Effects of a Myofascial Technique on the Stiffness and Thickness of the Thoracolumbar Fascia and Lumbar Erector Spinae Muscles in Adults with Chronic Low Back Pain: A Randomized before-and-after Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Participants
2.3. Randomization and Blinding
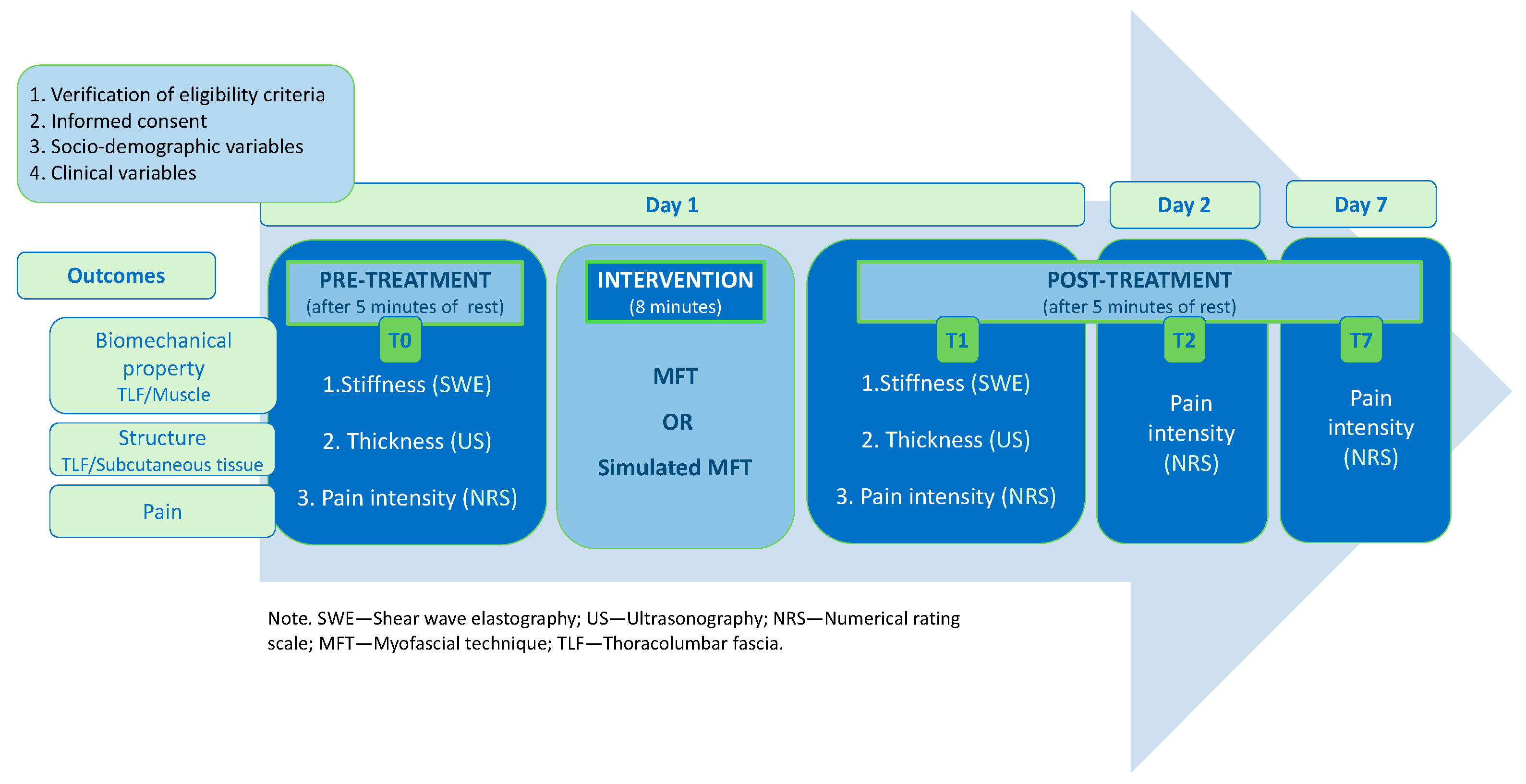
2.4. Procedures
2.5. Treatment
2.6. Outcomes and Instruments
2.6.1. Primary Outcome—Stiffness
2.6.2. Secondary Outcome—Thickness
2.6.3. Secondary Outcome—Pain Intensity
2.7. Statistical Analysis
3. Results
3.1. Participants
3.2. Stiffness
3.3. Thickness
3.4. Pain Intensity
4. Discussion
4.1. Tissue Stiffness
4.2. Tissue Thickness
4.3. Pain Intensity
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A Systematic Review of the Global Prevalence of Low Back Pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Balagué, F.; Mannion, A.F.; Pellisé, F.; Cedraschi, C. Non-Specific Low Back Pain. Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, R.; Blyth, F.M.; March, L.M.; Brooks, P.; Woolf, A.D.; Hoy, D.G. Placing the Global Burden of Low Back Pain in Context. Best Pract. Res. Clin. Rheumatol. 2013, 27, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years Lived with Disability (YLDs) for 1160 Sequelae of 289 Diseases and Injuries 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Husky, M.M.; Ferdous Farin, F.; Compagnone, P.; Fermanian, C.; Kovess-Masfety, V. Chronic Back Pain and Its Association with Quality of Life in a Large French Population Survey. Health Qual. Life Outcomes 2018, 16, 195. [Google Scholar] [CrossRef]
- Maetzel, A.; Li, L. The Economic Burden of Low Back Pain: A Review of Studies Published between 1996 and 2001. Best Pract. Res. Clin. Rheumatol. 2002, 16, 23–30. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-Specific Low Back Pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Langevin, H.M.; Sherman, K.J. Pathophysiological Model for Chronic Low Back Pain Integrating Connective Tissue and Nervous System Mechanisms. Med. Hypotheses 2007, 68, 74–80. [Google Scholar] [CrossRef]
- Langevin, H.M.; Stevens-Tuttle, D.; Fox, J.R.; Badger, G.J.; Bouffard, N.A.; Krag, M.H.; Wu, J.; Henry, S.M. Ultrasound Evidence of Altered Lumbar Connective Tissue Structure in Human Subjects with Chronic Low Back Pain. BMC Musculoskelet. Disord. 2009, 10, 151. [Google Scholar] [CrossRef]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan- Naumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.-N.; Triano, J.J.; Henry, S.M. Reduced Thoracolumbar Fascia Shear Strain in Human Chronic Low Back Pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef]
- Wilke, J.; Schleip, R.; Klingler, W.; Stecco, C. The Lumbodorsal Fascia as a Potential Source of Low Back Pain: A Narrative Review. BioMed Res. Int. 2017, 2017, 5349620. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Bishop, J.; Maple, R.; Badger, G.J.; Fox, J.R. Effect of Stretching on Thoracolumbar Fascia Injury and Movement Restriction in a Porcine Model. Am. J. Phys. Med. Rehabil. 2018, 97, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M. Fascia Mobility, Proprioception, and Myofascial Pain. Life 2021, 11, 668. [Google Scholar] [CrossRef]
- Casato, G.; Stecco, C.; Busin, R. Role of Fasciae in Nonspecific Low Back Pain. Eur. J. Transl. Myol. 2019, 29, 8330. [Google Scholar] [CrossRef]
- Tesarz, J.; Hoheisel, U.; Wiedenhöfer, B.; Mense, S. Sensory Innervation of the Thoracolumbar Fascia in Rats and Humans. Neuroscience 2011, 194, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Mense, S. Innervation of the Thoracolumbar Fascia. Eur. J. Transl. Myol. 2019, 29, 8297. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Petrelli, L.; Guidolin, D.; Porzionato, A.; Pirri, C.; Fan, C.; De Caro, R.; Stecco, C. Evidence of a New Hidden Neural Network into Deep Fasciae. Sci. Rep. 2021, 11, 12623. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Rodriguez, V.; Fede, C.; Pirri, C.; Lucia, P.; Loro-Ferrer, J.; Rodriguez-Ruiz, D.; Caro, R.; Stecco, C. Fascial Innervation: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 5674. [Google Scholar] [CrossRef] [PubMed]
- Schilder, A.; Hoheisel, U.; Magerl, W.; Benrath, J.; Klein, T.; Treede, R.-D. Sensory Findings after Stimulation of the Thoracolumbar Fascia with Hypertonic Saline Suggest Its Contribution to Low Back Pain. Pain 2014, 155, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Schilder, A.; Magerl, W.; Hoheisel, U.; Klein, T.; Treede, R.-D. Electrical High-Frequency Stimulation of the Human Thoracolumbar Fascia Evokes Long-Term Potentiation-like Pain Amplification. Pain 2016, 157, 2309–2317. [Google Scholar] [CrossRef]
- Sinhorim, L.; Amorim, M.d.S.; Ortiz, M.E.; Bittencourt, E.B.; Bianco, G.; da Silva, F.C.; Horewicz, V.V.; Schleip, R.; Reed, W.R.; Mazzardo-Martins, L.; et al. Potential Nociceptive Role of the Thoracolumbar Fascia: A Scope Review Involving In Vivo and Ex Vivo Studies. J. Clin. Med. 2021, 10, 4342. [Google Scholar] [CrossRef]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The Thoracolumbar Fascia: Anatomy, Function and Clinical Considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C. Deep Fascia. In Functional Atlas of the Human Fascial System; Elsevier: Amsterdam, The Netherlands, 2015; p. 385. ISBN 978-7020-4430-4. [Google Scholar]
- Stecco, C.; Stern, R.; Porzionato, A.; Macchi, V.; Masiero, S.; Stecco, A.; De Caro, R. Hyaluronan within Fascia in the Etiology of Myofascial Pain. Surg. Radiol. Anat. 2011, 33, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 622. [Google Scholar] [CrossRef]
- Koppenhaver, S.; Gaffney, E.; Oates, A.; Eberle, L.; Young, B.; Hebert, J.; Proulx, L.; Shinohara, M. Lumbar Muscle Stiffness Is Different in Individuals with Low Back Pain than Asymptomatic Controls and Is Associated with Pain and Disability, but Not Common Physical Examination Findings. Musculoskelet. Sci. Pract. 2020, 45, 102078. [Google Scholar] [CrossRef]
- Bishop, J.H.; Fox, J.R.; Maple, R.; Loretan, C.; Badger, G.J.; Henry, S.M.; Vizzard, M.A.; Langevin, H.M. Ultrasound Evaluation of the Combined Effects of Thoracolumbar Fascia Injury and Movement Restriction in a Porcine Model. PLoS ONE 2016, 11, e0147393. [Google Scholar] [CrossRef]
- Nelson-Wong, E.; Glinka, M.; Noguchi, M.; Langevin, H.; Badger, G.J.; Callaghan, J.P. Acute Surgical Injury Alters the Tensile Properties of Thoracolumbar Fascia in a Porcine Model. J. Biomech. Eng. 2018, 140, 101012. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.F. Myofascial Release: The Search for Excellence—A Comprehensive Evaluatory and Treatment Approach, 10th ed.; Rehabilitation Services, Inc.: Laurel, MD, USA, 1990; ISBN 978-1-929894-00-0. [Google Scholar]
- Chen, Z.; Wu, J.; Wang, X.; Wu, J.; Ren, Z. The Effects of Myofascial Release Technique for Patients with Low Back Pain: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2021, 59, 102737. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Y.; Ye, X.; Chen, Z.; Zhou, R.; Ye, Z.; Huang, J.; Zhu, Y.; Chen, G.; Xu, X. Myofascial Release for Chronic Low Back Pain: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 697986. [Google Scholar] [CrossRef]
- Stecco, A.; Meneghini, A.; Stern, R.; Stecco, C.; Imamura, M. Ultrasonography in Myofascial Neck Pain: Randomized Clinical Trial for Diagnosis and Follow-Up. Surg. Radiol. Anat. 2014, 36, 243–253. [Google Scholar] [CrossRef]
- Ichikawa, K.; Takei, H.; Usa, H.; Mitomo, S.; Ogawa, D. Comparative Analysis of Ultrasound Changes in the Vastus Lateralis Muscle Following Myofascial Release and Thermotherapy: A Pilot Study. J. Bodyw. Mov. Ther. 2015, 19, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.-K.; Chai, H.-M.; Chen, Y.-J.; Wang, C.-L.; Shau, Y.-W.; Wang, S.-F. Mechanical Deformation of Posterior Thoracolumbar Fascia after Myofascial Release in Healthy Men: A Study of Dynamic Ultrasound Imaging. Musculoskelet. Sci. Pract. 2017, 27, 124–130. [Google Scholar] [CrossRef]
- Gao, J.; Caldwell, J.; McLin, K.; Zhang, M.; Park, D. Ultrasound Shear Wave Elastography to Assess Osteopathic Manipulative Treatment on the Iliocostalis Lumborum Muscle: A Feasibility Study: Shear Wave Elastography to Assess Osteopathic Manipulative Treatment. J. Ultrasound Med. 2019, 39, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Lohr, C.; Medina-Porqueres, I. Immediate Effects of Myofascial Release on Neuromechanical Characteristics in Female and Male Patients with Low Back Pain and Healthy Controls as Assessed by Tensiomyography. A Controlled Matched-Pair Study. Clin. Biomech. 2021, 84, 105351. [Google Scholar] [CrossRef]
- Tamartash, H.; Bahrpeyma, F.; Mokhtari Dizaji, M. Comparative Effect of Lumbar Myofascial Release with Electrotherapy on the Elastic Modulus of Lumbar Fascia and Pain in Patients with Non-Specific Low Back Pain. J. Bodyw. Mov. Ther. 2022, 29, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ilahi, S.T.; Masi, A.; White, A.; Devos, A.; Henderson, J.; Nair, K. Quantified Biomechanical Properties of Lower Lumbar Myofascia in Younger Adults with Chronic Idiopathic Low Back Pain and Matched Healthy Controls. Clin. Biomech. 2020, 73, 78–85. [Google Scholar] [CrossRef]
- Langevin, H.M.; Konofagou, E.E.; Badger, G.J.; Churchill, D.L.; Fox, J.R.; Ophir, J.; Garra, B.S. Tissue Displacements during Acupuncture Using Ultrasound Elastography Techniques. Ultrasound Med. Biol. 2004, 30, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lei, D.; Li, L.; Leng, Y.; Yu, Q.; Wei, X.; Lo, W.L.A. Quantifying Paraspinal Muscle Tone and Stiffness in Young Adults with Chronic Low Back Pain: A Reliability Study. Sci. Rep. 2018, 8, 14343. [Google Scholar] [CrossRef]
- Nair, K.; Masi, A.T.; Andonian, B.J.; Barry, A.J.; Coates, B.A.; Dougherty, J.; Schaefer, E.; Henderson, J.; Kelly, J. Stiffness of Resting Lumbar Myofascia in Healthy Young Subjects Quantified Using a Handheld Myotonometer and Concurrently with Surface Electromyography Monitoring. J. Bodyw. Mov. Ther. 2016, 20, 388–396. [Google Scholar] [CrossRef]
- Educational Council on Osteopathic Principles (ECOP). Glossary of Osteopathic Terminology, 3rd Edition. Available online: https://www.aacom.org/docs/default-source/default-document-library/glossary2017.pdf?sfvrsn=a41c3b97_0 (accessed on 26 September 2019).
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. RadioGraphics 2017, 37, 855–870. [Google Scholar] [CrossRef]
- Eby, S.F.; Song, P.; Chen, S.; Chen, Q.; Greenleaf, J.F.; An, K.-N. Validation of Shear Wave Elastography in Skeletal Muscle. J. Biomech. 2013, 46, 2381–2387. [Google Scholar] [CrossRef]
- Koo, T.K.; Guo, J.-Y.; Cohen, J.H.; Parker, K.J. Relationship between Shear Elastic Modulus and Passive Muscle Force: An Ex-Vivo Study. J. Biomech. 2013, 46, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhao, H.; Liao, L.; Zhang, Z.; Liu, C. Reliability of Shear-Wave Elastography in Assessing Thoracolumbar Fascia Elasticity in Healthy Male. Sci. Rep. 2020, 10, 19952. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; Koppenhaver, S.L.; Michener, L.A.; Proulx, L.; Bisagni, F.; Cleland, J.A. Characterization of Tissue Stiffness of the Infraspinatus, Erector Spinae, and Gastrocnemius Muscle Using Ultrasound Shear Wave Elastography and Superficial Mechanical Deformation. J. Electromyogr. Kinesiol. 2018, 38, 73–80. [Google Scholar] [CrossRef]
- Stecco, C. Subcutaneous Tissue and Superficial Fascia. In Functional Atlas of the Human Fascial System; Churchill Livingstone: Edinburgh, UK; Elsevier: New York, NY, USA, 2015; ISBN 978-0-7020-4430-4. [Google Scholar]
- Haefeli, M.; Elfering, A. Pain Assessment. Eur. Spine J. 2006, 15, S17–S24. [Google Scholar] [CrossRef]
- Younger, J.; McCue, R.; Mackey, S. Pain Outcomes: A Brief Review of Instruments and Techniques. Curr. Pain Headache Rep. 2009, 13, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Yahia, L.H.; Pigeon, P.; DesRosiers, E.A. Viscoelastic Properties of the Human Lumbodorsal Fascia. J. Biomed. Eng. 1993, 15, 425–429. [Google Scholar] [CrossRef]
- Schleip, R.; Duerselen, L.; Vleeming, A.; Naylor, I.L.; Lehmann-Horn, F.; Zorn, A.; Jaeger, H.; Klingler, W. Strain Hardening of Fascia: Static Stretching of Dense Fibrous Connective Tissues Can Induce a Temporary Stiffness Increase Accompanied by Enhanced Matrix Hydration. J. Bodyw. Mov. Ther. 2012, 16, 94–100. [Google Scholar] [CrossRef]
- Schleip, R.; Findley, T.; Chaitow, L.; Huijing, P.A. Fascia: The Tensional Network of the Human Body: The Science and Clinical Applications in Manual and Movement Therapy; Churchill Livingstone: Edinburgh, UK; Elsevier: New York, NY, USA, 2012; ISBN 978-0-7020-3425-1. [Google Scholar]
- Fede, C.; Petrelli, L.; Pirri, C.; Neuhuber, W.; Tiengo, C.; Biz, C.; De Caro, R.; Schleip, R.; Stecco, C. Innervation of Human Superficial Fascia. Front. Neuroanat. 2022, 16, 981426. [Google Scholar] [CrossRef]





| Parameters | Sample (n = 48) | MFT (n = 24) | Simulated MFT (n = 24) | p Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 37.4 ± 13.3 | 37.9 ± 14.2 | 37.0 ± 12.7 | 0.81 a |
| Sex (male/female), n, % | 24/24 (50%/50%) | 12/12 (50%/50%) | 12/12 (50%/50%) | 1.00 b |
| Dominance (right/left), n, % | 46/2 (91.7%/8.3%) | 24/0 (100%/0%) | 22/2 (91.7%/8.3%) | 0.49 c |
| Duration of pain (months), median (IQR 25th–75th) | 48 (36–69) | 42 (15–60) | 60 (42–90) | 0.09 d |
| BMI (kg/cm2), median (IQR 25th–75th) | 24.5 (22.6–29.6) | 24.7 (22.4–30) | 24.3 (22.6–28.5) | 0.89 d |
| Schober index (cm), mean ± SD | 6.8 ± 1.6 | 6.5 ± 1.6 | 7.1 ± 1.5 | 0.18 a |
| SF-MPQ total score (/45), median (IQR 25th–75th) | 11 (9–15.8) | 11 (10–14) | 11 (8.3–17.8) | 0.95 d |
| SF-MPQ sensory score (/33), median (IQR 25th–75th) | 9 (7.3–12) | 8 (7–13.5) | 8 (7–13.5) | 0.72 d |
| SF-MPQ affective score (/12), median (IQR, 25th–75th) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.69 d |
| SF-MPQ VAS (cm), mean ± SD | 44.0 ± 19.1 | 49.3 ± 19.4 | 38.7 ± 17.6 | 0.05 a |
| SF-MPQ present pain index (0–5), n, % | 1:18 (37.5%) | 1:7 (29.2%) | 1:11 (45.8%) | 0.16 d |
| 2:23 (47.9%) | 2:12 (50%) | 2:11 (45.3%) | ||
| 3:5 (10.4%) | 3:4 (16.7%) | 3:1 (4.2%) | ||
| 4:1 (2.1%) | 4:0 | 4:1 (4.2%) | ||
| 5:1 (2.1%) | 5:1 (4.17%) | 5:0 | ||
| ODS (%), mean ± SD | 21.4 ± 8.5 | 21.4 ± 9.3 | 21.3 ± 7.8 | 0.97 a |
| IPAQ (MET/week), median (IQR 25th–75th) | 3197.8 (1575–6004) | 2435 (1599–6975) | 3496 (1502–5220) | 0.78 d |
| (n = 46) | (n = 23) | (n = 23) | ||
| IPAQ categorical score (low/moderate/high), n, % | Low: 8 (17.4%) | Low: 4 (17.4%) | Low: 4 (17.4%) | 1.00 d |
| Mod: 10 (21.7%) | Mod: 5 (21.7%) | Mod: 5 (21.7%) | ||
| High: 28 (60.9%) | High: 14 (60.9%) | High: 14 (60.9%) | ||
| (n = 46) | (n = 23) | (n = 23) | ||
| IPAQ sitting (hours/week) | 39.2 ± 18.3 | 39.7 ± 17.6 | 38.6 ± 19.4 | 0.83 a |
| Structures | Descriptive Statistics | Generalized Estimating Equation Models | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MFT | Simulated MFT | Mean Difference between Groups | 95% CI | p Value | ||||||
| n | Mean | (SD) | n | Mean | (SD) | |||||
| TFL-R | ||||||||||
| Pre-treatment | 24 | 75.3 | 37.2 | 24 | 59.6 | 24.8 | ||||
| Post-treatment | 24 | 74.4 | 37.4 | 23 | 71.6 | 35.4 | −13.0 | −32.5 | 6.5 | 0.191 |
| TFL-L | ||||||||||
| Pre-treatment | 24 | 73.4 | 38.6 | 24 | 60.6 | 35.3 | ||||
| Post-treatment | 24 | 70.9 | 40.0 | 23 | 59.3 | 17.5 | −1.2 | −17.7 | 15.2 | 0.880 |
| Muscle-R | ||||||||||
| Pre-treatment | 24 | 52.5 | 32.7 | 22 | 44.1 | 25.1 | ||||
| Post-treatment | 24 | 50.6 | 42.4 | 21 | 51.6 | 35.5 | −9.2 | −26.4 | 7.9 | 0.290 |
| Muscle-L | ||||||||||
| Pre-treatment | 23 | 54.0 | 37.8 | 23 | 39.5 | 18.2 | ||||
| Post-treatment | 23 | 41.5 | 17.7 | 22 | 43.1 | 14.6 | −16.1 | −31.1 | −1.14 | 0.035 * |
| Structures | Descriptive Statistics | Generalized Estimating Equation Models | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MFT | Simulated MFT | Mean Difference between Groups | 95% CI | p Value | ||||||
| n | Mean | (SD) | n | Mean | (SD) | |||||
| TFL-R | ||||||||||
| Pre-treatment | 24 | 2.00 | 0.64 | 24 | 1.80 | 0.45 | ||||
| Post-treatment | 24 | 1.95 | 0.62 | 23 | 1.77 | 0.41 | −0.02 | −0.10 | 0.06 | 0.640 |
| TFL-L | ||||||||||
| Pre-treatment | 24 | 1.90 | 0.50 | 24 | 1.78 | 0.42 | ||||
| Post-treatment | 24 | 1.88 | 0.52 | 23 | 1.83 | 0.43 | −0.07 | −0.13 | 0.00 | 0.039 * |
| SC tissue-R | ||||||||||
| Pre-treatment | 24 | 10.7 | 5.55 | 24 | 9.62 | 6.37 | ||||
| Post-treatment | 24 | 11.8 | 5.84 | 23 | 9.58 | 6.67 | 1.15 | 0.60 | 1.71 | <0.0001 * |
| SC tissue-L | ||||||||||
| Pre-treatment | 24 | 10.4 | 5.48 | 24 | 9.48 | 6.38 | ||||
| Post-treatment | 24 | 11.7 | 5.71 | 23 | 9.37 | 6.39 | 1.46 | 0.41 | 2.52 | 0.007 * |
| Descriptive Statistics | Generalized Estimating Equation Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | MFT | Simulated MFT | Mean Difference between Groups | 95% CI | p Value | |||||
| n | Mean | (SD) | n | Mean | (SD) | |||||
| Pain | ||||||||||
| Pre-treatment (T0) | 24 | 3.3 | 1.6 | 24 | 3.0 | 1.5 | ||||
| Post-treatment (T1) | 24 | 1.9 | 1.7 | 24 | 2.9 | 1.6 | −1.3 | −2.1 | 0.6 | <0.0001 * |
| Post-treatment (T2) | 24 | 2.3 | 1.6 | 24 | 2.7 | 1.3 | −0.8 | −1.5 | 0.0 | 0.043 * |
| Post-treatment (T7) | 24 | 2.5 | 1.5 | 24 | 3.1 | 1.7 | −0.9 | −1.9 | 0.1 | 0.081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devantéry, K.; Morin, M.; Grimard, J.; Gaudreault, N. Effects of a Myofascial Technique on the Stiffness and Thickness of the Thoracolumbar Fascia and Lumbar Erector Spinae Muscles in Adults with Chronic Low Back Pain: A Randomized before-and-after Experimental Study. Bioengineering 2023, 10, 332. https://doi.org/10.3390/bioengineering10030332
Devantéry K, Morin M, Grimard J, Gaudreault N. Effects of a Myofascial Technique on the Stiffness and Thickness of the Thoracolumbar Fascia and Lumbar Erector Spinae Muscles in Adults with Chronic Low Back Pain: A Randomized before-and-after Experimental Study. Bioengineering. 2023; 10(3):332. https://doi.org/10.3390/bioengineering10030332
Chicago/Turabian StyleDevantéry, Karine, Mélanie Morin, Julien Grimard, and Nathaly Gaudreault. 2023. "Effects of a Myofascial Technique on the Stiffness and Thickness of the Thoracolumbar Fascia and Lumbar Erector Spinae Muscles in Adults with Chronic Low Back Pain: A Randomized before-and-after Experimental Study" Bioengineering 10, no. 3: 332. https://doi.org/10.3390/bioengineering10030332
APA StyleDevantéry, K., Morin, M., Grimard, J., & Gaudreault, N. (2023). Effects of a Myofascial Technique on the Stiffness and Thickness of the Thoracolumbar Fascia and Lumbar Erector Spinae Muscles in Adults with Chronic Low Back Pain: A Randomized before-and-after Experimental Study. Bioengineering, 10(3), 332. https://doi.org/10.3390/bioengineering10030332






