The Role of Hydrogel in Cardiac Repair and Regeneration for Myocardial Infarction: Recent Advances and Future Perspectives
Abstract
:1. Introduction
2. Biomaterials in Cardiac Regeneration Medicine
3. The Property of Biomaterials for Cardiac Regeneration Medicine
4. Hydrogel in Cardiac Repair and Regeneration for MI
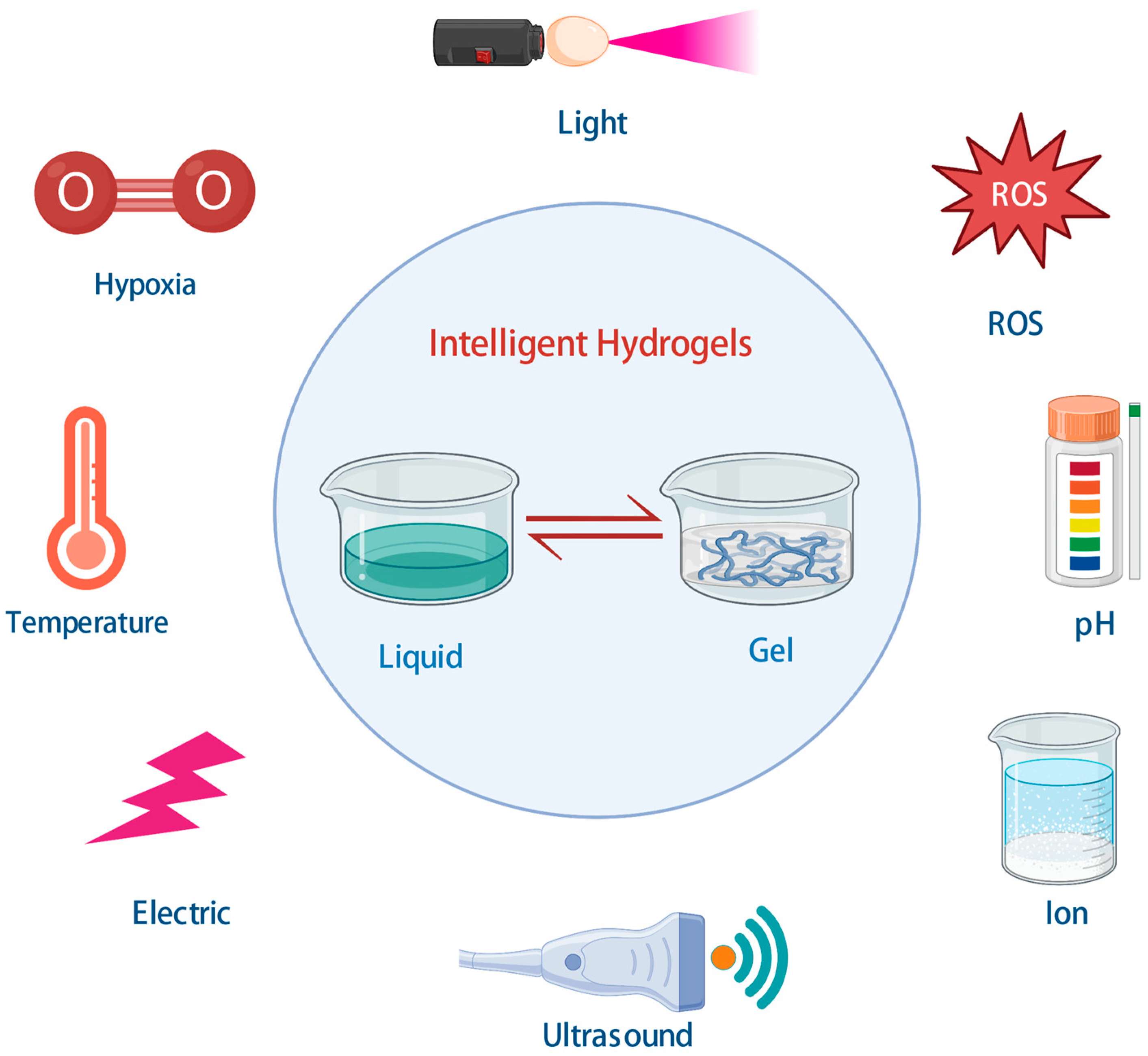
5. Intelligent Hydrogels and Cardiac Tissue Engineering
5.1. Temperature-Responsive Hydrogels
5.2. pH-Responsive Hydrogel
5.3. Ion-Sensitive Hydrogels
5.4. Hypoxia-Responsive Hydrogels
5.5. ROS-Responsive Hydrogels
6. Approaches of Hydrogel-Based Cardiac Regeneration
6.1. Injectable Hydrogels
6.2. Hydrogel-Based Cardiac Patches
6.3. Potential Application of Hydrogels in Cardiac Repair and Regeneration after MI
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foinquinos, A.; Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Gyongyosi, M.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat. Commun. 2020, 11, 633. [Google Scholar] [CrossRef] [Green Version]
- Gladka, M.M.; Kohela, A.; Molenaar, B.; Versteeg, D.; Kooijman, L.; Monshouwer-Kloots, J.; Kremer, V.; Vos, H.R.; Huibers, M.; Haigh, J.J.; et al. Cardiomyocytes stimulate angiogenesis after ischemic injury in a ZEB2-dependent manner. Nat. Commun. 2021, 12, 84. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Anderson, R.N. The Leading Causes of Death in the US for 2020. JAMA J. Am. Med. Assoc. 2021, 325, 1829–1830. [Google Scholar] [CrossRef]
- Walli-Attaei, M.; Rosengren, A.; Rangarajan, S.; Breet, Y.; Abdul-Razak, S.; Sharief, W.A.; Alhabib, K.F.; Avezum, A.; Chifamba, J.; Diaz, R.; et al. Metabolic, behavioural, and psychosocial risk factors and cardiovascular disease in women compared with men in 21 high-income, middle-income, and low-income countries: An analysis of the PURE study. Lancet 2022, 400, 811–821. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Tariq, U.; Gupta, M.; Pathak, S.; Patil, R.; Dohare, A.; Misra, S.K. Role of Biomaterials in Cardiac Repair and Regeneration: Therapeutic Intervention for Myocardial Infarction. ACS Biomater. Sci. Eng. 2022, 8, 3271–3298. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.P.; Martin, J.F. Cardiomyocyte Proliferation for Therapeutic Regeneration. Curr. Cardiol. Rep. 2018, 20, 63. [Google Scholar] [CrossRef]
- Nguyen, P.D.; de Bakker, D.; Bakkers, J. Cardiac regenerative capacity: An evolutionary afterthought? Cell.d Mol. Life Sci. 2021, 78, 5107–5122. [Google Scholar] [CrossRef] [PubMed]
- Prag, H.A.; Aksentijevic, D.; Dannhorn, A.; Giles, A.V.; Mulvey, J.F.; Sauchanka, O.; Du, L.; Bates, G.; Reinhold, J.; Kula-Alwar, D.; et al. Ischemia-Selective Cardioprotection by Malonate for Ischemia/Reperfusion Injury. Circ. Res. 2022, 131, 528–541. [Google Scholar] [CrossRef]
- Li, H.; Bao, M.; Nie, Y. Extracellular matrix-based biomaterials for cardiac regeneration and repair. Heart Fail. Rev. 2021, 26, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Hu, S.; Cheng, K. A New Era of Cardiac Cell Therapy: Opportunities and Challenges. Adv. Healthc. Mater. 2019, 8, e1801011. [Google Scholar] [CrossRef]
- Giacca, M. Cardiac Regeneration After Myocardial Infarction: An Approachable Goal. Curr. Cardiol. Rep. 2020, 22, 122. [Google Scholar] [CrossRef]
- Schlickewei, C.W.; Kleinertz, H.; Thiesen, D.M.; Mader, K.; Priemel, M.; Frosch, K.H.; Keller, J. Current and Future Concepts for the Treatment of Impaired Fracture Healing. Int. J. Mol. Sci. 2019, 20, 5805. [Google Scholar] [CrossRef] [Green Version]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Mao, Y.; Wang, D.; Zhao, Q.; Wang, S. Multi-stimuli responsive nanosystem modified by tumor-targeted carbon dots for chemophototherapy synergistic therapy. J. Colloid Interf. Sci. 2019, 552, 639–650. [Google Scholar] [CrossRef]
- Ueki, K.; Yanagihara, S.; Ueda, K.; Nakai, M.; Nakano, T.; Narushima, T. Overcoming the strength-ductility trade-off by the combination of static recrystallization and low-temperature heat-treatment in Co-Cr-W-Ni alloy for stent application. Mat. Sci. Eng. A Struct. 2019, 766, 138400. [Google Scholar] [CrossRef]
- Chua, K.; Khan, I.; Malhotra, R.; Zhu, D. Additive manufacturing and 3D printing of metallic biomaterials. Engin. Regen. 2021, 2, 288–299. [Google Scholar] [CrossRef]
- Taniguchi, I.; Yoshida, S.; Hiraga, K.; Miyamoto, K.; Kimura, Y.; Oda, K. Biodegradation of PET: Current Status and Application Aspects. ACS Catal. 2019, 9, 4089–4105. [Google Scholar] [CrossRef]
- Bone, J.M.; Childs, C.M.; Menon, A.; Poczos, B.; Feinberg, A.W.; LeDuc, P.R.; Washburn, N.R. Hierarchical Machine Learning for High-Fidelity 3D Printed Biopolymers. ACS Biomater. Sci. Eng. 2020, 6, 7021–7031. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Huang, K.; Ma, H.; Liang, H.; Dinh, P.U.; Chen, J.; Shen, D.; Allen, T.A.; Qiao, L.; Li, Z.; et al. Platelet-Inspired Nanocells for Targeted Heart Repair After Ischemia/Reperfusion Injury. Adv. Funct. Mater. 2019, 29, 1803567. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Li, Z.; Huang, K.; Caranasos, T.G.; Rossi, J.S.; Cheng, K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat. Commun. 2021, 12, 1412. [Google Scholar] [CrossRef]
- Wang, C.G.; Surat’Man, N.; Chang, J.J.; Ong, Z.L.; Li, B.; Fan, X.; Loh, X.J.; Li, Z. Polyelectrolyte Hydrogels for Tissue Engineering and Regenerative Medicine. Chem. Asian J. 2022, 17, e202200604. [Google Scholar] [CrossRef]
- Whitehead, K.M.; Hendricks, H.; Cakir, S.N.; de Castro, B.L. ECM roles and biomechanics in cardiac tissue decellularization. Am. J. Physiol. Heart C 2022, 323, H585–H596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Esworthy, T.; Mei, D.; Wang, Y.; Zhang, L.G. Emerging 4D Printing Strategies for Next-Generation Tissue Regeneration and Medical Devices. Adv. Mater. 2022, 34, e2109198. [Google Scholar] [CrossRef]
- Han, X.; Alu, A.; Liu, H.; Shi, Y.; Wei, X.; Cai, L.; Wei, Y. Biomaterial-assisted biotherapy: A brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact. Mater. 2022, 17, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef]
- Liang, W.; Chen, J.; Li, L.; Li, M.; Wei, X.; Tan, B.; Shang, Y.; Fan, G.; Wang, W.; Liu, W. Conductive Hydrogen Sulfide-Releasing Hydrogel Encapsulating ADSCs for Myocardial Infarction Treatment. ACS Appl. Mater. Inter. 2019, 11, 14619–14629. [Google Scholar] [CrossRef]
- Jin, J.; Jeong, S.I.; Shin, Y.M.; Lim, K.S.; Shin, H.S.; Lee, Y.M.; Koh, H.C.; Kim, K. Transplantation of mesenchymal stem cells within a poly(lactide-co-ɛ-caprolactone) scaffold improves cardiac function in a rat myocardial infarction model. Eur. J. Heart Fail. 2009, 11, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejleri, D.; Streeter, B.W.; Nachlas, A.; Brown, M.E.; Gaetani, R.; Christman, K.L.; Davis, M.E. A Bioprinted Cardiac Patch Composed of Cardiac-Specific Extracellular Matrix and Progenitor Cells for Heart Repair. Adv. Healthc. Mater. 2018, 7, e1800672. [Google Scholar] [CrossRef]
- Rodrigues, I.; Kaasi, A.; Maciel, F.R.; Jardini, A.L.; Gabriel, L.P. Cardiac tissue engineering: Current state-of-the-art materials, cells and tissue formation. Einstein-Sao Paulo 2018, 16, B4538. [Google Scholar] [CrossRef]
- Curley, C.J.; Dolan, E.B.; Otten, M.; Hinderer, S.; Duffy, G.P.; Murphy, B.P. An injectable alginate/extra cellular matrix (ECM) hydrogel towards acellular treatment of heart failure. Drug Deliv. Transl. Res.d 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Pok, S.; Vitale, F.; Eichmann, S.L.; Benavides, O.M.; Pasquali, M.; Jacot, J.G. Biocompatible Carbon Nanotube–Chitosan Scaffold Matching the Electrical Conductivity of the Heart. ACS Nano 2014, 8, 9822–9832. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Dai, K.; Gao, Z.; Tang, W.; Shen, T.; Yuan, Y.; Wang, J.; Liu, C. Sulfated polysaccharide directs therapeutic angiogenesis via endogenous VEGF secretion of macrophages. Sci. Adv. 2021, 7, eabd8217. [Google Scholar] [CrossRef]
- Huang, Q.; Zou, Y.; Arno, M.C.; Chen, S.; Wang, T.; Gao, J.; Dove, A.P.; Du, J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017, 46, 6255–6275. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhu, Y.; Hu, Y.; Zeng, G.; Zhang, Y.; Zhang, C.; Feng, C. How to Construct DNA Hydrogels for Environmental Applications: Advanced Water Treatment and Environmental Analysis. Small 2018, 14, e1703305. [Google Scholar] [CrossRef]
- Mamidi, N.; Velasco, D.R.; Barrera, E.V. Covalently Functionalized Carbon Nano-Onions Integrated Gelatin Methacryloyl Nanocomposite Hydrogel Containing gamma-Cyclodextrin as Drug Carrier for High-Performance pH-Triggered Drug Release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Han, H.; Zheng, H.; Xu, W.; Wang, Z. Dopamine-Triggered Hydrogels with High Transparency, Self-Adhesion, and Thermoresponse as Skinlike Sensors. ACS Nano 2021, 15, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekar, S.; Kasi, G.; He, X.; Zhang, K.; Xu, L.; Kang, E.T. Recent advances in engineered polymeric materials for efficient photodynamic inactivation of bacterial pathogens. Bioact. Mater. 2023, 21, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Liu, X.; Steiger, C.; Lin, S.; Parada, G.A.; Liu, J.; Chan, H.F.; Yuk, H.; Phan, N.V.; Collins, J.; Tamang, S.; et al. Ingestible hydrogel device. Nat. Commun. 2019, 10, 493. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.; Hong, Y.J.; Jung, J.; Shin, Y.; Sunwoo, S.H.; Baik, S.; Park, O.K.; Choi, S.H.; Hyeon, T.; Kim, J.H.; et al. Tissue-like skin-device interface for wearable bioelectronics by using ultrasoft, mass-permeable, and low-impedance hydrogels. Sci. Adv. 2021, 7, eabd3716. [Google Scholar] [CrossRef] [PubMed]
- Saludas, L.; Pascual-Gil, S.; Prósper, F.; Garbayo, E.; Blanco-Prieto, M. Hydrogel based approaches for cardiac tissue engineering. Int. J. Pharm. 2017, 523, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Nunes, S.S. Overview of hydrogel-based strategies for application in cardiac tissue regeneration. Biomed. Mater. 2015, 10, 34005. [Google Scholar] [CrossRef]
- Hastings, C.L.; Roche, E.T.; Ruiz-Hernandez, E.; Schenke-Layland, K.; Walsh, C.J.; Duffy, G.P. Drug and cell delivery for cardiac regeneration. Adv. Drug Deliv. Rev. 2015, 84, 85–106. [Google Scholar] [CrossRef]
- Davari, N.; Bakhtiary, N.; Khajehmohammadi, M.; Sarkari, S.; Tolabi, H.; Ghorbani, F.; Ghalandari, B. Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers 2022, 14, 986. [Google Scholar] [CrossRef]
- Cheng, G.; Zhu, D.; Huang, K.; Caranasos, T.G. Minimally invasive delivery of a hydrogel-based exosome patch to prevent heart failure. J. Mol. Cell Cardiol. 2022, 169, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel drug delivery system with predictable and tunable drug release and degradation rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.H.; Chung, J.J.; Mealy, J.E.; Zaman, S.; Li, E.C.; Arisi, M.F.; Atluri, P.; Burdick, J.A. Injectable Supramolecular Hydrogel/Microgel Composites for Therapeutic Delivery. Macromol. Biosci. 2019, 19, e1800248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufaihah, A.J.; Seliktar, D. Hydrogels for therapeutic cardiovascular angiogenesis. Adv. Drug Deliv. Rev. 2016, 96, 31–39. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Zhang, J.; Shen, S.; Yin, W.; Ye, G.; Wang, L.; Hou, H.; Qiu, X. A tunable self-healing ionic hydrogel with microscopic homogeneous conductivity as a cardiac patch for myocardial infarction repair. Biomaterials 2021, 273, 120811. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.Y.; Tian, Y.; Liu, Z.J. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chu, W.; Zhuo, X.; Zhang, Y.; Gou, J.; Ren, T.; He, H.; Yin, T.; Tang, X. Modified PLGA-PEG-PLGA thermosensitive hydrogels with suitable thermosensitivity and properties for use in a drug delivery system. J. Mater. Chem. B 2017, 5, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 thermosensitive injectable smart hydrogels for controlled drug delivery system development. J. Colloid Interf. Sci. 2020, 565, 119–130. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Kharlampieva, E. Self-Assemblies of Thermoresponsive Poly(N-vinylcaprolactam) Polymers for Applications in Biomedical Field. ACS Appl. Polym. Mater. 2020, 2, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Boyaci, T.; Orakdogen, N. Poly(N,N-dimethylaminoethyl methacrylate-co-2-acrylamido-2-methyl-propanosulfonic acid)/Laponite nanocomposite hydrogels and cryogels with improved mechanical strength and rapid dynamic properties. Appl. Clay Sci. 2016, 121–122, 162–173. [Google Scholar] [CrossRef]
- Aliakbar, A.Z.; Khosravimelal, S.; Eftekhari, B.S.; Mehrabi, S.; Hashemi, A.; Eftekhari, S.; Brouki, M.P.; Mobaraki, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Thermo-responsive chitosan hydrogel for healing of full-thickness wounds infected with XDR bacteria isolated from burn patients: In vitro and in vivo animal model. Int. J. Biol. Macromol. 2020, 164, 4475–4486. [Google Scholar] [CrossRef]
- Rodell, C.B.; Lee, M.E.; Wang, H.; Takebayashi, S.; Takayama, T.; Kawamura, T.; Arkles, J.S.; Dusaj, N.N.; Dorsey, S.M.; Witschey, W.R.; et al. Injectable Shear-Thinning Hydrogels for Minimally Invasive Delivery to Infarcted Myocardium to Limit Left Ventricular Remodeling. Circ. Cardiovasc. Interv. 2016, 9, e004058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.; Xie, J.; Chen, Y.; Liu, J.; Liu, Y.; Bi, B.; Luo, J.; Li, S.; Jiang, X.; Li, J. A thermo-sensitive injectable hydroxypropyl chitin hydrogel for sustained salmon calcitonin release with enhanced osteogenesis and hypocalcemic effects. J. Mater. Chem. B 2020, 8, 270–281. [Google Scholar] [CrossRef]
- Li, Z.; Shim, H.; Cho, M.O.; Cho, I.S.; Lee, J.H.; Kang, S.W.; Kwon, B.; Huh, K.M. Thermo-sensitive injectable glycol chitosan-based hydrogel for treatment of degenerative disc disease. Carbohydr. Polym. 2018, 184, 342–353. [Google Scholar] [CrossRef]
- Lu, Y.J.; Lan, Y.H.; Chuang, C.C.; Lu, W.T.; Chan, L.Y.; Hsu, P.W.; Chen, J.P. Injectable Thermo-Sensitive Chitosan Hydrogel Containing CPT-11-Loaded EGFR-Targeted Graphene Oxide and SLP2 shRNA for Localized Drug/Gene Delivery in Glioblastoma Therapy. Int. J. Mol. Sci. 2020, 21, 7111. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, W.; Park, H.; Lee, D.K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Xu, Z.; Niu, H.; Sui, Y.; Li, H.; Ma, J.; Guan, J. Spatiotemporal delivery of basic fibroblast growth factor to directly and simultaneously attenuate cardiac fibrosis and promote cardiac tissue vascularization following myocardial infarction. J. Control. Release 2019, 311–312, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, Y.; Takai, H.; Mayumi, N.; Fujiwara, S.; Kuzuya, A.; Ohya, Y. Cellular therapy for myocardial ischemia using a temperature-responsive biodegradable injectable polymer system with adipose-derived stem cells. Sci. Technol. Adv. Mater. 2021, 22, 627–642. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Niu, S.; Lin, Z.; Li, L.; Yang, P.; Rao, P.; Yang, L.; Jiang, L.; Sun, L. Injectable hydrogel with dual-sensitive behavior for targeted delivery of oncostatin M to improve cardiac restoration after myocardial infarction. J. Mater. Chem. B 2022, 10, 6514–6531. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Tian, S.; Liu, Q.; Xiu, K.; Lei, I.; Wang, Z.; Ma, P.X. Biodegradable nanofibrous temperature-responsive gelling microspheres for heart regeneration. Adv. Funct. Mater. 2020, 30, 2000776. [Google Scholar] [CrossRef]
- Ding, F.; Shi, X.; Wu, S.; Liu, X.; Deng, H.; Du, Y.; Li, H. Flexible Polysaccharide Hydrogel with pH-Regulated Recovery of Self-Healing and Mechanical Properties. Macromol. Mater. Eng. 2017, 302, 1700221. [Google Scholar] [CrossRef]
- Garbern, J.C.; Minami, E.; Stayton, P.S.; Murry, C.E. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials 2011, 32, 2407–2416. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; He, C.; Chen, X. Hydrogels based on pH-responsive reversible carbon-nitrogen double-bond linkages for biomedical applications. Mater. Chem. Front. 2018, 2, 1765–1778. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, Z.; Yao, Y.; Stant, J.C.; Landrum, S.L.; Bortner, M.J.; Frazier, C.E.; Edgar, K.J. Oxidized hydroxypropyl cellulose/carboxymethyl chitosan hydrogels permit pH-responsive, targeted drug release. Carbohydr. Polym. 2023, 300, 120213. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Sun, T.; Tsou, Y.H.; Chen, H.; Xu, X. Dual-Functional Dextran-PEG Hydrogel as an Antimicrobial Biomedical Material. Macromol. Biosci. 2018, 18, 10–1002. [Google Scholar] [CrossRef]
- Bastings, M.M.; Koudstaal, S.; Kieltyka, R.E.; Nakano, Y.; Pape, A.C.; Feyen, D.A.; van Slochteren, F.J.; Doevendans, P.A.; Sluijter, J.P.; Meijer, E.W.; et al. A fast pH-switchable and self-healing supramolecular hydrogel carrier for guided, local catheter injection in the infarcted myocardium. Adv. Healthc. Mater. 2014, 3, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Zhou, X.; Guo, H. A Metal Ion and Thermal-Responsive Bilayer Hydrogel Actuator Achieved by the Asymmetric Osmotic Flow of Water between Two Layers under Stimuli. Polymers 2022, 14, 4019. [Google Scholar] [CrossRef]
- Ashtari, K.; Nazari, H.; Ko, H.; Tebon, P.; Akhshik, M.; Akbari, M.; Alhosseini, S.N.; Mozafari, M.; Mehravi, B.; Soleimani, M.; et al. Electrically conductive nanomaterials for cardiac tissue engineering. Adv. Drug Deliv. Rev. 2019, 144, 162–179. [Google Scholar] [CrossRef]
- Rodriguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Cationic cellulose hydrogels: Kinetics of the cross-linking process and characterization as pH-/ion-sensitive drug delivery systems. J. Control. Release 2003, 86, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Qi, X.; Li, J.; Zhong, Y.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Synthesis and characterization of a novel cationic hydrogel base on salecan-g-PMAPTAC. Int. J. Biol. Macromol. 2017, 101, 474–480. [Google Scholar] [CrossRef]
- Fan, Z.; Xu, Z.; Niu, H.; Gao, N.; Guan, Y.; Li, C.; Dang, Y.; Cui, X.; Liu, X.L.; Duan, Y.; et al. An Injectable Oxygen Release System to Augment Cell Survival and Promote Cardiac Repair Following Myocardial Infarction. Sci. Rep. 2018, 8, 1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemdar, N.; Leijten, J.; Camci-Unal, G.; Hjortnaes, J.; Ribas, J.; Paul, A.; Mostafalu, P.; Gaharwar, A.K.; Qiu, Y.; Sonkusale, S.; et al. Oxygen-Generating Photo-Cross-Linkable Hydrogels Support Cardiac Progenitor Cell Survival by Reducing Hypoxia-Induced Necrosis. ACS Biomater. Sci. Eng. 2017, 3, 1964–1971. [Google Scholar] [CrossRef]
- Bai, Q.; Zheng, C.; Sun, N.; Chen, W.; Gao, Q.; Liu, J.; Hu, F.; Zhou, T.; Zhang, Y.; Lu, T. Oxygen-releasing hydrogels promote burn healing under hypoxic conditions. Acta Biomater. 2022, 154, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, P.A.; Singh, A.; Kumar, A. Oxygen-Releasing Antioxidant Cryogel Scaffolds with Sustained Oxygen Delivery for Tissue Engineering Applications. ACS Appl. Mater. Inter. 2018, 10, 18458–18469. [Google Scholar] [CrossRef] [PubMed]
- Thi, P.L.; Lee, Y.; Tran, D.L.; Hoang, T.T.; Park, K.M.; Park, K.D. Calcium peroxide-mediated in situ formation of multifunctional hydrogels with enhanced mesenchymal stem cell behaviors and antibacterial properties. J. Mater. Chem. B 2020, 8, 11033–11043. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar] [CrossRef]
- Park, S.; Park, K.M. Hyperbaric oxygen-generating hydrogels. Biomaterials 2018, 182, 234–244. [Google Scholar] [CrossRef]
- Niu, H.; Li, C.; Guan, Y.; Dang, Y.; Li, X.; Fan, Z.; Shen, J.; Ma, L.; Guan, J. High oxygen preservation hydrogels to augment cell survival under hypoxic condition. Acta Biomater. 2020, 105, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Kosiol, M.; Mayr, M.; Schulz, R.; Rohrbach, S. Mitochondria and ageing: Role in heart, skeletal muscle and adipose tissue. J. Cachexia Sarcopeni 2017, 8, 349–369. [Google Scholar] [CrossRef] [Green Version]
- Criado-Gonzalez, M.; Mecerreyes, D. Thioether-based ROS responsive polymers for biomedical applications. J. Mater. Chem. B 2022, 10, 7206–7221. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Lu, Y.; Xin, L.; Gao, J.; Shang, C.; Jiang, Z.; Lin, H.; Fang, X.; Qu, Y.; Wang, Y.; et al. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid. Med. Cell Longev. 2021, 2021, 6614009. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yao, Y.; Wang, S.; Fan, L.; Ding, J.; Gao, Y.; Li, S.; Shen, L.; Zhu, Y.; Gao, C. Alleviating Oxidative Injury of Myocardial Infarction by a Fibrous Polyurethane Patch with Condensed ROS-Scavenging Backbone Units. Adv. Healthc. Mater. 2022, 11, e2101855. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Xu, P.; Liu, Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1699–1701. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shu, Y.; Hao, T.; Wang, Y.; Qian, Y.; Duan, C.; Sun, H.; Lin, Q.; Wang, C. A chitosan-glutathione based injectable hydrogel for suppression of oxidative stress damage in cardiomyocytes. Biomaterials 2013, 34, 9071–9081. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Lei, C.; Liu, H.; Jiang, M.; Zhou, Z.; Zhao, Y.; Yu, C.Y.; Wei, H. A ROS-Responsive Liposomal Composite Hydrogel Integrating Improved Mitochondrial Function and Pro-Angiogenesis for Efficient Treatment of Myocardial Infarction. Adv. Healthc. Mater. 2022, 11, e2200990. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Qian, M.; Zhang, Y.; Liu, Q.; Midgley, A.C.; Liu, Y.; Che, Y.; Hou, J.; Zhao, Q. An Injectable Dual-Function Hydrogel Protects Against Myocardial Ischemia/Reperfusion Injury by Modulating ROS/NO Disequilibrium. Adv. Sci. 2022, 9, e2105408. [Google Scholar] [CrossRef]
- Vong, L.B.; Bui, T.Q.; Tomita, T.; Sakamoto, H.; Hiramatsu, Y.; Nagasaki, Y. Novel angiogenesis therapeutics by redox injectable hydrogel—Regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials 2018, 167, 143–152. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Cheng, Y.; Zhang, Z.; Zhang, P.; Chen, X.; Wei, Y. In vitro study of electroactive tetraaniline-containing thermosensitive hydrogels for cardiac tissue engineering. Biomacromolecules 2014, 15, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Matsumura, Y.; Velayutham, M.; Foley, L.M.; Hitchens, T.K.; Wagner, W.R. Reactive oxygen species scavenging with a biodegradable, thermally responsive hydrogel compatible with soft tissue injection. Biomaterials 2018, 177, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yue, Z.; Shi, M.; Jiang, L.; Chen, S.; Yao, M.; Yu, Q.; Wu, X.; Zhang, H.; Yao, F.; et al. An Intrapericardial Injectable Hydrogel Patch for Mechanical-Electrical Coupling with Infarcted Myocardium. ACS Nano 2022, 16, 16234–16248. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Bai, A.; Cai, H.; Bai, Y.; Jiang, W.; Yang, H.; Wang, X.; Yang, L.; Sun, N.; et al. A viscoelastic adhesive epicardial patch for treating myocardial infarction. Nat. Biomed. Eng. 2019, 3, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Khattab, A.; Islam, M.A.; Hweij, K.A.; Zeitouny, J.; Waters, R.; Sayegh, M.; Hossain, M.M.; Paul, A. Injectable Hydrogels for Cardiac Tissue Repair after Myocardial Infarction. Adv. Sci. 2015, 2, 1500122. [Google Scholar] [CrossRef]
- Bar, A.; Cohen, S. Inducing Endogenous Cardiac Regeneration: Can Biomaterials Connect the Dots? Front. Bioeng. Biotechnol. 2020, 8, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, S.; McNeill, B.; Podrebarac, J.; Hosoyama, K.; Sedlakova, V.; Cron, G.; Smyth, D.; Seymour, R.; Goel, K.; Liang, W.; et al. Injectable human recombinant collagen matrices limit adverse remodeling and improve cardiac function after myocardial infarction. Nat. Commun. 2019, 10, 4866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Contessotto, P.; Orbanić, D.; Da Costa, M.; Jin, C.; Owens, P.; Chantepie, S.; Chinello, C.; Newell, J.; Magni, F.; Papy-Garcia, D.; et al. Elastin-like recombinamers-based hydrogel modulates post-ischemic remodeling in a non-transmural myocardial infarction in sheep. Sci. Transl. Med. 2021, 13, eaaz5380581. [Google Scholar] [CrossRef]
- Pupkaite, J.; Sedlakova, V.; Eren, C.C.; Bak, M.; McLaughlin, S.; Ruel, M.; Alarcon, E.I.; Suuronen, E.J. Delivering More of an Injectable Human Recombinant Collagen III Hydrogel Does Not Improve Its Therapeutic Efficacy for Treating Myocardial Infarction. ACS Biomater. Sci. Eng. 2020, 6, 4256–4265. [Google Scholar] [CrossRef] [PubMed]
- Cha, G.D.; Lee, W.H.; Sunwoo, S.H.; Kang, D.; Kang, T.; Cho, K.W.; Kim, M.; Park, O.K.; Jung, D.; Lee, J.; et al. Multifunctional Injectable Hydrogel for In Vivo Diagnostic and Therapeutic Applications. ACS Nano 2022, 16, 554–567. [Google Scholar] [CrossRef]
- Zhang, L.; Li, T.; Yu, Y.; Shi, K.; Bei, Z.; Qian, Y.; Qian, Z. An injectable conductive hydrogel restores electrical transmission at myocardial infarct site to preserve cardiac function and enhance repair. Bioact. Mater. 2023, 20, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lei, D.; Yang, Q.; Yang, Y.; Jiang, C.; Shi, H.; Qian, B.; Long, Q.; Chen, W.; Chen, Y.; et al. A perfusable, multifunctional epicardial device improves cardiac function and tissue repair. Nat. Med. 2021, 27, 480–490. [Google Scholar] [CrossRef]
- Whyte, W.; Roche, E.T.; Varela, C.E.; Mendez, K.; Islam, S.; O’Neill, H.; Weafer, F.; Shirazi, R.N.; Weaver, J.C.; Vasilyev, N.V.; et al. Sustained release of targeted cardiac therapy with a replenishable implanted epicardial reservoir. Nat. Biomed. Eng. 2018, 2, 416–428. [Google Scholar] [CrossRef]
- Taylor, D.L.; In Het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mat. Sci. Eng. R 2015, 93, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, Y.; Ye, G.; He, Y.; Li, B.; Guan, Y.; Gong, B.; Mequanint, K.; Xing, M.; Qiu, X. Injectable and conductive cardiac patches repair infarcted myocardium in rats and minipigs. Nat. Biomed. Eng. 2021, 5, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Sun, X.; Zhang, H.; Yu, Q.; Wang, X.; He, S.; Yao, F.; Li, J. A transparent, ultrastretchable and fully recyclable gelatin organohydrogel based electronic sensor with broad operating temperature. J. Mater. Chem. A 2020, 8, 4447–4456. [Google Scholar] [CrossRef]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 2021, 20, 229–236. [Google Scholar] [CrossRef]
- Yu, C.; Yao, F.; Li, J. Rational design of injectable conducting polymer-based hydrogels for tissue engineering. Acta Biomater. 2022, 139, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Zeglio, E.; Rutz, A.L.; Winkler, T.E.; Malliaras, G.G.; Herland, A. Conjugated Polymers for Assessing and Controlling Biological Functions. Adv. Mater. 2019, 31, 1806712. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, Y.; Wang, H.; Xu, Z.; Chen, J.; Bao, R.; Tan, B.; Cui, Y.; Fan, G.; Wang, W.; et al. Paintable and Rapidly Bondable Conductive Hydrogels as Therapeutic Cardiac Patches. Adv. Mater. 2018, 30, e1704235. [Google Scholar] [CrossRef] [PubMed]
- Pok, S.; Benavides, O.M.; Hallal, P.; Jacot, J.G. Use of myocardial matrix in a chitosan-based full-thickness heart patch. Tissue Eng. Part A 2014, 20, 1877–1887. [Google Scholar] [CrossRef] [Green Version]
- Rodness, J.; Mihic, A.; Miyagi, Y.; Wu, J.; Weisel, R.D.; Li, R. VEGF-loaded microsphere patch for local protein delivery to the ischemic heart. Acta Biomater. 2016, 45, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhang, X.; Liu, Y.; Cui, C.; Sun, Y.; Liu, W. Wet adhesive hydrogel cardiac patch loaded with anti-oxidative, autophagy-regulating molecule capsules and MSCs for restoring infarcted myocardium. Bioact. Mater. 2023, 21, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.; Patino-Guerrero, A.; Hasany, M.; Ansari, M.O.; Memic, A.; Dolatshahi-Pirouz, A.; Nikkhah, M. Electroconductive biomaterials for cardiac tissue engineering. Acta Biomater. 2022, 139, 118–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, W.; Radisic, M.; Vunjak-Novakovic, G. Can We Engineer a Human Cardiac Patch for Therapy? Circ. Res. 2018, 123, 244–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shadrin, I.Y.; Lam, J.; Xian, H.; Snodgrass, H.R.; Bursac, N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 2013, 34, 5813–5820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [Green Version]
- Cannata, A.; Petrella, D.; Russo, C.F.; Bruschi, G.; Fratto, P.; Gambacorta, M.; Martinelli, L. Postsurgical intrapericardial adhesions: Mechanisms of formation and prevention. Ann. Thorac. Surg. 2013, 95, 1818–1826. [Google Scholar] [CrossRef]
- Wang, Q.L.; Wang, H.J.; Li, Z.H.; Wang, Y.L.; Wu, X.P.; Tan, Y.Z. Mesenchymal stem cell-loaded cardiac patch promotes epicardial activation and repair of the infarcted myocardium. J. Cell. Mol. Med. 2017, 21, 1751–1766. [Google Scholar] [CrossRef]
- Han, M.; Yang, H.; Lu, X.; Li, Y.; Liu, Z.; Li, F.; Shang, Z.; Wang, X.; Li, X.; Li, J.; et al. Three-Dimensional-Cultured MSC-Derived Exosome-Hydrogel Hybrid Microneedle Array Patch for Spinal Cord Repair. Nano Lett. 2022, 22, 6391–6401. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, K.; Jiang, T.; Li, S.; Chen, J.; Wu, Z.; Li, W.; Tan, R.; Wei, W.; Yang, X.; et al. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing. J. Nanobiotechnol. 2022, 20, 147. [Google Scholar] [CrossRef] [PubMed]


| Author/Year | Animal | Patch Properties | Hydrogel Composition | Heart Function Tests | Biological Properties | Bioactivity |
|---|---|---|---|---|---|---|
| Yu/2022 [98] | Rat heart | Intrapericardial injectable mechanical−electrical coupled hydrogel patch | Hydrogel patch combined with adipose-derived stem cells | Hemodynamics, electrophysiology, echocardiography. Electrocardiography, histology, and immunofluorescence | Enhanced hydrophilicity and the flexibility of hydrogel preparation, modulated the conductive network, conductivity maintenance, rapid preparation and easy injection, tailored to the shape and dynamic characteristics of the pericardial cavity | Effectively inhibited malignant ventricular fibrosis, dilatation, and thinning, promoted revascularization in the infarcted region and assisted electrical conduction and synchronous pulsation functions |
| Cheng/2022 [48] | Rat and pig hearts | Hydrogel-based exosome-derived from MSC patch | Exosomes isolated from MSCs | Echocardiographic, Masson’s trichrome staining, and wheat germ agglutinin staining | Protected the heart from adverse remodeling, reduced the LV chamber size, improved LV wall thickness, and reduced interstitial fibrosis | Protected cardiomyocytes from hypertrophy, promoted cardiac cell proliferation, and reduced cardiac cell apoptosis |
| Rodness/2016 [119] | Rat heart | VEGF-loaded hydrogel-based microsphere patch | Hydrogel patch loaded VEGF | Masson’s trichrome staining, α-SMA staining, gsisolectin b4 staining, and VEGF staining | An average diameter of 3.2μm, nonporous, smooth dimpled surface, and prolonged releasement | Patches promote the sustained release of bioactive VEGF and augmented LV function by promoting angiogenesis |
| Wu/2023 [120] | Rat heart | Wet adhesive hydrogel cardiac patch loaded with anti-oxidative and autophagy-regulating molecule capsules and MSCs | Hp-β-cd and resveratrol synthesized hydrogel integrated with antioxidant and autophagy bioactivities | Echocardiographic, histological evaluation, and immunofluorescence staining | The scavenging ability of the hydrogel cardiac patch came from the loaded anti-oxidative agents | Hp-β-cd protected cardiomyocytes via the promotion of autophagy, the reduction of oxidative stress damage in cardiomyocytes, and the restoration of mitochondrial function |
| Wang/2021 [112] | Rat and minipig hearts | Injectable and conductive cardiac patches | Patches seeded with rat cardiomyocytes and patches incorporating cardiomyocytes differentiated from human pluripotent stem cells | Echocardiography, conductivity assessment, epicardial activation mapping, histology, and immunofluorescence evaluation | Maintained a constant storage modulus without any mechanical fatigue or failure | Functional repair after 4 weeks, as indicated by increases in fractional shortening, the ejection fraction, and by a decrease in the infarcted area |
| Applications/Potential Applications | Advantages | Challenges | |
|---|---|---|---|
| Injectable hydrogels | Administered intramyocardially, intracoronary, or intravenously as the candidates of translatable agent carriers for cardiac repair and regeneration | Minimally invasive procedure, acceptable physicochemical and mechanical properties, provides a supporting matrix to protect the encapsulated cells/agents | Low retention of cells and drugs, hydrogels hard to adhere to heart surface, poor mechanical properties, potential immunogenicity, and being washed away rapidly from the beating heart |
| Cardiac patches | Surgically sutured to the epicardial surface | Improving efficacy and reducing systemic toxicity, providing mechanical support, improving the interaction between the cardiac patch and the host myocardium, enhancing retention, and promoting the controlled release of bioactive agents | Surgical trauma impairs the contraction relaxation and electronic conductivity, and hard to enter the damaged tissue |
| Nanocarriers | Delivery carriers for cells, proteins, drugs, and nucleic acids | Improve the survival and proliferation of cardiomyocytes and stem cells, and ensure a sustained release at the target sites, thereby enhancing the therapeutic efficacy and reducing the systemic side effects | The uncontrolled balance between biodegradation and mechanical strength |
| Vascular grafts | Tissue-engineered vascular grafts supply blood directly to the infarcted tissue apart from cardiac regeneration | Degradable, modifiable biocompatibility, nontoxic, and excessively nonimmunogenic | Enough mechanical properties in the beating heart with high blood pressure, thrombosis risk, and resistible with long-term complications |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Hu, J.; Wang, J.; Zhang, J.; Wang, L.; Zhang, C. The Role of Hydrogel in Cardiac Repair and Regeneration for Myocardial Infarction: Recent Advances and Future Perspectives. Bioengineering 2023, 10, 165. https://doi.org/10.3390/bioengineering10020165
Li P, Hu J, Wang J, Zhang J, Wang L, Zhang C. The Role of Hydrogel in Cardiac Repair and Regeneration for Myocardial Infarction: Recent Advances and Future Perspectives. Bioengineering. 2023; 10(2):165. https://doi.org/10.3390/bioengineering10020165
Chicago/Turabian StyleLi, Ping, Jiajia Hu, Jian Wang, Junjie Zhang, Lu Wang, and Chengliang Zhang. 2023. "The Role of Hydrogel in Cardiac Repair and Regeneration for Myocardial Infarction: Recent Advances and Future Perspectives" Bioengineering 10, no. 2: 165. https://doi.org/10.3390/bioengineering10020165
APA StyleLi, P., Hu, J., Wang, J., Zhang, J., Wang, L., & Zhang, C. (2023). The Role of Hydrogel in Cardiac Repair and Regeneration for Myocardial Infarction: Recent Advances and Future Perspectives. Bioengineering, 10(2), 165. https://doi.org/10.3390/bioengineering10020165









