3D Printing and Its Current Status of Application in Obstetrics and Gynecological Diseases
Abstract
1. Introduction
1.1. Types and Characteristics of 3D Bioprinting Techniques
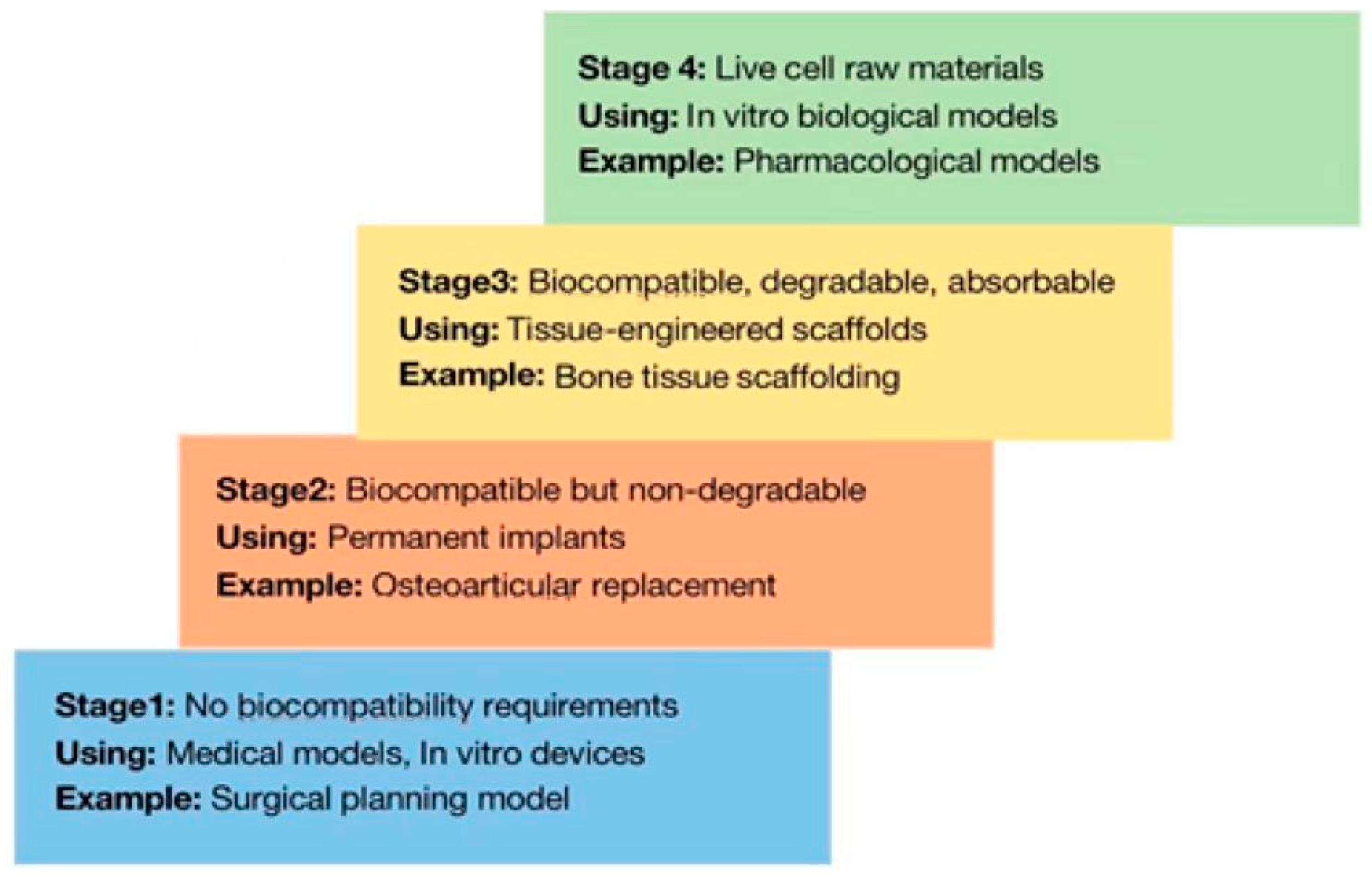
1.2. Selection of Materials for 3D Bioprinting
1.3. Application of 3D Printing Technology in Clinical Medicine
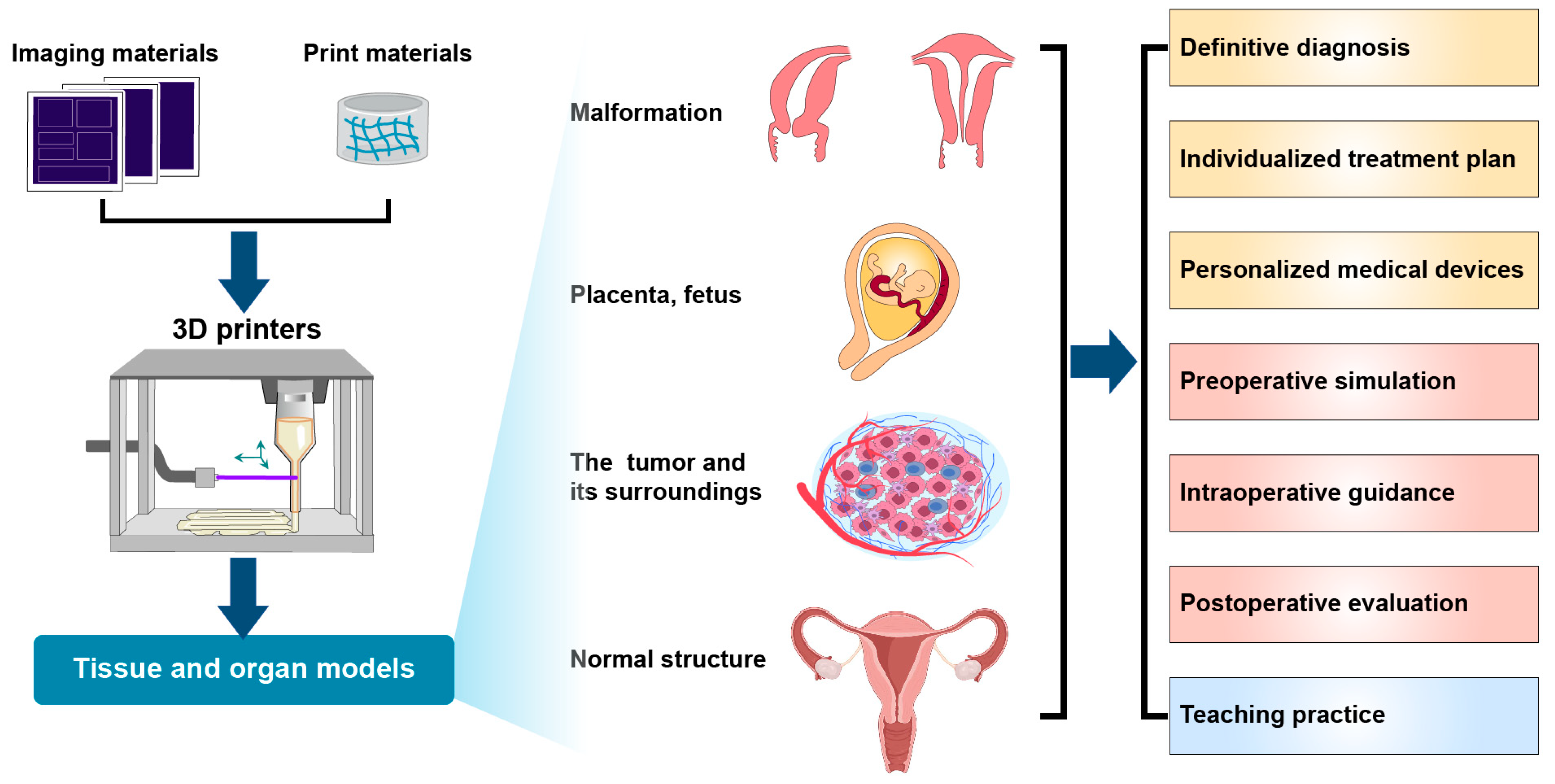
2. Applications of 3D Printing Technology in Obstetrics and Gynecology
2.1. Tumors
2.1.1. Uterine Fibroids
2.1.2. Malignancies
- (1)
- Cervical cancer
- (2)
- Endometrial cancer
- (3)
- Ovarian cancer
2.2. Premature Ovarian Failure
2.3. Intrauterine Adhesions
2.4. Malformations of the Genital Tract
2.5. Perinatal Medicine
2.5.1. Prenatal Diagnoses of Fetal Malformations
2.5.2. Prenatal Assessment of Placenta Accreta Spectrum
2.5.3. Clinical Teaching in Obstetrics and Gynecology
| Classification | Disease | Application | Models | Strength |
|---|---|---|---|---|
| Benign tumor | Uterine fibroids [14,26,27] | Preoperative simulation, intraoperative guidance, teaching | 3D-printed tumor models | Relationship between fibroid sites and surroundings can be clarified Different colored markers can be applied to distinguish different tissues |
| Malignancy | Cervical cancer [28,29] | Preoperative simulation, intraoperative guidance, teaching, radiotherapy and chemotherapy guidance, basic research | 3D-printed tumor and surrounding tissue models, in vitro cultured cell models, in vivo cultured animal models | Identification of the invasion of the lesion and surroundings Guidance for surgical planning to minimize physical injury Development of individualized chemotherapy regimens Provision of more models for basic research |
| Endometrial cancer [30] | ||||
| Ovarian cancer [33,34] | ||||
| Functional diseases | POF [35,36,38] | Treatment | 3D printing of ovarian tissue model | Radical treatment |
| Structural diseases | IUA [42,43,44,45,46] | Treatment, prevention | G-CSF-SRM hiMSC-loaded hydrogel scaffolds | Stable release Long duration of action |
| Malformations of the genital tract [9,49] | Diagnosis, teaching, individualized molds, preoperative simulation, intraoperative guidance | 3D printing of abnormal organ models | Individualized treatment plan | |
| Fetal malformations [52,53,54] | Diagnosis, treatment, teaching | 3D printed fetal models | Can be magnified at different scales | |
| PAS [55] | Diagnosis, preoperative simulation, intraoperative guidance | 3D printed tissue models of the uterus and placenta | Determine the extent of placental implantation, surrounding tissues and angry blood vessels to reduce intraoperative bleeding |
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arslan-Yildiz, A.; El Assal, R.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: Past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.S.; Boland, G.; Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2003, 5, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Pietrabissa, A.; Marconi, S.; Negrello, E.; Mauri, V.; Peri, A.; Pugliese, L.; Marone, E.M.; Auricchio, F. An overview on 3D printing for abdominal surgery. Surg. Endosc. 2020, 34, 1–13. [Google Scholar] [CrossRef]
- Hong, N.; Yang, G.H.; Lee, J.; Kim, G. 3D bioprinting and its in vivo applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Nam, L.; Ong, K.; Krishnan, A.; Huang, C.Y.; Fukunishi, T.; Hibino, N. 3D and 4D Bioprinting of the Myocardium: Current Approaches, Challenges, and Future Prospects. Biomed Res. Int. 2018, 2018, 6497242. [Google Scholar] [CrossRef]
- Tamay, D.G.; Dursun Usal, T.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D Printing of Polymers for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Kikano, E.; Grosse Hokamp, N.; Ciancibello, L. Utility of virtual monoenergetic images from spectral detector computed tomography in improving image segmentation for purposes of 3D printing and modeling. 3D Print. Med. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef]
- Tse, C.C.W.; Smith, P.J. Inkjet Printing for Biomedical Applications. Methods Mol. Biol. 2018, 1771, 107–117. [Google Scholar] [CrossRef]
- Mishra, A.; Srivastava, V. Biomaterials and 3D printing techniques used in the medical field. J. Med. Eng. Technol. 2021, 45, 290–302. [Google Scholar] [CrossRef]
- Roato, I.; Masante, B.; Putame, G.; Massai, D.; Mussano, F. Challenges of Periodontal Tissue Engineering: Increasing Biomimicry through 3D Printing and Controlled Dynamic Environment. Nanomaterials 2022, 12, 3878. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Leberfinger, A.N.; Ravnic, D.J.; Dhawan, A.; Ozbolat, I.T. Concise Review: Bioprinting of Stem Cells for Transplantable Tissue Fabrication. Stem Cells Transl. Med. 2017, 6, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Marga, F.; Jakab, K.; Khatiwala, C.; Shepherd, B.; Dorfman, S.; Hubbard, B.; Colbert, S.; Gabor, F. Toward engineering functional organ modules by additive manufacturing. Biofabrication 2012, 4, 022001. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Williams, D.; Patrich, T.; Hector, M.; Erik, J.; Ali, K. A perspective on the physical, mechanical and biological specifications of bioinks and the development of functional tissues in 3D bioprinting. Bioprinting 2018, 9, 19–36. [Google Scholar] [CrossRef]
- Cadena, I.; Chen, A.; Arvidson, A.; Fogg, K.C. Biomaterial strategies to replicate gynecological tissue. Biomater. Sci. 2021, 9, 1117–1134. [Google Scholar] [CrossRef]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef]
- Wei, F.; Li, Z.; Liu, Z.; Liu, X.; Jiang, L.; Yu, M.; Xu, N.; Wu, F.; Dang, L.; Zhou, H.; et al. Upper cervical spine reconstruction using customized 3D-printed vertebral body in 9 patients with primary tumors involving C2. Ann. Transl. Med. 2020, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Valverde, I.; Sarnago, F.; Prieto, R.; Zunzunegui, J.L. Three-dimensional printing in vitro simulation of percutaneous pulmonary valve implantation in large right ventricular outflow tract. Eur. Heart J. 2017, 38, 1262–1263. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.W.; Lee, D.Y.; Pang, C.H.; Kim, J.E.; Park, C.K.; Lee, D.; Park, S.J.; Cho, W.S. Clinical application of 3D virtual and printed models for cerebrovascular diseases. Clin. Neurol. Neurosurg. 2021, 206, 106719. [Google Scholar] [CrossRef] [PubMed]
- Crafts, T.D.; Ellsperman, S.E.; Wannemuehler, T.J.; Bellicchi, T.D.; Shipchandler, T.Z.; Mantravadi, A.V. Three-Dimensional Printing and Its Applications in Otorhinolaryngology-Head and Neck Surgery. Otolaryngol. Neck Surg. 2017, 156, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Towner, M.N.; Lozada-Capriles, Y.; LaLonde, A.; Ertefaie, A.; Stone, J.; Bhagavath, B.; Ghazi, A. Creation and Piloting of a Model for Simulating a Minimally Invasive Myomectomy. Cureus 2019, 11, e4223. [Google Scholar] [CrossRef]
- Ho, M.; Park, B.Y.; Rosenblum, N.G.; Al Mukaddam, M.; Kaplan, F.S.; Kucherov, V.; Hubosky, S.G.; Kane, G.; Desai, V.; Kramer, M.R.; et al. Surgical and Radiological Management of Complicated Uterine Leiomyoma Aided by 3D Models in a Patient with Fibrodysplasia Ossificans Progressiva. Am. J. Case Rep. 2021, 22, e931614. [Google Scholar] [CrossRef]
- Mackey, A.; Ng, J.I.; Core, J.; Nguyen, L.; Cross, D.; Lim, P.; Woodfield, C.; Pugliese, R.; Ku, B. Three-Dimensional-Printed Uterine Model for Surgical Planning of a Cesarean Delivery Complicated by Multiple Myomas. Obstet. Gynecol. 2019, 133, 720–724. [Google Scholar] [CrossRef]
- Molander, D.; Sbirkov, Y.; Sarafian, V. 3D bioprinting as an emerging standard for cancer modeling and drug testing. Folia Med. 2022, 64, 559–565. [Google Scholar] [CrossRef]
- Li, P.; Liu, P.; Chen, C.; Duan, H.; Qiao, W.; Ognami, O.H. The 3D reconstructions of female pelvic autonomic nerves and their related organs based on MRI: A first step towards neuronavigation during nerve-sparing radical hysterectomy. Eur. Radiol. 2018, 28, 4561–4569. [Google Scholar] [CrossRef]
- Sayed Aluwee, S.A.Z.B.; Zhou, X.; Kato, H.; Makino, H.; Muramatsu, C.; Hara, T.; Matsuo, M.; Fujita, H. Evaluation of pre-surgical models for uterine surgery by use of three-dimensional printing and mold casting. Radiol. Phys. Technol. 2017, 10, 279–285. [Google Scholar] [CrossRef]
- Cho, H.; Jammalamadaka, U.; Tappa, K.; Egbulefu, C.; Prior, J.; Tang, R.; Achilefu, S. 3D Printing of Poloxamer 407 Nanogel Discs and Their Applications in Adjuvant Ovarian Cancer Therapy. Mol. Pharm. 2019, 16, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Dadashzadeh, A.; Moghassemi, S.; Shavandi, A.; Amorim, C.A. A review on biomaterials for ovarian tissue engineering. Acta Biomater. 2021, 135, 48–63. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, X.; Yang, X.; Yuan, M.; Xian, S.; Zhang, L.; Yang, D.; Liu, S.; Dai, F.; Tan, Z.; et al. The role of a drug-loaded poly (lactic co-glycolic acid) (PLGA) copolymer stent in the treatment of ovarian cancer. Cancer Biol. Med. 2020, 17, 237–250. [Google Scholar] [CrossRef]
- Yee, C.; Dickson, K.A.; Muntasir, M.N.; Ma, Y.; Marsh, D.J. Three-Dimensional Modelling of Ovarian Cancer: From Cell Lines to Organoids for Discovery and Personalized Medicine. Front. Bioeng. Biotechnol. 2022, 10, 836984. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.T.; Dubey, P.K.; Meur, S.K. Survival and developmental competence of buffalo preantral follicles using three-dimensional collagen gel culture system. Anim. Reprod. Sci. 2009, 114, 115–124. [Google Scholar] [CrossRef]
- Joo, S.; Oh, S.H.; Sittadjody, S.; Opara, E.C.; Jackson, J.D.; Lee, S.J.; Yoo, J.J.; Atala, A. The effect of collagen hydrogel on 3D culture of ovarian follicles. Biomed. Mater. 2016, 11, 065009. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, Y.; Wei, S.; Xue, L.; Tang, W.; Chen, D.; Xiong, J.; Huang, Y.; Fu, F.; Wu, C.; et al. Biomaterials and advanced technologies for the evaluation and treatment of ovarian aging. J. Nanobiotechnology 2022, 20, 1–41. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.Y.; Su, J.; Tang, X.N.; Chen, Q.; Ma, L.W.; Zhang, J.J.; Wu, J.M.; Wang, S.X. Three-dimensional bioprinting of artificial ovaries by an extrusion-based method using gelatin-methacryloyl bioink. Climacteric 2022, 25, 170–178. [Google Scholar] [CrossRef]
- Li, X.; Lv, H.F.; Zhao, R.; Ying, M.F.; Samuriwo, A.T.; Zhao, Y.Z. Recent developments in bio-scaffold materials as delivery strategies for therapeutics for endometrium regeneration. Mater. Today Bio 2021, 11, 100101. [Google Scholar] [CrossRef] [PubMed]
- Benor, A.; Gay, S.; DeCherney, A. An update on stem cell therapy for Asherman syndrome. J. Assist. Reprod. Genet. 2020, 37, 1511–1529. [Google Scholar] [CrossRef]
- Li, H.; Shen, S.; Fu, H.; Wang, Z.; Li, X.; Sui, X.; Yuan, M.; Liu, S.; Wang, G.; Guo, Q. Immunomodulatory Functions of Mesenchymal Stem Cells in Tissue Engineering. Stem Cells Int. 2019, 2019, 9671206. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Hou, B.; Lin, W.; Guo, F.; Cheng, M.; Zheng, J.; He, P.; Ji, W. 3D-printed hydrogel scaffold-loaded granulocyte colony-stimulating factor sustained-release microspheres and their effect on endometrial regeneration. Biomater. Sci. 2022, 10, 3346–3358. [Google Scholar] [CrossRef] [PubMed]
- Salama, N.M.; Zaghlol, S.S.; Mohamed, H.H.; Kamar, S.S. Suppression of the inflammation and fibrosis in Asherman syndrome rat model by mesenchymal stem cells: Histological and immunohistochemical studies. Folia Histochem. Cytobiol. 2020, 58, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Hou, B.; Lin, W.; Wang, L.; Zheng, W.; Li, W.; Zheng, J.; Wen, X.; He, P. 3D Bioprinting a human iPSC-derived MSC-loaded scaffold for repair of the uterine endometrium. Acta Biomater. 2020, 116, 268–284. [Google Scholar] [CrossRef]
- Xin, L.; Lin, X.; Pan, Y.; Zheng, X.; Shi, L.; Zhang, Y.; Ma, L.; Gao, C.; Zhang, S. A collagen scaffold loaded with human umbilical cord-derived mesenchymal stem cells facilitates endometrial regeneration and restores fertility. Acta Biomater. 2019, 92, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Hu, S.; Qin, W.; Tang, Y.; Guo, R.; Han, L. Bioprinting of a Blue Light-Cross-Linked Biodegradable Hydrogel Encapsulating Amniotic Mesenchymal Stem Cells for Intrauterine Adhesion Prevention. ACS Omega 2021, 6, 23067–23075. [Google Scholar] [CrossRef]
- Azizi, R.; Aghebati-Maleki, L.; Nouri, M.; Marofi, F.; Negargar, S.; Yousefi, M. Stem cell therapy in Asherman syndrome and thin endometrium: Stem cell-based therapy. Biomed Pharmacother. 2018, 102, 333–343. [Google Scholar] [CrossRef]
- Sun, H.; Lu, J.; Li, B.; Chen, S.; Xiao, X.; Wang, J.; Wang, J.; Wang, X. Partial regeneration of uterine horns in rats through adipose-derived stem cell sheets. Biol. Reprod. 2018, 99, 1057–1069. [Google Scholar] [CrossRef]
- Hou, C.; Zheng, J.; Li, Z.; Qi, X.; Tian, Y.; Zhang, M.; Zhang, J.; Huang, X. Printing 3D vagina tissue analogues with vagina decellularized extracellular matrix bioink. Int. J. Biol. Macromol. 2021, 180, 177–186. [Google Scholar] [CrossRef]
- Chen, S.A.; Ong, C.S.; Hibino, N.; Baschat, A.A.; Garcia, J.R.; Miller, J.L. 3D printing of fetal heart using 3D ultrasound imaging data. Ultrasound Obstet. Gynecol. 2018, 52, 808–809. [Google Scholar] [CrossRef]
- Huang, J.; Shi, H.; Chen, Q.; Hu, J.; Zhang, Y.; Song, H.; Zhou, Q. Three-Dimensional Printed Model Fabrication and Effectiveness Evaluation in Fetuses With Congenital Heart Disease or With a Normal Heart. J. Ultrasound Med. 2021, 40, 15–28. [Google Scholar] [CrossRef]
- Daniilidis, A.; Theodoros, D.; Grimbizis, G.F. 3D printing in gynecology and obstetrics. In 3D Printing: Applications in Medcineand Surgery Volume 2; Elsevier: Amsterdam, The Netherlands, 2022; pp. 141–157. [Google Scholar]
- Ionov, L. 4D Biofabrication: Materials, Methods, and Applications. Adv. Healthc. Mater. 2018, 7, e1800412. [Google Scholar] [CrossRef]
- Ruedinger, K.L.; Zhou, H.; Trampe, B.; Heiser, T.; Srinivasan, S.; Iruretagoyena, J.I.; Roldán-Alzate, A. Modeling Fetal Cardiac Anomalies from Prenatal Echocardiography with 3-Dimensional Printing and 4-Dimensional Flow Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2018, 11, e007705. [Google Scholar] [CrossRef] [PubMed]
- Coté, J.J.; Badura-Brack, A.S.; Walters, R.W.; Dubay, N.G.; Bredehoeft, M.R. Randomized Controlled Trial of the Effects of 3D-Printed Models and 3D Ultrasonography on Maternal-Fetal Attachment. J. Obstet. Gynecol. Neonatal Nurs. 2020, 49, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, K.M.; Bartels, H.C.; Armstrong, F.; Immel, E.; Corcoran, S.; Walsh, J.M.; McAuliffe, F.; McParland, P.; Carroll, S.; Higgins, S.; et al. Comparing three-dimensional models of placenta accreta spectrum with surgical findings. Int. J. Gynaecol. Obstet. 2022, 157, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Goudie, C.; Shanahan, J.; Gill, A.; Murphy, D.; Dubrowski, A. Investigating the Efficacy of Anatomical Silicone Models Developed from a 3D Printed Mold for Perineal Repair Suturing Simulation. Cureus 2018, 10, e3181. [Google Scholar] [CrossRef]
- Yu, J.C.; Cassidy, P.; Cory, G.; Jennifer, S.; Noah, L.; Lisa, L. “Build an embryo”: Educational efficacy of. 3D printed embryo models in pieces or enbloc in enhancing spatial & temporal anatomy learning. FASEB J. 2020, 34, 1-1. [Google Scholar]
- Kudryavtseva, E.; Popov, V.; Muller-Kamskii, G.; Zakurinova, E.; Kovalev, V. Advantages of 3D printing for gynecology and obstetrics: Brief review of applications, technologies, and prospects. In Proceedings of the IEEE 10th International Conference Nanomaterials: Applications & Properties (NAP), Sumy, Ukraine, 9–13 November 2020. [Google Scholar]
- Yasli, M.; Dabbagh, S.R.; Tasoglu, S.; Aydin, S. Additive manufacturing and three-dimensional printing in obstetrics and gynecology: A comprehensive review. Arch. Gynecol. Obstet. 2023, 1–12, Epub ahead of print. [Google Scholar] [CrossRef]

| Characteristic | Inkjet Bioprinting | Extrusion Bioprinting | Laser-Assisted Bioprinting |
|---|---|---|---|
| Principle | Droplet form of thermal or sonic methods | Linearly applied pneumatic pressure or mechanical force | Laser-induced forward transfer |
| Strengths | Fast speed, low cost, wide availability, and high cell viability | Deposition of high-density cells with high structural integrity | Enables the printing of different living cells and biological materials with precision and micron-level resolution |
| Limitations | Low pressure and easily blocked nozzle | Pressure is higher and cell viability is reduced during printing | Effects of laser light on cells are not well studied; high printing costs and complex print control systems |
| Resolution | Medium | Low | High |
| Application | Irregular or complex three-dimensional structures | Design of complex structures | Different types of organizational structures |
| Print speed | Fast | Medium | Slow |
| Cell viability | Medium | Low | High |
| Cost | Low | Medium | High |
| Cell density | Low | High | Medium |
| Materials | Classification | Composition | Strength | Limitations |
|---|---|---|---|---|
| Polyethylene glycol (PEG) and PEG copolymer | Synthetic biomaterials | One of the most studied and widely used biomaterials | Can be modified or combined with other biomaterials to design in vitro models Biocompatible, biodegradable, and reproducible Possesses experimental control properties | Failiure to support desired cell behaviors and tissue formation |
| Collagen | Natural biological materials | Main component of ECM, including Type I and Type IV collagen | Porous structure Strong hydrophilicity | Protein concentration is affected by the biological origin No chemical modifications Biochemical cues cannot be provided |
| Matrix | Natural biological materials | Tumor-derived product extracted from mouse sarcomas composed of basement membrane components | Provides growth factor information Stimulates cell–matrix interactions Induces differentiation | Lack of biomimetic function in vivo |
| Complex biomaterials | Complex biomaterials | Consists of natural and synthetic biomaterials | Leads to the formation of advanced in vitro models that resemble in vivo tissues | More expensive Difficult to determine the optimal ratio |
| Characteristic | Natural Biomaterials | Synthetic Biomaterials | Composite Biomaterials |
|---|---|---|---|
| Definition | Composed of naturally occurring substances | Composed of synthetic biological materials | Combination of different kinds of biomaterials |
| Classification | Protein biomaterials Polysaccharide biomaterials Natural nanomaterials | Polyethylene glycol Polyethylene glycol copolymer | Nanopolymer biomaterials Non-nanopolymer biomaterials |
| Strength | Better biocompatibility Better biodegradability Provides biological cues Replicates specific ECMs | Easy to control Biocompatible, biodegradable and reproducible A combination of materials is used Cost is relatively low | Better biocompatibility Better thermal stability and antibacterial efficacy Associated applications function more effectively |
| Limitations | Difficult to control Lacks mechanical integrity Difficult to separate | Toxicity Immune-related issues | More complicated process |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Zhang, W.; Li, P. 3D Printing and Its Current Status of Application in Obstetrics and Gynecological Diseases. Bioengineering 2023, 10, 299. https://doi.org/10.3390/bioengineering10030299
Hu C, Zhang W, Li P. 3D Printing and Its Current Status of Application in Obstetrics and Gynecological Diseases. Bioengineering. 2023; 10(3):299. https://doi.org/10.3390/bioengineering10030299
Chicago/Turabian StyleHu, Caihong, Weishe Zhang, and Ping Li. 2023. "3D Printing and Its Current Status of Application in Obstetrics and Gynecological Diseases" Bioengineering 10, no. 3: 299. https://doi.org/10.3390/bioengineering10030299
APA StyleHu, C., Zhang, W., & Li, P. (2023). 3D Printing and Its Current Status of Application in Obstetrics and Gynecological Diseases. Bioengineering, 10(3), 299. https://doi.org/10.3390/bioengineering10030299






