Abstract
Tissue Engineering (TE) is an interdisciplinary field that encompasses materials science in combination with biological and engineering sciences. In recent years, an increase in the demand for therapeutic strategies for improving quality of life has necessitated innovative approaches to designing intelligent biomaterials aimed at the regeneration of tissues and organs. Polymeric porous scaffolds play a critical role in TE strategies for providing a favorable environment for tissue restoration and establishing the interaction of the biomaterial with cells and inducing substances. This article reviewed the various polymeric scaffold materials and their production techniques, as well as the basic elements and principles of TE. Several interesting strategies in eight main TE application areas of epithelial, bone, uterine, vascular, nerve, cartilaginous, cardiac, and urinary tissue were included with the aim of learning about current approaches in TE. Different polymer-based medical devices approved for use in clinical trials and a wide variety of polymeric biomaterials are currently available as commercial products. However, there still are obstacles that limit the clinical translation of TE implants for use wide in humans, and much research work is still needed in the field of regenerative medicine.
1. Introduction
Human beings are made up of multiple complex tissues assembled in hierarchical structures that range from macro to nanoscale and fulfill specific roles to maintain the proper functioning of the body. These biological characteristics and structures inspired many scientists to design advanced multifunctional materials for the replacement of organs and tissues [1]. Every year, millions of patients suffer total or partial damage to their organs and tissues, and they are potential candidates for studies in the field of regenerative medicine. On the other hand, human life expectancy has quadrupled in the last three centuries, and the great drawback of conventional treatments lies mainly in the difficulty of finding donors and the rejection of the transplanted organ/tissue by the recipient organism [2,3]. This is how the field of TE in biomedicine was born, in order to develop functional tissues capable of regenerating and/or improving damaged tissue, and do so requires the contribution of several fields: TE, cell therapy, molecular therapy (e.g., gene and drug delivery), and artificial and bio-organ technology. TE started as a branch of regenerative medicine, and it is a rapidly growing research field in recent times. It combines engineering and biological science principles to create functional substitutes for native tissue and facilitate the maintenance, repair, and restoration of damaged tissue. In recent years, it has received considerable attention, as it is a promising field with a likely profound impact in field of medicine [4,5].
The term TE was officially created at a National Science Foundation workshop in 1988. TE consists of “the application of engineering and life science principles and methods toward the fundamental understanding of the structure-function relationship in normal and pathological mammalian tissues and the development of biological substitutes to restore, maintain, or improve tissue function” [6]. The roots of TE as a modern discipline lie in Boston, and the first recorded use of the term TE, as applied today, was published in an article titled “Functional Organ Replacement: The New Tissue Engineering Technology” in “Surgical Technology International” in 1991 [7]. Although the field of TE appears to be relatively new, the idea of replacing one tissue with another is as old as history itself. Examples are the Greek legend of “Prometheus” and the eternal regeneration of his liver, the miracle of the creation of Eve in “Genesis”, and the miraculous transplant of a member of the Holy Cosmos and Damian. With the introduction of the scientific method and advancements in our knowledge of traumatic injuries and diseases, the secrets of biology are better understood now [8].
TE and regenerative medicine are interdisciplinary fields that have evolved rapidly in recent years (Figure 1), and TE focuses on repairing and restoring the structural function of damaged tissue using various materials such as decellularized matrices, cells, scaffolds, and others [9]. A scaffold is a three-dimensional platform that can mimic the extracellular matrix (ECM) and is capable of providing mechanical, spatial, and biological signals to regulate and guide cellular responses [10] (Figure 2). Many researchers [11,12,13,14,15,16] use polymeric matrices composed of a mixture of one or more polymers (natural or synthetic) to solve the mechanical, thermal, and biological challenges that arise when they are used as scaffolds. Currently, the focus is on developing smart material devices that combine the benefits of different components, taking into account the specific biological, clinical, and medical aspects of the tissue defect.
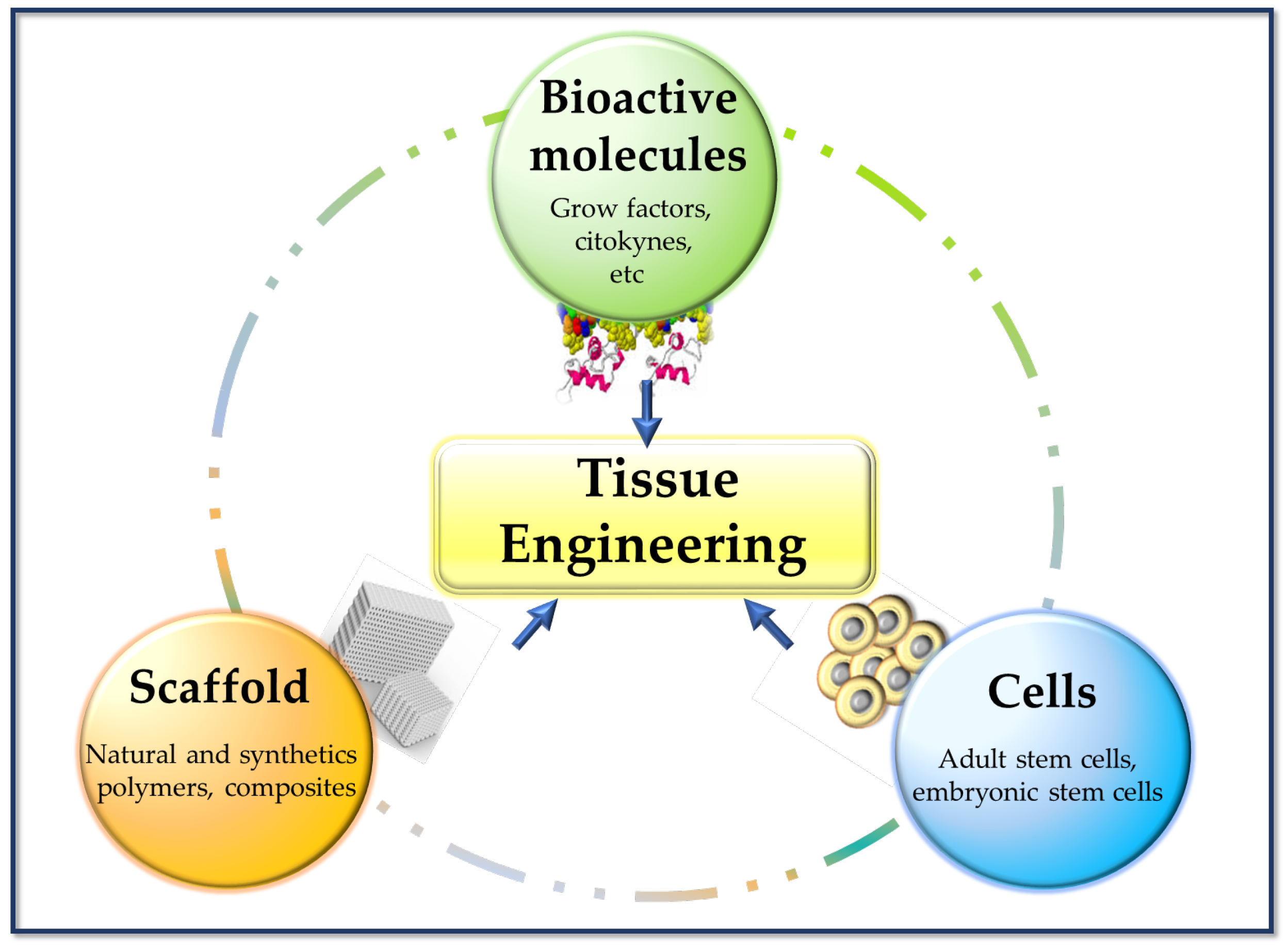
In regenerative medicine, cell management strategies can help replace lost cells in cases where the endogenous cells are insufficient or dysfunctional (e.g., regeneration of nerve tissue). TE tries to combine aspects of biomaterial scaffolds with cell replacement techniques to create a 3D environment that influences cell behavior (of native cells and cells previously cultured in the implant), such as their phenotype, architecture, migration, and survival. Biomaterials can provide structures for host cell infiltration, differentiation, and organization, and can serve as a means for drug delivery (e.g., controlled release of nanomedicines) [17]. These composites (scaffold-cells) combined with suitable biomolecules of interest (cytokines, growth factors, adhesion proteins, peptides, etc.), can be cultured in vitro to prepare a tissue that will subsequently be implanted in the damaged area and regenerate tissue in vivo. This combination of cells, signal molecules, and scaffolds is known as the tissue engineering triad [6] (Figure 3).
In this review, we highlight the importance of the use of polymers in ET, followed by brief descriptions of different methodologies for their fabrication. In addition, we put emphasis on the description of polymeric biomaterials, since they are mainly chosen for the design and construction of scaffolds due to their wide range of properties, availability, and low cost. Moreover, we provide a concise review of recent advances in the preclinical applications for different tissue types (epithelial, skeletal, cartilage, urinary, uterine, nervous, cardiac, and adipose). Finally, we gather some relevant data for the application of polymeric matrices in clinical trials, and we make a brief description of the current state of the polymers available as commercial products.
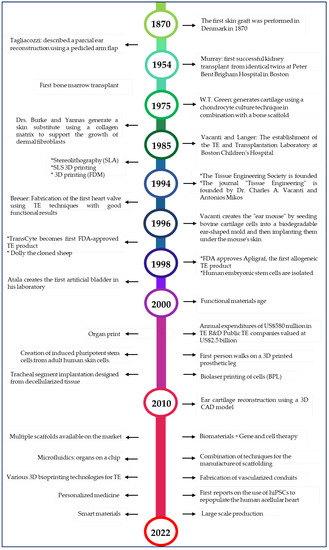
Figure 1.
Timeline of tissue engineering [6,7,8,18,19,20,21,22,23,24,25,26].
Figure 1.
Timeline of tissue engineering [6,7,8,18,19,20,21,22,23,24,25,26].
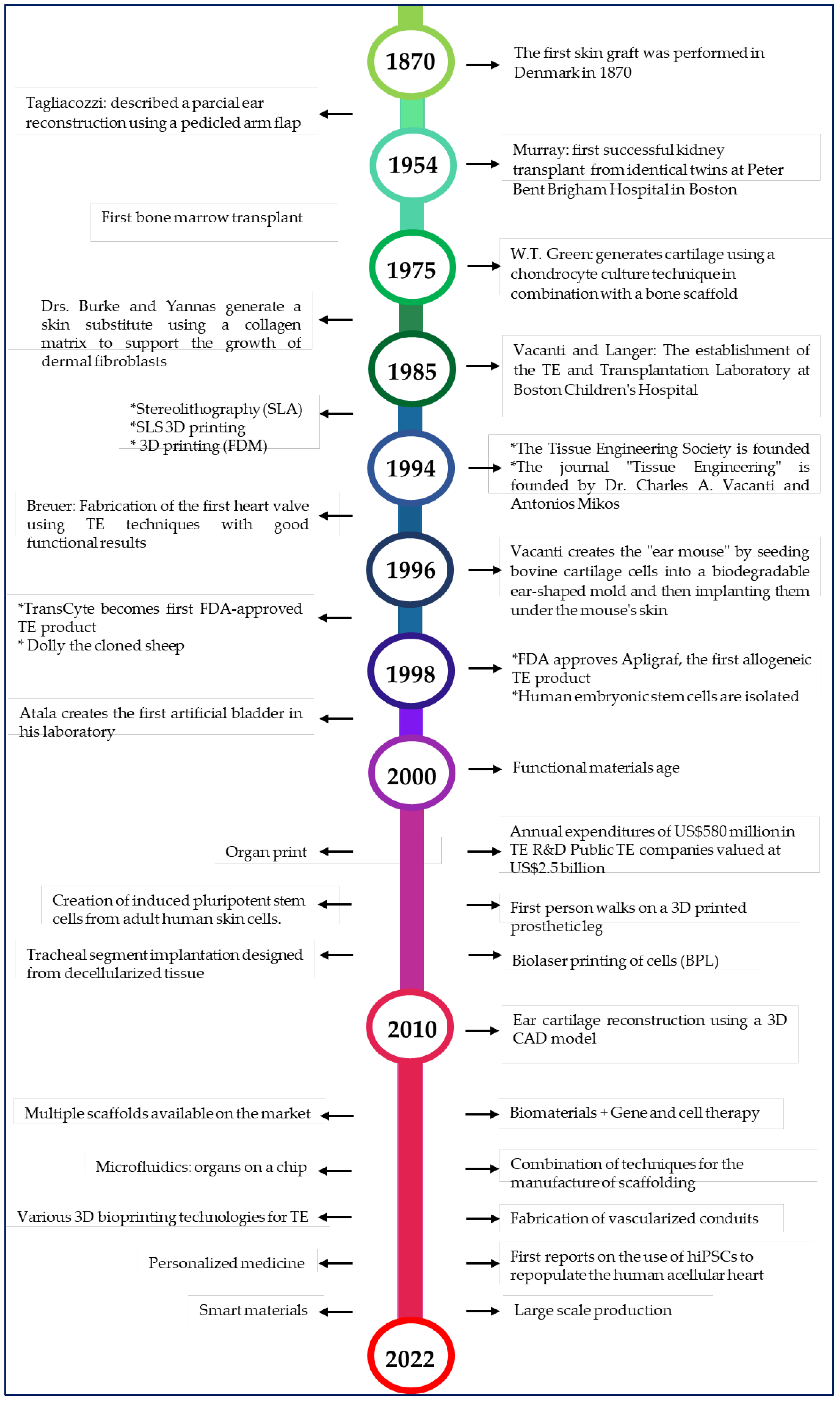
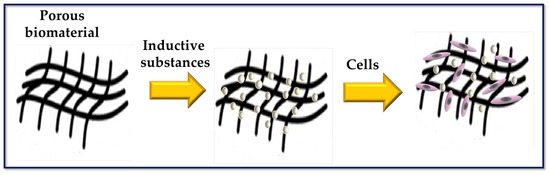
Figure 2.
Scheme to represent a porous biomaterial used as a scaffold for tissue engineering. The surface of the scaffold is functionalized with biomolecules capable of inducing cellular responses.
Figure 2.
Scheme to represent a porous biomaterial used as a scaffold for tissue engineering. The surface of the scaffold is functionalized with biomolecules capable of inducing cellular responses.
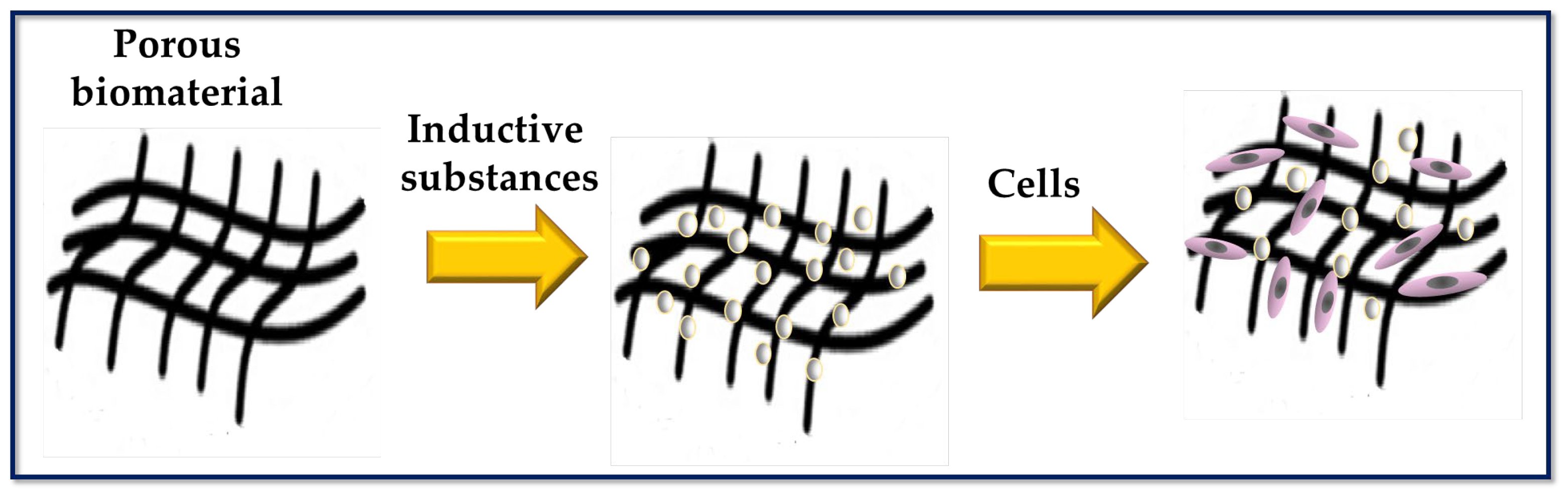
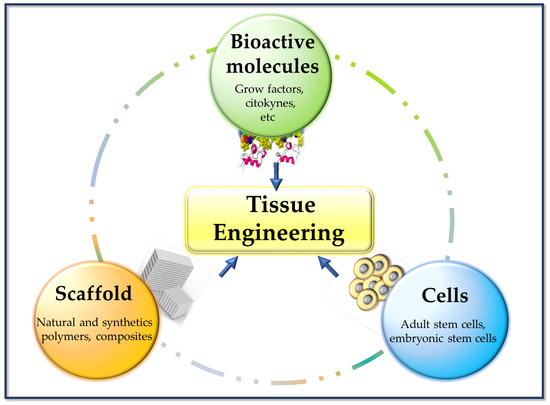
Figure 3.
Basic pillars of tissue engineering.
Figure 3.
Basic pillars of tissue engineering.
2. Biomaterials for Tissue Engineering Scaffolds Fabrication
The definition of biomaterial emerged in 1976 at the First Consensus Conference of the European Society for Biomaterials (ESB). A biomaterial was defined as “a non-viable material used as a medical device, intended to interact with biological systems”. However, the current definition of biomaterial by ESB is “material intended to interact with biological systems to evaluate, treat, augment, or replace any tissue, organ, or function in the body” [6]. This subtle change in definition is indicative of how ESB has evolved in the field of biomaterials over time.
The repair and healing of the tissue usually involves the autograft technique as a conventional method, but it depends mainly on the availability of other donor tissue and on factors such as graft failure, pain, persistent bleeding, the risk of associated infectious diseases in the patients, etc. [27,28]. Current TE technologies have an advantage over traditional technology, since studies have focused on choosing materials with beneficial characteristics to build scaffolds that are highly compatible with the human body and its tissues. This is of great importance as it reduces the risk of immune rejection and the susceptibility to infection [29]. Biomaterials can be used as implants in the form of sutures, bone, joint replacements, ligaments, vascular grafts, heart valves, intraocular lenses, dental implants, and medical devices such as pacemakers, biosensors, etc. [30]. Therefore, bio-based materials are changing the dynamics of 21st century materials. The use of bio-based materials in various research areas is becoming more frequent, as they have potential applications in health care to improve the quality of life of many people [31].
An important concept in TE strategies is that of the scaffold, defined as a highly porous three-dimensional (3D) matrix capable of providing an adequate surface for cells adhesion and interacting with the biomolecules of interest (Figure 4). The composition and internal architecture of the scaffolds control cell behavior and well-being [6,32] by acting as a supporting prosthesis in vivo or as a cell adhesion substrate for TE in vitro [33]. The scaffolds shape the macroscopic level of the organs and tissues to be replaced without recreating the details that are observed at the nanoscale in real organs (Figure 5). However, the nanoarchitecture of the ECM provides an intricate fibrillar system in which specific molecular interactions occur between various ratios, isoforms, and geometric shapes of elastins, collagens, proteoglycans, and adhesion proteins such as laminins and fibronectins. This creates an environment with informational signals and instructions that guide cellular behavior to form complex tissues such as bone, liver, heart, and kidney tissue [34].
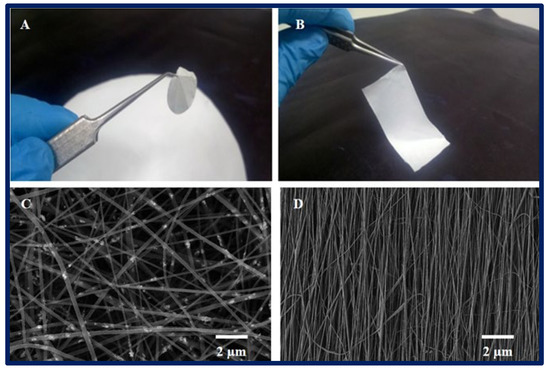
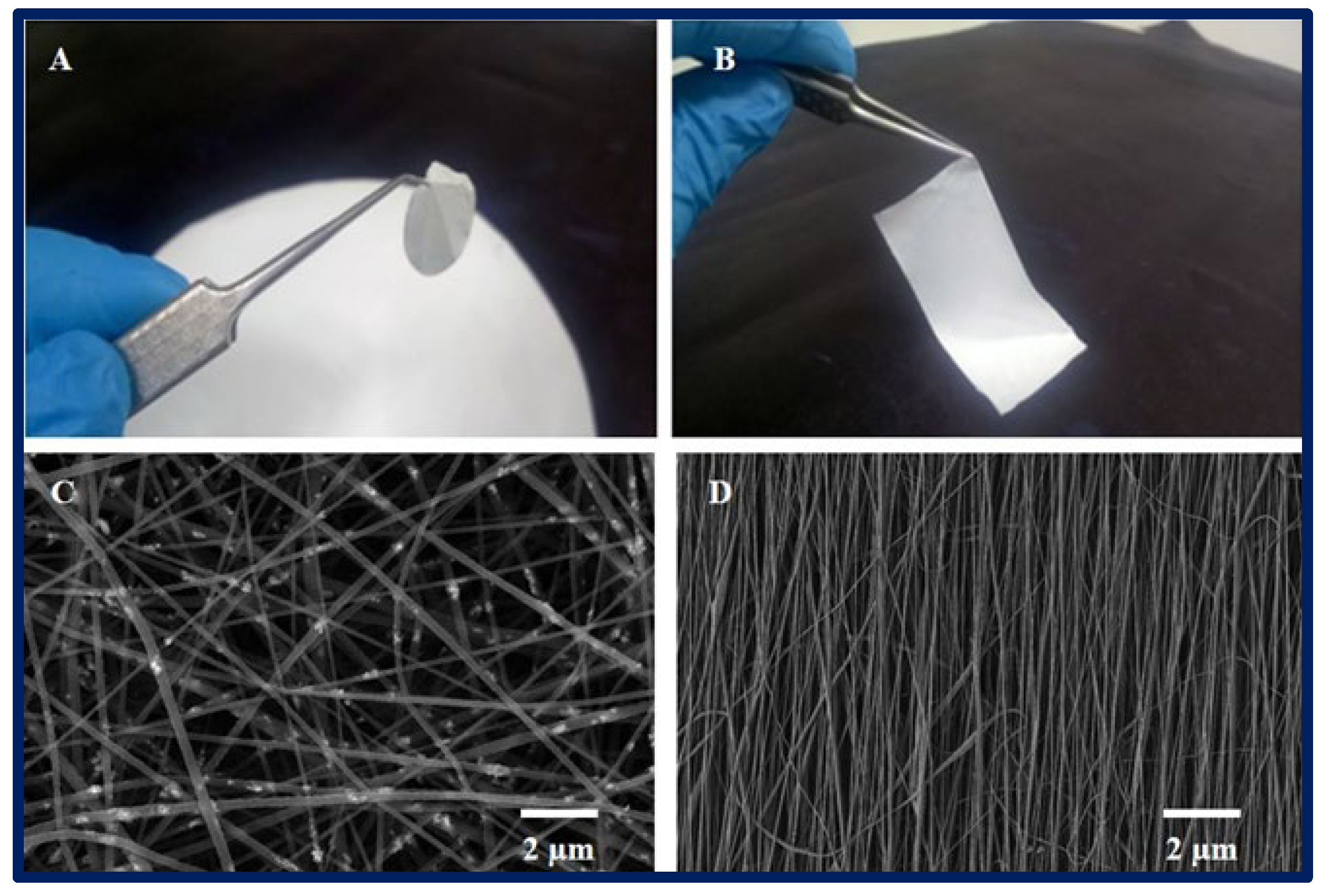
Figure 4.
(A,B): macroscopic appearance of a 2D PCL scaffold fabricated by electrospinning technique. (C,D): microarchitecture of scaffolds observed under scanning electron microscopy, (C): randomly oriented nanofibers, and (D): nanofibers with parallel orientation.
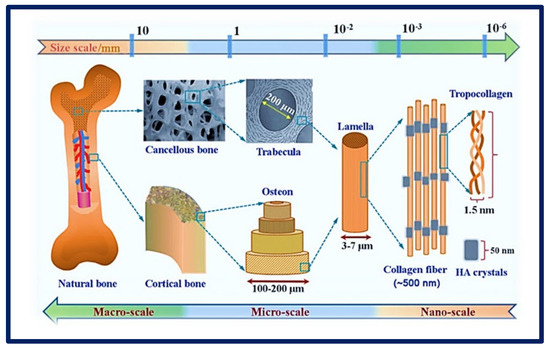
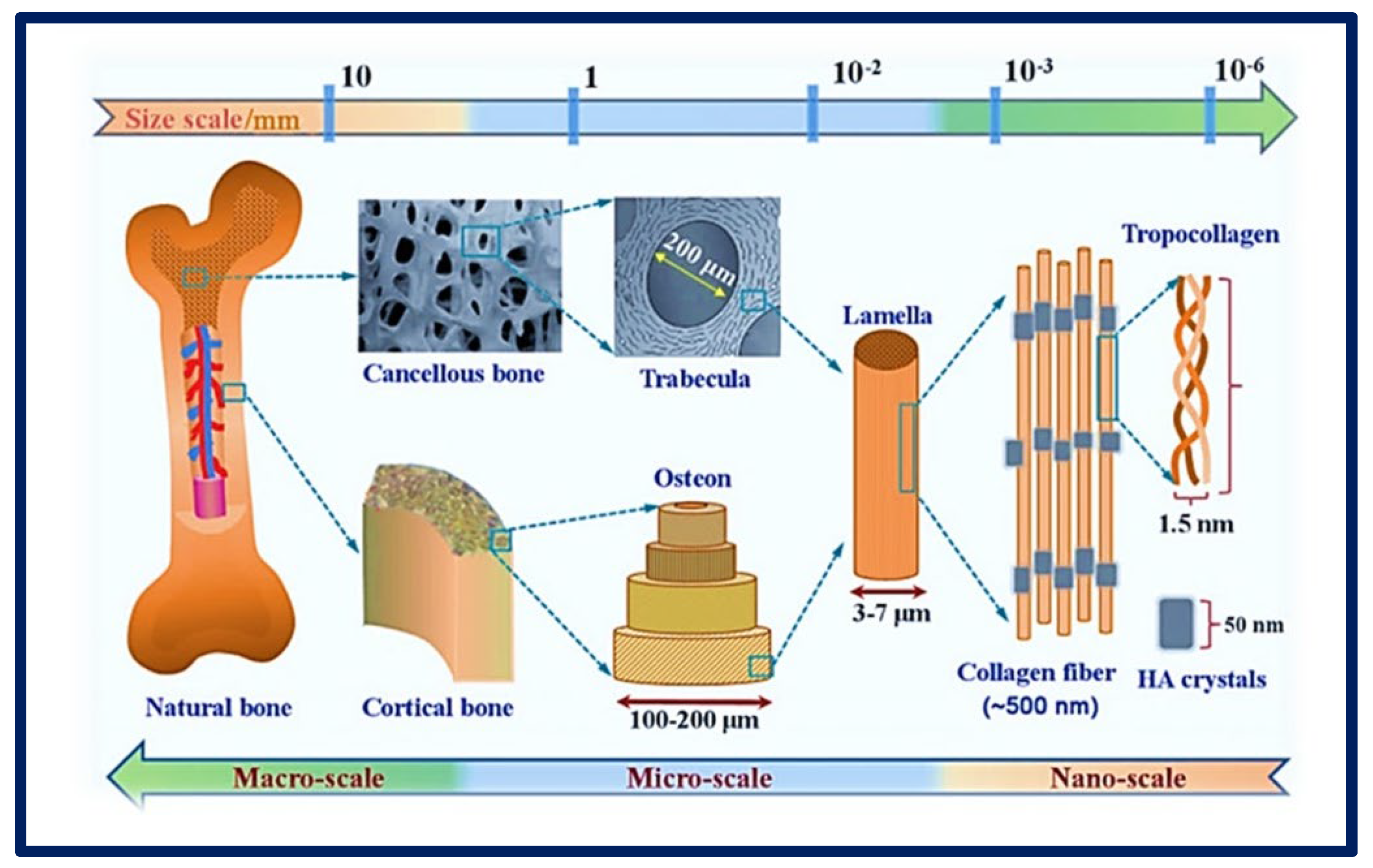
Figure 5.
Complexity of an organ showing its macro, micro, and nanoarchitecture. Chemical composition and multi−scale structure of natural bone. Bibliography consulted [35] is licensed under CC BY 2.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ (accessed on 15 December 2022).
Currently, various approaches can be applied to improve the biological and mechanical characteristics of scaffolds by combining design and manufacturing strategies with new materials. For the design of a good scaffold, it is of great importance to evaluate certain criteria before choosing a technique or its manufacture (Table 1). Parameters such as the surface characteristics (topography and roughness) and porosity (pore shape and size) of the scaffold must be controlled to increase cell migration into and on the surface of the scaffold [36,37] and to favor the efficient transport of metabolites without significantly compromising its mechanical stability [38]. The final applications of the scaffolds can vary significantly. They can be implanted empty (acellular) when they are expected to be colonized and invaded by host cells in a short time, or they may need to be previously seeded with appropriate cells before implantation or pre-cultured with cells in vitro in a suitable culture medium [39]. It is important to note that most mammalian cells depend on anchorage. Cell adhesion plays a crucial role in the development and maintenance of tissues, since it stimulates the signals that regulate the cell cycle, migration, differentiation, and cell survival. Therefore, it is important to design scaffold matrices that increase cell affinity to promote an ideal environment for cell attachment [40,41].
An ideal TE biomaterial not only mimics the native ECM of the tissue and provides mechanical support but also allows vascularization and integration with the host tissue, and gradually biodegrades and is remodeled with time as new tissues are formed. In this way, the native tissue can integrate with the scaffold and gradually replace the area originally occupied by it [42,43].

Table 1.
Criteria for building an ideal scaffold.
Table 1.
Criteria for building an ideal scaffold.
| Feature | Description |
|---|---|
| Adequate intrinsic physical and mechanical properties | This is defined by the microarchitecture and surface microtextures (surface topography). An ideal microarchitecture should be highly porous, with defined and interconnected pore sizes and a high surface area to volume ratio to allow for better vascularization, mass transfer, and cell growth. |
| Biocompatibility | It must produce the desired effect, be safe, and cause the minimum degree of inflammation once implanted. |
| Bioactivity | The biomaterial-cell interaction favors cell adhesion and proliferation, facilitating contact between cells and their migration over a prolonged period. Therefore, scaffolds can include biological molecules on their surface to promote cell adhesion or can also serve as a delivery vehicle or reservoir for growth-stimulating substances such as growth factors to accelerate regeneration. |
| Mimic EMC | It must be capable of mimicking the native tissue, providing an environment of optimal protection and nutrition. |
| Bioabsorption | It must be bioabsorbed in a controlled and appropriate time so that the new tissue replaces the space initially occupied by the biomaterials. |
| Versatility | They must be adaptable to different manufacturing techniques. |
| Translational perspective | The scaffold must be reproducible, accessible, and scalable to enable its use in high-demand applications for large tissues. |
Bibliography consulted [4,30,39,44].
3. Polymers for Tissue Engineering
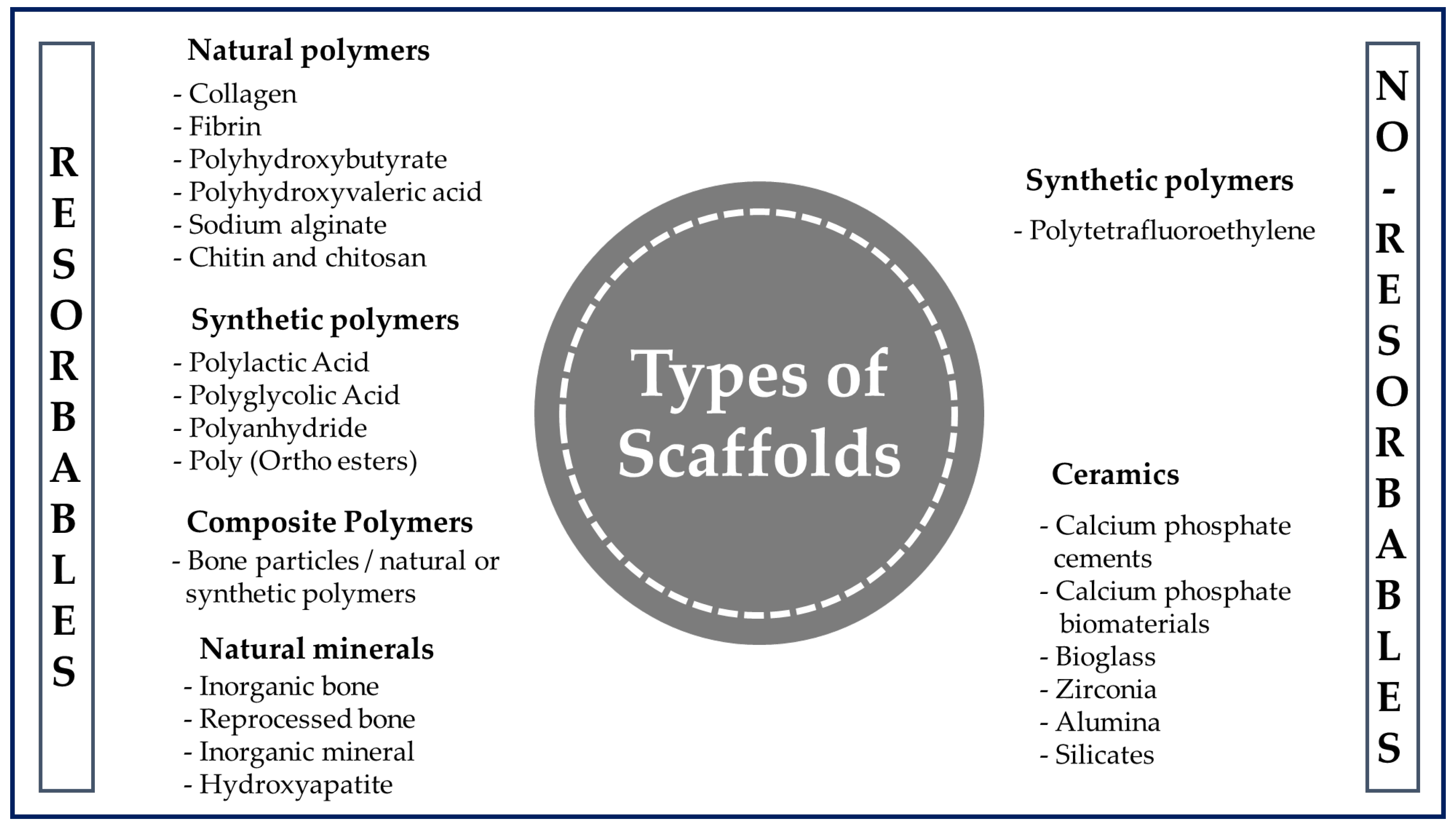
When the tissue is damaged after an accident or illness, it is not always possible to fully recover or have access to a donor in a short time. This is where polymer-based scaffolds can play a vital role in improving the quality of life of the patient by restoring tissue and maintaining a suitable environment to produce faster healing [29]. Polymer engineering represents a growing area that is often tailored to specific needs in terms of the design and manufacturing for a given application [45]. Although there are various materials available to produce scaffolds (Figure 6), they are usually chosen for the design and construction of scaffolds due to their wide range of properties, availability, and low cost [46]. In addition, they stand out as a special class of materials due to their flexibility and versatility. They have been used for many types of biomedical devices, such as dental [47], orthopedic [48], and cardiovascular [49] implants.
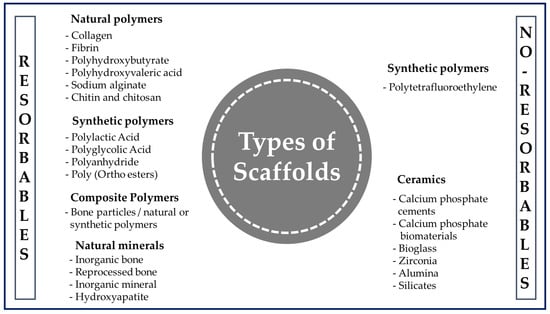
Figure 6.
General classification of polymers for medical use.
A polymer is a long-chain macromolecule composed of repeating subunits called monomers that are connected by covalent bonds, and knowledge of the synthesis and manufacture of products from them is essential in the biomedical field [50]. Among the various classes of biomaterials available for medical uses (Figure 6), natural polymers (NP) and synthetic polymers (SP) are used the most often for the manufacture of scaffolds.
NPs are produced by biological systems and have been used in human applications such as pharmaceutical excipients, drug delivery, cosmetics, prostheses, and biomedical scaffolds [51]. NPs are components of the ECM, and these bioactive properties allow them to be recognized and degraded by the biological environment, increasing cell interaction with the biomaterial [52]. Also, they present less toxicity from chronic inflammation or immunological reactions than those observed with the use of SP [53] and can undergo chemical modifications and be potentially biodegradable and biocompatible [54]. For all of these reasons, as well as for their cost effectiveness and ready availability, the use of NPs is attractive in biomedical applications. Their disadvantages are their sensitivity to temperature increase, which causes these polymers to be destroyed before reaching their melting point; their complex structure, which makes them difficult to process; and the possibility of the transmission of diseases to humans from other species due to their sources of origin [52]. There are several examples of NPs that are used in clinical applications. Among the proteins are silk fibroin, collagen, gelatin, albumin, keratin, fibrinogen, elastin, and actin; the polysaccharides include chitosan, chitin, alginate, gellan gum, and derivatives; and the glycosaminoglycans include hyaluronic acid [30,53].
SPs appeared much later than NPs but have been playing crucial roles in recent times. They are more diverse and versatile for biomedical applications due to the ease of creating custom designs with them and making more controlled chemical modifications [53]. They also offer several further advantages over other materials used in developing TE scaffolds, such as the ability to tailor their mechanical properties and their more controlled degradation kinetics for various applications [55]. Often cheaper than biological scaffolds, they can be produced uniformly in large quantities and have a long storage time. Many commercially available SPs exhibit physical, chemical, and mechanical properties comparable to those of biological tissues [51]. The most widely used SPs approved by the FDA (Food and Drug Administration of the United States) are aliphatic, e.g., poly (lactic acid) (PLA), poly (glycolic acid) (PGA), and its copolymers poly (lactic-co-glycolic acid) (PLGA). They are linear aliphatic polyesters and degrade by the hydrolysis of their ester linkages, resulting in non-toxic products and metabolites that can be removed by the natural metabolism of the host [56]. Other linear aliphatic polyesters, such as poly (ε-caprolactone) (PCL) and poly (hydroxybutyrate) (PHB), are also used in TE research. PCL degrades at a significantly slower rate than PLA, PGA, and PLGA, making PCL less attractive for general TE applications, but more attractive for long-term implantation and the controlled release of substances [57]. Other SPs widely applied in TE to manufacture scaffolds are poly (propylene fumarate) (PPF) and poly (glycerol sebacate) (PGS). PPF is a good candidate for bone TE application as it is biocompatible, biodegradable, and osteoconductive and can be strengthened by cross-linking reactions [56]. Polymers are generally degraded by hydrolysis, producing intermediate natural metabolites, and have controllable degradation rates ranging from months to years. Therefore, scaffolds could be designed according to the requirement of each tissue by adjusting the initial proportion of the monomers. In addition, this is an advantage when polymers are required to release a molecule of interest, such as hormones and growth factors, in a controlled manner [19]. Despite all these advantages, many SPs tend to present an immune response or toxicity when combined with certain polymers that the host tissue cannot incorporate [53]. Therefore, one possible solution is to combine synthetic and natural polymers to overcome the deficiencies of each and obtain a scaffold that exploits the great merits of both [58].
4. Strategies for Fabrication of Scaffolds in Tissue Engineering
To obtain biomedical devices with improved diagnostic and therapeutic capabilities, there are modern technologies in their design and manufacturing methods that promote remarkable alternative approaches in the field of regenerative medicine and TE. These include new micro- and nano-fabrication technologies, tools based on the use of medical images, computer-aided design and manufacturing, rapid prototyping and manufacturing technologies, and current advances in materials science [59]. Some of the most widely used techniques for scaffold fabrication in TE are described below.
4.1. Electrospinning
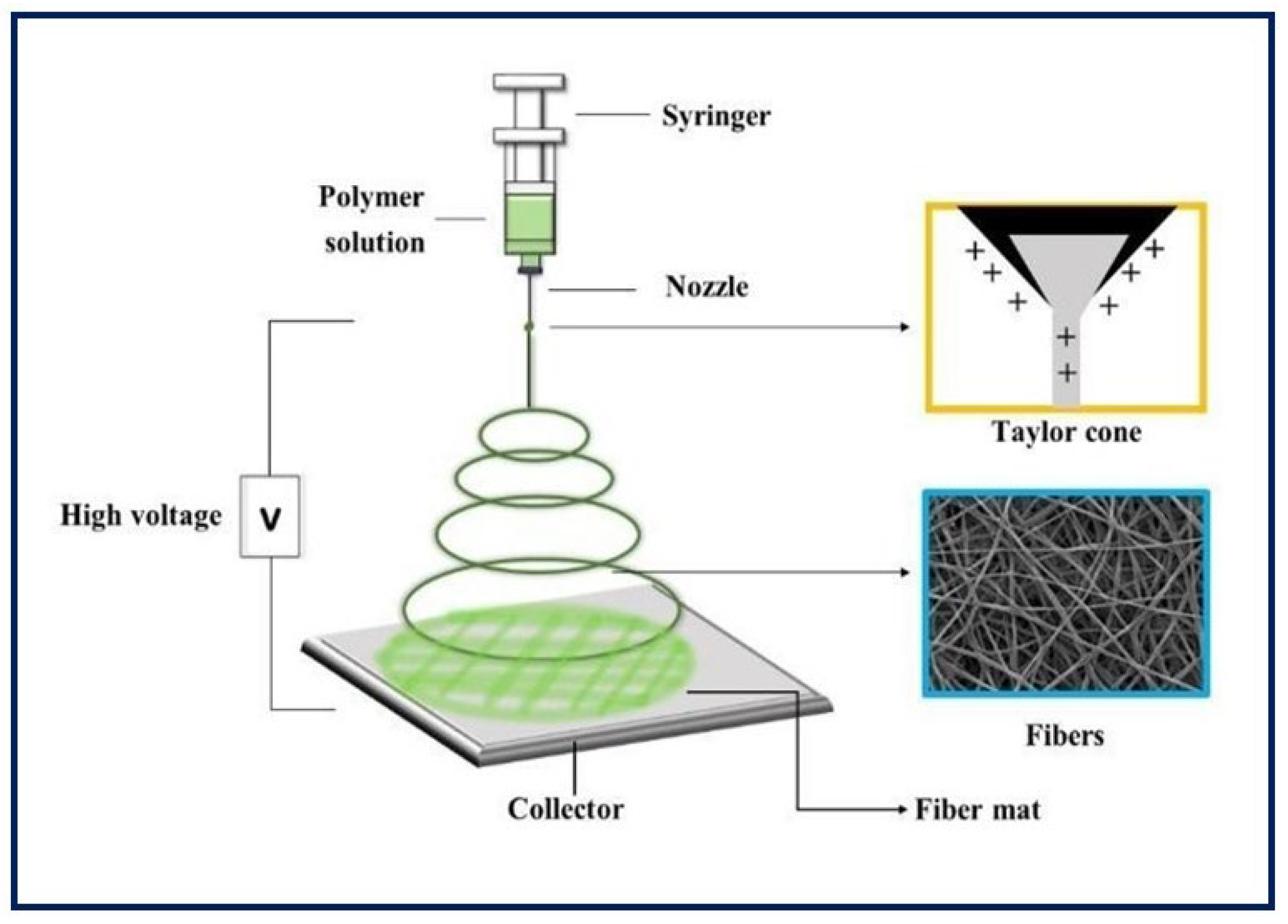
It is a unique, simple, cost-effective, and efficient technique for manufacturing continuous polymeric, ceramic and hybrid fibers. The diameters are in the range of hundreds of nanometers. Although it has many other potential applications, electrospinning has taken on great importance in TE, since it can be applied in the production of large-scale nanostructured scaffolds for tissue growth [60,61]. As for the equipment, the main components include a power supply of high voltage (can be direct or alternating current), an infusion pump coupled to a syringe, a capillary (usually a blunt-tipped hypodermic needle), and a conductive manifold [62] (Figure 7). During the electrospinning process, a high voltage is applied to the polymeric fluid (several tens of Kv), which is delivered to the tip of the capillary. This causes an accumulation of charges on the free surface of the fluid that protrudes from the tip of the needle and interacts with the external electric field. When the same polarity charge is reached as in the solution, a repulsive Coulomb force is generated which, when it overcomes the surface tension of the drop, allows the formation of a Taylor cone. From that, a continuous fluid jet is expelled which is elongated and accelerated by the applied electric field, travels a short distance between the electrodes, and is finally deposited on the collector of the opposite charge. As a result, the process leads to the formation of fine solid fibers when the solvent evaporates [63,64,65,66]. Random fiber ordering is generally achieved, but aligned nanofibers can also be obtained using a fast-rotating collector or a pair of parallel electrodes. This arrangement is useful in nerve or skeletal muscle TE applications [67]. Moreover, there is coaxial electrospinning that allows the combination of solutions with different properties into a single fiber, and it can be useful for the encapsulation and protection of functional molecules and their controlled release [68].
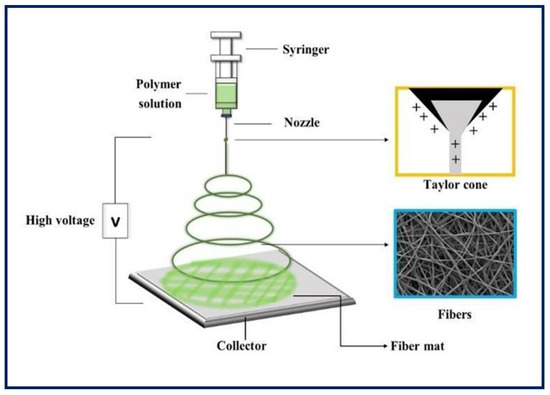
Figure 7.
Basic electrospinning equipment.
An important factor to control in electrospinning is the diameter of the fibers, which depends on the processing parameters and conditions (injection flow, applied voltage, needle-collector distance, etc.), the properties of the polymer solution (viscosity, conductivity, polymer type, concentration, surface tension, etc.), and the environmental conditions (relative humidity, temperature, and atmospheric pressure) [69,70]. All of these can dramatically influence the resulting diameter, shape, and spatial distribution of the electrospun fibers [71]. Various benefits associated with electrospun fibrous structures are their nanometric structure that mimic the architecture of the ECM, their large surface/volume ratio for binding cells and bioactive factors, their highly interconnected porous architecture, their high load capacity, their encapsulation efficiency, and their capability of transporting various drugs to specific sites of action [72,73,74].
4.2. Molecular Self-Assembly
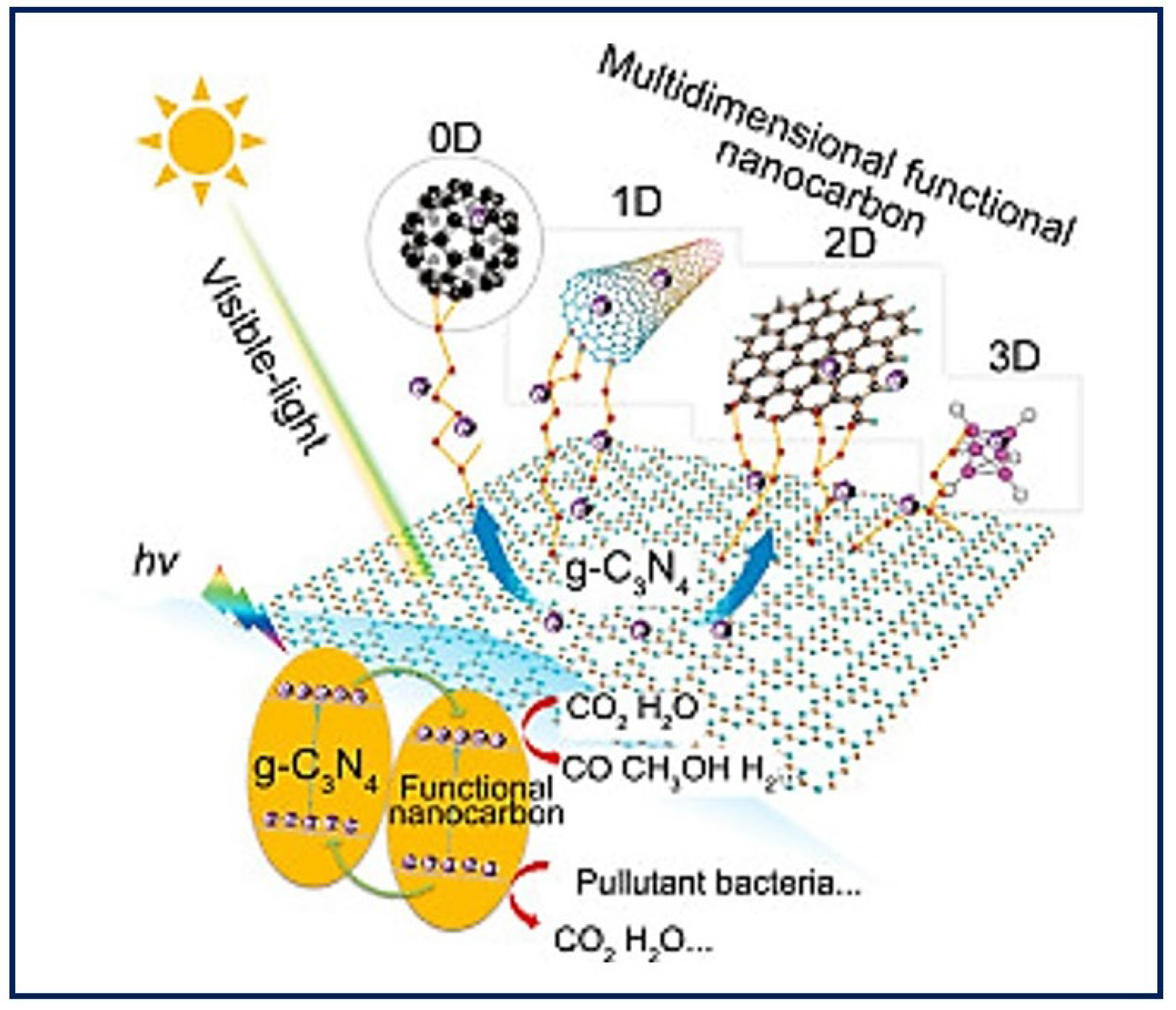
Self-assembly describes the autonomous organization of individual components into ordered patterns or structures. It is characterized by the specific association of molecules through non-covalent interactions, including hydrogen bonding and ionic bonding, and hydrophobic and Van der Waals interactions. Individually, such interactions are weak, but, in large numbers, they dominate the structural and conformational behavior of the assembly [75]. The concept of self-assembly has been applied to the construction of synthetic nanostructures for TE that aim to mimic the ECM [76]. The building blocks of construction interact one by one at the atomic and molecular scale, to organize into more complex supramolecular structures that can further reorganize into functional native tissue structures [77]. Thus, a further understanding of the biomolecular process could inspire the assembly of nanoparticles to promote the design and manufacture of new functional nanomaterials [78] with personalized morphologies from a single molecule. To obtain a final product with the desired properties, it is necessary to control the modulation of the individual monomeric building blocks [79] and other parameters (self-assembly time, temperature, solution concentration, pH, metal ions, etc.) [80]. Highly ordered biomaterials can be obtained from zero-dimensional (0D) structures such as the nanoparticles, nanospheres, and rings that are base structures for protein formation; one-dimensional (1D) structures such as nanofibers or nanotubes; two-dimensional (2D) structures such as nanofilms or 2D DNA arrays; and three-dimensional (3D) structures such as 3D hydrogels [81]. Figure 8 shows an example of the different dimensions that materials can take on nanocarbon as modifiers to enhance the visible light photocatalytic activity of g-C3N4 [82]. The two types of natural materials usually used in manufacturing processes are collagen and elastin. Both molecules are found abundantly in nature because they are components of all connective tissues and the ECM. These molecules have been used as the basis for the design of new materials that resemble collagen and elastin, though they gave way to the de novo design of synthetic peptides. Currently, peptide-based hydrogels are used to provide extracellular environments that mimic ECM and open up great opportunities for biomedical applications in fields such as TE, drug delivery, and wound healing [83].
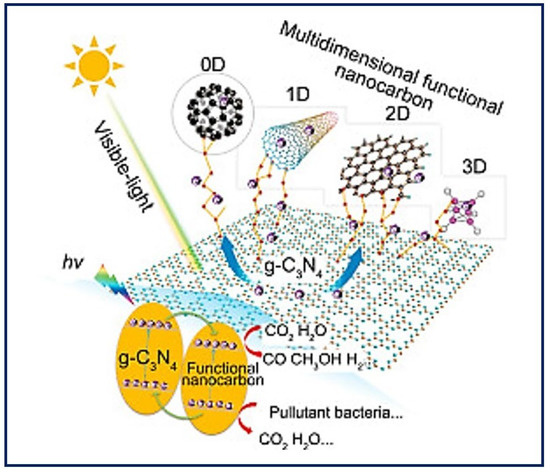
Figure 8.
Utilization of multidimensional functional nanocarbon materials (0D–3D) as modifiers to enhance the visible light photocatalytic activity of g-C3N4. Bibliography consulted [82] is licensed under CC BY 2.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/, (accessed on 15 December 2022).
4.3. Phase Separation
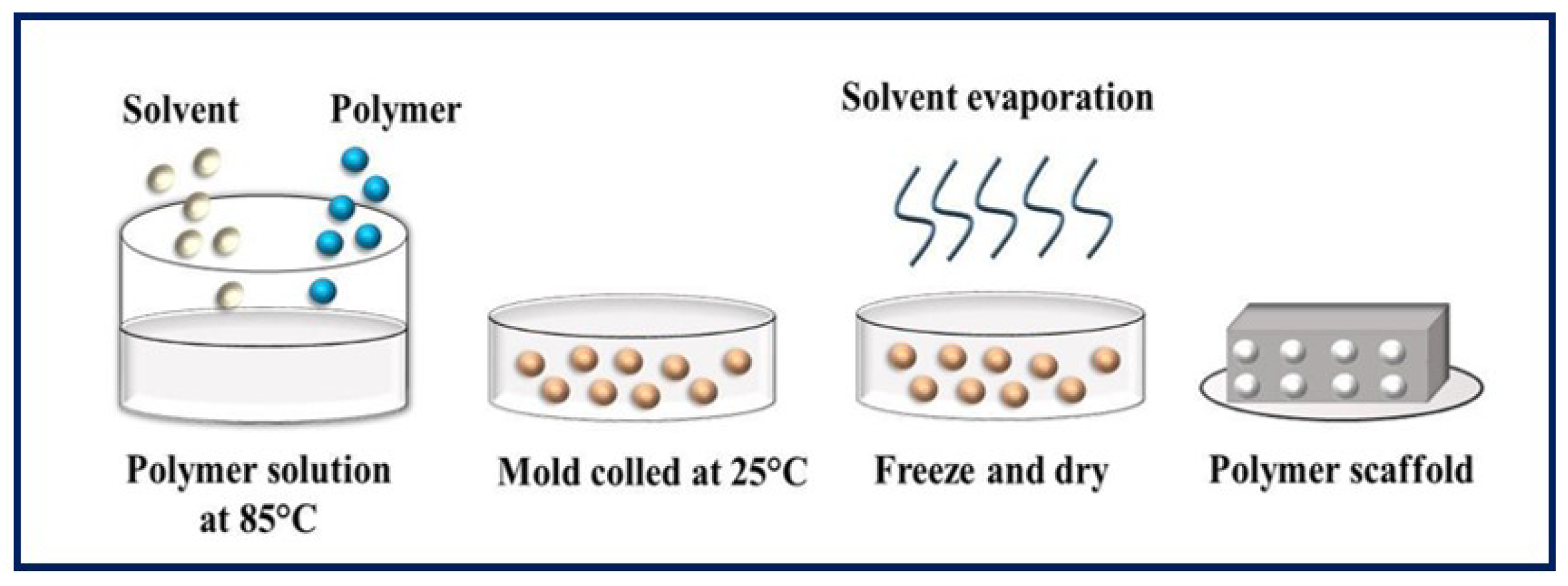
In this process, the polymer dissolves and phase separation is induced, either thermally or by addition of an immiscible solvent to the polymer solution, leading to the formation of a gel [84,85]. The term TIPS refers to temperature-induced phase separation, and, in this process, polymer precipitation is caused by a decrease in temperature, normally provoked by immersion in a quenching bath. It is a conventional technique for manufacturing highly porous scaffolds by forming networks of interconnected pores [86,87]. It has the advantage of being a fast, inexpensive, scalable, and controllable procedure [88,89], but its application is limited to certain polymers compatible with the use of organic solvents [28]. The TIPS process can be divided into solid-liquid phase separation (SLPS) (Figure 9) and liquid-liquid phase separation (LLPS), depending on the crystallization temperature of the solvent [90]. In the first case, a homogeneous solution of polymer is exposed to a certain temperature and becomes thermodynamically unstable, resulting in two separate phases. The solution is then freeze-dried, and the polymer-rich phase forms a matrix, while the lean phase produces pores when the solvents are removed. The SLPS method involves the production of porous scaffolds with good cell interconnectivity and permeability [91]. In the second case, a non-solvent is added to produce two different morphologies: (1) the solution separates into two phases (one rich and one lean in polymer) giving an emulsion when cooled below the binodal curve, and (2) the solution separates into a bicontinuous polymer-rich phase and a lean phase which, when cooled below the spinodal curve, produces a polymer with a highly porous structure, once the solvent is removed by leaching or cold drying [87,92]. Theoretically, the polymer-rich phase consists of the polymer and a fraction of the solvent, and the polymer-lean phase contains a non-solvent and the remaining solvent [93]. It is important to control certain parameters, such as the temperature and rate of cooling, crystallinity of the polymer, polymer concentration, and presence of ceramic powders, to manipulate and control the pore size, the pore geometry, and the interconnectivity of the pores in the scaffold [94].
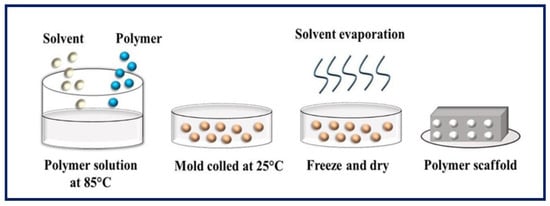
Figure 9.
Schematic diagram of solid-liquid phase separation. Bibliography consulted [88].
4.4. Particulate Leaching
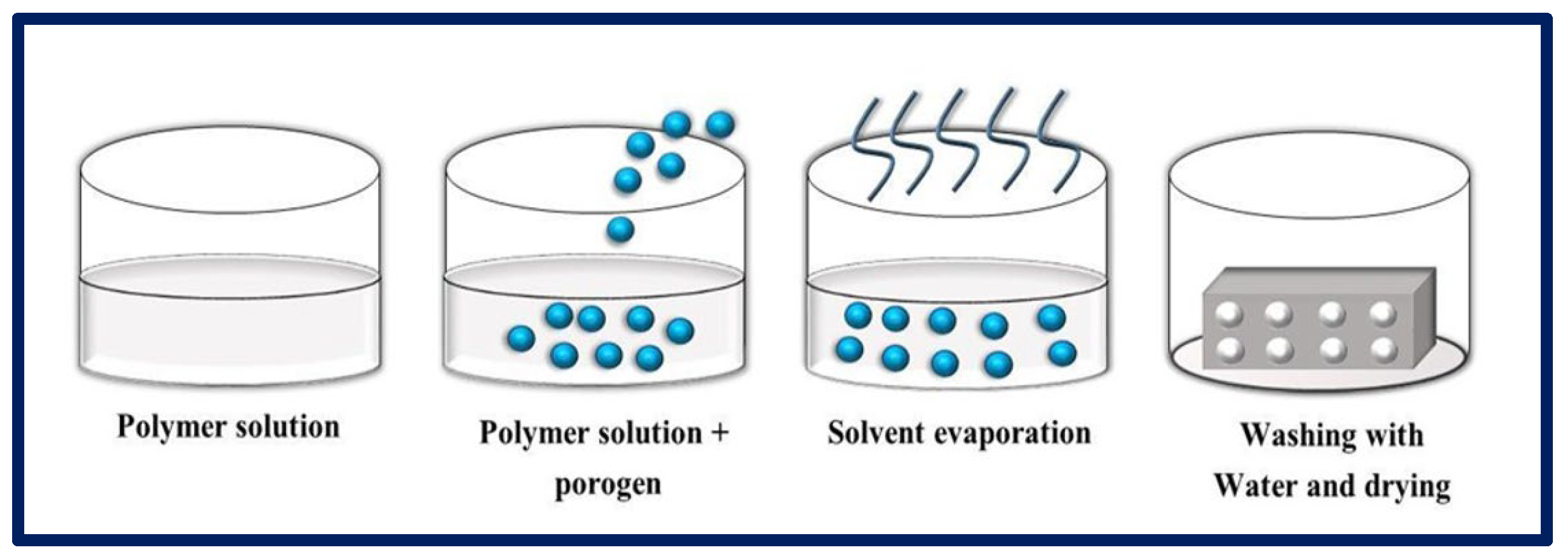
Particulate leaching consists of two categories: (1) solvent casting—particulate leaching (SC-PL) and (2) melt molding—particulate leaching (MM-PL). In SC-PL, a polymer solution is mixed with salt particles in a mold; then, the solvent is evaporated and the salt particles are removed by washing with water, resulting in the formation of a porous scaffold. In the MM-PL, the polymer is formed by introducing it into a mold with the embedded solid porogen (Figure 10). Pressure and heat are then applied to evaporate the solvent, and the particles are leached by washing the resulting product with water [95,96]. The SC-PL method is relatively simple and does not require specific and expensive equipment; it is also possible to control the final porosity, the pore size, and the interconnectivity of the pores by making a suitable selection of the initial proportions of the polymer and the porogen [97]. One of the advantages is that highly porous scaffolds (some reaching 93%) can be obtained with average pore sizes of up to 500 µm. However, the distribution of salt particles may be non-uniform inside due to the density difference between liquid polymer and solid salt. The number of salt particles in direct contact with each other is not well controlled, and salt residues may remain despite washings, which will lead to the poor interconnectivity of the pores [98].
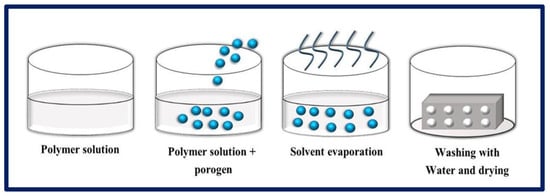
Figure 10.
MM-PL process, where a polymer is mixed with a porogen to obtain porous scaffolds. Bibliography consulted [88].
4.5. Gas Foaming
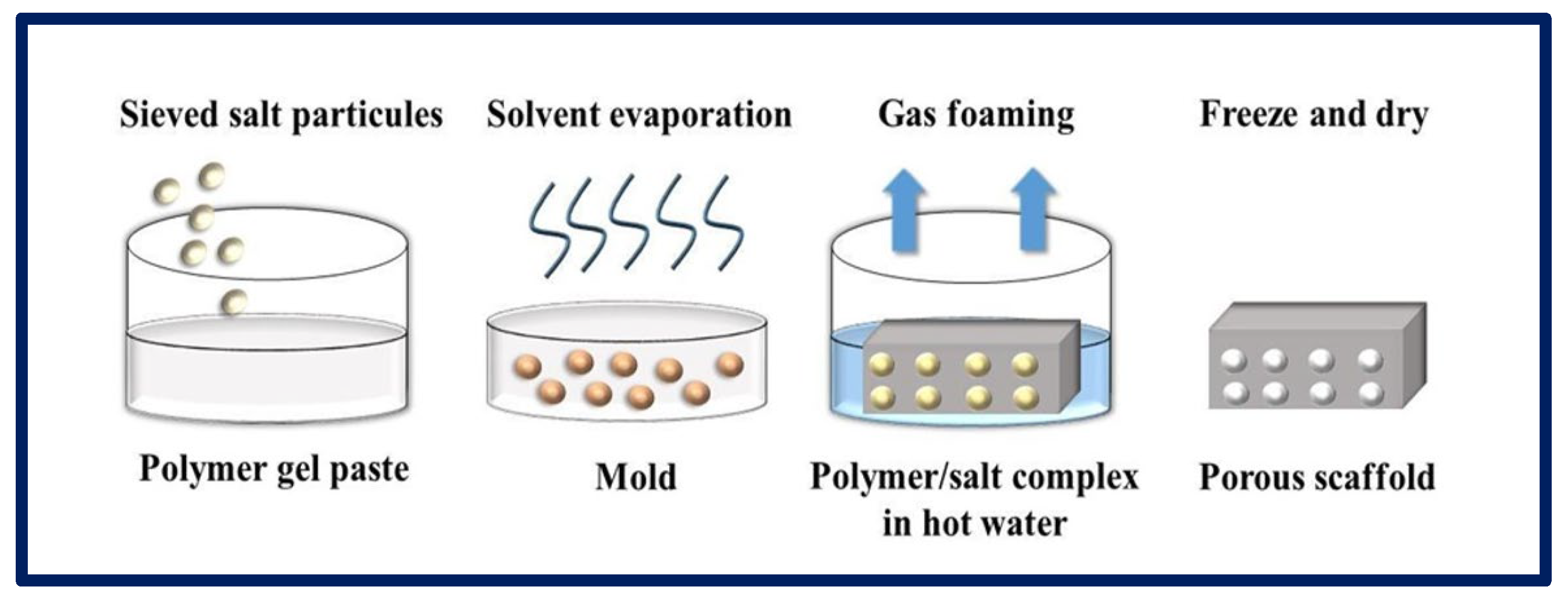
In this process, gases or blowing agents are mixed with polymeric solutions to manufacture porous matrices. Gas foaming can be carried out using chemical or physical methods. In the first case, the gases are formed from a chemical reaction or the decomposition (Figure 11) of a polymer salt complex in hot water, while, in the physical method, gases inert to the polymer are directly injected into the solution. The physical blowing agents are often volatile liquids that evaporate and cause the foam to expand, or pressurized gases or air injected directly into the foaming medium [99]. The polymers go through a pressurization process with gases such as N2 or CO2 over an extended period, until the full saturation level is reached. During the dissolution process, a monophasic homogeneous solution is formed. In the second stage, the nucleation process occurs due to thermodynamic instability, achieved by changes in temperature or pressure, leading to a decrease in the solubility of the gas, which escapes and forms bubbles within the polymeric matrix. Finally, the solution is stabilized by cooling [95,100]. Thus, it is possible to obtain pores that vary from 100 to 50 µm. When the gas formation is induced by a chemical reaction during the mixing process (e.g., N2), foam formation is rapidly induced, leading to the formation of a highly porous network. Although it is a relatively easy technique that does not require the use of solvents, most scaffolds made by this process have poorly connected pores and a non-porous outer surface, making them less suitable for TE applications [81].
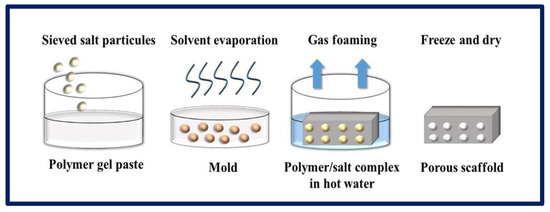
Figure 11.
Gas formation process by chemical method for the production of porous scaffolds. Bibliography consulted [88].
Tang and collaborators proposed a new simple and cost-effective gas-foam method for fabricating hydrogels with a macroporous structure for application in bone defects in which they mixed a cell-laden hydrogel with Mg particles. The production of H2 gas occurred in situ during their degradation, generating a hydrogel with an optimized porosity and greater bioactivity [101].
4.6. Rapid Prototyping
Rapid prototyping (RP) is a group of dynamic and evolutionary technologies that create complex three-dimensional (3D) objects additively, layer by layer, from a predefined design file. The development of RP is closely related to the development of the computer and software industry. In particular, the development of computer-aided design (CAD) plays a critical role in the emergence of almost all RP systems today [32,102]. RP technologies play an increasingly important role in biomedical applications for TE, and they have also been applied in surgical planning, drug delivery devices, implants, and custom prosthetics. These systems are broadly classified into three categories based on the initial state of the material used, liquid, solid, and powder [103], and apply to polymers, ceramics, and metals [104].
Printing techniques for polymeric materials mainly include the Stereolithography Apparatus (SLA) [105], Fused Deposition Modeling (FDM) [106], 3D Printing [107], and Selective Laser Synthesis (SLS) [108]. The general scheme of RP is composed of (a) a computer with software to carry out the design, (b) machinery to carry out the additive process (or printers), and (c) suitable materials [109].
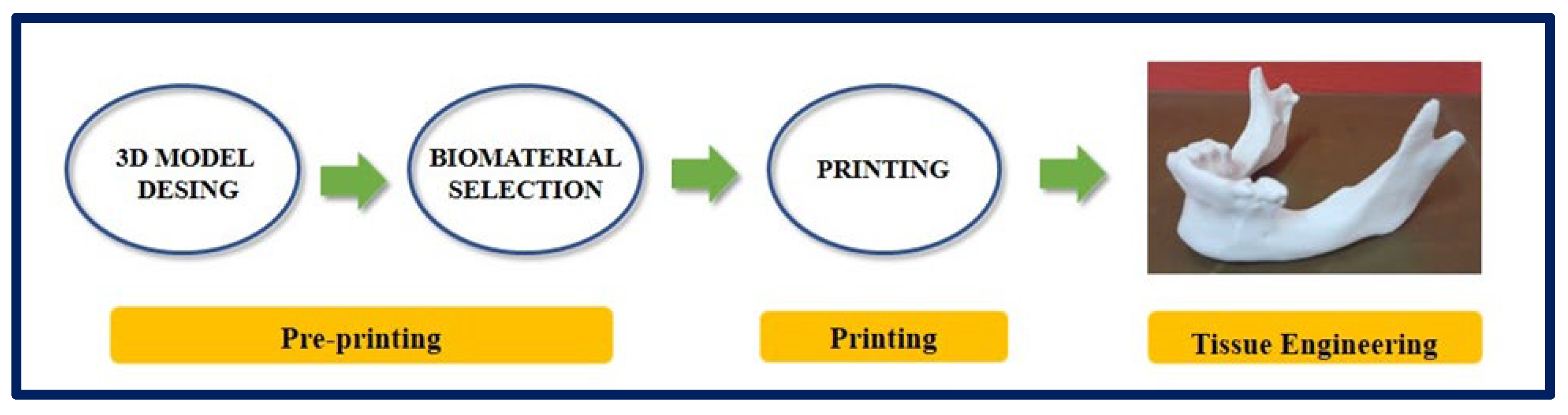
There is a variant within 3D printing, and it is an emerging technology with a great impact in the area of TE and regenerative medicine: 3D Bioprinting [110,111]. In conventional 3D printing for the fabrication of non-cellular scaffolds, strategies such as microextrusion, inkjet, and laser-assisted printing are used [112]. However, when we refer to bioprinting, biomaterials and cells are deposited simultaneously in an additive manner and can be considered as “bioinks” [113]. For a correct maturation of the tissue, the deposition of cells and biomaterials must be precise to facilitate cell-cell and cell-biomaterial interactions [114]. Due to advances in 3D printing technology and the increasing availability of user-friendly software, almost any shape can be designed, and this is highly attractive for the design and fabrication of custom surgical implants [115] (Figure 12 and Figure 13).
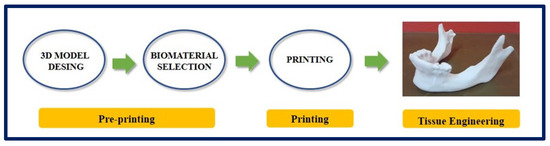
Figure 12.
Process of design and manufacture of a jaw by means of 3D printing. Bibliography consulted [116].
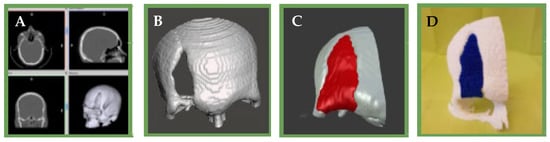
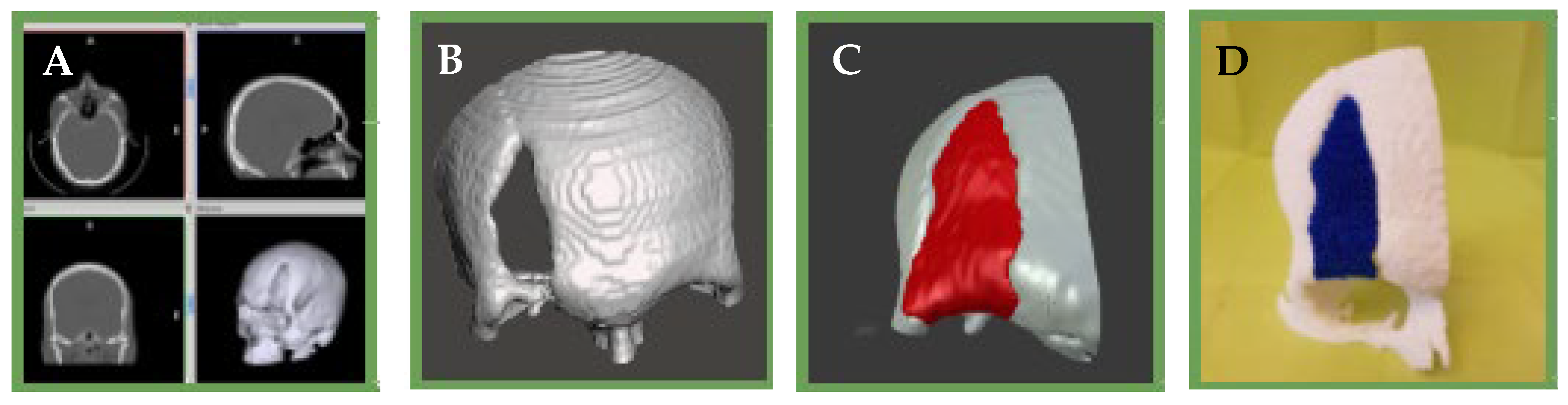
Figure 13.
(A): Tomographic image of the patient’s skull (B): 3D reconstruction of the skull and the bone defect and surrounding tissue (C): 3D computational model of the bone defect (D): 3D impression of the scaffold and surrounding bone tissue.
4.7. Decellularization
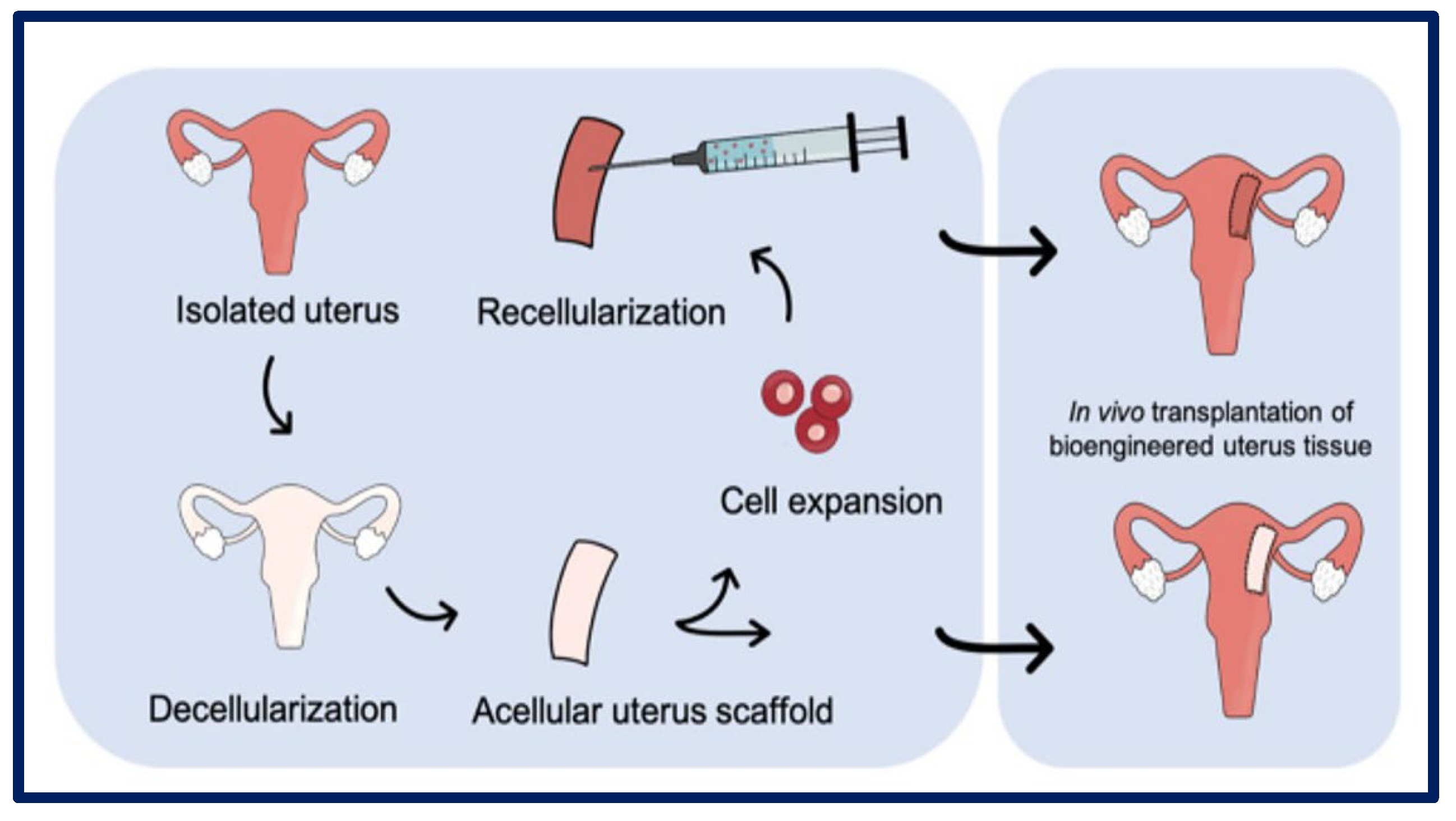
Decellularization is a technique that has been intensively studied in the last decade, and its objective is to produce artificial organs and tissues from the elimination of all their cells, while fully preserving the ultrastructure and composition of the native ECM [23] (Figure 14). With this aim, scaffolds of decellularized extracellular matrix (dECM) are achieved that serve as three-dimensional biological supports with vascular conduits that facilitate future grafting through vascular anastomosis. To produce these dECMs, cells can be removed from donor tissue using physical, chemical, enzymatic, or combined methods, resulting in the removal of the immunoreactive intracellular components [117,118,119]. These are obtained from various allogeneic or xenogeneic tissues, including the bladder matrix, dermis, heart valves, small intestinal submucosa, urinary bladder mucosa, skeletal muscle, mesothelium, or pericardium of different species using different methods [120].
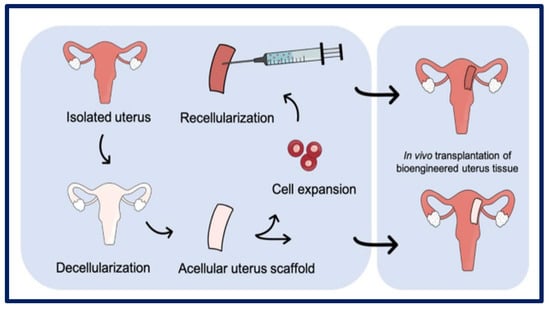
Figure 14.
Uterus decellularization process and subsequent cellularization. Bibliography consulted [118] is licensed under CC BY 2.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ (accessed on 15 December 2022).
Currently, numerous clinical products are composed of ECM and are used as surgical materials. These are obtained from various allogeneic or xenogeneic tissues, including the bladder matrix, dermis, heart valves, small intestine, mesothelium, or pericardium of different species [121]. As can be seen, the dECM is completely integrated with natural components, which gives it great advantages such as appropriate mechanical properties and biocompatibility [23]. In general, the host recognizes xenogeneic and allogeneic cellular antigens, and they are capable of inducing an inflammatory response, or the host’s immune system may reject the tissue. However, ECM components are generally conserved between species, so implantation can be tolerated [122]. The bioscaffolds (e.g., scaffold of biological origin) can be recellularized to create potentially functional constructs as a therapeutic strategy in regenerative medicine [120]. Although successful decellularization has been achieved for many organs, scientists and engineers are still making great efforts to design functional arrays and establish standardized decellularization protocols for clinical applications. To achieve those standardized protocols, it is essential to have solid knowledge of topics such as biodegradation, cytocompatibility, pathogenicity, and immunogenicity [123].
Interestingly, Aulino et al. mention a new concept, the multipotency of scaffolds, because the skeletal muscle acellular scaffolds offer a multipotent environment. These scaffolds were implanted at the interface of the tibialis anterior/tibial bone and the masseter muscle/mandible bone in a murine model and promoted muscle, bone, and cartilage formation at the interface with long and flat bone [124].
4.8. Cell Sheets
The effectiveness of tissue regeneration is based on a good integration of the scaffold with functional cells. However, despite extensive research efforts, some conventional applications of TE have not produced the desired results due to certain limitations such as a low cell survival rate, the inability of the injected cells to adhere to the injured site, the difficulty of vascularization, and an immunological rejection against the scaffold that may occur. To address this problem, several research groups have developed new TE approaches based on cell layer technology (CST). The CST is a novel technique that allows cells to be preserved in a sheet format without the need to use proteolytic enzymes or other destructive methods, enabling the preservation of ECM components [125]. These methods are promising for use in regenerative medicine because they preserve cell–ECM interactions, cell–cell contacts, and the composition of key proteins such as fibronectin, leukemia inhibitory factor receptor (LIFR), integrin-5, stromal cell-derived factor 1 (SDF-1), myosin heavy chain (MHC), endothelial growth factor (VEGF), and β-actin from cell membranes [126].
Sekine and Okano describe a method for creating tubular cardiac tissue for in vitro implanting and culturing cardiac cell sheets on the inner wall of a perfused segment of the small intestine [127], and Nishida et al. performed ocular surface reconstruction in four patients using autologous oral mucosal epithelial cells. They used a carrier-free cell sheet by exploiting the temperature-responsive culture surfaces by lowering the temperature; they thus managed to obtain an intact cell sheet to be transplanted without the need for a support [128].
With the CST system, numerous films of cells can be applied directly to a defective area as a coating creating a three-dimensional structure, or even shrunk into a cell pellet, which can be applied as a graft to the area of interest. [125]. Despite the great advantages offered by the CST technique, it is still difficult to obtain intact cell layers due to the low stability and low strength of the ECM. On the other hand, multilayer stratification can limit cells’ growth and nutrient supply, which reduces cells’ viability and differentiation potential. Therefore, new strategies combining CST systems with porous membrane supports are proposed [129]. In conclusion, CTS is a promising approach that should prove useful as a fundamental and widely used technique in next-generation ET and regenerative medicine [130].
5. Smart Materials
Materials that are described as “smart” or “functional” are usually part of a “smart system” that can sense and respond to its environment. They are designed to have one or more properties that can be significantly changed in a controlled fashion by external stimuli. The stimuli can be thermal, electrical, magnetic, mechanical, chemical, etc. Smart materials are the basis of many applications, such as textiles [131], food and beverage packaging [132], and biomaterials for tissue engineering [133], among others. In the present article, the focus is the use smart polymers to develop porous structures for tissue engineering. Thus, Mountaki et al. reported the synthesis of “smart”, pH-responsive, biodegradable polyesters bearing alkene/carboxylic acid side groups. The carboxylic acid side groups provide pH-responsive properties to the polymers and render them water soluble, whereas the alkene groups can be utilized for the formation of stable hydrogels and cross-linked polymer films [133].
One of the conventional methods for obtaining CST is using temperature-sensitive culture plates with poly (N-isopropylacrylamide) (PIPAAm). Smart interfaces have been developed to control the attachment of cells by manipulating the temperature. This method allows cells to be detached from the culture surface without the use of proteolytic enzymes, while maintaining the integrity of adhesion proteins such as E-cadherin and laminin and preserving the ECM components secreted by the cells [125]. Other excellent example are the temperature-responsive polymer brush coatings (TRPBCs). There is a commercially available product—Nunc™ Multidishes with UpCell™ Surface—that allows the adhesion of the cells of the culture solution at high temperatures and the detachment of the entire cell sheet at a lower temperature [134].
6. Functionalization Strategies to Promote Bioactivity in Polymeric Supports
Most polymers are not very reactive, and surface modifications that generate different chemical groups of interest are needed, or bioactive compounds (proteins, peptides, growth factors, hormones, enzymes, or other regulators of cell behavior) are added. The methodology used can be physical adsorption, chemical immobilization, or encapsulation. The surface properties of an implantable device are of critical importance, since the first contact with the organism is mediated by the cell–biomaterial interface. For this reason, many research groups are studying the optimal chemical and physical configurations of the surfaces of new biomaterials to promote cell interaction and to produce efficient tissue engineering constructs. Instructional biointerfaces could determine the type of cell to attach to the polymer and direct their behavior through the scaffold. The common purpose of surface treatment is to modify the outermost layer of a polymer to improve properties such as its wettability, sealability, adhesion to other materials, and interaction with a biological environment while keeping the mechanical properties of the polymer intact. When a scaffold is modified, the surface, chemical, and topological interactions that usually occur drive subsequent cellular and tissue events such as cell adhesion, cell orientation, cell motility, surface antigen display, cytoskeleton condensation, tyrosine kinase activation, cell modulation, and modification of intracellular signaling pathways that regulate transcriptional activity, gene expression, and inflammatory responses, etc. [34,40,135,136,137].
On the other hand, scaffolds can encapsulate and act as reservoirs for soluble bioactive molecules such as cytokines, growth factors, chemokines, or hormones that are subsequently released and act in a paracrine manner. In addition, the development of intelligent biomaterials that are capable of responding to specific stimuli such as pH, electrical signals, light, temperature, and metabolites e.g., adenosine triphosphate (ATP), and glucose is being explored. Such properties can be used to control cell adhesion, drug release, phase behavior, and mechanical parameters such as electrical conductivity, permeability, and tissue volume [138].
In conclusion, determining the design characteristics of a scaffold poses a great challenge, and it must be considered at the chemical-molecular level, taking into account the planned specific replacement therapy. Currently, the design of biomaterials can also be achieved by combining computer-aided design and nano-fabrication techniques for extreme precision design [8].
In Table 2, we tabulated examples of the polymers that have been subjected to both physical and chemical surface treatments and those in which biomolecules have been encapsulated for controlled release and that have thus achieved a more efficient scaffold.

Table 2.
Different strategies for modification of scaffolds to promote their bioactivity.
7. Cells for Tissue Engineering: Stem Cells
Stem cells have the ability to self-renew and resume their undifferentiated state and in turn produce specialized progeny under the appropriate physiological conditions. These properties of stem cells present them as a crucial strategy for tissue regeneration [154,155]. Self-renewal is a characteristic fundamental to the survival of stem cells. Stem cells can vary their differentiation potential depending on the organ where they are found. In any case, there must be a regulated balance between cell self-renewal and differentiation [156]. An important concept to keep in mind is the cell niche. The cellular niche is made up of signaling molecules and other cells that perform nutritional functions that are responsible for maintaining tissue homeostasis [157]. Stem cells are extremely sensitive to the physical nature of their environments, and the cell niche is extremely important not only in directing the development and behavior of stem cells, but also in maintaining their state of differentiation [158]. Knowledge and understanding of the environment of stem cells is essential if one wants to recreate biomimetic structures seeded with stem cells in TE.
8. Stem Cell Classification
Table 3 shows the different types of stem cells classified according to their differentiation potential.

Table 3.
Classification of stem cells according to their plasticity.
9. Cell Replacement Therapies Combined with Tissue Engineering Strategies: More Complex Than Is Believed
Cell therapy encompasses a broad clinical spectrum in which transplanted cells are applied in disease processes, such as cardiovascular disease, neurodegenerative disease, diabetes, autoimmune disorders, and cancer. In the last decade, there has been an exponential advance in the treatment of hematological malignancies, with promising preclinical results that produce a substantial clinical benefit. Despite this, other cell therapy approaches have not been successful, and this can be attributed to poor cell adhesion and survival at the site of injury [167]. It is here that TE plays a fundamental role, being able to promote cell regeneration using materials that serve as templates for cell and tissue growth and other approaches that rely on exogenous cells that have been implanted to become part of a pre-designed device [168].
As seen above, the mechanical properties of a scaffold can exert a significant influence on seeded stem cell differentiation, and also on the behavior of many mature cell types such as epithelial cells, fibroblasts, muscle, and neuronal cells. These are capable of preserving the rigidity of the substrate and displaying different morphological and adhesive characteristics against it [138]. The cells are capable of invading the matrix and organizing themselves in the form of function tissue by proliferating and migrating through the matrix architecture. This reorganization is followed by ECM synthesis to regenerate damaged tissue [168].
In the last decade stem, a cell science revolution emerged with the advent of human pluripotent stem cell (hiPSC) technologies and, more recently, the CRISPR/Cas9 genetic engineering technique and organoid technologies. The scope of this field is intended to encompass biomimetic engineering, to provide and design cells, tissues, and organs for the precise study of numerous diseases in the near future [169]. Recently, genetically modified stem and vascular cells were generated by improving their efficacy and safety from editing longevity genes and tumor suppressors. This same approach could be used to activate tissue regeneration by optimizing the study of cellular senescence and regeneration pathways [170]. An example of this is the use TE vascular grafts as an attractive alternative for creating blood vessels in vitro that maintain their function after the implantation. Generally, grafts have been manufactured using vascular smooth muscle cells (VSMC), but these cells are limited in quantity and proliferation potential. In recent years, numerous researchers have optimized the method of obtaining VSMC from the genetic engineering of IPSc [171].
In cell therapies, cells are isolated from a donor and transplanted into a recipient (Figure 15). When the donor and the recipient are the same, the procedure is referred to as an autologous transplant, and, when they are different individuals, the transplant is allogeneic. However, in vitro findings have not always been replicated in humans. Several reports note that many therapies appeal to potentially vulnerable individuals, raising ethical and safety concerns about using cells in an unregulated manner [172]. In the case of iPSCs generated from human cells, there are major hurdles preventing the use of these cells in the development of safe and efficient therapies, since there is a risk of tumor formation [161]. Therefore, a critical point is to guarantee a complete and irreversible differentiation of the stem cells to the desired progenitor cells or terminal target cell type. This could be possible if the appropriate trophic factors are added to the culture medium or incorporated as reservoirs in the scaffolds so that they are released in a controlled manner [18].
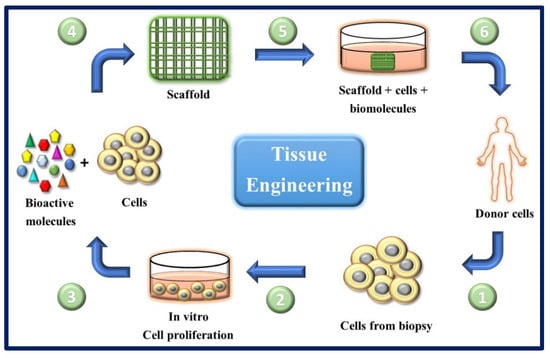
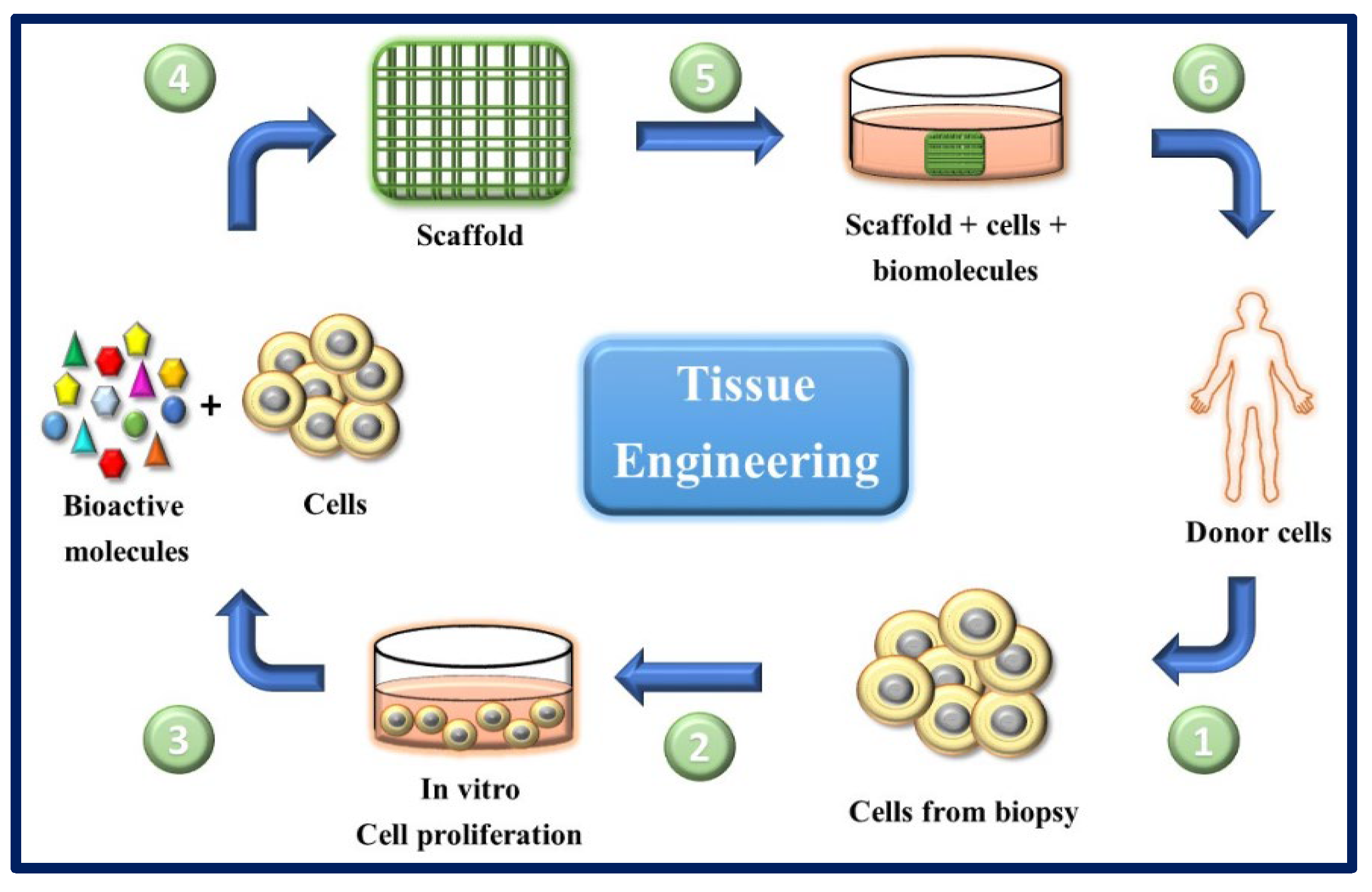
Figure 15.
General scheme of the ideal circuit in regenerative therapies combining cells with appropriate molecules and biomaterials. (1) Obtaining cells from the patient (for example, from a skin biopsy). (2) In vitro culture and expansion of autologous cells.
(3) and (4) cells are seeded on scaffolds along with biomolecules of interest. (5) The scaffold-molecules-cells system is pre-incubated under optimal conditions. (6) The scaffold is implanted in the patient.
Next-generation stem cell therapies also face manufacturing and regulatory hurdles, as the cells are exposed to complex and sophisticated genetic engineering modifications, which are costly and require skilled labor. This is because the product must be customized and designed for each individual patient in appropriate facilities to allow viral transduction or gene editing. For large-scale commercial therapies, manufacturing systems must be optimized to generate commercial-scale quantities of cells without altering their therapeutic properties, or to generate engineering components for product(s) that are stable over time [164].
Cellular behaviors are so complex that it is necessary to have a thorough understanding of the mechanisms of morphogenesis and normal wound healing before designing a scaffold that will be combined with cell therapy, since cells use preprogrammed information and signaling to create or recreate functional structures [8]. For this, a critical point is to know what characteristics of biomaterials can affect the components of the cellular microenvironment to allow the design of scaffolds that act as extrinsic regulators of stem cells’ fates and regulate their differentiation towards the desired tissue [154].
Overall, it can be seen that there are still many limitations when choosing a suitable cell therapy for tissue regeneration. On the other hand, it is necessary to combine these therapies with TE strategies, where the immobilization of endogenous cells from a patient must be carried out in porous matrices after accounting for exhaustive selection criteria, and, finally, the device must be directed to where it can exert a beneficial effect for a given pathology [173].
10. Applications of Polymers for Tissue Engineering in Experimental Models
As described above, three basic elements are required in TE: bioactive scaffolds, cells with a compromised differentiation potential, and adequate biochemical signals that promote cell differentiation and angiogenesis. Furthermore, for the successful design of the scaffold, different techniques can be evaluated for use in its manufacturing. Next, reviews of several interesting strategies in eight main TE application areas, epithelial, bone, uterine, vascular, nerve, cartilaginous, cardiac, and urinary tissue, will be made with the aim of learning more about current approaches in TE.
10.1. Epithelial Tissue Engineering
The skin is the largest and fastest growing organ in the body. It plays a vital role in protecting the body against external damages from common skin injuries such as abrasions, burns, or lacerations. Traumatic wounds such as third degree burns and defects in the dermis layer are more difficult to treat due to limited self-healing capacity. Therefore, bioengineered human skin donors or constructs are required to support the regeneration process. However, skin regeneration remains a major challenge due to the scarcity of donor skin and poor long-term viability [174]. For skin regeneration, TE has used techniques ranging from simple autologous epidermal sheets to more complex double-layer skin substitutes such as grafts to achieve rapid wound closure and an aesthetic and functional scar. It must be porous to allow the diffusion of water, nutrients, and waste, and to promote cell migration, and have mechanical properties similar to those of the native tissue, with an adequate rate of degradation that matches the rate of regeneration. Finally, the scaffold must prevent bacterial infection. To lead to the regeneration of the dermis and epidermis, polymers with biomimetic properties are required, containing chemical signals, soluble growth factors, and physical and mechanical properties similar to the ECM of the native tissue [175].
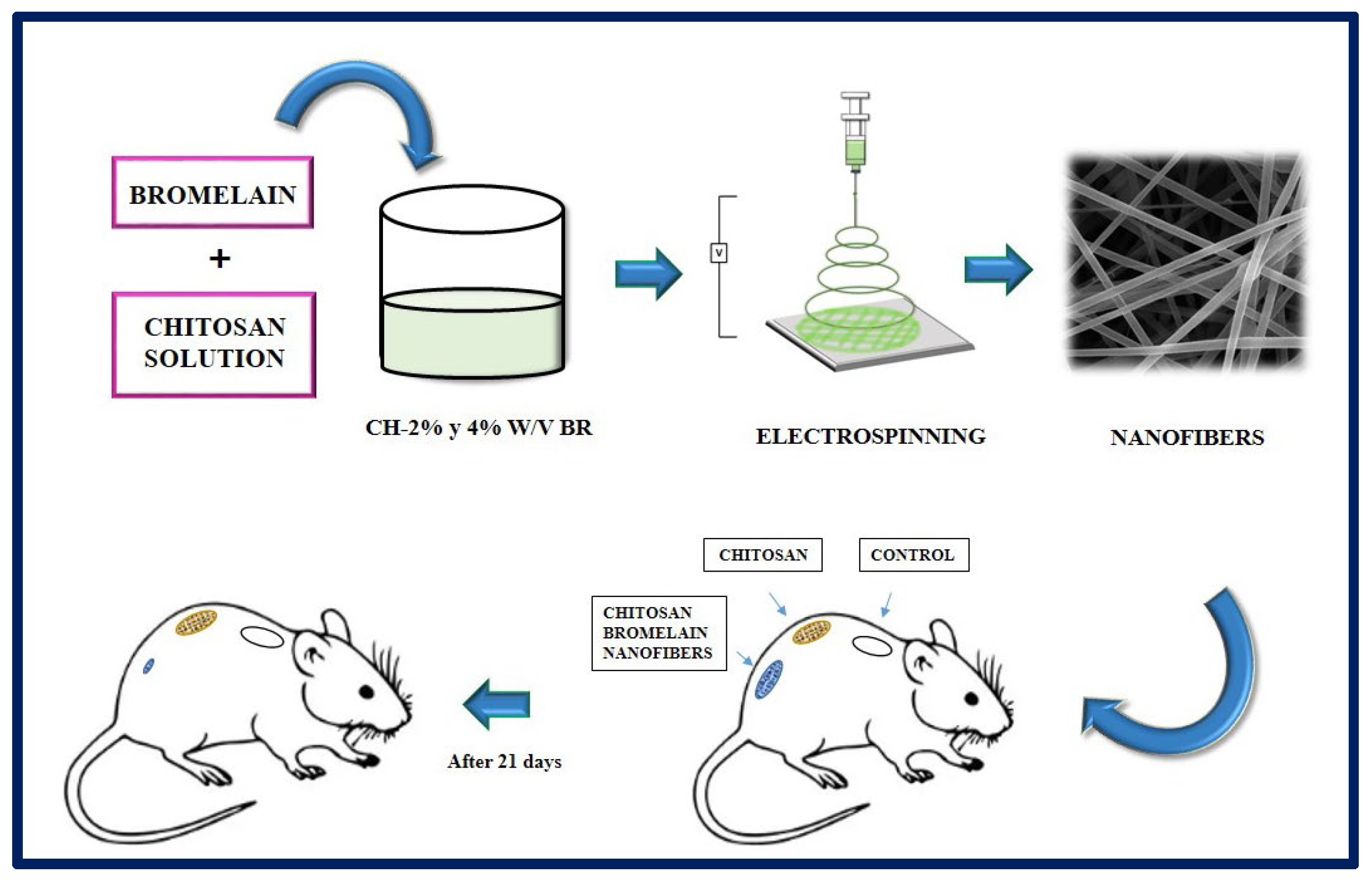
Bayat et al. manufactured chitosan nanofibers containing bromelain at 2 and 4% w/v by the electrospinning method. Bromelain, a mixture of proteolytic enzymes present in all the tissues of the pineapple (Ananas comosus), has application in the treatment of wounds and inflammations, and it is used as a debriding agent in burns. In this study, the physicochemical properties of the nanofibers (viscosity, electrical conductivity, tension, swelling test), the drug release profile, enzymatic activity, cytotoxicity, and the potential of the synthesized fibers were evaluated. According to the results obtained, the chitosan-bromelain (Ch-Br) nanofibers at 2% w/v presented better physicochemical properties, a more accelerated release profile, and lower cytotoxicity than the 4% w/v counterpart, for which they were selected for in vivo studies. In conclusion, it was observed that Ch-Br nanofibers accelerate the recovery process in burn injuries in animal models (Figure 16). Therefore, this system has the potential to be proposed as a therapy to heal burns in humans [176].
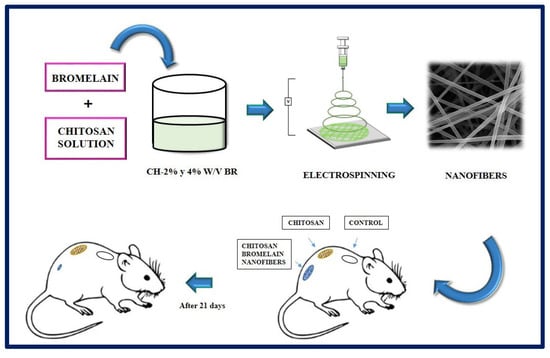
Figure 16.
Chitosan nanofibers containing bromelain (2% and 4% w/v) were prepared by electrospinning method. The burn wounds of the rats were treated with chitosan nanofibers, and chitosan-Bromelalin nanofibers. The results indicated that chitosan-2% w/v bromelain nanofiber was more efficient to heal burn skin compared to chitosan nanofiber alone in the animal model tested. Bibliography consulted [176].
10.2. Bone Tissue Engineering
Bone is a complex and dynamically active tissue in the body. It has the natural ability to regenerate when minor damage or injury occurs. When fractures and injuries do occur, long periods of time are usually required for recovery. Traumatic bone defects are considered a significant socioeconomic burden, primarily because these severe bone injuries lack the ability to fully reconstitute collapsed bone. For example, in the area of periodontics and maxillofacial surgery, there is a growing need to regenerate alveolar bone that has been lost due to chronic diseases, malignant neoplasms, or trauma. The demand occurs from patients seeking to prolong the longevity of their natural teeth or implant assisted prosthetics. Importantly, the regenerative capacity of injured bone is diminished due to the absence of a template for the regeneration of new tissue. To overcome this problem, the field of bone TE has advanced in the design of efficient therapeutic mechanisms that include the incorporation of cells, biomaterials, and the corresponding growth factors to promote osteogenesis, osteoinduction, and osteoconduction, as well as bone mineralization [177,178].
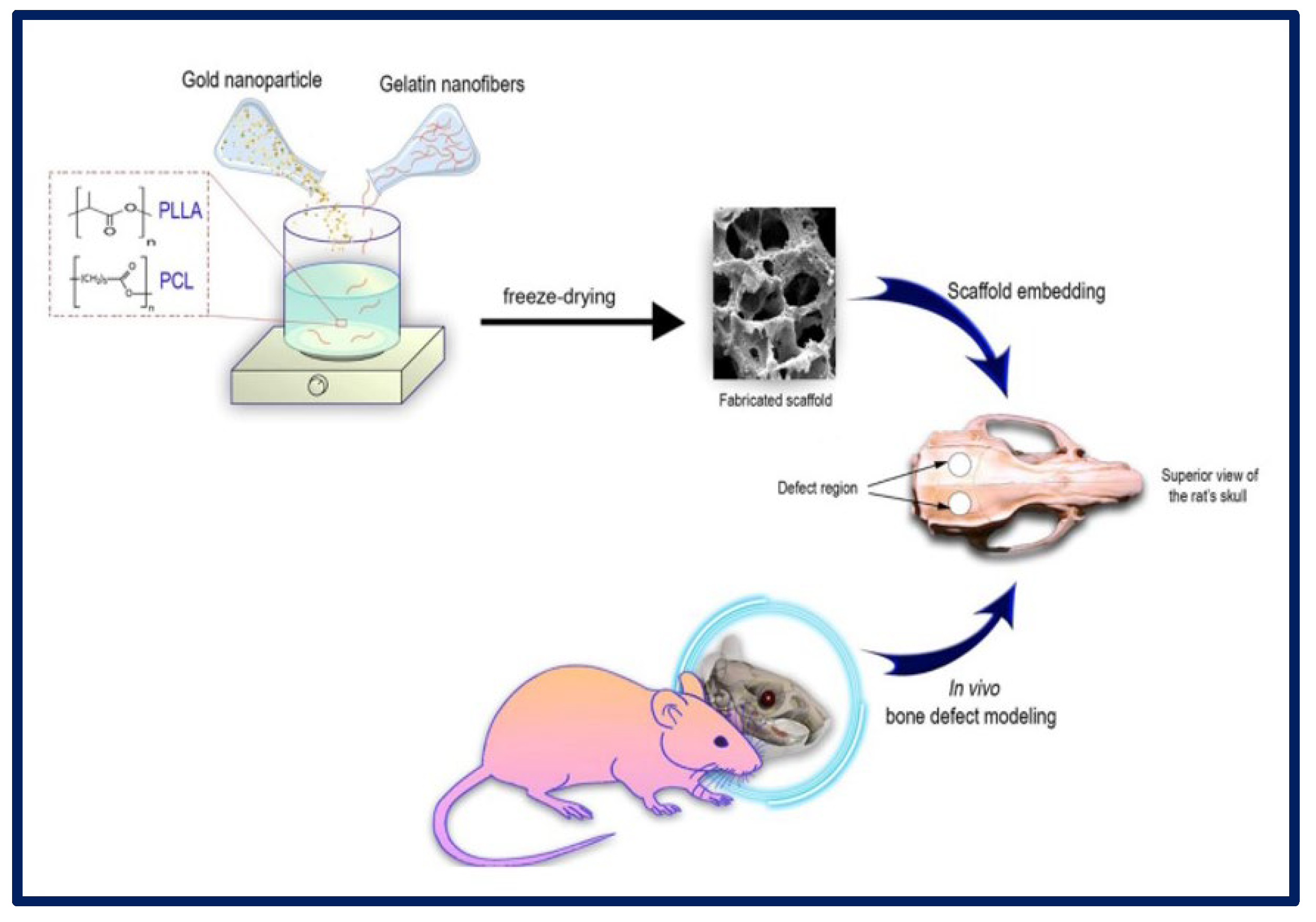
H. Samadian et al. manufactured a 3D scaffold based on the following components: poly (l-lactic acid) (PLLA)/Polycaprolactone (PCL) that act as a polymeric matrix, gelatin nanofibers (GNFs) that mimic the collagen fibers of the bone ECM, and gold nanoparticles (AuNPs) to mimic hydroxyapatite crystals and act as a healing agent for application in bone TE. To carry out cytotoxicity studies, MG-63 cells were cultured on the scaffolds, and it was observed that the greatest proliferation occurred in those membranes that contained PCL/PLLA/GNF/AuNPs (80 ppm) at 72 h. AuNPs are considered non-toxic and biocompatible structures at optimal concentrations. Regarding the in vivo tests, the samples with PLLA/PCL/GNF/AuNPs (80 ppm) induced the highest bone regeneration (Figure 17). Therefore, it was concluded that the combination of 3D scaffolds containing GNFs and AuNPs could mimic the native structure of the tissue and promote the healing process of the bone [179].
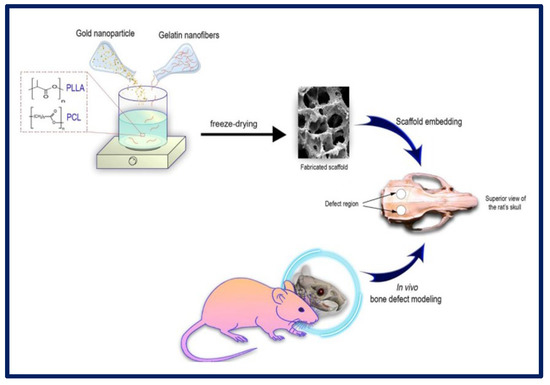
Figure 17.
Schematic representation of the in vitro and in vivo experiments using PLLA-PCL-GELATIN scaffolds with gold nanoparticles for bone tissue regeneration. Bibliography consulted [179] is licensed under CC BY 2.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ (accessed on 15 December 2022).
10.3. Urinary Tissue Engineering
Various reconstructive approaches have been used to treat and restore function in congenital and acquired pathologies that can affect the human urinary tract, such as hypospadias, strictures, fistulas, trauma, and cancer. However, there are certain factors that complicate the repair. Due to the poor quality of local tissues, additional tissues must be used for replacement, including genital skin and buccal and lingual mucosa. As these are available in limited quantities, their use is restricted and alternative approaches need to be sought [180]. Furthermore, mimicking urinary tissue is not easy due to the complexities of the native system. The field of urology has recently turned to TE and regenerative technology, and, although it is still in its infancy in clinical applications, a great deal of research is under way in search of solutions for urinary reconstruction [181].
Adamowicz et al. proposed a new composite biomaterial derived from the amniotic membrane (Am) covered with a layer of graphene. Graphene creates an interface between cells and external stimuli that replaces the neural network in the bladder. The main objective is to obtain a biomaterial with response to electrical stimuli for the application of urological TE in neurogenic bladder. In this case, porcine-derived smooth muscle cells (SMC) and urothelial cells (UC) were used to evaluate the properties of the developed biomaterial (Figure 18). The presence of the graphene layer significantly increased the electrical conductivity of the biocomposite. UC and SMC showed an organized growth pattern on graphene-coated surfaces and contractile response of SMC was observed when electrically stimulated [182].
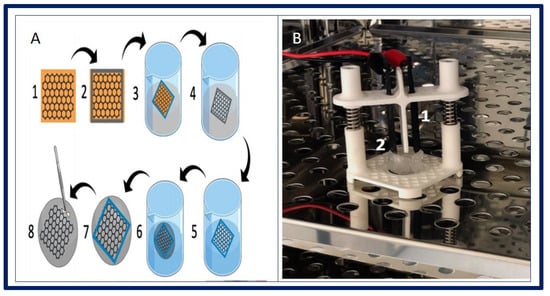
Figure 18.
(A): fabrication of biocomposite. (1) Graphene layer on copper foil. (2) PDMS frame adjusted to desired biomaterial shape. (3) Etching Cu foil by ammonium persulfate solution. (4) Floating graphene layer in PDMS frame. (5) Washing out ammonium persulfate. (6) Fishing graphene layer posited in PDMS frame with Am. (7) Graphene placed on Am surface. (8) Carful mechanical removing of PDMS frame. (B): 3D-printed stimulator in CO2 incubator. (1) Graphene based electrodes (2) Biocomposite fixed in cell crown. Bibliography consulted [182] is licensed under CC BY 2.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ (accessed on 15 December 2022).
10.4. Uterus Tissue Engineering
In mammalian reproduction, the uterus fulfills essential complex biological functions. These functions can be affected by congenital anomalies or acquired diseases that affect the integrity of the uterus. This can result in the woman’s inability to conceive or carry a viable fetus to term. Allogeneic uterine transplantation is a possible treatment strategy, but it requires donors and the use of anti-rejection therapies. Regenerative medicine and TE technologies become attractive options to overcome these limitations [183].
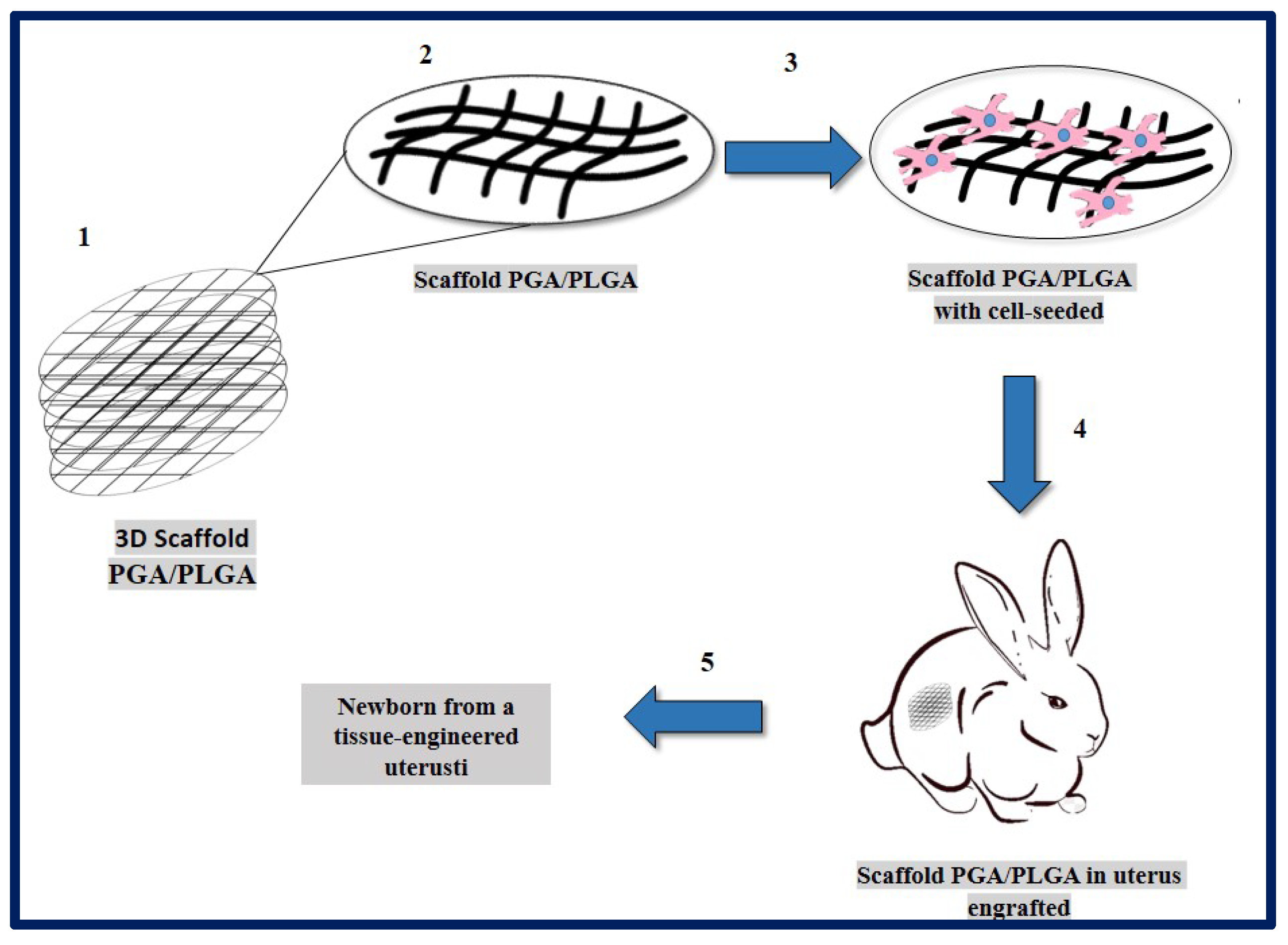
Magalhaes and collaborators described the design and fabrication of uterine tissue in vitro and its subsequent application in a rabbit model, performing a subtotal uterine reconstruction. They fabricated PGA/PLGA scaffolds in semicircular shapes and successfully seeded autologous myometrial-derived cells on the outer layer of the scaffold, and endometrial-derived cells on the interior of the scaffold using a sequential seeding method. To carry out the implantation, three experimental groups were taken into account: 1—rabbits with a subtotal semicircular excision in their remaining uterine horn that received the scaffold with cells, 2—those that received an acellular scaffold, and 3—those in which they were sutured the remaining uterine margins.
To investigate the in vivo functionality of the engineered uterine tissue, reproductive studies were carried out using fertile males for natural mating six months after graft implantation. Only the group that received the scaffold with cells, developed structures similar to native tissues (organized luminal/glandular epithelium, stroma, vascularized mucosa, and two-layer myometrium). Furthermore, it was compatible with pregnancy and the fetus had a normal development within the length of the segment where the reconstruction was carried out, which resulted in well-formed offspring with body weights similar to normal rabbits (Figure 19) [183].
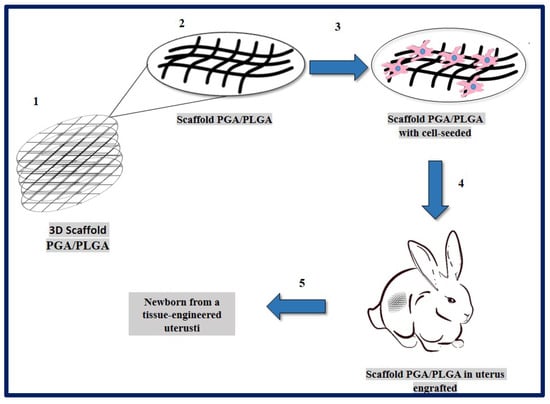
Figure 19.
PGA/PLGA scaffold fabrication and in vivo implantation to tissue-engineered uterus. (1) PGA/PLGA scaffold macroscopic image. (2) PGA/PLGA scaffold details that shows its microstructure. (3) Scaffold SEM image with cell-seeded before implantation. (4) Scaffold engrafted to uterus tissue in a rabbit model. (5) Fetus was obtained then tissue-engineered uterus. Adapted from bibliography [183].
10.5. Vascular Tissue Engineering
The functions of the organs are maintained by the vascular systems, which are responsible for the exchange of substances (nutrients, oxygen, and transport of waste products). The construction of channels and capillaries similar to the real ones is a great challenge, since they must promote the exchange of substances in vitro and, at the same time, preserve their structure and function [184]. In addition, the cells of the blood vessels have a complex structure and are functionally dynamic, but with minimal regeneration potential. The various functions they perform are due to the presence of a complex extracellular matrix that varies in composition, thickness, and general architecture, and the function differs between arteries, capillaries, and veins. For all these reasons, it is ideal to find a consistent approach in choosing appropriate materials for tissue-engineered vascular grafts [153].
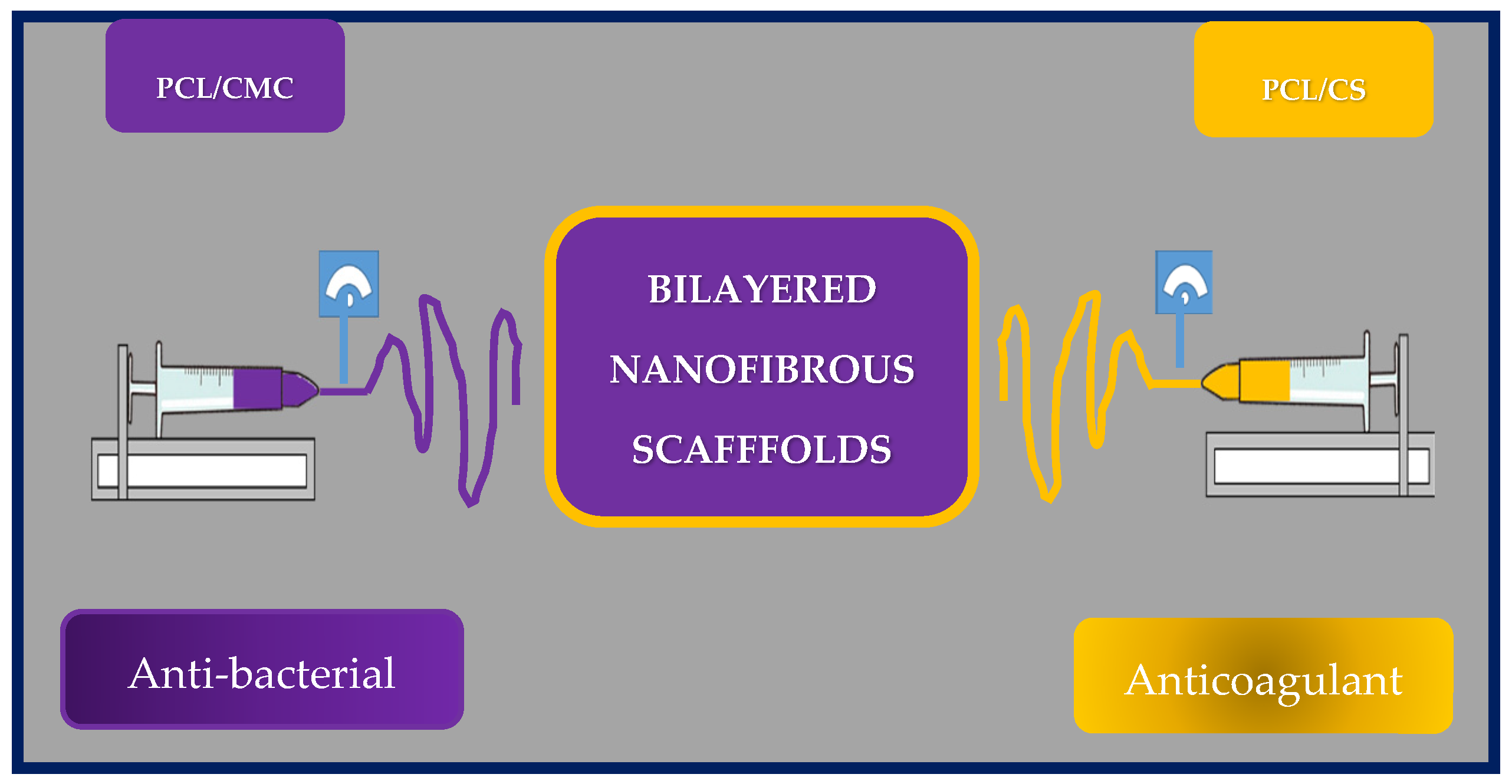
Yilin Wang et al. fabricated an asymmetric two-layer scaffold by co-electrospinning poly(e-caprolactone) and chitosan (PCL/CS) and poly(e-caprolactone) with carboxymethylchitosan (PCL/CMC). Chitosan and carboxymethylchitosan were selected as the antibacterial and antithrombotic components, respectively. The inner layer corresponds to PCL/CMC, while the outer layer to PCL/CS (Figure 20).
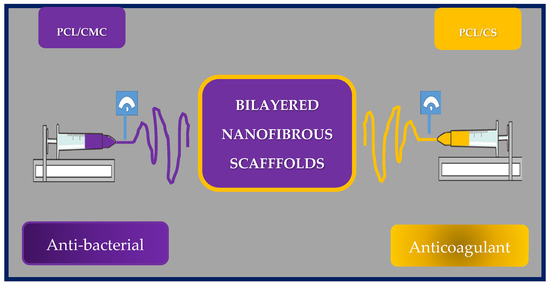
Figure 20.
Schematic representation of the manufacture of asymmetric two-layer scaffold by co-electrospinning of poly(e-caprolactone) and chitosan (PCL/CS) and poly(e-caprolactone) with carboxymethylchitosan (PCL/CMC) scaffolds. Bibliography consulted [185].
To assess blood compatibility, the hemolysis ratio was investigated using red blood cells on the PCL nanofibrous scaffolds. All hemolysis rates were less than 2%, indicating that the scaffold could be safe for clinical use. Furthermore, the nanofibers showed good compatibility with red blood cells, since the morphology of those cells does not change, apparently. The anticoagulant activity of the grafts was also evaluated as a function of coagulation times. The inner layer exhibited thrombosis inhibitory activity, because the structure of CMC is similar to that of heparin, and the presence of carboxyl groups seems to prolong coagulation times and could effectively prevent restenosis. In addition, the whole blood circulation was evaluated, and the results showed that the asymmetric nanofibrous scaffold has good blood compatibility and can inhibit thrombus formation. On the other hand, only the PCL/CS outer membrane exhibited good bactericidal properties against S. aureus, and E. coli, indicating that this vascular graft could reduce the risk of postoperative infection.
To assess cell viability and adhesion, they seeded HUVEC on the nanofibrous membranes, and the results showed that the cells have good affinity for the scaffold. Furthermore, PCL/CMC nanofibrous membranes could enhance rapid endothelialization after implantation.
In conclusion, the researchers designed and fabricated an asymmetric bilayer scaffold with biodegradable properties, excellent mechanical properties, and good blood compatibility. Therefore, these artificial grafts show promise as substitutes for native blood vessels and could reduce the rate of restenosis and the risk of postoperative infection [185].
10.6. Cardiac Tissue Engineering
Despite significant advances in medicine and technology, heart failure is still associated with a high mortality rate throughout the world. Therefore, it is essential to apply new concepts for the development of new treatments and therapeutic alternatives [186].
Myocardial infarction is a very common heart disease worldwide. For that reason, cardiac patches gained great interest as therapeutic candidates to stimulate myocardial regeneration after their implantation. Thus, Fiamingo et al. studied Chitosan-based hydrogels implanted onto the epicardial surfaces of the infarcted myocardium of rats. The result revealed that chitosan hydrogels prepared from 1.5% polymer solutions were perfectly incorporated onto the epicardial surface of the heart and presented partial degradation accompanied by mononuclear cell infiltration [187]. Later, in order to determine the impact of the acetylation degree of chitosan in the degradation and biological activity, acellular chitosan hydrogels with different degrees of acetylation were implanted in a murine model of both ischemic and non-ischemic cardiomyopathies. This study demonstrated the beneficial effects of chitosan hydrogels, particularly with polymers with an acetylation degree of 24%, on cardiac remodeling in two cardiomyopathy models [188].
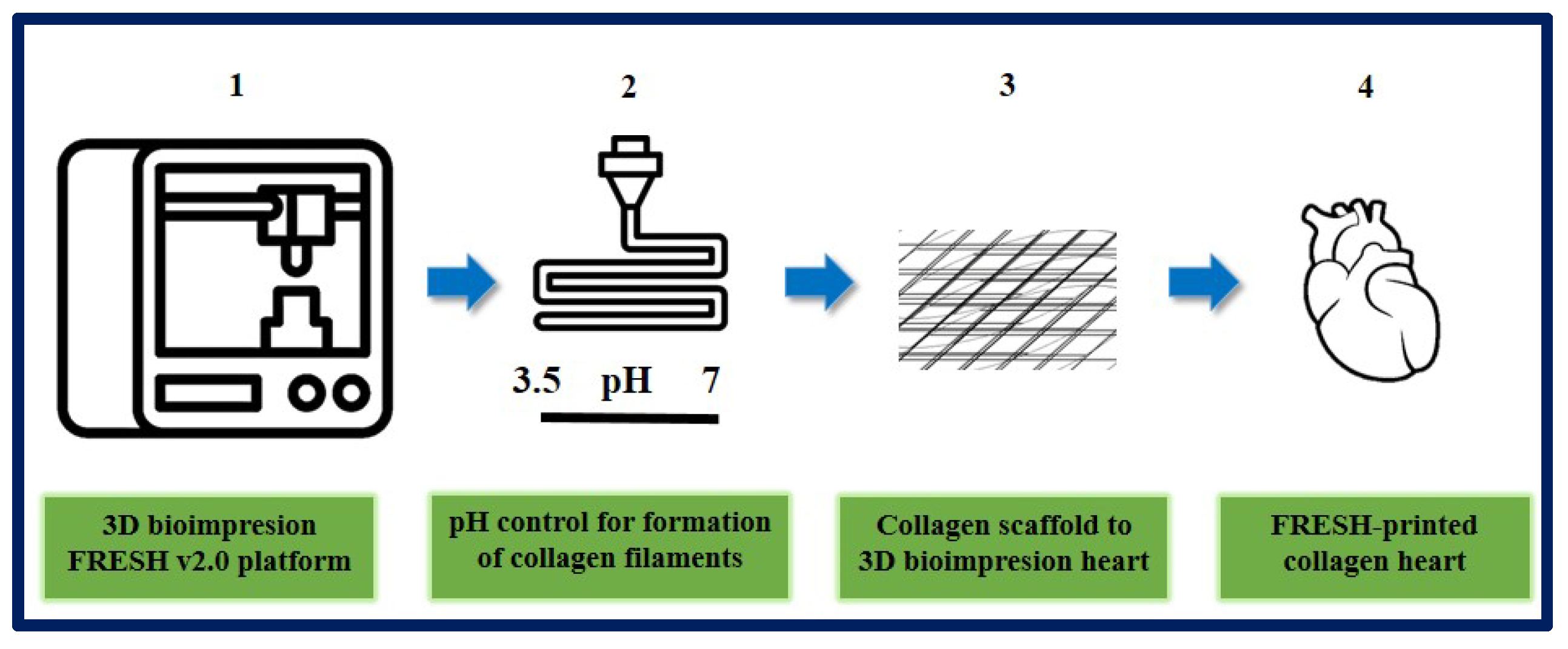
On the other hand, Lee et al. designed and fabricated different components of the human heart with complex collagen scaffolds at various scales, from capillaries to the entire organ. To achieve this, they used 3D bioprinting using the “FRESH” platform (freeform reversible embedding of suspended hydrogels). They managed to control the degree of gelation by adjusting the pH to obtain microfilaments and a porous structure that favor microvascularization, cell infiltration, and mechanical resistance for the fabrication and perfusion of multi-scale vasculature. Thanks to microcomputed tomography, hearts can be bioprinted more precisely, resembling the specific anatomical structure of each patient. In this study, they succeeded in printing cardiac ventricles using human cardiomyocytes, and these showed directional action potential propagation, synchronized contractions, and wall thickening during systole. Although the human heart was used as a proof of concept in this work, the collagen FRESH v2.0 platform could have potential for scaffold fabrication and applications in other tissue and organ systems. This study shows an important advance in the manufacture of scaffolds with structural, mechanical, and biological properties similar to those of native tissues, but there are still many challenges to overcome in order to reach the stage of clinical application, since the 3D bioprinting of a fully functional organ has not yet been achieved [189] (Figure 21).
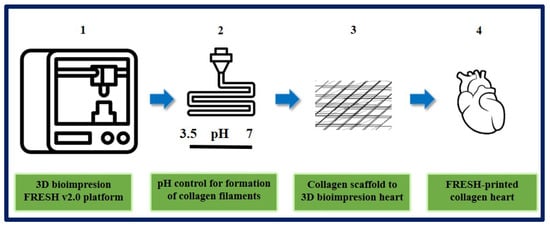
Figure 21.
Organ-scale FRESH v2.0 3D bioprinting. (1) 3D bioprinting of collagen using FRESH v2.0 in high resolution. (2) pH control for rapid neutralization causing gelation to collagen filament formation process. (3) Single filaments of collagen obtained to this system were smooth with 20 to 200 nm diameter. (4) In the first instance, the ventricle was printed with a central section of cardiac cells, internal and external collagen shell, and a collagen-only section. Then Tri-leaflet heart valve 3D model at adult human scale was printed with the same system. Finally, a FRESH v2.0 collagen heart was printed at a neonatal scale. Bibliography consulted [189].
10.7. Cartilage Tissue Engineering
There is an increase in degenerative joint diseases (e.g., osteoarthritis) as the general population continues to age. Overweight and sports injuries may occur in young and healthy people in whom spontaneous cartilage repair is limited. This is why there has been an increase in the demand for regenerative engineering approaches to cartilage. One of the causes could be the lack of vascularization of the articular cartilage, which would not allow an inflammatory response after the injury and the resulting repair. Another reason would be the intrinsic inability to self-repair due to the low proliferative capacity of chondrocytes, resulting in scar tissue with inferior mechanical properties and durability [31].
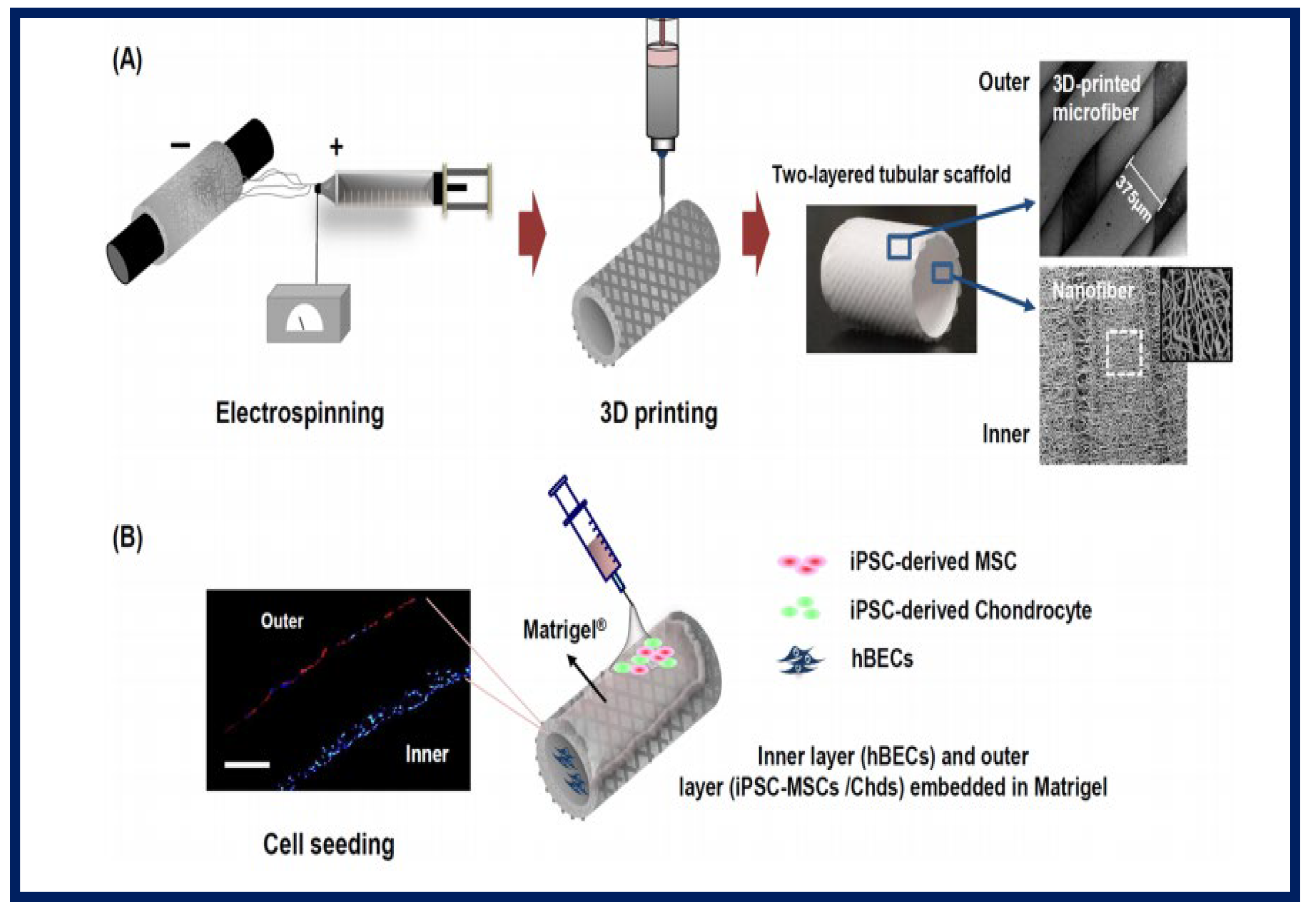
Gul Kim et al. designed and fabricated an artificial trachea with mechanical properties similar to the native trachea. For this, a two-layer tubular scaffold was fabricated: the inner layer with electrospun PCL nanofibers and the outer layer of 3D-printed PCL microfibers. To enhance the regeneration of the tracheal mucosa and cartilage, they used human induced pluripotent stem cells (iPSCs), human bronchial epithelial cells (hBECs), iPSC-derived mesenchymal stem cells (iPSC-MSCs), and iPSC-derived chondrocytes (iPSC-Chds) (Figure 22). Using a bioreactor system, the artificial tracheae were transplanted into a rabbit with a tracheal defect (1.5 cm in length). Endoscopy did not reveal granulation growth in the tracheal lumen. Alcian blue staining clearly showed the formation of ciliated columnar epithelium in iPSC-MSC clusters. Furthermore, micro-CT analysis showed that the iPSC-Chd groups formed neocartilage at the defective sites. Therefore, this study describes a promising long-term approach in the functional reconstruction of a segmental tracheal defect [31].
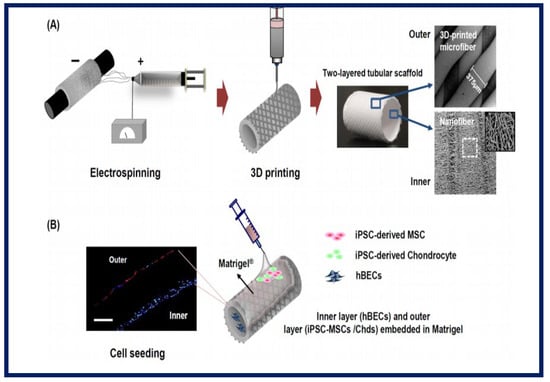
Figure 22.
Illustrative schematic of the fabrication of three-dimensional tubular artificial tracheal scaffolds (A): The inner layer was fabricated from electrospun polycaprolactone (PCL) nanofibers and the outer layer from 3D-printed PCL strands. (B): Scaffolds were seeded with induced pluripotent stem cell-derived chondrocytes (iPSC-Chds in the outer layer and human bronchial epithelial cells (hBEC) in the inner layer of the scaffold. Identification was by PKH-26 staining (red color; iPSC-chds) and DAPI staining (blue color; hBEC). Bibliography consulted [31] is licensed under CC BY 2.0. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ (accessed on 15 December 2022).
10.8. Neural Tissue Engineering
The nervous system is the most complex in the human body. When damage occurs, the motor and sensory functions are affected. Injuries to the central nervous system (CNS), which consists of the brain and spinal cord, generally lead to permanent disability, as spontaneous tissue regeneration is limited, and this leads to considerable socioeconomic problems [190]. The CNS can be affected by certain disorders, such as neurodegenerative diseases and ischemic injuries. While there are numerous approaches and treatments, very few result in a complete recovery or resolution. It is important to reduce the pathogenesis of the ongoing disease and prevent further tissue damage and recurrence [191]. In this regard, TE scaffolds are an attractive approach to replace damaged neural tissue or promote the regeneration of existing tissue to regain the functionality of the injured nerves [192].
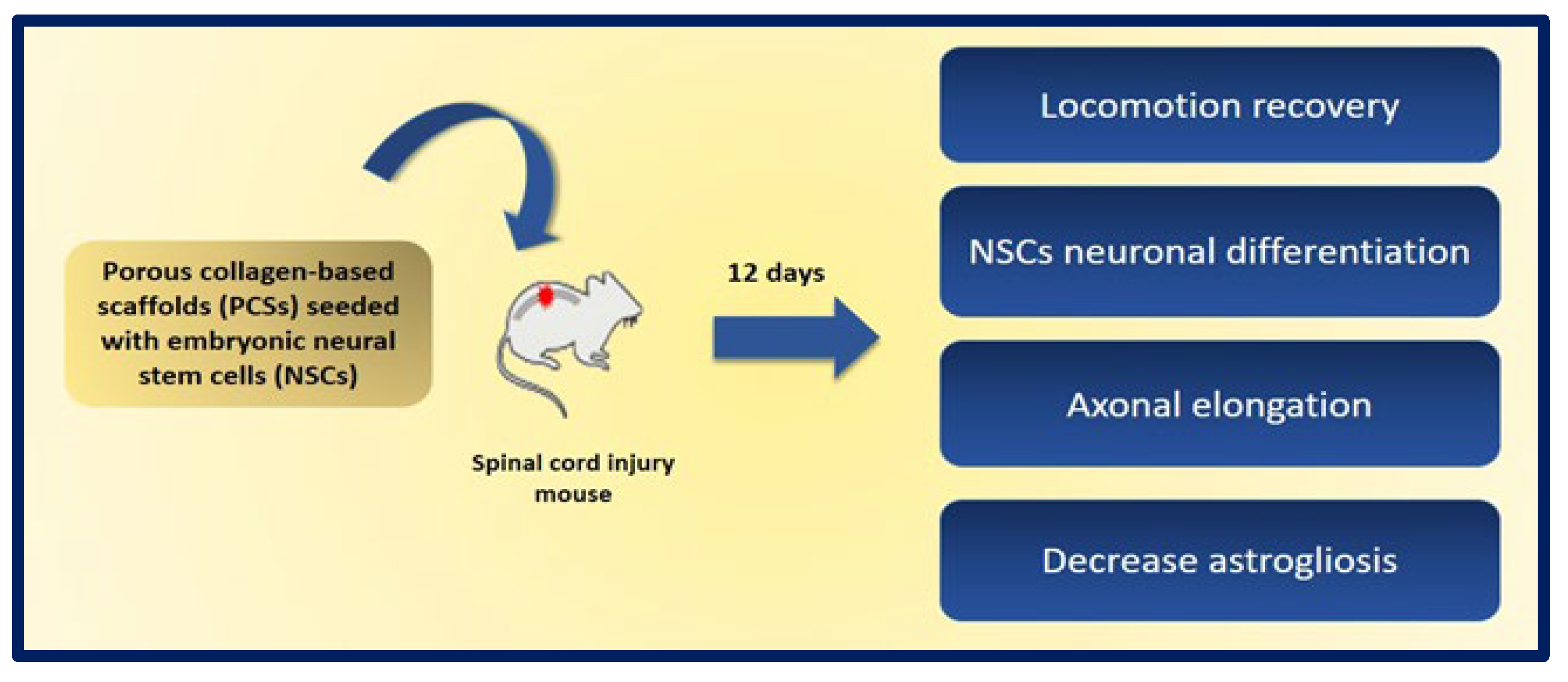
A promising approach is the use of neural stem cells (NSCs), as they can differentiate into both neurons and glial cells and secrete neurotrophic factors, and their use does not raise the safety concerns of pluripotent stem cells. The combination of NSCs with biomaterials can improve the availability of cells at the site of injury and can fill the volume of the lesion cavity. The cells are protected from immunological attack, and axonal elongation is favored by providing adhesion and regulation signals to reduce inflammation and scar formation. Kourgiantaki et al. evaluated grafts based on collagen-based porous scaffolds (PCS) similar to the biomaterials used in clinical regenerative medicine already approved by the FDA. For this, 3D cultures of neural stem cells (NSCs) were combined with cylindrical collagen structures, and the graft was implanted in an animal model after generating a crush injury in the spinal cord. The evidence suggests that this type of support protects and regulates the cellular activity of the NSCs at injury sites, since PCS grafts seeded with NSCs favor the modulation of key processes such as cell differentiation, axonal elongation at the site of lesion and across the lesion boundary, the migration of NSC to the surroundings, and the reduction of astrogliosis. In addition, the in vivo functional integration of the implant was observed by means of histological tests. On the other hand, the results show improvements in recovery of locomotion in mice after 12 weeks of injury, and their locomotion performance does not differ statistically from that of uninjured control animals. These findings demonstrate the potential of PCS combined with NSCs as future therapies to induce nerve tissue regeneration in spinal cord injuries (Figure 23) [193].
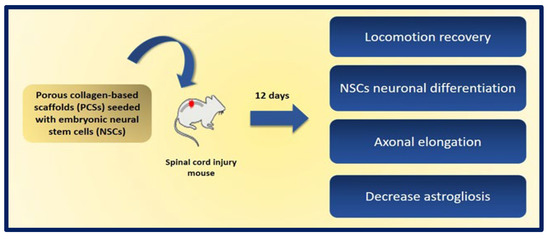
Figure 23.
Graphic representation of the benefits obtained after 12 days of the implantation of porous collagen-based scaffolds (PCSs) seeded with NSCs in a murine model of spinal cord injury. Bibliography consulted [193].
10.9. Adipose Tissue Engineering
Adipose tissue is one of the most abundant tissues in the human body, but its regeneration in vitro and in vivo still presents substantial challenges. Currently, autologous fat grafting is used, but necrosis-induced volume loss occurs in the long term. One strategy is the use of primary cells and cell lines seeded on synthetic and natural polymer spheroids or scaffolds for the regeneration of adipose tissue [194]. In addition, adipose tissue is an important source of mesenchymal stem cells, and this makes it an interesting alternative for use in tissue engineering and cell regeneration [195].
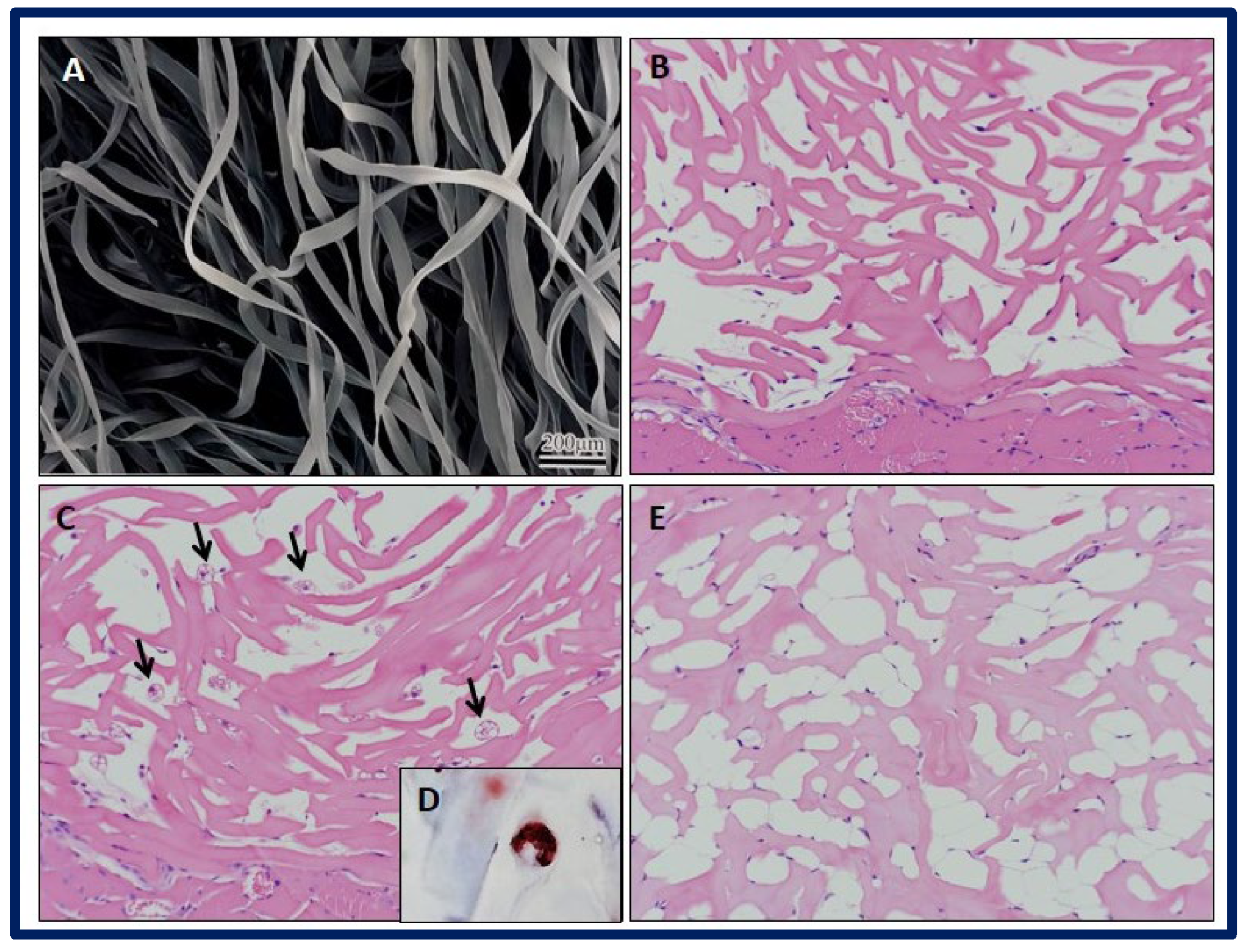
Rodriguez et al. evaluated two types of scaffolds with different microstructures, one made of fibrous structured atelocollagen (FSA) and the other made of honeycomb-structured atelocollagen (HCA). The aim of the study was to carry out in vivo adipogenesis induction by implanting the scaffolds in four-week-old male severe combined immunodeficiency (SCID) mice. To evaluate the results, histological and immunohistochemical tests were performed after one, two, and four weeks of implantation. After these procedures, it was verified that the FSA scaffold had a greater capacity to induce adipogenesis in vivo without the incorporation of preadipocytes or adipogenic factors, while the HCA scaffold induced a severe acute inflammatory response. Therefore, it appears that the fibril organization of the FSA scaffolds is a key factor in the development of the adipogenic response. In addition, it was possible to obtain primary adipose tissue in the FSA scaffolds, composed of a mixture of white and brown adipocytes at week 2, and only white adipocytes at week 4. After carrying out immunostaining with a positive Pax7 result at weeks 1 and 2, the authors suggested the existence of a common myogenic progenitor shared by the observed brown and white adipocytes. For all this, the FSA scaffolds are presented as a promising structure for the engineering of brown and white adipose tissue (Figure 24) [196].
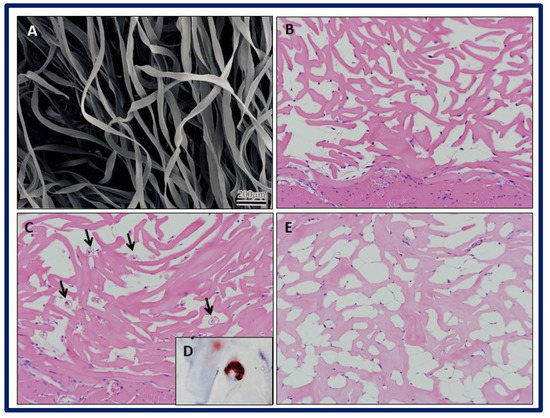
Figure 24.
SEM micrograph showing the (A) FSA scaffolds. Histological examination of implant specimens of FSA scaffolds at different weeks (Hematoxylin and Eosin staining). (B) Week 1: Note the presence of stained spindle cells attached to scaffold fibers. (C) Week 2: Black narrows indicate brown-like adipocytes within the scaffold, with multiple cytoplasmic lipid droplets. Note the presence of some small spherical cells close to scaffold fibers. No inflammatory reaction is observed. (D) Week 2: Multiple cytoplasmic lipid droplets within the cells are stained with Oil Red O staining. (E) Week 4: Multiple white-like adipocytes within scaffold bulk are observed, with large cytoplasmic lipid droplets. Bibliography consulted [196].
11. Tissue Engineering Polymers in Clinical Applications
The objective of TE is to continue expanding as a scientific platform to advance in the regeneration and repair of different types of tissues. For certain products to reach the clinical phase, where applications and studies are carried out in human beings, they must go through a rigorous control process after successful results have been obtained in preclinical studies. Currently, modern medicine uses polymer-based implantable medical devices for diagnosis or regenerative therapies in various clinical applications for tissue repair, and biomaterials often cause an immediate response in the host after being implanted that includes tissue injury, inflammation, cell proliferation, and tissue remodeling. The host response affects the degradation process, which results in changes in the structure, morphology, and mechanical properties of the scaffold, and it is reflected in a decrease in yield. To develop functional and biodegradable devices for the replacement or repair of damaged tissues, a deep understanding of the degradation process of the material and the specific interaction between it and the host system is required [69].
There are important factors to take into account, such as differences in the age, nutritional status, physical activity, and disease status of patients, since, for a bioengineered tissue to be clinically relevant, it needs to integrate seamlessly into the body after implantation in vivo and play an active role in the development of tissue repair [28]. Thanks to specific biological and technological advances, it is possible to design and build tissues with a predictive performance based on previously identified patient needs. This will result in high quality and robust TE product under regulatory approvals. At the same time, they become attractive products for investors, since, by reducing the risks associated with product failure in the market stage, they become a new sector associated with medicine capable of revolutionizing the future of health care [197].
Advances in this area can be found at ClinicalTrials.gov, a database of publicly and privately funded clinical trials conducted around the world. Most of the records on ClinicalTrials.gov describe clinical trials in which human volunteers are assigned to interventions (for example, a medical product, behavior, or procedure) based on a protocol, and then their effects on the biomedical or health outcomes are evaluated. Table 4 briefly describes some approaches used in clinical trials using composite scaffolds as starting material.

Table 4.
Different polymer-based medical devices approved to use in clinical trials.
12. Tissue Engineering Polymers in the Market
Costs related to organ replacement represent 8% of global health spending, and it is estimated that, by 2040, 25% of US GDP will be linked to health. The developments and progress in the design and engineering of biomaterials have made possible a new generation of attractive materials for regenerative medicine, but a question arises: which of these materials will successfully position itself in the market? That will depend on a combination of clinical results, performance, commercialization, and profitability. The materials used must contain complex information encoded in their physical and chemical structures, since one drawback is their ability to influence cell behavior. On the other hand, complexity must be minimized to obtain financial profitability. There should be several phases of product development, beginning with the identification of the simplest functional performance required to solve a defined clinical problem. The initial ambitious goals of rebuilding entire organs have largely given way to smaller, more achievable goals: for example, instead of trying to replace an entire heart, clinical advances in cardiac repair focus on manufacturing coronary arteries, valves, and myocardium [20].
Numerous companies have developed TE scaffolds of different materials and shapes that have reached the commercialization stage and can now be found as off-the-shelf products that have already undergone adequate testing and controls. The Nanotechnology Products Database (NPD) provides a trusted source of information on the nanotechnology products currently used in a wide range of industrial applications.
We can find products with nanofibers being used as artificial arteries, 3D biological inks, pre-loaded well scaffolds, or biocompatible polymers sheets as a replacement for traditional lab plasticware for tissue culture, custom 3D scaffolds for bioreactor scale up and regenerative medicine, or resorbable synthetic hydrogel composed of repetitive amino acid sequences of arginine-alanine-acid and aspartic-alanine prepared in aqueous solutions.
13. Conclusions
Herein, we provided a comprehensive overview of recent advances in the biological applications of biodegradable polymers in tissue engineering, ranging from basic scientific concepts to clinical application. Currently, different polymer-based medical devices have been approved for use in clinical trials, and a wide variety of polymeric biomaterials are available as commercial products.
At the same time, the choice of a good scaffold is critical, and the polymeric composites must exhibit properties such as the porosity, permeability, fibrousness, and mechanical stability necessary to successfully mimic extracellular matrices and provide the necessary structural support for the subsequent cell adhesion and tissue regeneration. Despite their many advantages, including biocompatibility, biodegradability, and others, these materials are rather inert and lack the specific functionalities that would endow them with additional biological and responsive properties. This is why numerous researchers are searching for new, smart functional materials that promote cell functions and tissue regeneration.
We are in an era of biological renaissance that poses multiple challenges to the scientific community in the areas of regenerative medicine and organ and tissue engineering. Despite great advances in science and technology, there is still a lot of research work ahead, and numerous efforts are currently underway to improve the mechanical properties of scaffolds with the aim of increasing the number of commercially and clinically successful trials. Knowledge and technology related to polymeric scaffolds are growing exponentially, and improvements in regenerative medicine will undoubtedly lead to safer and more integrated tissue-engineered systems to treat human disease. In the future, there could be “tissue and organ banks” that are available to any patient. To achieve this goal, regenerative medicine and tissue engineering technologies will require substantial and ongoing interdisciplinary research leading to successful results.
Author Contributions
Conceptualization, M.C.S. and A.P.R.; software, S.F.; validation, C.J.F., M.C.S. and A.P.R.; writing—original draft preparation, M.C.S.; writing—review and editing, G.R.; visualization, E.O.; supervision, A.P.R.; project administration, K.T.; funding acquisition, H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI Grant Numbers JP20H03888, JP20K10178, and JP19K19159.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, T.; Chang, J.; Zhu, Y.; Wu, C. 3D printing of bioinspired biomaterials for tissue regeneration. Adv. Health Mater. 2020, 27, e2000208. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G. Diseño y preparación de matrices poliméricas porosas para ingeniería de tejidos biológicos. Anales. Acad. Nac. CS Ex. Fis. Nat. 2017, 59, 115–130. [Google Scholar]
- Del Barrio Cortés, E.; Matutano Molina, C.; Rodríguez-Lorenzo, L.; Cubo-Mateo, N. Generation of Controlled Micrometric Fibers inside Printed Scaffolds Using Standard FDM 3D Printers. Polymers 2023, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, V.D.; Zeng, P.; Li, B.; Zhang, Y. In vitro cell culture in hollow microfibers with porous structures. Biomater. Sci. 2020, 8, 2175–2188. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.H.; Min, B.H.; Chun, H.J.; Han, D.K.; Lee, H.B. Polymeric scaffolds for regenerative medicine. Polym. Rev. 2011, 51, 23–52. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Vacanti, C.A. The history of tissue engineering. J. Cell. Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef]
- Vacanti, J.P.; Vacanti, C.A. The history and scope of tissue engineering. In Principles of Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–8. [Google Scholar]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.-H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Bio-medical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef]
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B 2014, 2, 5319–5338. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Khan, M.R.; Azam, M.; Al Ammari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2021, 13, 135. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Serrà, A.; Bhawani, S.A.; Ibrahim, M.N.M.; Khan, A.; Alorfi, H.S.; Asiri, A.M.; Hussein, M.A.; Khan, I.; Umar, K. Utilizing Biomass-Based Graphene Oxide–Polyaniline–Ag Electrodes in Microbial Fuel Cells to Boost Energy Generation and Heavy Metal Removal. Polymers 2022, 14, 845. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jung, J.-Y.; Shin, H.-W.; Park, J.-W.; Bang, J.; Huh, J. Loop and Bridge Conformations of ABA Triblock Comb Co-polymers: A Conformational Assessment for Molecular Composites. Polymers 2022, 14, 2301. [Google Scholar] [CrossRef] [PubMed]
- Hansapaiboon, S.; Bulatao, B.P.; Sorasitthiyanukarn, F.N.; Jantaratana, P.; Nalinratana, N.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Fabrication of Curcumin Diethyl γ-Aminobutyrate-Loaded Chitosan-Coated Magnetic Nanocarriers for Improvement of Cytotoxicity against Breast Cancer Cells. Polymers 2022, 14, 5563. [Google Scholar] [CrossRef]
- El-Dessouky, H.M.; McHugh, C. Multifunctional auxetic and honeycomb composites made of 3D woven carbon fibre preforms. Sci. Rep. 2022, 12, 22593. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.I.; Astudillo, S.; Mina Hernandez, J.H.; Saavedra, M.; Zapata, P.A.; Valencia-Llano, C.H.; Chaur, M.N.; Grande-Tovar, C.D. Synthesis, Characterization, and Optimization Studies of Polycaprolactone/Polylactic Acid/Titanium Dioxide Nanoparti-cle/Orange Essential Oil Membranes for Biomedical Applications. Polymers 2023, 15, 135. [Google Scholar] [CrossRef]
- Harris, J.P.; Burrell, J.C.; Struzyna, L.A.; Chen, H.I.; Serruya, M.D.; Wolf, J.A.; Duda, J.; Cullen, K. Emerging regenerative medicine and tissue engineering strategies for Parkinson’s disease. NPJ Park. Dis. 2020, 6, 4. [Google Scholar] [CrossRef]
- Diego Rodriguez, E.; Villanueva Peña, A.; Roca Edreira, A.; Martín García, B.; Meana Infiesta, A.; Gómez Llames, S. Estado actual de la ingeniería de tejidos en urología: Revisión de la literatura. Actas Urol. Esp. 2004, 28, 636–645. [Google Scholar] [CrossRef]
- Chaignaud, B.E.; Langer, R.; Vacanti, J.P. The history of tissue engineering using synthetic biodegradable polymer scaffolds and cells. In Synthetic Biodegradable Polymer Scaffolds; Atala, A., Mooney, D.J., Eds.; Birkhäuser Boston: Boston, MA, USA, 1996; pp. 1–14. [Google Scholar]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Kengla, C.; Lee, S.J.; Yoo, J.J. AtalaA. 3-D bioprinting technologies for tissue engineering applications. In Rapid Prototyping of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–288. [Google Scholar]
- Woodfield, T.; Lim, K.; Morouço, P.; Levato, R.; Malda, J.; Melchels, F. Biofabrication in tissue engineering. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 236–266. [Google Scholar]
- Garreta, E.; Oria, R.; Tarantino, C.; Pla-Roca, M.; Prado, P.; Fernández-Avilés, F.; Campistol, J.M.; Samitier, J.; Montserrat, N. Tissue engineering by decellularization and 3D bioprinting. Mater. Today 2017, 20, 166–178. [Google Scholar] [CrossRef]
- Meyer, U. The history of tissue engineering and regenerative medicine in perspective. In Fundamentals of Tissue Engineering and Regenerative Medicine; Meyer, U., Handschel, J., Wiesmann, H.P., Meyer, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 5–12. [Google Scholar]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue engineering and regenerative medicine: History, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef]
- Vacanti, C.A. History of tissue engineering and a glimpse into its future. Tissue Eng. 2006, 12, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Yousefi, A.-M. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.S.; Kandasubramanian, B. Progress in the advancement of porous biopolymer scaffold: Tissue engineering application. Ind. Eng. Chem. Res. 2019, 58, 6163–6194. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Kim, I.G.; Park, S.A.; Lee, S.-H.; Choi, J.S.; Cho, H.; Lee, S.J.; Kwon, Y.-W.; Kwon, S.K. Transplantation of a 3D-printed tracheal graft combined with iPS cell-derived MSCs and chondrocytes. Sci. Rep. 2020, 10, 4326. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef]
- Chung, H.J.; Park, T.G. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 249–262. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef]
- Moreno, M.; Amaral, M.H.; Lobo, J.M.S.; Silva, A.C. Scaffolds for bone regeneration: State of the art. Curr. Pharm. Des. 2016, 22, 2726–2736. [Google Scholar] [CrossRef] [PubMed]
- Moreno Madrid, A.P.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Sabino, M.A.; Loaiza, M.; Dernowsek, J.; Rezende, R.; Da Silva, J.V.L. Técnicas para la fabricación de andamios poliméricos con aplicaciones en ingenierÍa de tejidos. Rev. LatinAm. Metal. Mat. 2017, 37, 1–27. [Google Scholar]
- Perniconi, B.; Costa, A.; Aulino, P.; Teodori, L.; Adamo, S.; Coletti, D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials 2011, 32, 7870–7882. [Google Scholar] [CrossRef]
- Aguero Luztonó, L.; Zaldivar Silva, D.; Escobar Ivirico, J.L. Liberación de cefalexina a partir de hidrogeles de poli (acrilamida-co-ácido metacrílico). Biomecánica 2000, 18, 58–62. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Chan, J.P.; Battiston, K.G.; Santerre, J.P. Synthesis and characterization of electrospun nanofibrous tissue engineering scaffolds generated from in situ polymerization of ionomeric polyurethane composites. Acta Biomater. 2019, 96, 161–174. [Google Scholar] [CrossRef]
- Pierre, J.; Maria, K.; Solene-Emmanuelle, B.; Hany, N.; Valerie, V.; Mathieu, P.; Jerome, L.; Patricia, F.; Yong, C.; Philippe, M.; et al. Nanofibrous clinical-grade collagen scaffolds seeded with human cardiomyocytes induces cardiac remodeling in dilated cardiomyopathy. Biomaterials 2016, 80, 157–168. [Google Scholar]
- Mata, A.; Kim, E.J.; Boehm, C.A.; Fleischman, A.J.; Muschler, G.F.; Roy, S. A three-dimensional scaffold with precise micro-architecture and surface micro-textures. Biomaterials 2009, 30, 4610–4617. [Google Scholar] [CrossRef]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer- and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef]
- Wang, M.; Guo, L.; Sun, H. Manufacture of Biomaterials. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 116–134. [Google Scholar]
- Yu, T.-T.; Cui, F.-Z.; Meng, Q.-Y.; Wang, J.; Wu, D.-C.; Zhang, J.; Kou, X.-X.; Yang, R.-L.; Liu, Y.; Zhang, Y.S.; et al. Influence of surface chemistry on adhesion and osteo/odontogenic differentiation of dental pulp stem cells. ACS Biomater. Sci. Eng. 2017, 3, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Boey, F.; Lao, L.L. Implanted cardiovascular polymers: Natural, synthetic and bio-inspired. Prog. Polym. Sci. 2008, 33, 853–874. [Google Scholar] [CrossRef]
- Hamid Akash, M.S.; Rehman, K.; Chen, S. Natural and synthetic polymers as drug carriers for delivery of therapeutic proteins. Polym. Rev. 2015, 55, 371–406. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–89. [Google Scholar]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni Vishakha, S.; Butte Kishor, D.; Rathod Sudha, S. Natural polymers—A comprehensive review. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 1597–1613. [Google Scholar]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Int. Mater. Rev. 2018, 64, 1–36. [Google Scholar] [CrossRef]
- Qian, Z.; Radke, D.; Jia, W.; Tahtinen, M.; Wang, G.; Zhao, F. Bioengineering scaffolds for regenerative engineering. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 444–461. [Google Scholar]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109768. [Google Scholar] [CrossRef]
- Gómez Perez, C.A. Desarrollo de Implantes Craneofaciales Utilizando Técnicas de Diseño y Manufactura Avanzadas. Master’s Thesis, Facultad de Ingeniería, Universidad Autonoma de San Luis Potosí, Cuernavaca, Mexico, 2015. [Google Scholar]
- Coluzza, I.; Pisignano, D.; Gentili, D.; Pontrelli, G.; Succi, S. Ultrathin Fibers from Electrospinning Experiments under Driven Fast-Oscillating Perturbations. Phys. Rev. Appl. 2014, 2, 054011. [Google Scholar] [CrossRef]
- Ye, K.; Kuang, H.; You, Z.; Morsi, Y.; Mo, X. Electrospun Nanofibers for Tissue Engineering with Drug Loading and Release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ma, Q.; Yu, W.; Li, D.; Dong, X.; Liu, G.; Yu, H. Comparison of different electrospinning technologies for the production of arrays with multifunctional properties: Fluorescence, conduction and magnetism. J. Phys. D Appl. Phys. 2020, 53, 155301. [Google Scholar] [CrossRef]
- Fridrikh, S.V.; Yu, J.H.; Brenner, M.P.; Rutledge, G.C. Controlling the fiber diameter during electrospinning. Phys. Rev. Lett. 2003, 90, 144502. [Google Scholar] [CrossRef]
- Dzenis, Y. Material science. Spinning continuous fibers for nanotechnology. Science 2004, 304, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Ewaldz, E.; Brettmann, B. Molecular interactions in electrospinning: From polymer mixtures to supramolecular assemblies. ACS Appl. Polym. Mater. 2019, 1, 298–308. [Google Scholar] [CrossRef]
- Jana, S.; Cooper, A.; Ohuchi, F.; Zhang, M. Uniaxially aligned nanofibrous cylinders by electrospinning. ACS Appl. Mater. Interfaces 2012, 4, 4817–4824. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yu, X.; Chai, Q.; Ayres, N.; Steckl, A.J. Stimuli-Responsive Self-Immolative Polymer Nanofiber Membranes Formed by Coaxial Electrospinning. ACS Appl. Mater. Interfaces 2017, 9, 11858–11865. [Google Scholar] [CrossRef]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2019, 5, 61–81. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Chan, C.-H.; Tang, W.C. Alignment of multiple electrospun piezoelectric fiber bundles across serrated gaps at an incline: A method to generate textile strain sensors. Sci. Rep. 2017, 7, 15436. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Ezazi, N.Z.; Liu, D.; Santos, H.A. Electrospun fibrous architectures for drug delivery, tissue engineering and cancer therapy. Adv. Funct. Mater. 2019, 29, 1802852. [Google Scholar] [CrossRef]
- Tan, H.-L.; Kai, D.; Pasbakhsh, P.; Teow, S.-Y.; Lim, Y.-Y.; Pushpamalar, J. Electrospun cellulose acetate butyrate/polyethylene glycol (CAB/PEG) composite nanofibers: A potential scaffold for tissue engineering. Colloids Surf. B Biointerfaces 2020, 188, 110713. [Google Scholar] [CrossRef] [PubMed]
- Prabu, G.T.V.; Dhurai, B. A Novel Profiled Multi-Pin Electrospinning System for Nanofiber Production and Encapsulation of Nanoparticles into Nanofibers. Sci. Rep. 2020, 10, 4302. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.; Aggeli, A.; Ingham, E.; McPherson, M.J. Production of self-assembling biomaterials for tissue engineering. Trends Biotechnol. 2009, 27, 423–433. [Google Scholar] [CrossRef]
- Hughes, E.A.B.; Chipara, M.; Hall, T.J.; Williams, R.L.; Grover, L.M. Chemobrionic structures in tissue engineering: Self-assembling calcium phosphate tubes as cellular scaffolds. Biomater. Sci. 2020, 8, 812–822. [Google Scholar] [CrossRef]
- Ebrahimi, M. Biomimetic principle for development of nanocomposite biomaterials in tissue engineering. In Applications of Nanocomposite Materials in Orthopedics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 287–306. [Google Scholar]
- Gong, C.; Sun, S.; Zhang, Y.; Sun, L.; Su, Z.; Wu, A.; Wei, G. Hierarchical nanomaterials via biomolecular self-assembly and bioinspiration for energy and environmental applications. Nanoscale 2019, 11, 4147–4182. [Google Scholar] [CrossRef]
- Panda, J.J.; Chauhan, V.S. Short peptide based self-assembled nanostructures: Implications in drug delivery and tissue engineering. Polym. Chem. 2014, 5, 4431–4449. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, C.; Akakuru, O.U.; Su, Z.; Wu, A.; Wei, G. The design and biomedical applications of self-assembled two-dimensional organic biomaterials. Chem. Soc. Rev. 2019, 48, 5564–5595. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Bio 2020, 6, 100043. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Feng, M.; Chen, X.; Sun, J. Multidimensional (0D-3D) functional nanocarbon: Promising material to strengthen the photocatalytic activity of graphitic carbon nitride. Green Energy Environ. 2021, 6, 823–845. [Google Scholar] [CrossRef]
- Saunders, L.; Ma, P.X. Self-Healing Supramolecular Hydrogels for Tissue Engineering Applications. Macromol. Biosci. 2019, 19, e1800313. [Google Scholar] [CrossRef]
- Nakamatsu, J.; Torres, F.G.; Troncoso, O.P.; Min-Lin, Y.; Boccaccini, A.R. Processing and characterization of porous structures from chitosan and starch for tissue engineering scaffolds. Biomacromolecules 2006, 7, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.A.; Rao, P.S. Synthesis of polymeric nanomaterials for biomedical applications. In Nanomaterials in Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2013; pp. 27–63. [Google Scholar]
- Nam, Y.S.; Park, T.G. Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J. Biomed. Mater. Res. 1999, 47, 8–17. [Google Scholar] [CrossRef]
- Rana, D.; Ratheesh, G.; Ramakrishna, S.; Ramalingam, M. Nanofiber composites in cartilage tissue engineering. In Nanofiber Composites for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 325–344. [Google Scholar]
- Rowlands, A.S.; Lim, S.A.; Martin, D.; Cooper-White, J.J. Polyurethane/poly (lactic-co-glycolic) acid composite scaffolds fabricated by thermally induced phase separation. Biomaterials 2007, 28, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.B.; Reddy, G.S.M.; Sivanjineyulu, V.; Jayaramudu, J.; Varaprasad, K.; Sadiku, E.R. Hydrophobic/hydrophilic nanostructured polymer blends. In Design and Applications of Nanostructured Polymer Blends and Nanocomposite Systems; Elsevier: Amsterdam, The Netherlands, 2016; pp. 385–411. [Google Scholar]
- Ghalia, M.A.; Dahman, Y. Advanced nanobiomaterials in tissue engineering. In Nanobiomaterials in Soft Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 141–172. [Google Scholar]
- Salehi, M.; Farzamfar, S.; Bozorgzadeh, S.; Bastami, F. Fabrication of Poly(L-Lactic Acid)/Chitosan Scaffolds by Solid-Liquid Phase Separation Method for Nerve Tissue Engineering: An In Vitro Study on Human Neuroblasts. J. Craniofac. Surg. 2019, 30, 784–789. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; McNulty, J.; Salick, M.R.; Peng, X.-F.; Turng, L.-S. Approaches to Fabricating Multiple-Layered Vascular Scaffolds Using Hybrid Electrospinning and Thermally Induced Phase Separation Methods. Ind. Eng. Chem. Res. 2016, 55, 882–892. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.-Y.; Huh, M.; Yun, S.I. Porous Poly (3-hydroxybutyrate) Scaffolds Prepared by Non-Solvent-Induced Phase Separation for Tissue Engineering. Macromol. Res. 2020, 28, 835–843. [Google Scholar] [CrossRef]
- Yousefi, A.-M.; Liu, J.; Sheppard, R.; Koo, S.; Silverstein, J.; Zhang, J.; James, P.F. I-Optimal Design of Hierarchical 3D Scaffolds Produced by Combining Additive Manufacturing and Thermally Induced Phase Separation. ACS Appl. Bio Mater. 2019, 2, 685–696. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L. Fabrication of scaffolds in tissue engineering: A review. Front. Mech. Eng. 2018, 13, 107–119. [Google Scholar] [CrossRef]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.C.; Suhaimin, I.; Kassim, S.; Zubir, S.; Abdullah, T. Effect of modified solvent casting/particulate leaching (SCPL) technique on the properties of bioactive glass reinforced polyurethane scaffold for biomedical applications. JPS 2019, 30, 115–126. [Google Scholar] [CrossRef]
- Costantini, M.; Barbetta, A. Gas foaming technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Elsevier: Amsterdam, The Netherlands, 2018; pp. 127–149. [Google Scholar]
- Dugad, R.; Radhakrishna, G.; Gandhi, A. Recent advancements in manufacturing technologies of microcellular polymers: A review. J. Polym. Res. 2020, 27, 182. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, S.; Yin, S.; Jiang, F.; Zhou, M.; Yang, G.; Sun, N.; Zhang, W.; Jiang, X. In situ gas foaming based on magnesium particle degradation: A novel approach to fabricate injectable macroporous hydrogels. Biomaterials 2020, 232, 119727. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F.; An, J. Introduction to rapid prototyping of biomaterials. In Rapid Prototyping of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–15. [Google Scholar]
- Touri, M.; Kabirian, F.; Saadati, M.; Ramakrishna, S.; Mozafari, M. Additive manufacturing of biomaterials—The evolution of rapid prototyping. Adv. Eng. Mater. 2019, 21, 1800511. [Google Scholar] [CrossRef]
- Ahmed, N. Direct metal fabrication in rapid prototyping: A review. J. Manuf. Process. 2019, 42, 167–191. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Bertoldi, K.; Gabbrielli, R.; Velders, A.H.; Feijen, J.; Grijpma, D.W. Mathematically defined tissue engineering scaffold architectures prepared by stereolithography. Biomaterials 2010, 31, 6909–6916. [Google Scholar] [CrossRef]
- Mazzanti, V.; Malagutti, L.; Mollica, F. FDM 3D Printing of Polymers Containing Natural Fillers: A Review of their Mechanical Properties. Polymers 2019, 11, 1094. [Google Scholar] [CrossRef]
- Kundu, A.; Mccoy, L.; Azim, N.; Nguyen, H.; Rajaraman, S. Fabrication and Characterization of 3D Printed, 3D Microelectrode Arrays for Interfacing with a Peripheral Nerve-on-a-Chip. ACS Biomater. Sci. Eng. 2021, 7, 3018–3029. [Google Scholar] [CrossRef] [PubMed]
- Kruth, J.P.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef]
- Robinson, D.K.R.; Lagnau, A.; Boon, W.P.C. Innovation pathways in additive manufacturing: Methods for tracing emerging and branching paths from rapid prototyping to alternative applications. Technol. Forecast. Soc. Chang. 2019, 146, 733–750. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Varkey, M.; Visscher, D.O.; van Zuijlen, P.P.M.; Atala, A.; Yoo, J.J. Skin bioprinting: The future of burn wound reconstruction. Burn. Trauma 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016, 9, 012001. [Google Scholar] [CrossRef]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Ng, W.L.; An, J.; Chua, C.K.; Tan, L.P. Layer-by-layer ultraviolet assisted extrusion-based (UAE) bioprinting of hydrogel constructs with high aspect ratio for soft tissue engineering applications. PLoS ONE 2019, 14, e0216776. [Google Scholar] [CrossRef]
- Willemsen, K.; Nizak, R.; Noordmans, H.J.; Castelein, R.M.; Weinans, H.; Kruyt, M.C. Challenges in the design and regulatory approval of 3D-printed surgical implants: A two-case series. Lancet Digit. Health 2019, 1, e163–e171. [Google Scholar] [CrossRef]
- Ng, W.L.; Chua, C.K.; Shen, Y.-F. Print Me an Organ! Why We Are Not There Yet. Prog. Polym. Sci. 2019, 97, 101145. [Google Scholar] [CrossRef]
- Syed, O.; Walters, N.J.; Day, R.M.; Kim, H.-W.; Knowles, J.C. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater. 2014, 10, 5043–5054. [Google Scholar] [CrossRef]
- Sehic, E.; Brännström, M.; Hellström, M. Progress in Preclinical Research on Uterus Bioengineering That Utilizes Scaffolds Derived from Decellularized Uterine Tissue. Biomed. Mater. Devices 2022, 1–8. [Google Scholar] [CrossRef]
- Mayorca-Guiliani, E.A.; Willacy, O.; Madsen, C.D.; Rafaeva, M.; Heumüller, S.E.; Bock, F.; Sengle, G.; Koch, M.; Imhof, T.; Zaucke, F.; et al. Decellularization and antibody staining of mouse tissues to map native extracellular matrix structures in 3D. Nat. Protoc. 2019, 14, 3395–3425. [Google Scholar] [CrossRef]
- Teodori, L.; Costa, A.; Marzio, R.; Perniconi, B.; Coletti, D.; Adamo, S.; Gupta, B.; Tárnok, A. Native extracellular matrix: A new scaffolding platform for repair of damaged muscle. Front. Physiol. 2014, 5, 218. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Isenberg, B.; Wong, J.Y. Building structure into engineered tissues. Mater. Today 2006, 9, 54–60. [Google Scholar] [CrossRef]
- Aulino, P.; Costa, A.; Chiaravalloti, E.; Perniconi, B.; Adamo, S.; Coletti, D.; Marrelli, M.; Tatullo, M.; Teodori, L. Muscle Extracellular Matrix Scaffold Is a Multipotent Environment. Int. J. Med. Sci. 2015, 12, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Bousnaki, M.; Beketova, A.; Kontonasaki, E. A Review of in Vivo and Clinical Studies Application of cell sheet and scaffold technology for periodontal ligament regeneration. Biomolecules 2022, 12, 435. [Google Scholar] [CrossRef]
- You, Q.; Lu, M.; Li, Z.; Zhou, Y. Cell Sheet Technology as an Engineering-Based Approach to Bone Regeneration. Int. J. Nanomed. 2022, 17, 6491–6511. [Google Scholar] [CrossRef]
- Sekine, H.; Okano, T. Tubular Cardiac Tissue Bioengineered from Multi-Layered Cell Sheets for Use in the Treatment of Heart Failure. Card. Tissue Eng. 2022, 2485, 227–242. [Google Scholar]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liao, Y.; Zhang, Y.; Shekh, M.I.; Zhang, J.; You, Z.; Du, B.; Lian, C.; Hel, Q. Novel nanofibrous membrane-supporting stem cell sheets for plasmid delivery and cell activation to accelerate wound healing. Bioeng. Transl. Med. 2022, 7, e10244. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Okano, T. Cell Sheet Engineering. Mater. Today 2004, 7, 42–47. [Google Scholar] [CrossRef]
- Alhashmi Alamer, F.; Almalki, G.A. Fabrication of Conductive Fabrics Based on SWCNTs, MWCNTs and Graphene and Their Applications: A Review. Polymers 2022, 14, 5376. [Google Scholar] [CrossRef]
- Vallejos, S.; Trigo-López, M.; Arnaiz, A.; Miguel, Á.; Muñoz, A.; Mendía, A.; García, J.M. From Classical to Advanced Use of Polymers in Food and Beverage Applications. Polymers 2022, 14, 4954. [Google Scholar] [CrossRef]
- Mountaki, S.A.; Kaliva, M.; Loukelis, K.; Chatzinikolaidou, M.; Vamvakaki, M. Responsive Polyesters with Alkene and Carboxylic Acid Side-Groups for Tissue Engineering Applications. Polymers 2021, 13, 1636. [Google Scholar] [CrossRef]
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-Responsive Polymer Brush Coatings for Advanced Biomedical Applications. Polymers 2022, 14, 4245. [Google Scholar] [CrossRef]
- Chandy, T. Biocompatibility of materials and its relevance to drug delivery and tissue engineering. In Biointegration of Medical Implant Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 297–331. [Google Scholar]
- Hetemi, D.; Pinson, J. Surface functionalisation of polymers. Chem. Soc. Rev. 2017, 46, 5701–5713. [Google Scholar] [CrossRef]
- Custódio, C.A.; del Campo, A.; Reis, R.L.; Mano, J.F. Smart instructive polymer substrates for tissue engineering. In Smart Polymers and Their Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 411–438. [Google Scholar]
- Chai, C.; Leong, K.W. Biomaterials approach to expand and direct differentiation of stem cells. Mol. Ther. 2007, 15, 467–480. [Google Scholar] [CrossRef]
- Bolger, M.; Groynom, R.; Bogie, K.; Lavik, E. Reporter scaffolds for clinically relevant cell transplantation studies. Ann. Biomed. Eng. 2020, 48, 1982–1990. [Google Scholar] [CrossRef]
- Agrawal, N.K.; Allen, P.; Song, Y.H.; Wachs, R.A.; Du, Y.; Ellington, A.D.; Schmidt, C.E. Oligonucleotide-functionalized hydrogels for sustained release of small molecule (aptamer) therapeutics. Acta Biomater. 2020, 102, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, Y.; Chen, X.; Zeng, C.; Hu, Q.; Yin, W.; Li, W.; Xie, H.; Zhang, B.; Huang, X.; et al. Nanoparticle-modified chitosan-agarose-gelatin scaffold for sustained release of SDF-1 and BMP-2. Int. J. Nanomed. 2018, 13, 7395–7408. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Zhao, R.; Wang, C.; Qiu, F.; Sun, B.; Ji, H.; Qiu, J.; Wang, C. Enhanced adhesion and proliferation of human umbilical vein endothelial cells on conductive PANI-PCL fiber scaffold by electrical stimulation. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 106–112. [Google Scholar] [CrossRef]
- Alanezi, A.A.; Neau, S.H.; D’mello, A.P. Development and application of a modified method to determine the encapsulation efficiency of proteins in polymer matrices. AAPS PharmSciTech 2020, 21, 248. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-C.; Li, X.-R.; Wei, W.-J.; Wei, Z.-Y.; Zhang, C.-R.; Wang, F.; Dawes, H.; Guo, S.-C. Polymeric coating on β-TCP scaffolds provides immobilization of small extracellular vesicles with surface-functionalization and ZEB1-Loading for bone defect repair in diabetes mellitus. Biomaterials 2022, 283, 121465. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Peng, K.; Fu, X.; Zhang, E.; Lv, F.; Liu, L.; Zhang, N.; Wang, Y.; Wang, S.; et al. Supramolecular nanofibers for encapsulation and in situ differentiation of neural stem cells. Adv. Healthc. Mater. 2020, 9, e1901295. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Jain, Y.; Sinha, S.; Rastogi, A.; Pradeep, S. Design and evaluation of chitosan/chondroitin sulfate/nano-bioglass based composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 817–830. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Sirova, M.; Rossmann, P.; Thielecke, H.; Boterberg, V.; Rihova, B.; Schacht, E.; Dubruel, P. Surface modification of polyimide sheets for regenerative medicine applications. Biomacromolecules 2010, 11, 2731–2739. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Hong, Y.; Fu, Q.; He, Q.; Mechakra, A.; Zhu, Q.; Zhou, F.; Liang, R.; Li, C.; et al. Tissue-Adhesive Paint of Silk Microparticles for Articular Surface Cartilage Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 22467–22478. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Huang, W.; Wu, L.; An, Y.; Xuan, T.; He, H.; Ye, M.; Qi, L.; Wu, J. Bioactive ECM Mimic Hyaluronic Acid Dressing via Sustained Releasing of bFGF for Enhancing Skin Wound Healing. ACS Appl. Bio Mater. 2020, 3, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Kim, Y.S.; Xie, V.Y.; Smith, B.T.; Watson, E.; Lam, J.; Pearce, H.A.; Engel, P.S.; Mikos, A.G. Modular, tissue-specific, and biodegradable hydrogel cross-linkers for tissue engineering. Sci. Adv. 2019, 5, eaaw7396. [Google Scholar] [CrossRef]
- Lee, C.-S.; Kim, S.; Fan, J.; Hwang, H.S.; Aghaloo, T.; Lee, M. Smoothened agonist sterosome immobilized hybrid scaffold for bone regeneration. Sci. Adv. 2020, 6, eaaz7822. [Google Scholar] [CrossRef] [PubMed]
- Adipurnama, I.; Yang, M.-C.; Ciach, T.; Butruk-Raszeja, B. Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: A review. Biomater. Sci. 2016, 5, 22–37. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Chang, J. Bioceramics to regulate stem cells and their microenvironment for tissue regeneration. Mater. Today 2018, 24, 41–56. [Google Scholar] [CrossRef]
- Trucco, M. Regeneration of the pancreatic beta cell. J. Clin. Investig. 2005, 115, 5–12. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Moore, K.A.; Lemischka, I.R. Stem cells and their niches. Science 2006, 311, 1880–1885. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.G. Stem cells in tissue engineering. Nature 2001, 414, 118–121. [Google Scholar] [CrossRef]
- Cai, W. Engineering in Translational Medicine, 2014th ed.; Springer: London, UK, 2013. [Google Scholar]
- Evans, N.D.; Gentleman, E.; Polak, J.M. Scaffolds for stem cells. Mater. Today 2006, 9, 26–33. [Google Scholar] [CrossRef]
- Deng, W. Induced pluripotent stem cells: Paths to new medicines. A catalyst for disease modelling, drug discovery and regenerative therapy. EMBO Rep. 2010, 11, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Colman, A.; Dreesen, O. Induced pluripotent stem cells and the stability of the differentiated state. EMBO Rep. 2009, 10, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Kimbrel, E.A.; Lanza, R. Next-generation stem cells—Ushering in a new era of cell-based therapies. Nat. Rev. Drug Discov. 2020, 19, 463–479. [Google Scholar] [CrossRef]
- Fernández Vallone, V.B.; Romaniuk, M.A.; Choi, H.; Labovsky, V.; Otaegui, J.; Chasseing, N.A. Mesenchymal stem cells and their use in therapy: What has been achieved. Differentiation 2013, 85, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Falzarano, M.S.; Ferlini, A. Urinary stem cells as tools to study genetic disease: Overview of the literature. J. Clin. Med. 2019, 8, 627. [Google Scholar] [CrossRef]
- Duffy, G.P.; Robinson, S.T.; O’Connor, R.; Wylie, R.; Mauerhofer, C.; Bellavia, G.; Straino, E.; Cianfarani, F.; Mendez, K.; Beatty, R.; et al. Implantable therapeutic reservoir systems for diverse clinical applications in large animal models. Adv. Health Mater. 2020, 9, e2000305. [Google Scholar] [CrossRef]
- Chhabra, R.; Peshattiwar, V.; Pant, T.; Deshpande, A.; Modi, D.; Sathaye, S.; Tibrewala, A.; Dyawanapelly, S.; Jain, R.D.; Dandekar, P. In Vivo Studies of 3D Starch–Gelatin Scaffolds for Full-Thickness Wound Healing. ACS Appl. Bio Mater. 2020, 3, 2920–2929. [Google Scholar] [CrossRef]
- Del Sol, A.; Thiesen, H.J.; Imitola, J.; Carazo Salas, R.E. Big-Data-Driven Stem Cell Science and Tissue Engineering: Vision and Unique Opportunities. Cell Stem Cell 2017, 20, 157–160. [Google Scholar] [CrossRef]
- Little, M.; Liu, G.-H.; Shenoy, K.V.; Vunjak-Novakovic, G.; Radisic, M. Engineering tissues and organs: The road to the clinic. Cell 2020, 81, 22–23. [Google Scholar]
- Abutaleb, N.O.; Truskey, G.A. Human iPSCs Stretch to Improve Tissue-Engineered Vascular Grafts. Cell Stem Cell 2020, 26, 136–137. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. Stem cells, regenerative medicine, and Prometheus. Lancet 2018, 391, 814. [Google Scholar] [CrossRef]
- Cossu, G.; Birchall, M.; Brown, T.; De Coppi, P.; Culme-Seymour, E.; Gibbon, S.; Hitchcock, J.; Mason, C.; Montgomery, J.; Morris, S.; et al. Lancet Commission: Stem cells and regenerative medicine. Lancet 2018, 391, 883–910. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Hong, Y.; Liang, R.; Zhang, X.; Liao, Y.; Jiang, D.; Zhang, J.; Sheng, Z.; Xie, C.; Peng, Z.; et al. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration. Biomaterials 2020, 258, 120287. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Synthetic biodegradable polymers for tissue engineering and drug delivery. Curr. Opin. Solid State Mater. Sci. 1998, 3, 246–251. [Google Scholar] [CrossRef]
- Bayat, S.; Amiri, N.; Pishavar, E.; Kalalinia, F.; Movaffagh, J.; Hashemi, M. Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci. 2019, 229, 57–66. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Krishnakumar, G.S.; Sampath, S.; Muthusamy, S.; John, M.A. Importance of crosslinking strategies in designing smart biomaterials for bone tissue engineering: A systematic review. Mater. Sci. Eng. C 2018, 96, 941–954. [Google Scholar] [CrossRef]
- Samadian, H.; Khastar, H.; Ehterami, A.; Salehi, M. Bioengineered 3D nanocomposite based on gold nanoparticles and gelatin nanofibers for bone regeneration: In vitro and in vivo study. Sci. Rep. 2021, 11, 13877. [Google Scholar] [CrossRef]
- Abbas, T.O.; Ali, T.A.; Uddin, S. Urine as a Main Effector in Urological Tissue Engineering—A Double-Edged Sword. Cells 2020, 9, 538. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Bivalacqua, T.J. Regenerative medicine in urology: The future of urinary reconstruction. Trends Urol. Men’s Health 2020, 11, 9–12. [Google Scholar] [CrossRef]
- Adamowicz, J.; Pasternak, I.; Kloskowski, T.; Gniadek, M.; Van Breda, S.V.; Buhl, M.; Balcerczyk, D.; Gagat, M.; Grzanka, D.; Strupinski, W.; et al. Development of a conductive biocomposite combining graphene and amniotic membrane for replacement of the neuronal network of tissue-engineered urinary bladder. Sci. Rep. 2020, 10, 8524. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, R.S.; Williams, J.K.; Yoo, K.W.; Yoo, J.J.; Atala, A. A tissue-engineered uterus supports live births in rabbits. Nat. Biotechnol. 2020, 38, 1280–1287. [Google Scholar] [CrossRef]
- Takehara, H.; Sakaguchi, K.; Sekine, H.; Okano, T.; Shimizu, T. Microfluidic vascular-bed devices for vascularized 3D tissue engineering: Tissue engineering on a chip. Biomed. Microdevices 2019, 22, 9. [Google Scholar] [CrossRef]
- Wang, Y.; He, C.; Feng, Y.; Yang, Y.; Wei, Z.; Zhao, W.; Zhao, C. A chitosan modified asymmetric small-diameter vascular graft with anti-thrombotic and anti-bacterial functions for vascular tissue engineering. J. Mater. Chem. B 2019, 8, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Miyagawa, S.; Toyofuku, T.; Fukushima, S.; Kawamura, T.; Kawamura, A.; Kashiyama, N.; Nakamura, Y.; Toda, K. Syngeneic Mesenchymal Stem Cells Reduce Immune Rejection After Induced Pluripotent Stem Cell-Derived Allogeneic Cardiomyocyte Transplantation. Sci. Rep. 2020, 10, 4593. [Google Scholar] [CrossRef]
- Fiamingo, A.; Montembault, A.; Boitard, S.-E.; Naemetalla, H.; Agbulut, O.; Delair, T.; Campana-Filho, S.P.; Menasché, P.; David, L. Chitosan Hydrogels for the Regeneration of Infarcted Myocardium: Preparation, Physicochemical Characterization, and Biological Evaluation. Biomacromolecules 2016, 17, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Domengé, O.; Ragot, H.; Deloux, R.; Crépet, A.; Revet, G.; Boitard, S.E.; Simon, A.; Mougenot, N.; David, L.; Delair, T.; et al. Efficacy of epicardial implantation of acellular chitosan hydrogels in ischemic and nonischemic heart failure: Impact of the acetylation degree of chitosan. Acta Biomater. 2021, 119, 125–139. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Zaszczynska, A.; Sajkiewicz, P.; Gradys, A. Piezoelectric Scaffolds as Smart Materials for Neural Tissue Engineering. Polymers 2020, 12, 161. [Google Scholar] [CrossRef]
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17, 20190505. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Zamanian, A.; Kermanian, F.; Shamoosi, A. A bioinspired 3D shape olibanum-collagen-gelatin scaffolds with tunable porous microstructure for efficient neural tissue regeneration. Biotechnol. Prog. 2019, 36, e2918. [Google Scholar] [CrossRef] [PubMed]
- Kourgiantaki, A.; Tzeranis, D.S.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen. Med. 2020, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Louis, F.; Matsusaki, M. Adipose tissue engineering. In Biomaterials for Organ and Tissue Regeneration; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–423. [Google Scholar]
- Nagano, H.; Suematsu, Y.; Takuma, M.; Aoki, S.; Satoh, A.; Takayama, E.; Kinoshita, M.; Morimoto, Y.; Takeoka, S.; Fujie, T.; et al. Enhanced cellular engraftment of adipose-derived mesenchymal stem cell spheroids by using nanosheets as scaffolds. Sci. Rep. 2021, 11, 14500. [Google Scholar] [CrossRef]
- Rodríguez, A.P.; Felice, B.; Sánchez, M.A.; Tsujigiwa, H.; Felice, C.J.; Nagatsuka, H. In Vivo evaluation of adipogenic induction in fibrous and honeycomb-structured atelocollagen scaffolds. Mater. Sci. Eng. C 2016, 63, 125–130. [Google Scholar] [CrossRef]
- Geris, L.; Papantoniou, I. The Third Era of Tissue Engineering: Reversing the Innovation Drivers. Tissue Eng. Part A 2019, 25, 821–826. [Google Scholar] [CrossRef]
- Goh, B.T.; Teh, L.Y.; Tan, D.B.P.; Zhang, Z.; Teoh, S.H. Novel 3D polycaprolactone scaffold for ridge preservation—A pilot randomised controlled clinical trial. Clin. Oral Implant. Res. 2014, 26, 271–277. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, K.S.; Coric, D.; Harrop, J.S.; Theodore, N.; Toselli, R.M. Acute Implantation of a Bioresorbable Polymer Scaffold in Patients with Complete Thoracic Spinal Cord Injury: 24-Month Follow-Up from the INSPIRE Study. Neurosurgery 2022, 90, 668–675. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, K.S.; Coric, D.; Chang, J.J.; Harrop, J.S.; Theodore, N.; Toselli, R.M. A study of probable benefit of a bioresorbable polymer scaffold for safety and neurological recovery in patients with complete thoracic spinal cord injury: 6-month results from the INSPIRE study. J. Neurosurg. Spine 2021, 34, 808–817. [Google Scholar] [CrossRef]
- Suzuki, N.; Kozuma, K.; Nakamura, S.; Aramaki, K.; Saito, S.; Shibata, Y.; Nanasato, M.; Fujii, K.; Kusano, H.; Ediebah, D.; et al. Absorb GT1 Bioresorbable Vascular Scaffold System-1-Year Post-Marketing Surveillance in Japan. Circ. J. 2019, 83, 2460–2465. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kubo, S.; Sasaki, K.; Kawakami, Y.; Oka, S.; Sasaki, H.; Takayama, K.; Tei, K.; Matsushita, T.; Mifune, Y.; et al. Acceleration of Tendon-Bone Healing of Anterior Cruciate Ligament Graft Using Autologous Ruptured Tissue. Am. J. Sports Med. 2012, 40, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- DiDomenico, L.A.; Orgill, D.P.; Galiano, R.D.; Serena, T.E.; Carter, M.J.; Kaufman, J.P.; Young, N.J.; Zelen, C.M. Aseptically processed placental membrane improves healing of diabetic foot ulcerations: Prospective, randomized clinical trial. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1095. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Rinker, B.D.; Weber, R.V.; Chao, J.D.; Ingari, J.V.; Brooks, D.; Buncke, G.M. Functional Outcome Following Nerve Repair in the Upper Extremity Using Processed Nerve Allograft. J. Hand Surg. 2012, 37, 2340–2349. [Google Scholar] [CrossRef]
- El Shazley, N.; Hamdy, A.; El-Eneen, H.A.; El Backly, R.M.; Saad, M.M.; Essam, W.; Moussa, H.; El Tantawi, M.; Jain, H.; Marei, M.K. Bioglass in alveolar bone regeneration in orthodontic patients: Randomized controlled clinical trial. JDR Clin. Trans. Res. 2016, 1, 244–255. [Google Scholar] [CrossRef]
- Ribichini, F.; Pighi, M.; Faggian, G.; Vassanelli, C. Bioresorbable vascular scaffolds in cardiac allograft vasculopathy: A new therapeutic option. Am. J. Med. 2013, 126, e11–e14. [Google Scholar] [CrossRef]
- Pighi, M.; Tomai, F.; Petrolini, A.; De Luca, L.; Tarantini, G.; Barioli, A.; Colombo, P.; Klugmann, S.; Ferlini, M.; Ormezzano, M.F.; et al. Everolimus-Eluting Bioresorbable Vascular Scaffold System in the Treatment of Cardiac Allograft Vasculopathy: The CART (Cardiac Allograft Reparative Therapy) Prospective Multicenter Pilot Study. J. Cardiovasc. Transl. Res. 2015, 9, 40–48. [Google Scholar] [CrossRef]
- Fine, N.A.; Lehfeldt, M.; Gross, J.E.; Downey, S.; Kind, G.M.; Duda, G.; David, K.; Rebecc, H.; Jeff, I.; Mark, J. SERI surgical scaffold, prospective clinical trial of a silk-derived biological scaffold in two-stage breast reconstruction: 1-year data. Plast. Reconstr. Surg. 2015, 135, 339–351. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Gong, J.; Yang, L.; Niu, C.; Ni, X.; Wang, Y.; Peng, S.; Gu, X.; Sun, C.; et al. Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials 2017, 134, 64–77. [Google Scholar] [CrossRef]
- Diebold, G.; Lam, P.; Walton, J.; Murrell, G.A.C. Relationship between age and rotator cuff retear: A study of 1,600 consecutive rotator cuff repairs. J. Bone Jt. Surg. Am. 2017, 99, 1198–1205. [Google Scholar] [CrossRef]
- Serruys, P.W.; Chevalier, B.; Sotomi, Y.; Cequier, A.; Carrié, D.; Piek, J.J.; Van Boven, A.J.; Dominici, M.; Dudek, D.; McClean, D.; et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): A 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016, 388, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, K.; Ueki, Y.; Souteyrand, G.; Daemen, J.; Wiebe, J.; Nef, H.; Adriaenssens, T.; Loh, J.P.; Lattuca, B.; Wykrzykowska, J.J.; et al. Mechanisms of very late bioresorbable scaffold thrombosis: The INVEST registry. J. Am. Coll. Cardiol. 2017, 70, 2330–2344. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xu, B.; Chen, Y.; Zhou, Y.; Jia, S.; Zhong, Z.; Su, X.; Ma, Y.; Zhang, Q.; Liu, J.; et al. Thinner Strut Sirolimus-Eluting BRS Versus EES in Patients with Coronary Artery Disease: FUTURE-II Trial. JACC Cardiovasc. Interv. 2021, 14, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).