, L.; Abreu, P.; Buciumeanu, M.; Silva, F.; Gasik, M.; Guerrero, R.S.; Carvalho, O. Novel laser surface texturing for improved primary stability of titanium implants. J. Mech. Behav. Biomed. Mater. 2019, 98, 26–39. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef]
- Hori, N.; Att, W.; Ueno, T.; Sato, N.; Yamada, M.; Saruwatari, L.; Suzuki, T.; Ogawa, T. Age-dependent degradation of the protein adsorption capacity of titanium. J. Dent. Res. 2009, 88, 663–667. [Google Scholar] [CrossRef]
- Kido, D.; Komatsu, K.; Suzumura, T.; Matsuura, T.; Cheng, J.; Kim, J.; Park, W.; Ogawa, T. Influence of Surface Contaminants and Hydrocarbon Pellicle on the Results ofWettability Measurements of Titanium. Int. J. Mol. Sci. 2023, 24, 14688. [Google Scholar] [CrossRef] [PubMed]
- Dongre, G.; Rajurkar, A.; Raut, R.; Jangam, S. Preparation of super-hydrophobic textures by using nanosecond pulsed laser. Mater. Today Proc. 2021, 42, 1145–1151. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.P.; Tian, Y.L. Insights into the wettability transition of nanosecond laser ablated surface under ambient air exposure. J. Colloid Interface Sci. 2019, 533, 268–277. [Google Scholar] [CrossRef]
- Edachery, V.; Kailas, S.R.; Kailas, S.V. Influence of surface texture directionality and roughness on wettability, sliding angle, contact angle hysteresis, and lubricant entrapment capability. Tribol. Int. 2021, 158, 106932. [Google Scholar] [CrossRef]
- Dou, H.; Liu, H.; Xu, S.; Chen, Y.; Miao, X.; Lü, H.; Jiang, X. Influence of laser fluences and scan speeds on the morphologies and wetting properties of titanium alloy. Optik 2020, 224, 165443. [Google Scholar] [CrossRef]
- Liu, Z.; Niu, T.; Lei, Y.; Luo, Y. Metal surface wettability modification by nanosecond laser surface texturing: A review. Biosurf. Biotribol. 2022, 8, 95–120. [Google Scholar] [CrossRef]
- Rafiee, K.; Naffakh-Moosavy, H.; Tamjid, E. The effect of laser frequency on roughness, microstructure, cell viability and attachment of Ti6Al4V alloy. Mater. Sci. Eng. C 2020, 109, 110637. [Google Scholar] [CrossRef] [PubMed]
- Nanduru, V.S.P.S.; Ramakrishna, N.S.; Babu, R.S.; Babu, P.D.; Marimuthu, P.; Miryala, S.; Srinandan, C.S. Laser surface texturing inhibits Biofilm formation. Mater. Chem. Phys. 2021, 271, 124909. [Google Scholar] [CrossRef]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial retention on superhydrophobic titanium surfaces fabricated by femtosecond laser ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef]

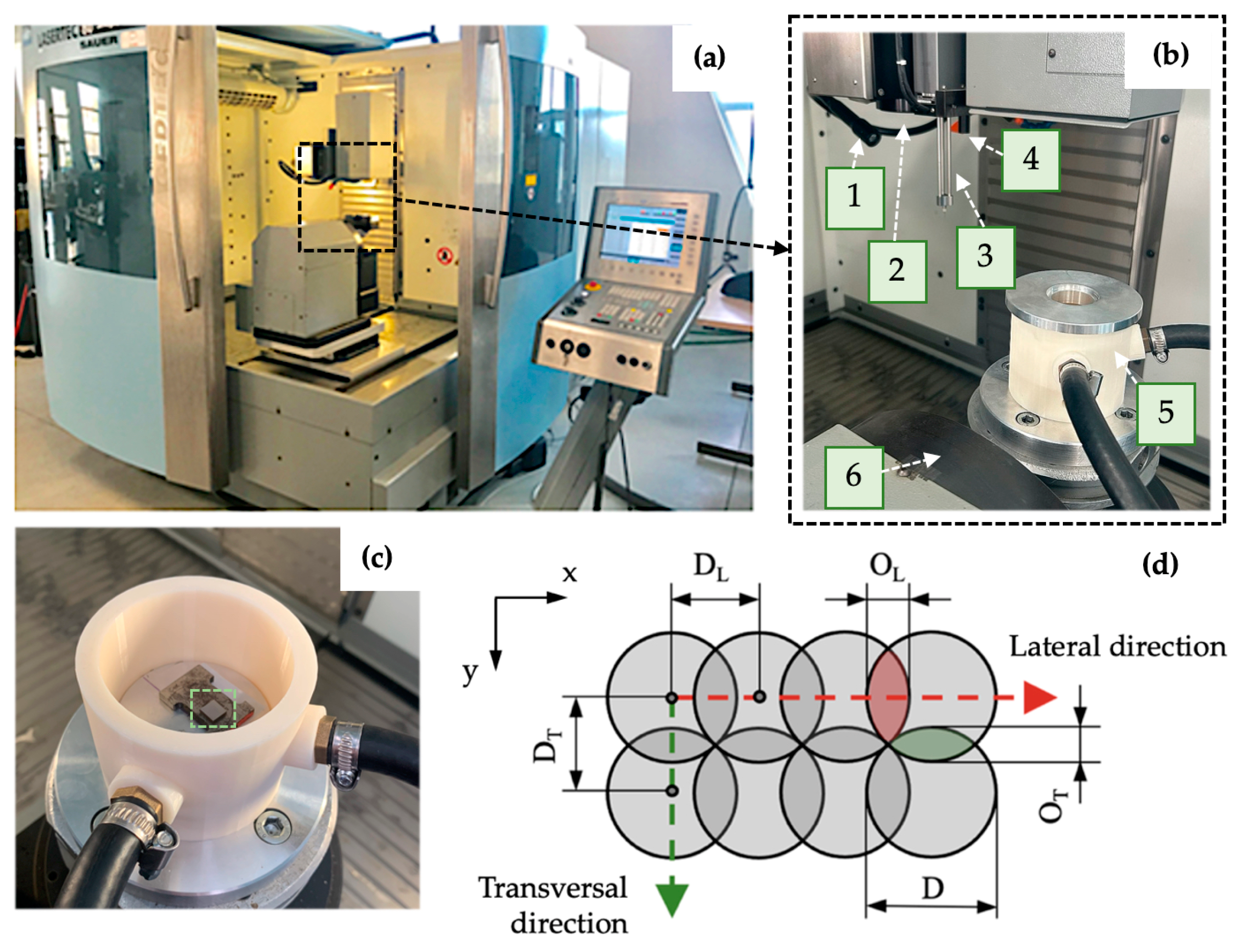





| Surface | Output Power (W) | vs (mm·s−1) | DL (μm) | EP (mJ) | ET (mJ) |
|---|---|---|---|---|---|
| P1 | 4 | 2000 | 100 | 0.2 | 0.5 |
| P2 | 8 | 0.4 | 1 | ||
| P3 | 12 | 0.6 | 1.5 | ||
| P4 | 16 | 0.8 | 2 | ||
| P5 | 20 | 1 | 2.5 | ||
| V1 | 4 | 500 | 25 | 0.2 | 2 |
| V2 | 1000 | 50 | 1 | ||
| V3 | 1500 | 75 | 0.67 | ||
| V4 | 2000 | 100 | 0.5 | ||
| V5 | 2500 | 125 | 0.4 | ||
| Constant parameters: Pulse frequency: f = 20 kHz; Transversal spacing: DT = 10 μm Laser beam movement strategy: cross-hatching; Number of ablated layers: 2; Argon flow rate: 20 L.min−1 | |||||
| Surface | P1 | P2 | P3 | P4 | P5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Ra (μm) | 2.37 | 0.10 | 3.22 | 0.18 | 3.83 | 0.20 | 3.94 | 0.12 | 3.24 | 0.18 |
| Rp (μm) | 6.86 | 0.52 | 10.00 | 0.45 | 11.48 | 0.32 | 11.44 | 0.83 | 10.45 | 0.85 |
| Rv (μm) | 7.49 | 0.45 | 9.21 | 0.83 | 10.57 | 0.90 | 10.77 | 0.94 | 9.93 | 0.31 |
| Rz (μm) | 14.35 | 0.53 | 19.22 | 0.64 | 22.05 | 0.87 | 22.20 | 1.48 | 20.38 | 0.63 |
| Rsk (-) | 0.00 | 0.16 | 0.12 | 0.20 | 0.18 | 0.09 | 0.14 | 0.07 | 0.10 | 0.15 |
| Rku (-) | 2.72 | 0.21 | 2.69 | 0.18 | 2.55 | 0.21 | 2.52 | 0.21 | 2.84 | 0.16 |
| Rsm (μm) | 92.00 | 3.94 | 89.68 | 1.09 | 88.68 | 2.57 | 93.62 | 9.22 | 93.54 | 4.19 |
| Rkp (μm) | 2.66 | 0.39 | 4.62 | 0.49 | 4.69 | 0.88 | 4.47 | 0.16 | 4.47 | 0.24 |
| Rkv (μm) | 2.58 | 0.55 | 3.58 | 0.89 | 3.74 | 0.44 | 3.79 | 0.59 | 4.11 | 0.54 |
| Surface | V1 | V2 | V3 | V4 | V5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Ra (μm) | 1.25 | 0.12 | 2.36 | 0.09 | 2.66 | 0.46 | 2.37 | 0.10 | 1.92 | 0.19 |
| Rp (μm) | 3.57 | 0.16 | 7.10 | 0.68 | 7.79 | 0.87 | 6.86 | 0.52 | 5.84 | 0.57 |
| Rv (μm) | 5.22 | 0.79 | 7.49 | 0.39 | 8.12 | 1.02 | 7.49 | 0.45 | 7.60 | 0.83 |
| Rz (μm) | 8.79 | 0.88 | 14.55 | 0.85 | 15.90 | 0.89 | 14.35 | 0.53 | 13.44 | 0.78 |
| Rsk (-) | −0.38 | 0.21 | −0.01 | 0.11 | 0.04 | 0.21 | 0.00 | 0.16 | −0.40 | 0.35 |
| Rku (-) | 3.58 | 0.55 | 2.72 | 0.12 | 2.67 | 0.43 | 2.72 | 0.21 | 3.62 | 0.76 |
| Rsm (μm) | 66.08 | 13.91 | 70.78 | 3.29 | 154.14 | 54.34 | 92.00 | 3.94 | 90.82 | 8.31 |
| Rkp (μm) | 1.29 | 0.04 | 2.74 | 0.34 | 3.09 | 0.71 | 2.66 | 0.39 | 2.50 | 0.42 |
| Rkv (μm) | 1.93 | 0.58 | 3.00 | 0.44 | 3.67 | 1.66 | 2.58 | 0.55 | 3.21 | 0.71 |
| Roughness Parameter | F-Value | p-Value | R2 |
|---|---|---|---|
| Ra | 76.62 | 0.000 * | 93.87 |
| Rp | 44.77 | 0.000 * | 89.95 |
| Rv | 16.35 | 0.000 * | 76.58 |
| Rz | 63.50 | 0.000 * | 92.70 |
| Rsk | 1.07 | 0.398 | 17.61 |
| Rku | 2.32 | 0.093 | 31.65 |
| Rsm | 1.00 | 0.430 | 16.69 |
| Rpk | 14.75 | 0.000 * | 74.68 |
| Rvk | 4.40 | 0.010 * | 46.83 |
| Roughness Parameter | F-Value | p-Value | R2 |
|---|---|---|---|
| Ra | 27.22 | 0.000 * | 84.48 |
| Rp | 36.66 | 0.000 * | 88.00 |
| Rv | 11.68 | 0.000 * | 70.02 |
| Rz | 31.25 | 0.000 * | 86.21 |
| Rsk | 5.00 | 0.006 * | 50.01 |
| Rku | 5.40 | 0.004 * | 51.91 |
| Rsm | 9.54 | 0.000 * | 65.61 |
| Rpk | 12.36 | 0.000 * | 71.20 |
| Rvk | 2.65 | 0.063 | 34.65 |
| Surface | P1 | P2 | P3 | P4 | P5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| CA (o) | 68.9 | 3.8 | 72.5 | 2.0 | 83.2 | 4.9 | 73.8 | 3.9 | 62.3 | 2.3 |
| Surface | V1 | V2 | V3 | V4 | V5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| CA (o) | 65.3 | 0.7 | 68.6 | 3.1 | 70.0 | 3.5 | 68.8 | 3.8 | 72.5 | 2.6 |
| CA (o) | F-Value | p-Value | R2 |
|---|---|---|---|
| P1–P5 | 13.79 | 0.000 * | 84.66 |
| V1–V5 | 2.38 | 0.121 | 48.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šugár, P.; Antala, R.; Šugárová, J.; Kováčik, J.; Pata, V. Study on Surface Roughness, Morphology, and Wettability of Laser-Modified Powder Metallurgy-Processed Ti-Graphite Composite Intended for Dental Application. Bioengineering 2023, 10, 1406. https://doi.org/10.3390/bioengineering10121406
Šugár P, Antala R, Šugárová J, Kováčik J, Pata V. Study on Surface Roughness, Morphology, and Wettability of Laser-Modified Powder Metallurgy-Processed Ti-Graphite Composite Intended for Dental Application. Bioengineering. 2023; 10(12):1406. https://doi.org/10.3390/bioengineering10121406
Chicago/Turabian StyleŠugár, Peter, Richard Antala, Jana Šugárová, Jaroslav Kováčik, and Vladimír Pata. 2023. "Study on Surface Roughness, Morphology, and Wettability of Laser-Modified Powder Metallurgy-Processed Ti-Graphite Composite Intended for Dental Application" Bioengineering 10, no. 12: 1406. https://doi.org/10.3390/bioengineering10121406
APA StyleŠugár, P., Antala, R., Šugárová, J., Kováčik, J., & Pata, V. (2023). Study on Surface Roughness, Morphology, and Wettability of Laser-Modified Powder Metallurgy-Processed Ti-Graphite Composite Intended for Dental Application. Bioengineering, 10(12), 1406. https://doi.org/10.3390/bioengineering10121406