Investigating Immunomodulatory Biomaterials for Preventing the Foreign Body Response
Abstract
:1. Introduction
2. Foreign Body Response (FBR)
2.1. Acute Inflammatory Response
2.2. Role of Macrophages in FBR
2.3. Fibrous Capsular Formation in FBR
2.4. Biomaterials in Surgery
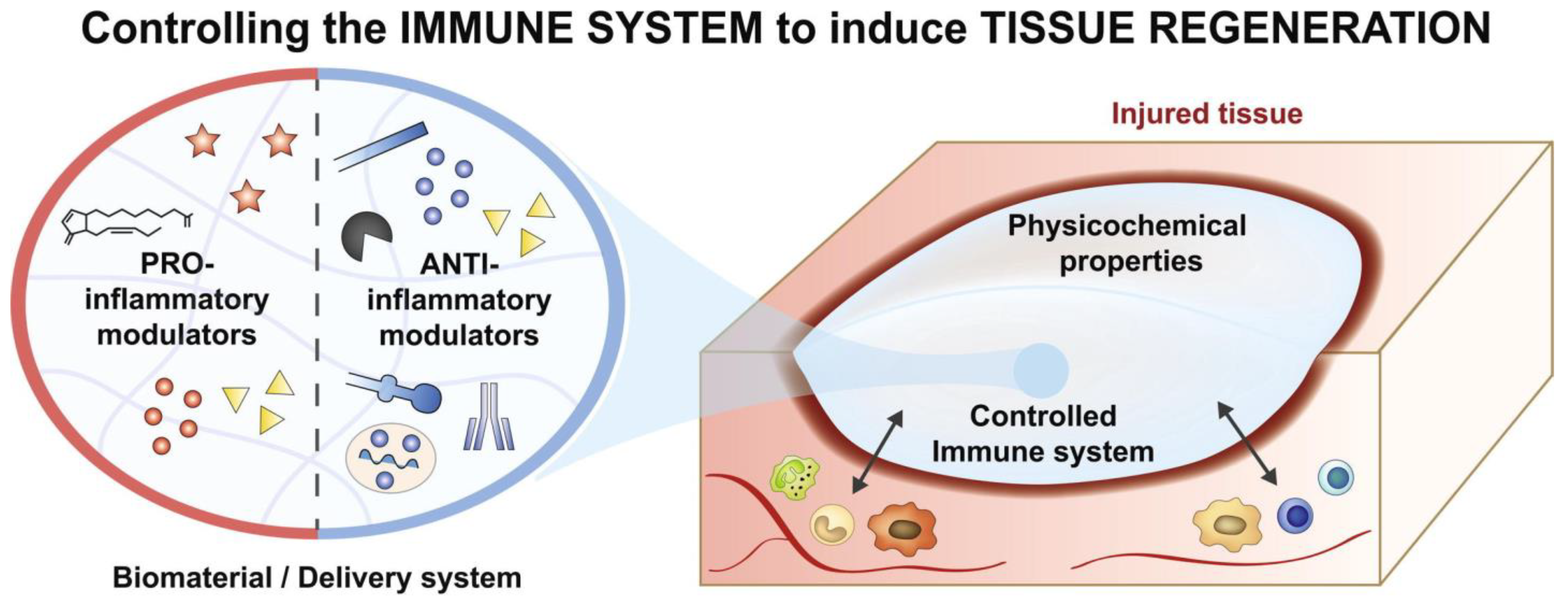
3. Immune System and Biomaterial Design
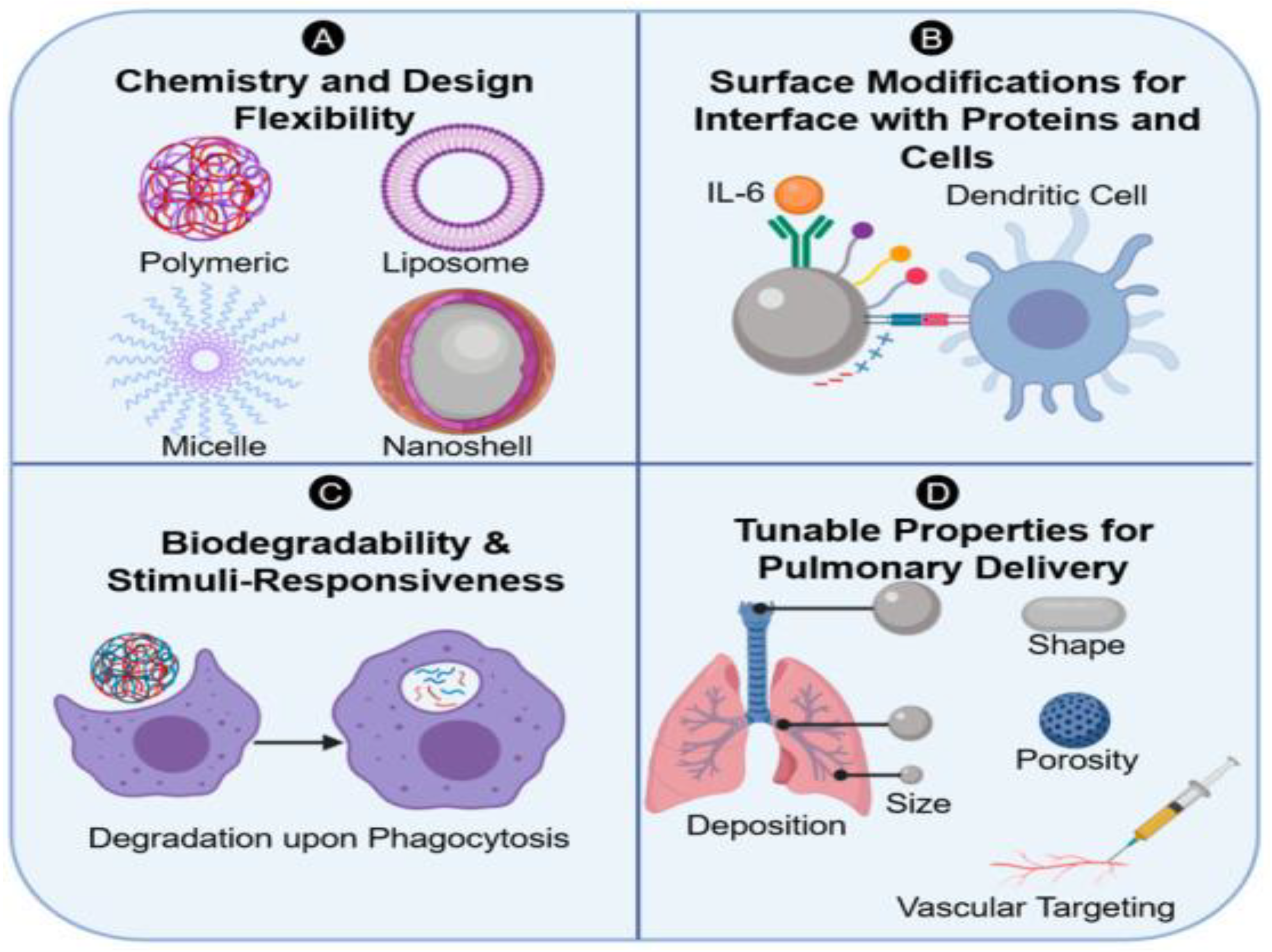
4. Surface Chemistry
4.1. Topography
4.2. Biomaterial Composition
4.3. Protein-Functionalized Biomaterials
4.4. Growth Factor-Releasing Biomaterials
4.5. Cell Therapy
5. Future Directions
Future Direction of Biomaterial Design
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parker, J.B.; Griffin, M.F.; Spielman, A.F.; Wan, D.C.; Longaker, M.T. Exploring the Overlooked Roles and Mechanisms of Fibroblasts in the Foreign Body Response. Adv. Wound Care 2023, 12, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Kumar, A. Biomaterials and bioengineering tomorrow’s healthcare. Biomatter 2013, 3, e24717. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Kenneth Ward, W. A review of the foreign-body response to subcutaneously-implanted devices: The role of macrophages and cytokines in biofouling and fibrosis. J. Diabetes Sci. Technol. 2008, 2, 768–777. [Google Scholar] [CrossRef]
- Frangogiannis, N. Transforming growth factor-β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.H.; Shores, L.S.; Votaw, N.L.; Collier, J.H. Biomaterial strategies for generating therapeutic immune responses. Adv. Drug Deliv. Rev. 2017, 114, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Chien, P.N.; Trinh, X.T.; Nam, S.Y.; Heo, C.Y. Comparison of Formation of Capsule Among Different Breast Silicone Implants. In Vivo 2022, 36, 2756–2766. [Google Scholar] [CrossRef]
- Margulies, I.G.; Salzberg, C.A. The use of acellular dermal matrix in breast reconstruction: Evolution of techniques over 2 decades. Gland Surg. 2019, 8, 3–10. [Google Scholar] [CrossRef]
- Lin, C.C.; Metters, A.T.; Anseth, K.S. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials 2009, 30, 4907–4914. [Google Scholar] [CrossRef]
- Elalouf, A. Immune response against the biomaterials used in 3D bioprinting of organs. Transpl. Immunol. 2021, 69, 101446. [Google Scholar] [CrossRef]
- Carnicer-Lombarte, A.; Chen, S.T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 622524. [Google Scholar] [CrossRef] [PubMed]
- Lotti, F.; Ranieri, F.; Vadalà, G.; Zollo, L.; Di Pino, G. Invasive Intraneural Interfaces: Foreign Body Reaction Issues. Front. Neurosci. 2017, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Bond, J.; Medina, M.A.; Chen, L.; Quiles, C.; Kokosis, G.; Bashirov, L.; Klitzman, B.; Levinson, H. Characterization of the Foreign Body Response to Common Surgical Biomaterials in a Murine Model. Eur. J. Plast. Surg. 2017, 40, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Su, Y.; Zhou, J.; Zheng, Y.; Zhu, D. Toward a Better Regeneration through Implant-Mediated Immunomodulation: Harnessing the Immune Responses. Adv. Sci. 2021, 8, e2100446. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, S.; Shin, B.H.; Heo, C.-Y.; Faruq, O.; Van Anh, L.T.; Dönmez, N.; Chien, P.N.; Shin, D.-S.; Nam, S.-Y.; et al. Silicone Implants Immobilized with Interleukin-4 Promote the M2 Polarization of Macrophages and Inhibit the Formation of Fibrous Capsules. Polymers 2021, 13, 2630. [Google Scholar] [CrossRef] [PubMed]
- Ksontini, R.; MacKay, S.L.; Moldawer, L.L. Revisiting the role of tumor necrosis factor alpha and the response to surgical injury and inflammation. Arch. Surg. 1998, 133, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Masuda, K.; Kishimoto, T. Regulation of IL-6 in Immunity and Diseases. Adv. Exp. Med. Biol. 2016, 941, 79–88. [Google Scholar]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Harada, A.S.N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leucoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Derler, M.; Basílio, J.; Schmid, J.A. NF-κB in monocytes and macrophages—An inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar] [CrossRef] [PubMed]
- Noskovicova, N.; Hinz, B.; Pakshir, P. Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction. Cells 2021, 10, 1794. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Peng, Z.; Tang, P.; Sun, H.; Lei, H.; Li, Z.; Hui, D.; Du, C.; Zhou, C.; Wang, Y. Review of Plastic Surgery Biomaterials and Current Progress in Their 3D Manufacturing Technology. Materials 2020, 13, 4108. [Google Scholar] [CrossRef] [PubMed]
- Jarai, B.M.; Stillman, Z.; Bomb, K.; Kloxin, A.M.; Fromen, C.A. Biomaterials-Based Opportunities to Engineer the Pulmonary Host Immune Response in COVID-19. ACS Biomater. Sci. Eng. 2021, 7, 1742–1764. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Dreaden, E.C.; Brown, D.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Whitaker, R.; Hernaez-Estrada, B.; Hernandez, R.M.; Santos-Vizcaino, E.; Spiller, K.L. Immunomodulatory Biomaterials for Tissue Repair. Chem. Rev. 2021, 121, 11305–11335. [Google Scholar] [CrossRef]
- Kämmerling, L.; Fisher, L.E.; Antmen, E.; Simsek, G.M.; Rostam, H.M.; Vrana, N.E.; Ghaemmaghami, A.M. Mitigating the foreign body response through ‘immune-instructive’ biomaterials. J. Immunol. Regen. Med. 2021, 12, 100040. [Google Scholar] [CrossRef]
- Kim, Y.; Wu, L.; Park, H.C.; Yang, H.C. Reduction of fibrous encapsulation by polyethylene glycol-grafted liposomes containing phosphatidylserine. Biomed. Mater. 2020, 15, 065007. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.; Su, Y.; Xi, Y.; Tian, L.; Xu, M.; Wang, Q.; Padidan, S.; Li, P.; Huang, W. Dual-Functional Polyethylene Glycol-b-polyhexanide Surface Coating with in Vitro and in Vivo Antimicrobial and Antifouling Activities. ACS Appl. Mater. Interfaces 2017, 9, 10383–10397. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, P.; Hu, W.; Tang, L. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [Google Scholar] [PubMed]
- Agarwal, A.; Weis, T.L.; Schurr, M.J.; Faith, N.G.; Czuprynski, C.J.; McAnulty, J.F.; Murphy, C.J.; Abbott, N.L. Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials 2010, 31, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Soto, R.J.; Merricks, E.P.; Bellinger, D.A.; Nichols, T.C.; Schoenfisch, M.H. Influence of diabetes on the foreign body response to nitric oxide-releasing implants. Biomaterials 2018, 157, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Harn, H.J.; Chiou, T.W. The Role of Biomaterials in Implantation for Central Nervous System Injury. Cell Transplant. 2018, 27, 407–422. [Google Scholar] [CrossRef]
- Ballo, A.; Agheli, H.; Lausmaa, J.; Thomsen, P.; Petronis, S. Nanostructured model implants for in vivo studies: Influence of well-defined nanotopography on de novo bone formation on titanium implants. Int. J. Nanomed. 2011, 6, 3415–3428. [Google Scholar] [CrossRef]
- McMurray, R.J.; Gadegaard, N.; Tsimbouri, P.M.; Burgess, K.V.; McNamara, L.E.; Tare, R.; Murawski, K.; Kingham, E.; Oreffo, R.O.C.; Dalby, M.J. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 2011, 10, 637–644. [Google Scholar] [CrossRef]
- Allan, C.; Ker, A.; Smith, C.A.; Tsimbouri, P.M.; Borsoi, J.; O’Neill, S.; Gadegaard, N.; Dalby, M.J.; Meek, R.M.D. Osteoblast response to disordered nanotopography. J. Tissue Eng. 2018, 9, 2041731418784098. [Google Scholar] [CrossRef]
- Curtis, A.; Wilkinson, C. Topographical control of cells. Biomaterials 1997, 18, 1573–1583. [Google Scholar] [CrossRef]
- Damiati, L.; Eales, M.G.; Nobbs, A.H.; Su, B.; Tsimbouri, P.M.; Salmeron-Sanchez, M.; Dalby, M.J. Impact of surface topography and coating on osteogenesis and bacterial attachment on titanium implants. J. Tissue Eng. 2018, 9, 2041731418790694. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.O.; Casanova, M.R.; Quinteira, R.; Fangueiro, J.F.; Reis, R.L.; Neves, N.M. Biomimetic surface topography as a potential modulator of macrophages inflammatory response to biomaterials. Biomater. Adv. 2022, 141, 213128. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, X.; Li, H.; Gelinsky, M.; Gu, Z. Tailoring Materials for Modulation of Macrophage Fate. Adv. Mater. 2021, 33, e2004172. [Google Scholar] [CrossRef] [PubMed]
- Refai, A.K.; Textor, M.; Brunette, D.M.; Waterfield, J.D. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J. Biomed. Mater. Res. A 2004, 70, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Takebe, J.; Champagne, C.M.; Offenbacher, S.; Ishibashi, K.; Cooper, L.F. Titanium surface topography alters cell shape and modulates bone morphogenetic protein 2 expression in the J774A.1 macrophage cell line. J. Biomed. Mater. Res. A 2003, 64, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Bota, P.C.; Collie, A.M.; Puolakkainen, P.; Vernon, R.B.; Sage, E.H.; Ratner, B.D.; Stayton, P.S. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J. Biomed. Mater. Res. A 2010, 95, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Kim, H.J.; Zheng, C.; Harkins, L.; Tao, W.; Deschenes, E. Foreign body response to synthetic polymer biomaterials and the role of adaptive immunity. Biomed. Mater. 2022, 17, 022007. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.E.; Griffin, M.; Berry, C.E.; Parker, J.B.; Downer, M.A.; Wan, D.C.; Longaker, M.T. Attenuating Chronic Fibrosis: Decreasing Foreign Body Response with Acellular Dermal Matrix. Tissue Eng. Part B Rev. 2023. [Google Scholar] [CrossRef]
- Yang, H.; Lai, C.; Xuan, C.; Muyuan, C.; Xuemin, L.; Yunhua, C.; Xuetao, S. Integrin-binding pro-survival peptide engineered silk fibroin nanosheets for diabetic wound healing and skin regeneration. Chem. Eng. J. 2020, 398, 125617. [Google Scholar] [CrossRef]
- Zhu, Y.; Cankova, Z.; Iwanaszko, M.; Lichtor, S.; Mrksich, M.; Ameer, G.A. Potent laminin-inspired antioxidant regenerative dressing accelerates wound healing in diabetes. Proc. Natl. Acad. Sci. USA 2018, 115, 6816–6821. [Google Scholar] [CrossRef]
- Cha, B.H.; Shin, S.R.; Leijten, J.; Li, Y.-C.; Singh, S.; Liu, J.C.; Annabi, N.; Abdi, R.; Dokmeci, M.R.; Vrana, N.E.; et al. Integrin-Mediated Interactions Control Macrophage Polarization in 3D Hydrogels. Adv. Healthc. Mater. 2017, 6, 1700289. [Google Scholar] [CrossRef] [PubMed]
- Wick, G.; Backovic, A.; Rabensteiner, E.; Plank, N.; Schwentner, C.; Sgonc, R. The immunology of fibrosis: Innate and adaptive responses. Trends Immunol. 2010, 3, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Morales, R.T.; Cui, X.; Huang, J.; Qian, W.; Tong, J.; Chen, W. A Photoresponsive Hyaluronan Hydrogel Nanocomposite for Dynamic Macrophage Immunomodulation. Adv. Healthc. Mater. 2019, 8, e1801234. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Kim, Y.; Seon, G.M.; Choi, S.H.; Park, H.C.; Son, G.; Kim, S.M.; Lim, B.-S.; Yang, H.-C. Effects of RGD-grafted phosphatidylserine-containing liposomes on the polarization of macrophages and bone tissue regeneration. Biomaterials 2021, 279, 121239. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, S.; Hayashida, K. Advances in surgical applications of growth factors for wound healing. Burns Trauma 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.W.; Chen, W.L.; Huang, S.M.; Chan, J.Y. Platelet-derived growth factor-AA is a substantial factor in the ability of adipose-derived stem cells and endothelial progenitor cells to enhance wound healing. FASEB J. 2019, 33, 2388–2395. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, I.; Pandey, H.; Patil, S.; Mishra, S.B.; Pandey, A.C.; Zamboni, P.; Ramteke, P.W.; Singh, A.V. In vivo diabetic wound healing with nanofibrous scaffolds modified with gentamicin and recombinant human epidermal growth factor. J. Biomed. Mater. Res. A 2018, 106, 641–651. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, X.; Jin, L.; Bai, J.; Liu, W.; Wang, Z. Stimulation of wound healing using bioinspired hydrogels with basic fibroblast growth factor (bFGF). Int. J. Nanomed. 2018, 13, 3897–3906. [Google Scholar] [CrossRef]
- Ito, R.; Morimoto, N.; Liem, P.H.; Nakamura, Y.; Kawai, K.; Taira, T.; Tsuji, W.; Toi, M.; Suzuki, S. Adipogenesis using human adipose tissue-derived stromal cells combined with a collagen/gelatin sponge sustaining release of basic fibroblast growth factor. J. Tissue Eng. Regen. Med. 2014, 8, 1000–1008. [Google Scholar] [CrossRef]
- Chen, M.; Tian, J.; Liu, Y.; Cao, H.; Li, R.; Wang, J.; Wu, J.; Zhang, Q. Dynamic covalent constructed self-healing hydrogel for sequential delivery of antibacterial agent and growth factor in wound healing. Chem. Eng. J. 2019, 373, 413–424. [Google Scholar] [CrossRef]
- Söderlund, Z.; Ibáñez-Fonseca, A.; Hajizadeh, S.; Rodríguez-Cabello, J.C.; Liu, J.; Ye, L.; Tykesson, E.; Elowsson, L. Westergren-Thorsson. Controlled release of growth factors using synthetic glycosaminoglycans in a modular macroporous scaffold for tissue regeneration. Commun. Biol. 2022, 5, 1349. [Google Scholar] [CrossRef] [PubMed]
- Ayala, P.; Caves, J.; Dai, E.; Siraj, L.; Liu, L.; Chaudhuri, O.; Haller, C.A.; Mooney, D.J.; Chaikof, E.L. Engineered composite fascia for stem cell therapy in tissue repair applications. Acta Biomater. 2015, 26, 1–12. [Google Scholar] [CrossRef]
- Prockop, D.J.; Oh, J.Y. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Vegas, A.J. Domesticating the foreign body response: Recent advances and applications. Adv. Drug Deliv. Rev. 2019, 144, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, W.; Tang, H.; Li, Y.; Li, C.; Wang, L.; Chen, J.; Lin, W.; Li, S.; Fan, Z.; et al. Interactions Between Immunomodulatory Biomaterials and Immune Microenvironment: Cues for Immunomodulation Strategies in Tissue Repair. Front. Bioeng. Biotechnol. 2022, 10, 820940. [Google Scholar] [CrossRef]
- Seok, S. Polymer-Based Biocompatible Packaging for Implantable Devices: Packaging Method, Materials, and Reliability Simulation. Micromachines 2021, 12, 1020. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.; Ella-Menye, J.R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]

| Cytokine | Function | Reference |
|---|---|---|
| Tumor necrosis factor-alpha (TNF-α) | Induces inflammation through activation of proinflammatory signaling pathways; activates apoptotic death | Ksontini et al. [16] |
| Interleukin-1β (IL-1β) | Induces inflammation through promoting recruitment and proliferation of innate immune cells; induces differentiation of type 17 T-helper (TH17) cells | Gabay et al. [17] Bent et al. [18] |
| Interleukin-6 (IL-6) | Induces inflammation through inducing synthesis of acute phase proteins and antibody production for elimination of infectious agents; supports differentiation of effector T cells | Tanaka et al. [19] Tanaka et al. [20] |
| Interleukin-8 (IL-8) | Inducing inflammation through recruiting and activating neutrophils | Harada et al. [21] |
| Interleukin-4, Interleukin-13 (IL-4, IL-13) | Prevents inflammation through directly inducing alternative (M2) activation of macrophages; promoting Th2 cell type response; and driving profibrotic activation and foreign body cell formation | Gordon & Martinez [22] |
| Interleukin-10 (IL-10) | Prevents inflammation through downregulating macrophage gene expression; directing Treg-mediated anti-inflammatory response; and preventing scar formation | Zhang et al. [14], Gordon & Martinez [22] |
| Protein | Function | Reference |
|---|---|---|
| Cytokines/Chemokines | Recruit immune response by recruiting immune cells to the implant site and promoting inflammation. | Veiseh et al. [32] |
| Fibronectin | A glycoprotein that can bind to surface of biomaterials and facilitate the adhesion of immune cells, particularly macrophages. This protein is involved in the initial recognition of the foreign material | Veiseh et al. [32] |
| Collagen | Collagen is a major component of the extracellular matrix and is produced during the FBR. It contributes to the formation of a fibrous capsule around the foreign material, isolating it from its surroundings. | Veiseh et al. [32] |
| TGF-β | TGF-β is a cytokine that plays a key role in tissue repair and fibrosis. Its production is often increased during the FBR and can contribute to the development of fibrous tissue around the implant. | Veiseh et al. [32] |
| Plasma Proteins | Various proteins such as fibrinogen can absorb into the surface of biomaterials promoting protein adhesion and cell attachment. | Veiseh et al. [32] |
| Growth Factors | Function | Reference |
|---|---|---|
| PDGF | PDGF is released by platelets and various cell types during the early stages of tissue injury and the FBR. It stimulates cell migration, proliferation, and the production of extracellular matrix components such as collagen, contributing to tissue repair fibrosis. | Yamakawa S. [55] |
| VEGF | VEGF is a critical growth factor in angiogenesis. It promotes the formation of new blood vessels, which can be important for supplying nutrients and oxygen to tissues surrounding the implant. | Yamakawa S. [55] |
| bFGFs | bFGFs are a family of growth factors that promote cell growth and angiogenesis. They can influence the tissue response of implanted materials by stimulation fibroblasts and endothelial. | Yamakawa S. [55] |
| TGF-β | TGF-β is involved in many aspects of wound healing. It promotes cell migration, angiogenesis, collagen synthesis, and tissue remodeling. There are multiple isoforms of TGF-β, and they can have distinct roles in different phases of wound healing. | Yamakawa S. [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, A.; Downer, M.A.; Berry, C.E.; Valencia, C.; Fazilat, A.Z.; Griffin, M. Investigating Immunomodulatory Biomaterials for Preventing the Foreign Body Response. Bioengineering 2023, 10, 1411. https://doi.org/10.3390/bioengineering10121411
Kim A, Downer MA, Berry CE, Valencia C, Fazilat AZ, Griffin M. Investigating Immunomodulatory Biomaterials for Preventing the Foreign Body Response. Bioengineering. 2023; 10(12):1411. https://doi.org/10.3390/bioengineering10121411
Chicago/Turabian StyleKim, Alexia, Mauricio A. Downer, Charlotte E. Berry, Caleb Valencia, Alex Z. Fazilat, and Michelle Griffin. 2023. "Investigating Immunomodulatory Biomaterials for Preventing the Foreign Body Response" Bioengineering 10, no. 12: 1411. https://doi.org/10.3390/bioengineering10121411
APA StyleKim, A., Downer, M. A., Berry, C. E., Valencia, C., Fazilat, A. Z., & Griffin, M. (2023). Investigating Immunomodulatory Biomaterials for Preventing the Foreign Body Response. Bioengineering, 10(12), 1411. https://doi.org/10.3390/bioengineering10121411








