Modelling and Simulation of the Combined Use of IABP and Impella as a Rescue Procedure in Cardiogenic Shock: An Alternative for Non-Transplant Centres?
Abstract
:1. Introduction
2. Materials and Methods
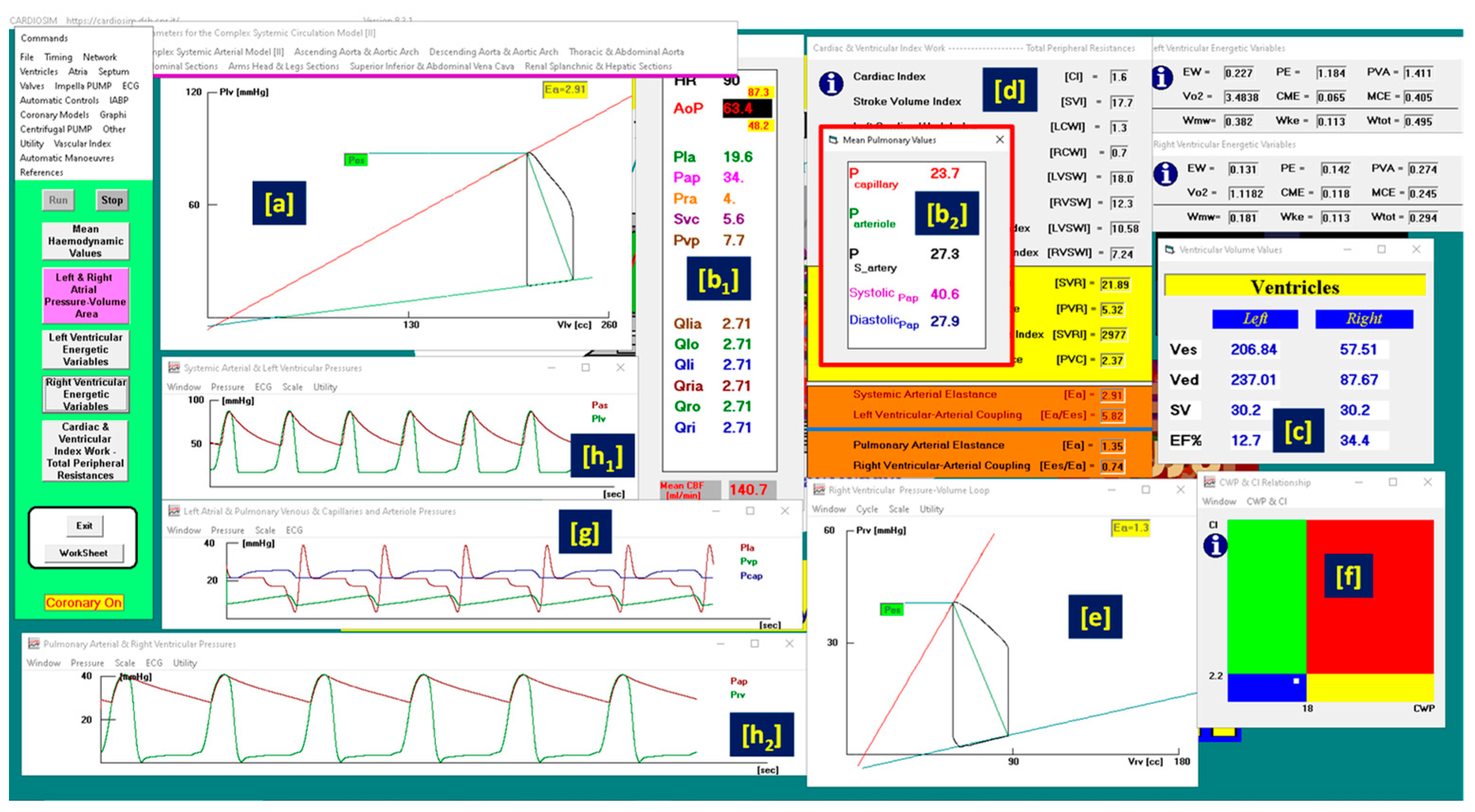
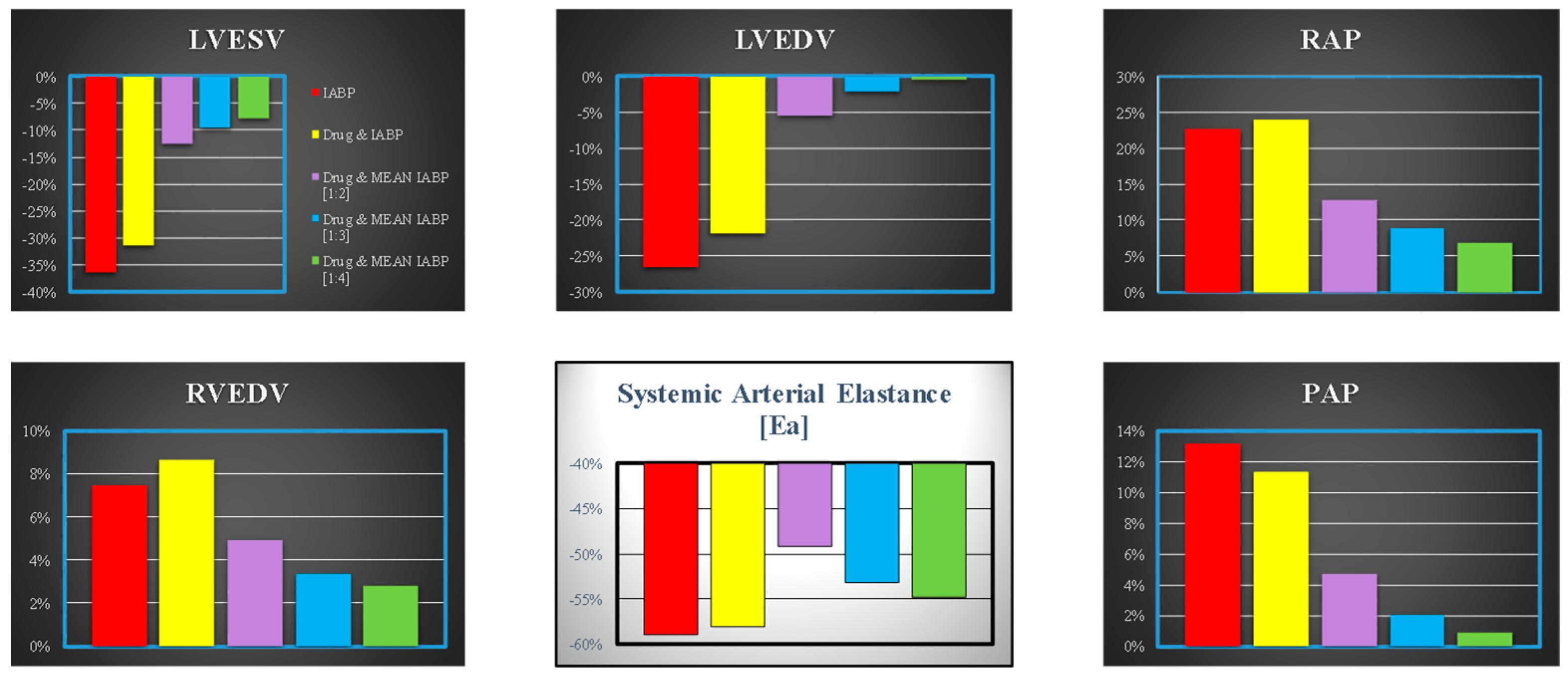
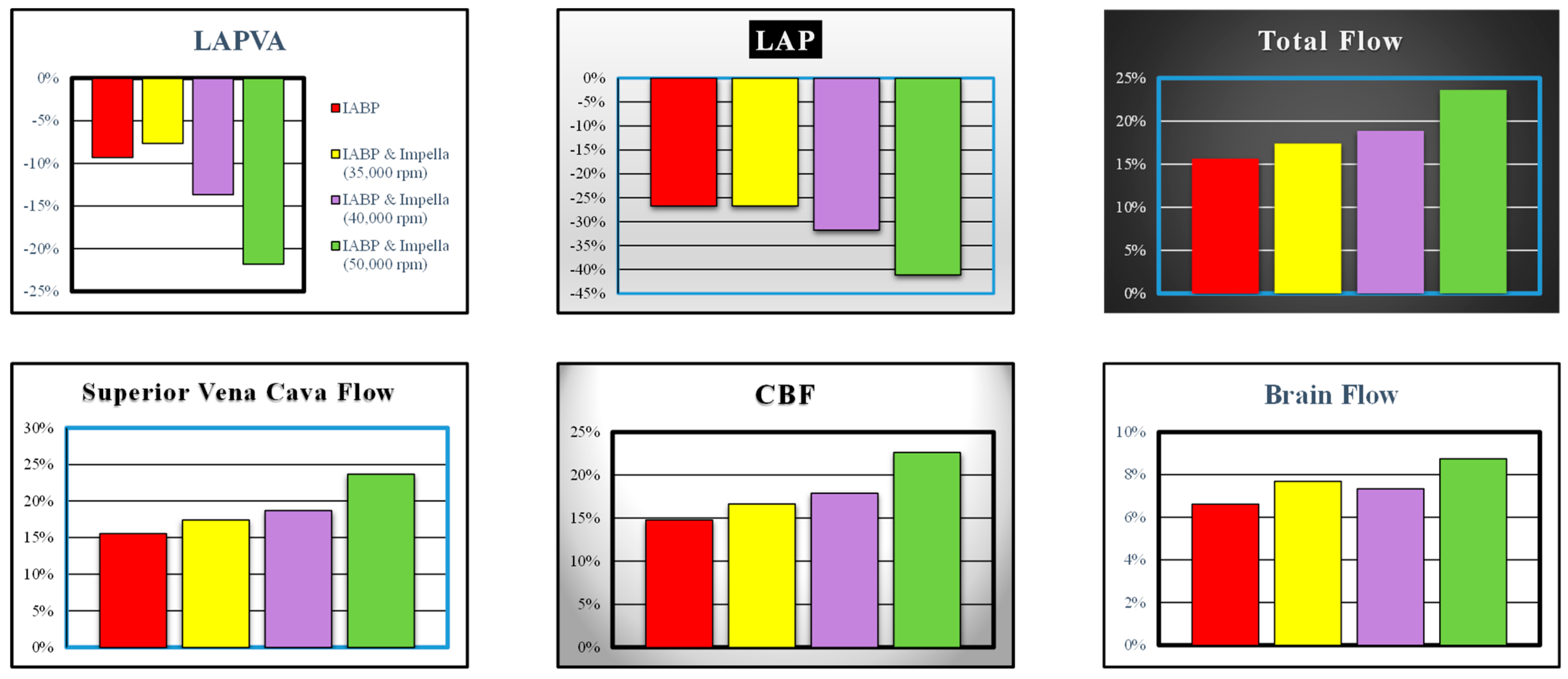
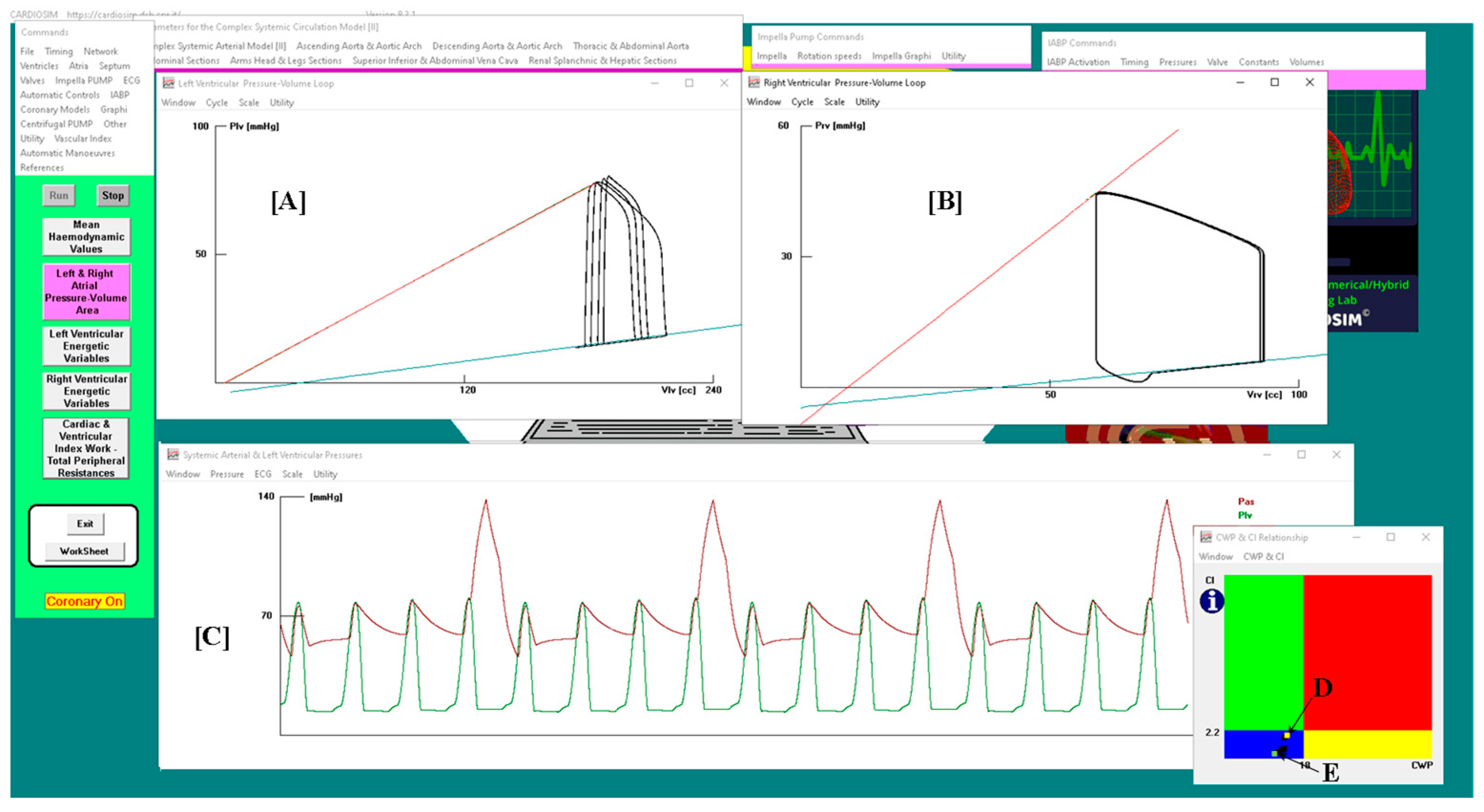
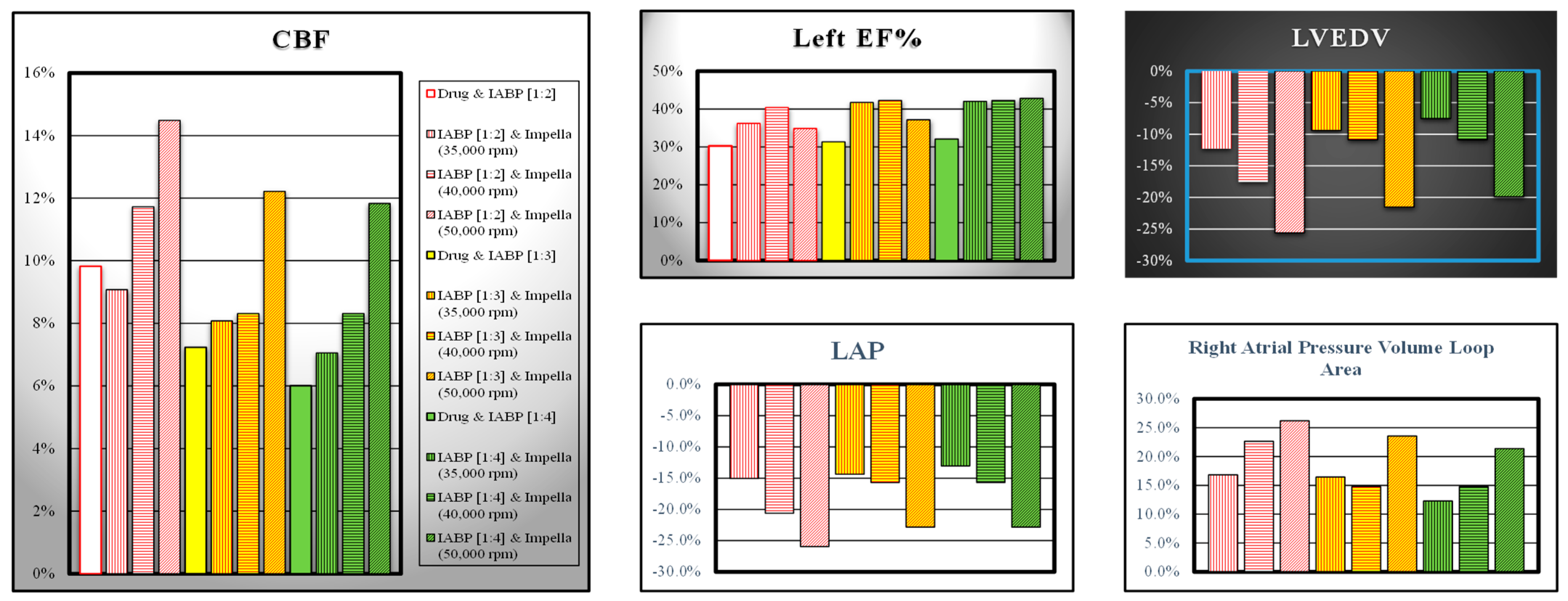
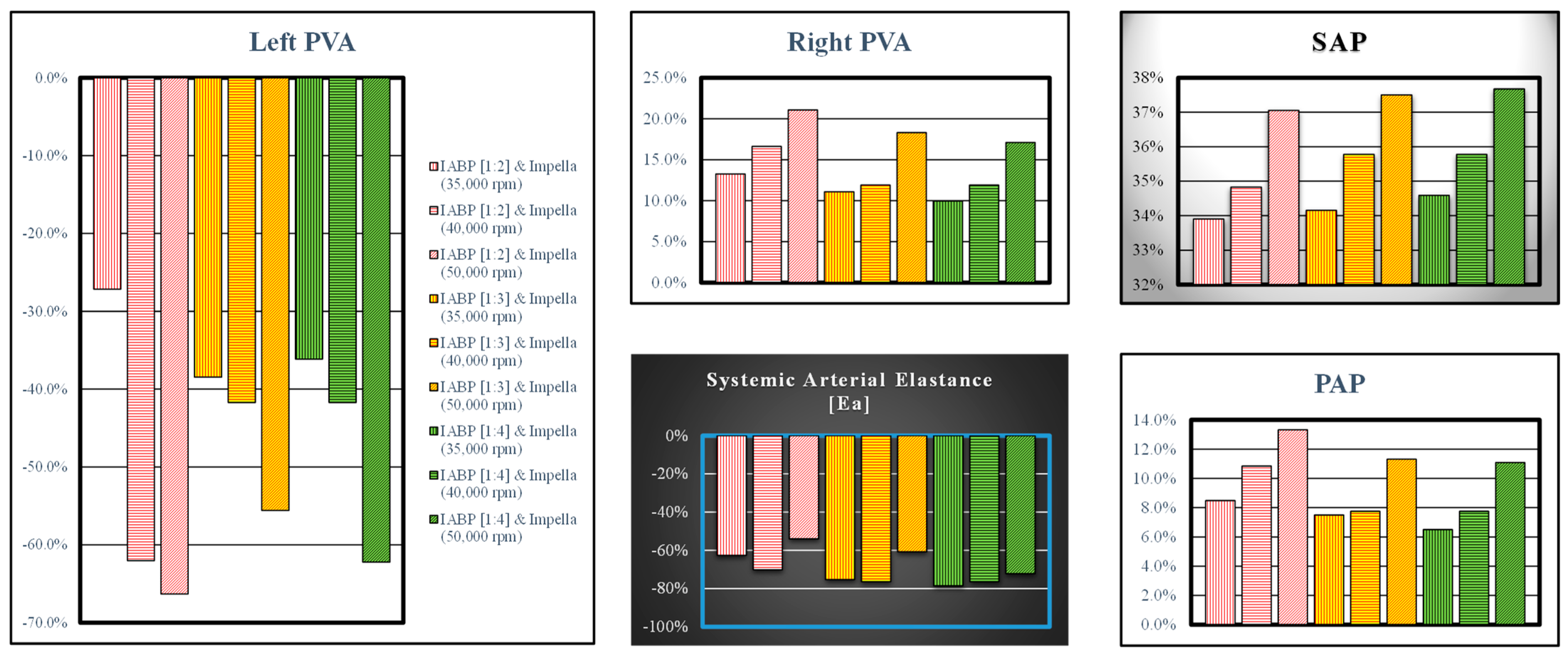
3. Results
- the normal haemodynamic condition (green rectangle);
- cardiorespiratory disease induced by insufficient CO (red rectangle);
- hypoperfusion state caused by fluid-volume depletion (blue rectangle);
- low flow rate (yellow rectangle) requiring either drug therapy or IABP device, or ventricular assist device (VAD) support.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhuiyan, R.; Bimal, T.; Fishbein, J.; Gandotra, P.; Selim, S.; Ong, L.; Gruberg, L. Percutaneous coronary intervention with Impella support with and without intra-aortic balloon in cardiogenic shock patients. Cardiovasc. Revascularization Med. 2023, 55, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.S.K.; Sin, S.W.C. Short-term mechanical circulatory support (intra-aortic balloon pump, Impella, extracorporeal membrane oxygenation, TandemHeart): A review. Ann. Transl. Med. 2020, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Merdji, H.; Levy, B.; Jung, C.; Ince, C.; Siegemund, M.; Meziani, F. Microcirculatory dysfunction in cardiogenic shock. Ann. Intensiv. Care 2023, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, B.; Capoccia, M.; Badagliacca, R.; Bozkurt, S.; De Lazzari, C. IABP versus Impella Support in Cardiogenic Shock: “In Silico” Study. J. Cardiovasc. Dev. Dis. 2023, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, B.; Iacovoni, A.; Mottaghy, K.; Capoccia, M.; Badagliacca, R.; Vizza, C.D.; De Lazzari, C. ECMO Assistance during Mechanical Ventilation: Effects Induced on Energetic and Haemodynamic Variables. Comput. Methods Programs Biomed. 2021, 202, 106003. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Shibasaki, I.; Matsuoka, T.; Niitsuma, K.; Hirota, S.; Kanno, Y.; Kanazawa, Y.; Tezuka, M.; Takei, Y.; Tsuchiya, G.; et al. Impella support as a bridge to heart surgery in patients with cardiogenic shock. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac088. [Google Scholar] [CrossRef]
- Ezad, S.M.; Ryan, M.; Donker, D.W.; Pappalardo, F.; Barrett, N.; Camporota, L.; Price, S.; Kapur, N.K.; Perera, D. Unloading the Left Ventricle in Venoarterial ECMO: In Whom, When, and How? Circulation 2023, 147, 1237–1250. [Google Scholar] [CrossRef]
- Møller, J.E.; Sionis, A.; Aissaoui, N.; Ariza, A.; Bělohlávek, J.; De Backer, D.; Färber, G.; Gollmann-Tepeköylu, C.; Mebazaa, A.; Price, S.; et al. Step by step daily management of short-term mechanical circulatory support for cardiogenic shock in adults in the intensive cardiac care unit: A clinical consensus statement of the Association for Acute CardioVascular Care of the European Society of Cardiology SC, the European Society of Intensive Care Medicine, the European branch of the Extracorporeal Life Support Organization, and the European Association for Cardio-Thoracic Surgery. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 475–485. [Google Scholar] [CrossRef]
- Russo, G.; Burzotta, F.; Aurigemma, C.; Pedicino, D.; Romagnoli, E.; Trani, C. Can we have a rationalized selection of intra-aortic balloon pump, Impella, and extracorporeal membrane oxygenation in the catheterization laboratory? Cardiol. J. 2022, 29, 115–132. [Google Scholar] [CrossRef]
- Cavayas, Y.A.; Noly, P.-E.; Singh, G.; Lamarche, Y. Controversies in extracorporeal membrane oxygenation: Immediate versus watchful waiting for venoarterial extracorporeal membrane oxygenation venting. JTCVS Open 2021, 8, 70–76. [Google Scholar] [CrossRef]
- Pavlidis, A.N.; Redwood, S.R.; Clapp, B.R. Combined hemodynamic support with the Impella 2.5 device and intra-aortic balloon pump for management of refractory cardiogenic shock. J. Invasive Cardiol. 2014, 26, E50–E51. [Google Scholar] [PubMed]
- Gupta, A.; Allaqaband, S.; Bajwa, T. Combined use of impella device and intra-aortic balloon pump to improve survival in a patient in profound cardiogenic shock post cardiac arrest. Catheter. Cardiovasc. Interv. 2009, 74, 975–976. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, D.M.; Sawlani, N.; Kanthi, Y.; Sipahi, I.; Fang, J.C.; Blitz, A. Successful combined use of Impella Recover 2.5 device and intra-aortic balloon pump support in cardiogenic shock from acute myocardial infarction. ASAIO J. 2010, 56, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, R.J.; Lago, R.; Horvath, S.A.; Vignola, P.A.; O’Neill, W.; Palacios, I.F. Use of the Impella 2.5 system alone, after and in combination with an intra-aortic balloon pump in patients with cardiogenic shock: Case description and review of the literature. EuroIntervention 2012, 7, 1453–1460. [Google Scholar] [CrossRef]
- Møller-Helgestad, O.K.; Poulsen, C.B.; Christiansen, E.H.; Lassen, J.F.; Ravn, H.B. Support with intra-aortic balloon pump vs. Impella2.5® and blood flow to the heart, brain and kidneys—An experimental porcine model of ischaemic heart failure. Int. J. Cardiol. 2015, 178, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Lansky, A.J.; Tirziu, D.; Moses, J.W.; Pietras, C.; Ohman, E.M.; O’Neill, W.W.; Ekono, M.M.; Grines, C.L.; Parise, H. Impella Versus Intra-Aortic Balloon Pump for High-Risk PCI: A Propensity-Adjusted Large-Scale Claims Dataset Analysis. Am. J. Cardiol. 2022, 185, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Meani, P.; Lorusso, R.; Pappalardo, F. ECPella: Concept, Physiology and Clinical Applications. J. Cardiothorac. Vasc. Anesth. 2022, 36, 557–566. [Google Scholar] [CrossRef]
- Weber, D.M.; Raess, D.H.; Henriques, J.; Siess, T. Principles of Impella Cardiac Support. Card. Interv. Today 2009, 3–16. [Google Scholar]
- De Lazzari, B.; Badagliacca, R.; Filomena, D.; Papa, S.; Vizza, C.D.; Capoccia, M.; De Lazzari, C. CARDIOSIM©: The First Italian Software Platform for Simulation of the Cardiovascular System and Mechanical Circulatory and Ventilatory Support. Bioengineering 2022, 9, 383. [Google Scholar] [CrossRef]
- Kaye, D.M.; Wolsk, E.; Nanayakkara, S.; Mariani, J.; Hassager, C.; Gustafsson, F.; Moller, J.E.; Sunagawa, K.; Burkhoff, D. Comprehensive Physiological Modeling Provides Novel Insights Into Heart Failure with Preserved Ejection Fraction Physiology. J. Am. Heart Assoc. 2021, 10, e021584. [Google Scholar] [CrossRef]
- De Lazzari, B.; Iacovoni, A.; Capoccia, M.; Papa, S.; Badagliacca, R.; Filomena, D.; De Lazzari, C. Ventricular and Atrial Pressure—Volume Loops: Analysis of the Effects Induced by Right Centrifugal Pump Assistance. Bioengineering 2022, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Manzi, G.; Miotti, C.; Mariani, M.V.; Papa, S.; Luongo, F.; Scoccia, G.; De Lazzari, B.; De Lazzari, C.; Benza, R.L.; Fedele, F.; et al. Computational Simulator Models and Invasive Hemodynamic Monitoring as Tools for Precision Medicine in Pulmonary Arterial Hypertension. J. Clin. Med. 2021, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Kozarski, M.; De Lazzari, C.; Ska, K.G.; Tosti, G.; Darowski, M. Development of a hybrid (numerical-hydraulic) circulatory model: Prototype testing and its response to IABP assistance. Int. J. Artif. Organs 2005, 28, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Kung, E.; Farahmand, M.; Gupta, A. A Hybrid Experimental-Computational Modeling Framework for Cardiovascular Device Testing. J. Biomech. Eng. 2019, 141, 051012. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, S.; Paracha, W.; Bakaya, K.; Schievano, S. Patient-Specific Modelling and Parameter Optimisation to Simulate Dilated Cardiomyopathy in Children. Cardiovasc. Eng. Technol. 2022, 13, 712–724. [Google Scholar] [CrossRef] [PubMed]
- De Lazzari, C.; Stalteri, D. 2011–2019, CARDIOSIM© Website. Available online: https://cardiosim.dsb.cnr.it (accessed on 29 May 2022).
- Vahdatpour, C.; Collins, D.; Goldberg, S. Cardiogenic Shock. J. Am. Heart Assoc. 2019, 8, e011991. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.Y.; Ma, T.S.K.; Ip, A.; Fang, S.; Li, A.C.C.; Wong, A.S.K.; Ngai, C.W.; Chan, W.M.; Sin, W.C. Effects of varying blood flow rate during peripheral veno-arterial extracorporeal membrane oxygen (V-A ECMO) on left ventricular function measured by two-dimensional strain. Front. Cardiovasc. Med. 2023, 10, 1147783. [Google Scholar] [CrossRef] [PubMed]
- Caldas, J.R.; Panerai, R.B.; Bor-Seng-Shu, E.; Almeida, J.P.; Ferreira, G.S.R.; Camara, L.; Nogueira, R.C.; Oliveira, M.L.; Jatene, F.B.; Robinson, T.G.; et al. Cerebral hemodynamics with intra-aortic balloon pump: Business as usual? Physiol. Meas. 2017, 38, 1349–1361. [Google Scholar] [CrossRef]
- Alkan, R.; De Lazzari, B.; Capoccia, M.; De Lazzari, C.; Bozkurt, S. Computational Evaluation of IABP, Impella 2.5, TandemHeart and Combined IABP and Impella 2.5 Support in Cardiogenic Shock. Mathematics 2023, 11, 3606. [Google Scholar] [CrossRef]
- De Lazzari, C. Interaction between the septum and the left (right) ventricular free wall in order to evaluate the effects on coronary blood flow: Numerical simulation. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 1359–1368. [Google Scholar] [CrossRef]
- Capoccia, M.; Marconi, S.; De Lazzari, C. Decision-making in advanced heart failure patients requiring LVAD insertion: Can preoperative simulation become the way forward? A case study. J. Biomed. Eng. Inform. 2018, 4, 8–20. [Google Scholar] [CrossRef]
- Schurtz, G.; Rousse, N.; Saura, O.; Balmette, V.; Vincent, F.; Lamblin, N.; Porouchani, S.; Verdier, B.; Puymirat, E.; Robin, E.; et al. IMPELLA® or Extracorporeal Membrane Oxygenation for Left Ventricular Dominant Refractory Cardiogenic Shock. J. Clin. Med. 2021, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Martinho, S.; Adão, R.; Leite-Moreira, A.F.; Brás-Silva, C. Persistent Pulmonary Hypertension of the Newborn: Pathophysiological Mechanisms and Novel Therapeutic Approaches. Front. Pediatr. 2020, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Abramov, D.; Haglund, N.A.; Di Salvo, T.G. Effect of Milrinone Infusion on Pulmonary Vasculature and Stroke Work Indices: A Single-Center Retrospective Analysis in 69 Patients Awaiting Cardiac Transplantation. Am. J. Cardiovasc. Drugs 2017, 17, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, A.; Pendela, V.S.; Hai, O. Cardiogenic Shock. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482255/ (accessed on 14 December 2023).
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef]
- Sauren, L.D.C.; Accord, R.E.; Hamzeh, K.; de Jong, M.; van der Nagel, T.; van der Veen, F.H.; Maessen, J.G. Combined Impella and Intra-aortic Balloon Pump Support to Improve Both Ventricular Unloading and Coronary Blood Flow for Myocardial Recovery: An Experimental Study. Artif. Organs 2007, 31, 839–842. [Google Scholar] [CrossRef]
- Sterling, L.H.; Fernando, S.M.; Talarico, R.; Qureshi, D.; van Diepen, S.; Herridge, M.S.; Price, S.; Brodie, D.; Fan, E.; Di Santo, P.; et al. Long-Term Outcomes of Cardiogenic Shock Complicating Myocardial Infarction. J. Am. Coll. Cardiol. 2023, 82, 985–995. [Google Scholar] [CrossRef]
- Bochaton, T.; Huot, L.; Elbaz, M.; Delmas, C.; Aissaoui, N.; Farhat, F.; Mewton, N.; Bonnefoy, E. Mechanical circulatory support with the Impella® LP5.0 pump and an intra-aortic balloon pump for cardiogenic shock in acute myocardial infarction: The IMPELLA-STIC randomi.sed study. Arch. Cardiovasc. Dis. 2020, 113, 237–243. [Google Scholar] [CrossRef]
- Rohm, C.L.; Gadidov, B.; Leitson, M.; Ray, H.E.; Prasad, R. Predictors of Mortality and Outcomes of Acute Severe Cardiogenic Shock Treated with the Impella Device. Am. J. Cardiol. 2019, 124, 499–504. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Engstrom, A.E.; Sjauw, K.D.; Hirsch, A.; Hill, J.M.; Gockel, B.; Tuseth, V.; van der Schaaf, R.J.; Henriques, J.P.S. Experience from a randomised controlled trial with Impella 2.5 versus IABP in STEMI patients with cardiogenic pre-shock. Lessons learnt from the IMPRESS in STEMI trial. Int. J. Cardiol. 2016, 202, 894–896. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lazzari, B.; Capoccia, M.; Badagliacca, R.; De Lazzari, C. Modelling and Simulation of the Combined Use of IABP and Impella as a Rescue Procedure in Cardiogenic Shock: An Alternative for Non-Transplant Centres? Bioengineering 2023, 10, 1434. https://doi.org/10.3390/bioengineering10121434
De Lazzari B, Capoccia M, Badagliacca R, De Lazzari C. Modelling and Simulation of the Combined Use of IABP and Impella as a Rescue Procedure in Cardiogenic Shock: An Alternative for Non-Transplant Centres? Bioengineering. 2023; 10(12):1434. https://doi.org/10.3390/bioengineering10121434
Chicago/Turabian StyleDe Lazzari, Beatrice, Massimo Capoccia, Roberto Badagliacca, and Claudio De Lazzari. 2023. "Modelling and Simulation of the Combined Use of IABP and Impella as a Rescue Procedure in Cardiogenic Shock: An Alternative for Non-Transplant Centres?" Bioengineering 10, no. 12: 1434. https://doi.org/10.3390/bioengineering10121434
APA StyleDe Lazzari, B., Capoccia, M., Badagliacca, R., & De Lazzari, C. (2023). Modelling and Simulation of the Combined Use of IABP and Impella as a Rescue Procedure in Cardiogenic Shock: An Alternative for Non-Transplant Centres? Bioengineering, 10(12), 1434. https://doi.org/10.3390/bioengineering10121434






