Application of a Magnetic Platform in α6 Integrin-Positive iPSC-TM Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human TM Cell Isolation and Culture
2.2. Human iPSC Culture and Differentiation
2.3. Immunohistochemistry (IHC) Analysis
2.4. Magnetic Bead-Based Separation
2.5. Trypan Blue Staining Assay
2.6. Immunopanning (IP) Separation
2.7. Flow Cytometry Analysis
2.8. Statistical Analysis
3. Results
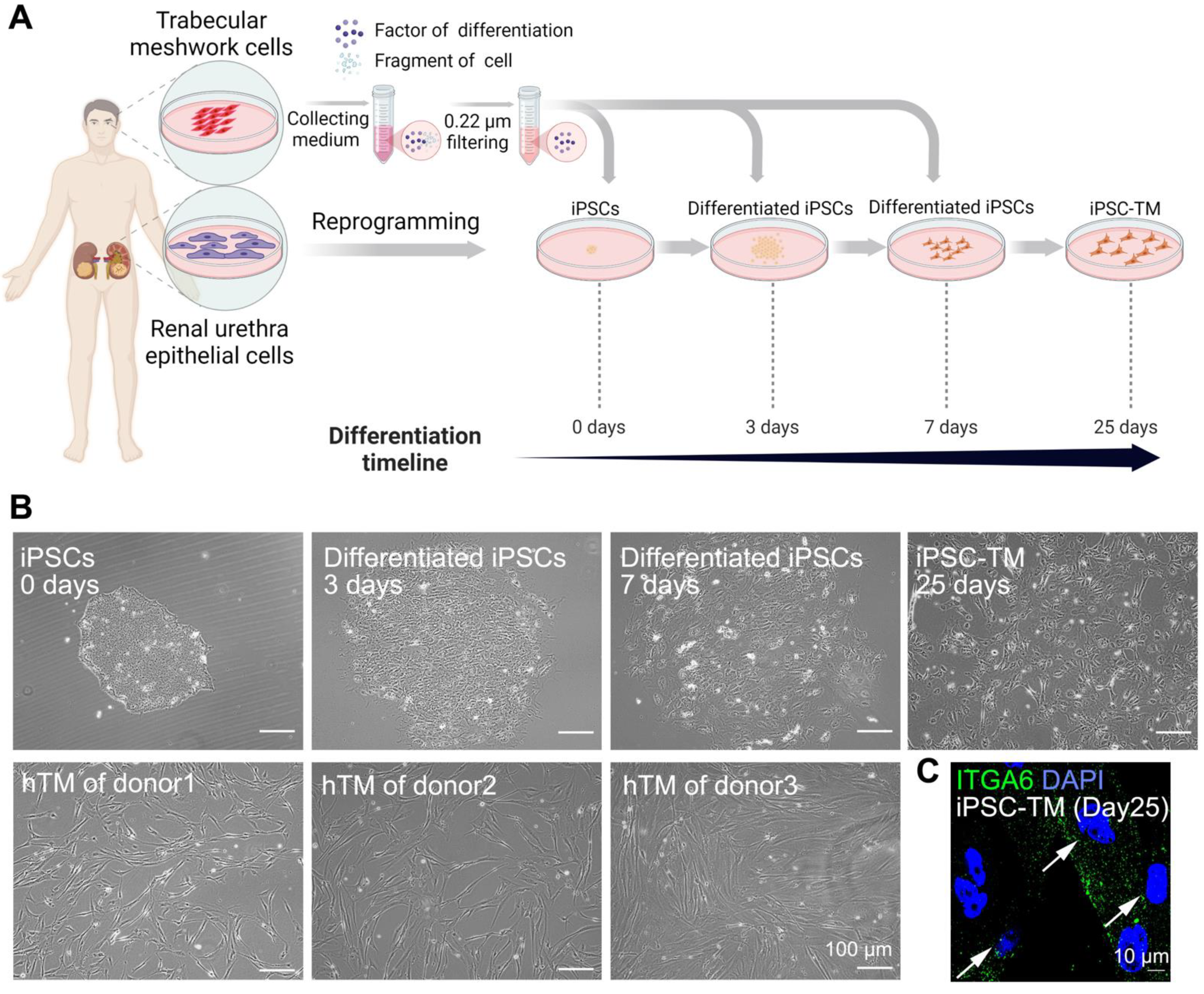
3.1. Differentiation of hiPSCs into iPSC-TM
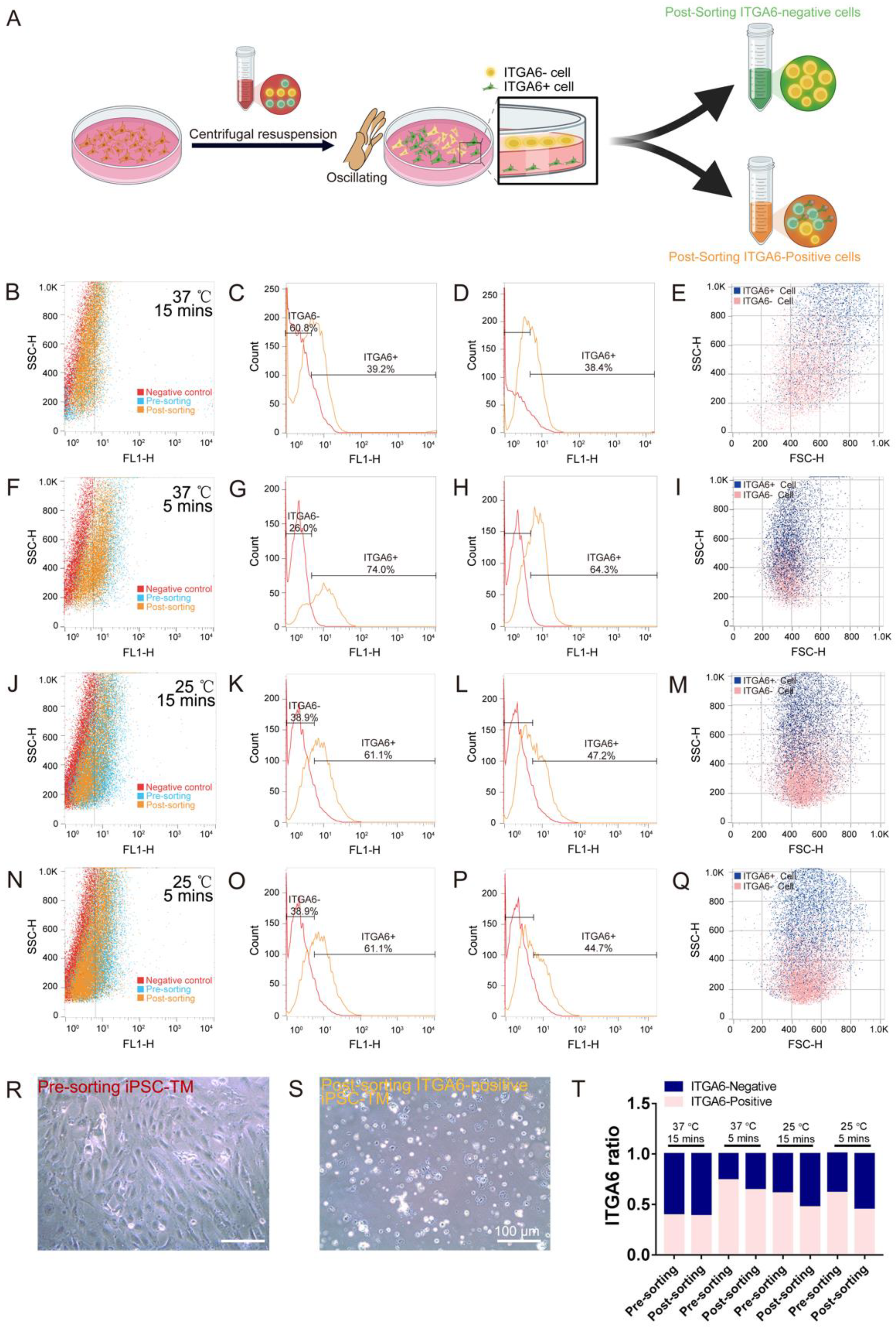
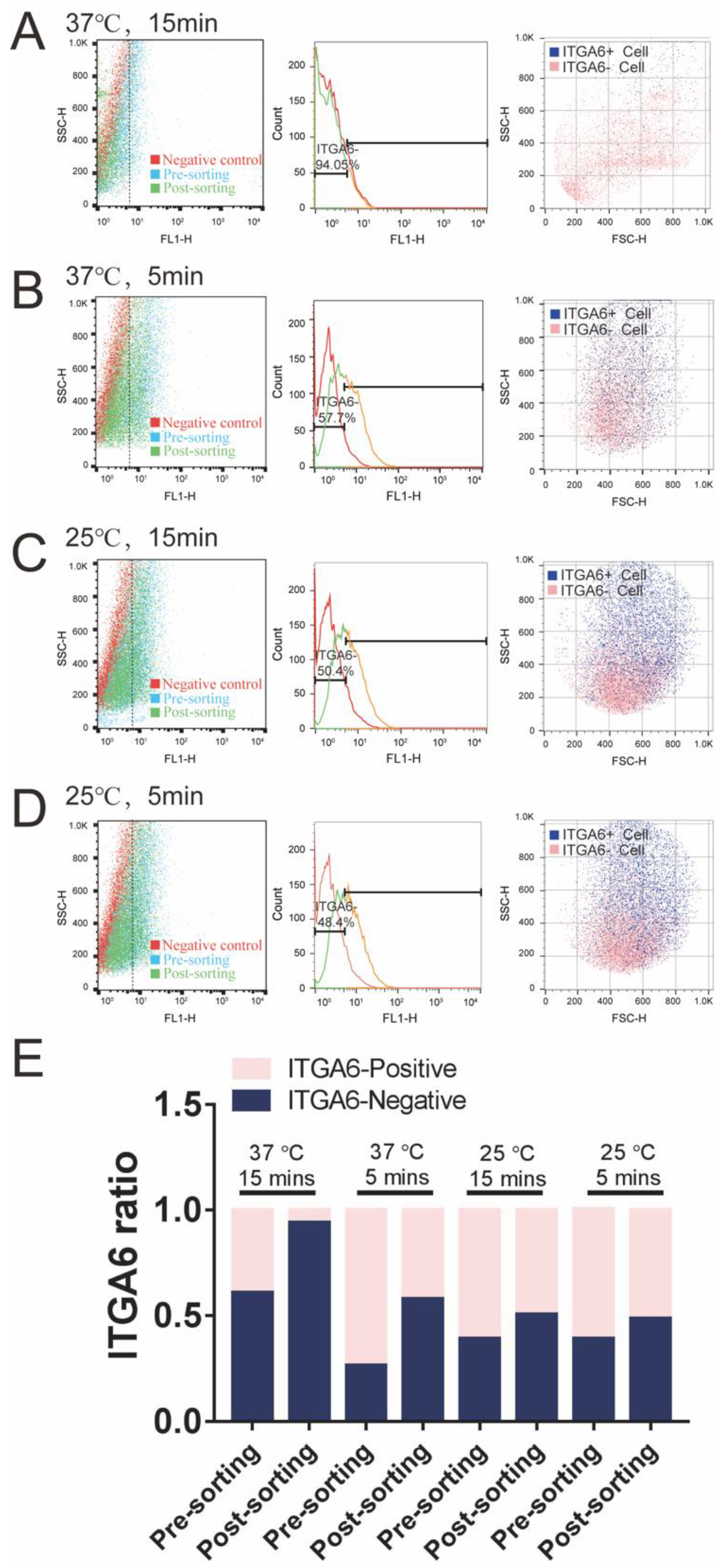
3.2. A Magnetic Platform to Purify ITGA6-Positive iPSC-TM
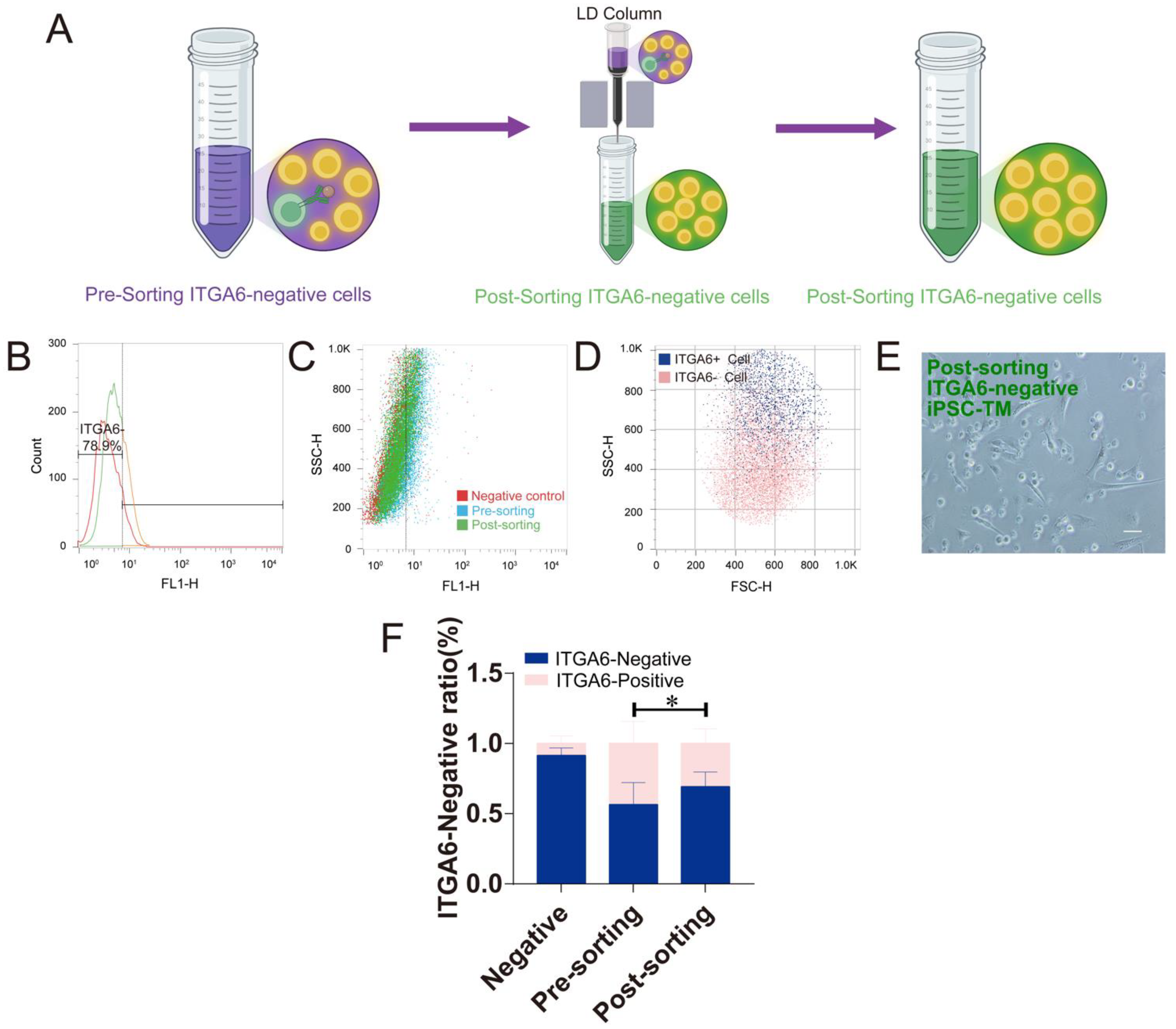
3.3. The Magnetic Platform to Purify ITGA6-Negative iPSC-TM
3.4. IP Method to Purify ITGA6-Positive/Negative iPSC-TM Cells
4. Discussion
- i.
- In the early 1950s, the TM was discovered to be an elaborate and complex tissue anatomically [31]. In short, it comprises three distinct layers with different structures and functions in aqueous humor drainage, indicating the heterogeneity of the TM. Recently, two new studies using scRNA-Seq have verified this heterogeneity, demonstrating 12 types of cells in the conventional outflow tissue [17,18]. Although researchers have investigated the different roles of cells in juxtacanalicular connective tissue or uveal meshwork [32,33], the functions of many other cell types such as Schwann cells, melanocytes, and T cells are still largely unknown. Our data using the MACS-based approach to purify ITGA6-positive iPSC-TM suggested that this purification method may be feasible to isolate the above subpopulations using different cell surface markers and investigate the roles of different cell clusters in controlling AH outflow and regulating IOP homeostasis.
- ii.
- Loss of TM cellularity, aberrant extracellular matrix remodeling, changes in the biomechanical properties of the TM, and mutations have been reported as risk factors for glaucoma [34,35]. For example, the first pathogenic mutation for primary open-angle glaucoma has been identified in the myocilin gene [36]. The aggregation of mutant myocilin can lead to a severe decline in TM cellularity due to endoplasmic reticulum stress [37,38]. To this end, it is very important to investigate how damage occurs in different TM subpopulations. Our MACS-based purification provides a simple method to generate TM subpopulations of glaucoma, which may facilitate us in determining the dysfunctional TM subpopulation at the earliest stage. The study can benefit not only the diagnoses but also the treatments for glaucoma patients in the early stages.
- iii.
- In recent times, some new glaucoma drugs have been identified that function primarily by modulating the TM cytoskeleton and the contractile tone of TM cells, their volume, and extracellular matrix deposition such as Rho kinase inhibitors [39], nitric oxide (NO) signaling regulators [40], latrunculins [41], and ion channel regulators [42]. Aside from pharmacologic treatments, gene therapy also holds a great promise in rescuing TM dysfunction [43,44,45,46]. However, which subpopulations of the TM that could be efficiently regulated by these new treatments are still elusive. The other side of answering this question would benefit the discovery of the proper delivery approaches for these new drugs/gene therapies. To this end, our MACS-based approach is feasible to address this question.
- iv.
- Moreover, we applied iPSC-TM in regenerating the damaged TM of several glaucoma models including Tg-MYOCY437H mice [13,14], GCα1-/- mice, and aged human eyes [15]. As previously investigated, a common phenomenon of iPSC-TM after cell transplantation is that endogenous TM cells could be stimulated to proliferate. Aside from iPSCs, mesenchymal stem cells (MSCs) are also used in TM regeneration [47,48]. MSCs exist in the TM and are identified by analyzing the expressions of stem cell biomarkers [49]. In glaucoma animal models, the transplanted MSCs exhibit positive therapeutic effects on TM regeneration [47,50,51] including migration into the TM, secretion factors to recruit nesting-positive progenitors, and the stimulation of the cell proliferation of endogenous TM cells. Although encouraging, it is still elusive as to how the transplanted cells stimulate endogenous cell proliferation. Thus, our MACS-based purification could efficiently isolate different iPSC-TM or MSC subpopulations that are of interest and facilitate a better understanding of the mechanism of stem cell-based therapy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ju, W.K.; Perkins, G.A.; Kim, K.Y.; Bastola, T.; Choi, W.Y.; Choi, S.H. Glaucomatous optic neuropathy: Mitochondrial dynamics, dysfunction and protection in retinal ganglion cells. Prog. Retin. Eye Res. 2022, 787, 101136. [Google Scholar] [CrossRef]
- Madeira, M.H.; Ortin-Martinez, A.; Nadal-Nicolas, F.; Ambrosio, A.F.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Santiago, A.R. Caffeine administration prevents retinal neuroinflammation and loss of retinal ganglion cells in an animal model of glaucoma. Sci. Rep. 2016, 6, 27532. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, Y.; Ma Khin Pyi, S.; Malihi, M.; Hodge, D.O.; Sit, A.J. Clinical results of ab interno trabeculotomy using the trabectome for open-angle glaucoma: The Mayo Clinic series in Rochester, Minnesota. Am. J. Ophthalmol. 2013, 156, 927–935. [Google Scholar] [CrossRef]
- Braunger, B.M.; Fuchshofer, R.; Tamm, E.R. The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm. 2015, 95, 173–181. [Google Scholar] [CrossRef]
- Keller, K.E.; Bhattacharya, S.K.; Borras, T.; Brunner, T.M.; Chansangpetch, S.; Clark, A.F.; Dismuke, W.M.; Du, Y.; Elliott, M.H.; Ethier, C.R.; et al. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res. 2018, 171, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.; Murphy, C.; Polansky, J.; Juster, R. Age-related changes in trabecular meshwork cellularity. Investig. Opthalmol. Vis. Sci. 1981, 21, 714–727. [Google Scholar]
- Alvarado, J.; Murphy, C.; Juster, R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology 1984, 91, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, M.H.; Vranka, J.A.; Wadkins, D.; Jackson, T.; Cheng, L.; Ledolter, J. Circumferential trabecular meshwork cell density in the human eye. Exp. Eye Res. 2021, 205, 108494. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Moodley, Y. Introduction to stem cells and regenerative medicine. Respiration 2013, 85, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.J.; Zhu, W.; Cook, A.C.; Anfinson, K.R.; Tucker, B.A.; Kuehn, M.H. Induction of trabecular meshwork cells from induced pluripotent stem cells. Investig. Opthalmol. Vis. Sci. 2014, 55, 7065–7072. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Miao, Y.; Sui, S.; Wang, Y.; Wu, S.; Cao, Q.; Duan, H.; Qi, X.; Zhou, Q.; Pan, X.; et al. Xeno- and Feeder-Free Differentiation of Human iPSCs to Trabecular Meshwork-Like Cells by Recombinant Cytokines. Transl. Vis. Sci. Technol. 2021, 10, 27. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Yang, X.; Chen, W.; Yang, X.; Pan, X.; Xu, P.; Zhu, W.; Han, Y.; Chen, X. ITGA8 positive cells in the conventional outflow tissue exhibit Schlemm’s canal endothelial cell properties. Life Sci. 2021, 278, 119564. [Google Scholar] [CrossRef]
- Zhu, W.; Gramlich, O.W.; Laboissonniere, L.; Jain, A.; Sheffield, V.C.; Trimarchi, J.M.; Tucker, B.A.; Kuehn, M.H. Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, E3492–E3500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Jain, A.; Gramlich, O.W.; Tucker, B.A.; Sheffield, V.C.; Kuehn, M.H. Restoration of Aqueous Humor Outflow Following Transplantation of iPSC-Derived Trabecular Meshwork Cells in a Transgenic Mouse Model of Glaucoma. Investig. Opthalmol. Vis. Sci. 2017, 58, 2054–2062. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Godwin, C.R.; Cheng, L.; Scheetz, T.E.; Kuehn, M.H. Transplantation of iPSC-TM stimulates division of trabecular meshwork cells in human eyes. Sci. Rep. 2020, 10, 2905. [Google Scholar] [CrossRef] [Green Version]
- Sui, S.; Yu, H.; Wang, X.; Wang, W.; Yang, X.; Pan, X.; Zhou, Q.; Xin, C.; Du, R.; Wu, S.; et al. iPSC-Derived Trabecular Meshwork Cells Stimulate Endogenous TM Cell Division Through Gap Junction in a Mouse Model of Glaucoma. Investig. Opthalmol. Vis. Sci. 2021, 62, 28. [Google Scholar] [CrossRef]
- Patel, G.; Fury, W.; Yang, H.; Gomez-Caraballo, M.; Bai, Y.; Yang, T.; Adler, C.; Wei, Y.; Ni, M.; Schmitt, H.; et al. Molecular taxonomy of human ocular outflow tissues defined by single-cell transcriptomics. Proc. Natl. Acad. Sci. USA 2020, 117, 12856–12867. [Google Scholar] [CrossRef] [PubMed]
- van Zyl, T.; Yan, W.; McAdams, A.; Peng, Y.R.; Shekhar, K.; Regev, A.; Juric, D.; Sanes, J.R. Cell atlas of aqueous humor outflow pathways in eyes of humans and four model species provides insight into glaucoma pathogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 10339–10349. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, B.; Radbruch, A.; Kummel, T.; Wickenhauser, C.; Korb, H.; Hansmann, M.L.; Thiele, J.; Fischer, R. Magnetic activated cell sorting (macs)—A new immunomagnetic method for megakaryocytic cell isolation—Comparison of different separation techniques. Eur. J. Haematol. 1994, 52, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Rosental, B.; Kozhekbaeva, Z.; Fernhoff, N.; Tsai, J.M.; Traylor-Knowles, N. Coral cell separation and isolation by fluorescence-activated cell sorting (FACS). BMC Cell Biol. 2017, 18, 30. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Li, T.; Hu, J.; Zhou, X.; Wu, J.; Wu, Q. Comparative analysis of three purification protocols for retinal ganglion cells from rat. Mol. Vis. 2016, 22, 387–400. [Google Scholar]
- Zahler, S.; Kowalski, C.; Brosig, A.; Kupatt, C.; Becker, B.F.; Gerlach, E. The function of neutrophils isolated by a magnetic antibody cell separation technique is not altered in comparison to a density gradient centrifugation method. J. Immunol. Methods 1997, 200, 173–179. [Google Scholar] [CrossRef]
- Sutermaster, B.A.; Darling, E.M. Considerations for high-yield, high-throughput cell enrichment: Fluorescence versus magnetic sorting. Sci. Rep. 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, J.J.; Zborowski, M.; Sun, L.; Moore, L. Flow through, immunomagnetic cell separation. Biotechnol. Prog. 1998, 14, 141–148. [Google Scholar] [CrossRef]
- Yin, Y.; Dong, Y.; Vu, S.; Yang, F.; Yarov-Yarovoy, V.; Tian, Y.; Zheng, J. Structural mechanisms underlying activation of TRPV1 channels by pungent compounds in gingers. Br. J. Pharmacol. 2019, 176, 3364–3377. [Google Scholar] [CrossRef]
- Gill, K.P.; Hewitt, A.W.; Davidson, K.C.; Pébay, A.; Wong, R.C.B. Methods of Retinal Ganglion Cell Differentiation From Pluripotent Stem Cells. Transl. Vis. Sci. Technol. 2014, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Cahan, P.; Daley, G.Q. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat. Rev. Mol. Cell Biol. 2013, 14, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Nagelkerke, A.; Ojansivu, M.; van der Koog, L.; Whittaker, T.E.; Cunnane, E.M.; Silva, A.M.; Dekker, N.; Stevens, M.M. Extracellular vesicles for tissue repair and regeneration: Evidence, challenges and opportunities. Adv. Drug Deliv. Rev. 2021, 175, 113775. [Google Scholar] [CrossRef]
- Weber, S.C.; Gratopp, A.; Akanbi, S.; Rheinlaender, C.; Sallmon, H.; Barikbin, P.; Koehne, P.S. Isolation and culture of fibroblasts, vascular smooth muscle, and endothelial cells from the fetal rat ductus arteriosus. Pediatr. Res. 2011, 70, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Andersen, N.D.; Srinivas, S.; Pinero, G.; Monje, P.V. A rapid and versatile method for the isolation, purification and cryogenic storage of Schwann cells from adult rodent nerves. Sci. Rep. 2016, 6, 31781. [Google Scholar] [CrossRef]
- Flocks, M. The anatomy of the trabecular meshwork as seen in tangential section. AMA Arch. Ophthalmol. 1956, 56, 708–718. [Google Scholar] [CrossRef]
- Kubota, T.; Schlötzer-Schrehardt, U.; Inomata, H.; Naumann, G.O. Immunoelectron microscopic localization of the HNK-1 carbohydrate epitope in the anterior segment of pseudoexfoliation and normal eyes. Curr. Eye Res. 1997, 16, 231–238. [Google Scholar] [CrossRef]
- Overby, D.R.; Zhou, E.H.; Vargas-Pinto, R.; Pedrigi, R.M.; Fuchshofer, R.; Braakman, S.T.; Gupta, R.; Perkumas, K.M.; Sherwood, J.M.; Vahabikashi, A.; et al. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc. Natl. Acad. Sci. USA 2014, 111, 13876–13881. [Google Scholar] [CrossRef] [Green Version]
- Mallick, S.; Sharma, M.; Kumar, A.; Du, Y. Cell-Based Therapies for Trabecular Meshwork Regeneration to Treat Glaucoma. Biomolecules 2021, 11, 1258. [Google Scholar] [CrossRef]
- Fan, X.; Bilir, E.K.; Kingston, O.A.; Oldershaw, R.A.; Kearns, V.R.; Willoughby, C.E.; Sheridan, C.M. Replacement of the Trabecular Meshwork Cells-A Way Ahead in IOP Control? Biomolecules 2021, 11, 1317. [Google Scholar] [CrossRef]
- Stone, E.M.; Fingert, J.H.; Alward, W.L.; Nguyen, T.D.; Polansky, J.R.; Sunden, S.L.; Nishimura, D.; Clark, A.F.; Nystuen, A.; Nichols, B.E.; et al. Identification of a gene that causes primary open angle glaucoma. Science 1997, 275, 668–670. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Zhang, Z.; Xue, H.; Chen, X.; Ji, Y. Physiological function of myocilin and its role in the pathogenesis of glaucoma in the trabecular meshwork. Int. J. Mol. Med. 2019, 43, 671–681. [Google Scholar] [CrossRef] [Green Version]
- Tanji, T.; Cohen, E.; Shen, D.; Zhang, C.; Yu, F.; Coleman, A.L.; Zheng, J.J. Age at Glaucoma Diagnosis in Germline Myocilin Mutation Patients: Associations with Polymorphisms in Protein Stabilities. Genes 2021, 12, 1802. [Google Scholar] [CrossRef]
- Wang, S.K.; Chang, R.T. An emerging treatment option for glaucoma: Rho kinase inhibitors. Clin. Ophthalmol. 2014, 8, 883–890. [Google Scholar] [CrossRef] [Green Version]
- Sharif, N.A. Ocular Hypertension and Glaucoma: A Review and Current Perspectives. Int. J. Ophthalmol. Vis. Sci. 2017, 2, 22–36. [Google Scholar] [CrossRef]
- Rasmussen, C.A.; Kaufman, P.L.; Ritch, R.; Haque, R.; Brazzell, R.K.; Vittitow, J.L. Latrunculin B Reduces Intraocular Pressure in Human Ocular Hypertension and Primary Open-Angle Glaucoma. Transl. Vis. Sci. Technol. 2014, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Carreon, T.A.; Castellanos, A.; Gasull, X.; Bhattacharya, S.K. Interaction of cochlin and mechanosensitive channel TREK-1 in trabecular meshwork cells influences the regulation of intraocular pressure. Sci. Rep. 2017, 7, 452. [Google Scholar] [CrossRef] [Green Version]
- De Groef, L.; Van Hove, I.; Dekeyster, E.; Stalmans, I.; Moons, L. MMPs in the trabecular meshwork: Promising targets for future glaucoma therapies? Investig. Opthalmol. Vis. Sci. 2013, 54, 7756–7763. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.P.; Samples, J.R.; Van Buskirk, E.M.; Acott, T.S. Expression of matrix metalloproteinases and inhibitor by human trabecular meshwork. Investig. Opthalmol. Vis. Sci. 1991, 32, 172–180. [Google Scholar]
- Tan, J.; Fan, N.; Wang, N.; Feng, B.; Yang, M.; Liu, G.; Wang, Y.; Zhu, X.; Kaufman, P.L.; Pang, I.H.; et al. Effects of Lentivirus-Mediated C3 Expression on Trabecular Meshwork Cells and Intraocular Pressure. Investig. Opthalmol. Vis. Sci. 2018, 59, 4937–4944. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Zode, G.; Kasetti, R.B.; Ran, F.A.; Yan, W.; Sharma, T.P.; Bugge, K.; Searby, C.C.; Fingert, J.H.; Zhang, F.; et al. CRISPR-Cas9-based treatment of myocilin-associated glaucoma. Proc. Natl. Acad. Sci. USA 2017, 114, 11199–11204. [Google Scholar] [CrossRef] [Green Version]
- Manuguerra-Gagné, R.; Boulos, P.R.; Ammar, A.; Leblond, F.A.; Krosl, G.; Pichette, V.; Lesk, M.R.; Roy, D.C. Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. Stem Cells 2013, 31, 1136–1148. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Arsenijevic, A.; Markovic, B.S.; Djonov, V.; Volarevic, V. Therapeutic Potential of Mesenchymal Stem Cells and Their Secretome in the Treatment of Glaucoma. Stem Cells Int. 2019, 2019, 7869130. [Google Scholar] [CrossRef]
- Nadri, S.; Yazdani, S.; Arefian, E.; Gohari, Z.; Eslaminejad, M.B.; Kazemi, B.; Soleimani, M. Mesenchymal stem cells from trabecular meshwork become photoreceptor-like cells on amniotic membrane. Neurosci. Lett. 2013, 541, 43–48. [Google Scholar] [CrossRef]
- Sathiyanathan, P.; Tay, C.Y.; Stanton, L.W. Transcriptome analysis for the identification of cellular markers related to trabecular meshwork differentiation. BMC Genom. 2017, 18, 383. [Google Scholar] [CrossRef] [Green Version]
- Roubeix, C.; Godefroy, D.; Mias, C.; Sapienza, A.; Riancho, L.; Degardin, J.; Fradot, V.; Ivkovic, I.; Picaud, S.; Sennlaub, F.; et al. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res. Ther. 2015, 6, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Kasus, T.; Schechter, B.; Lavi, S.; Yarden, Y.; Sela, M. Persistent elimination of ErbB-2/HER2-overexpressing tumors using combinations of monoclonal antibodies: Relevance of receptor endocytosis. Proc. Natl. Acad. Sci. USA 2009, 106, 3294–3299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byron, A.; Humphries, J.D.; Askari, J.A.; Craig, S.E.; Mould, A.P.; Humphries, M.J. Anti-integrin monoclonal antibodies. J. Cell Sci. 2009, 122, 4009–4011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurashina, Y.; Imashiro, C.; Hirano, M.; Kuribara, T.; Totani, K.; Ohnuma, K.; Friend, J.; Takemura, K. Enzyme-free release of adhered cells from standard culture dishes using intermittent ultrasonic traveling waves. Commun. Biol. 2019, 2, 393. [Google Scholar] [CrossRef] [Green Version]

| Donor | Age | Gender | Race | Cause of Death |
|---|---|---|---|---|
| Donor5 | 80 | Male | Caucasian | Acute respiratory distress |
| Donor6 | 37 | Female | Caucasian | Acute liver failure |
| Donor8 | N/A | N/A | Chinese | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, P.; Wang, W.; Xu, W.; Cao, Q.; Zhu, W. Application of a Magnetic Platform in α6 Integrin-Positive iPSC-TM Purification. Bioengineering 2023, 10, 410. https://doi.org/10.3390/bioengineering10040410
Feng P, Wang W, Xu W, Cao Q, Zhu W. Application of a Magnetic Platform in α6 Integrin-Positive iPSC-TM Purification. Bioengineering. 2023; 10(4):410. https://doi.org/10.3390/bioengineering10040410
Chicago/Turabian StyleFeng, Pengchao, Wenyan Wang, Wenhua Xu, Qilong Cao, and Wei Zhu. 2023. "Application of a Magnetic Platform in α6 Integrin-Positive iPSC-TM Purification" Bioengineering 10, no. 4: 410. https://doi.org/10.3390/bioengineering10040410




