3D Tortuosity and Diffusion Characterization in the Human Mineralized Collagen Fibril Using a Random Walk Model
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, Y.; Du, T.; Liu, Y. Biomechanical Characteristics and Analysis Approaches of Bone and Bone Substitute Materials. J. Funct. Biomater. 2023, 14, 212. [Google Scholar] [CrossRef]
- Liebi, M.; Georgiadis, M.; Menzel, A.; Schneider, P.; Kohlbrecher, J.; Bunk, O.; Guizar-Sicairos, M. Nanostructure Surveys of Macroscopic Specimens by Small-Angle Scattering Tensor Tomography. Nature 2015, 527, 349–352. [Google Scholar] [CrossRef]
- Fielder, M.; Nair, A.K. Effects of Hydration and Mineralization on the Deformation Mechanisms of Collagen Fibrils in Bone at the Nanoscale. Biomech. Model. Mechanobiol. 2019, 18, 57–68. [Google Scholar] [CrossRef]
- Chen, X.; Qian, T.; Hang, F.; Chen, X. Water Promotes the Formation of Fibril Bridging in Antler Bone Illuminated by in Situ AFM Testing. J. Mech. Behav. Biomed. Mater. 2021, 120, 104580. [Google Scholar] [CrossRef] [PubMed]
- Surowiec, R.K.; Allen, M.R.; Wallace, J.M. Bone Hydration: How We Can Evaluate It, What Can It Tell Us, and Is It an Effective Therapeutic Target? Bone Rep. 2022, 16, 101161. [Google Scholar] [CrossRef]
- Wang, Y.; Von Euw, S.; Fernandes, F.M.; Cassaignon, S.; Selmane, M.; Laurent, G.; Pehau-Arnaudet, G.; Coelho, C.; Bonhomme-Coury, L.; Giraud-Guille, M.M.; et al. Water-Mediated Structuring of Bone Apatite. Nat. Mater. 2013, 12, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.K.; Sinha, N. Dehydration-Induced Structural Changes in the Collagen-Hydroxyapatite Interface in Bone by High-Resolution Solid-State NMR Spectroscopy. J. Phys. Chem. C 2011, 115, 14219–14227. [Google Scholar] [CrossRef]
- Cowin, S.C. Bone Mechanics Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001; ISBN 9780429125447. [Google Scholar]
- Burr, D.B. Changes in Bone Matrix Properties with Aging. Bone 2019, 120, 85–93. [Google Scholar] [CrossRef]
- Maghsoudi-Ganjeh, M.; Wang, X.; Zeng, X. Computational Investigation of the Effect of Water on the Nanomechanical Behavior of Bone. J. Mech. Behav. Biomed. Mater. 2020, 101, 103454. [Google Scholar] [CrossRef]
- Fritton, S.P.; Weinbaum, S. Fluid and Solute Transport in Bone: Flow-Induced Mechanotransduction. Annu. Rev. Fluid Mech. 2009, 41, 347–374. [Google Scholar] [CrossRef]
- Fernández-Seara, M.A.; Wehrli, S.L.; Wehrli, F.W. Diffusion of Exchangeable Water in Cortical Bone Studied by Nuclear Magnetic Resonance. Biophys. J. 2002, 82, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.; Nyman, J.S.; Wang, X.; De Los Santos, A.; Nicolella, D.P. Assessment of Water Distribution Changes in Human Cortical Bone by Nuclear Magnetic Resonance. Meas. Sci. Technol. 2007, 18, 715–723. [Google Scholar] [CrossRef]
- Samuel, J.; Park, J.; Almer, J.; Wang, X. Effect of Water on Nanomechanics of Bone Is Different between Tension and Compression. J. Mech. Behav. Biomed. Mater. 2016, 57, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Van Tol, A.F.; Schemenz, V.; Wagermaier, W.; Roschger, A.; Razi, H.; Vitienes, I.; Fratzl, P.; Willie, B.M.; Weinkamer, R. The Mechanoresponse of Bone Is Closely Related to the Osteocyte Lacunocanalicular Network Architecture. Proc. Natl. Acad. Sci. USA 2020, 117, 32251–32259. [Google Scholar] [CrossRef] [PubMed]
- Marinozzi, F.; Bini, F.; Marinozzi, A. Water Uptake and Swelling in Single Trabeculæ from Human Femur Head. Biomatter 2014, 4, e28237. [Google Scholar] [CrossRef]
- Marinozzi, F.; Bini, F.; Quintino, A.; Corcione, M.; Marinozzi, A. Experimental Study of Diffusion Coefficients of Water through the Collagen: Apatite Porosity in Human Trabecular Bone Tissue. Biomed Res. Int. 2014, 2014, 796519. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. A Nanostructured Look of Collagen Apatite Porosity into Human Mineralized Collagen Fibril. Biocell 2022, 46, 2225–2229. [Google Scholar] [CrossRef]
- Hrabe, J.; Hrabětová, S.; Segeth, K. A Model of Effective Diffusion and Tortuosity in the Extracellular Space of the Brain. Biophys. J. 2004, 87, 1606–1617. [Google Scholar] [CrossRef]
- Nicholson, C. Diffusion and Related Transport Mechanisms in Brain Tissue. Rep. Prog. Phys. 2001, 64, 815–884. [Google Scholar] [CrossRef]
- Carneiro, I.; Carvalho, S.; Henrique, R.; Oliveira, L.M.; Tuchin, V.V. A Robust Ex Vivo Method to Evaluate the Diffusion Properties of Agents in Biological Tissues. J. Biophotonics 2019, 12, e201800333. [Google Scholar] [CrossRef]
- Lemaire, T.; Pham, T.T.; Capiez-Lernout, E.; de Leeuw, N.H.; Naili, S. Water in Hydroxyapatite Nanopores: Possible Implications for Interstitial Bone Fluid Flow. J. Biomech. 2015, 48, 3066–3071. [Google Scholar] [CrossRef] [PubMed]
- Bini, F.; Pica, A.; Novelli, S.; Marinozzi, A.; Marinozzi, F.; Fabiano Bini, Andrada Pica, Simone Novelli, Andrea Marinozzi, F. 3D-FEM Modeling of Iso-Concentration Maps in Single Trabecula from Human Femur Head. In VipIMAGE 2019. VipIMAGE 2019. Lecture Notes in Computational Vision and Biomechanics; Tavares, J.M.R.S., Jorge, R.M.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; Volume 34, ISBN 978-3-030-32039-3. [Google Scholar]
- Bini, F.; Pica, A.; Novelli, S.; Pecci, R.; Bedini, R.; Marinozzi, A.; Marinozzi, F. 3D FEM Model to Simulate Brownian Motion inside Trabecular Tissue from Human Femoral Head. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2022, 10, 500–507. [Google Scholar] [CrossRef]
- van Brakel, J.; Heertjes, P.M. Analysis of Diffusion in Macroporous Media in Terms of a Porosity, a Tortuosity and a Constrictivity Factor. Int. J. Heat Mass Transf. 1974, 17, 1093–1103. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. A 3D Model of the Effect of Tortuosity and Constrictivity on the Diffusion in Mineralized Collagen Fibril. Sci. Rep. 2019, 9, 2658. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarian, B.; Hunt, A.G.; Ewing, R.P.; Sahimi, M. Tortuosity in Porous Media: A Critical Review. Soil Sci. Soc. Am. J. 2013, 77, 1461–1477. [Google Scholar] [CrossRef]
- Moussatov, A.; Ayrault, C.; Castagnède, B. Porous Material Characterization-Ultrasonic Method for Estimation of Tortuosity and Characteristic Length Using a Barometric Chamber. Ultrasonics 2001, 39, 195–202. [Google Scholar] [CrossRef]
- Rottreau, T.J.; Parlett, C.M.A.; Lee, A.F.; Evans, R. Diffusion NMR Characterization of Catalytic Silica Supports: A Tortuous Path. J. Phys. Chem. C 2017, 121, 16250–16256. [Google Scholar] [CrossRef]
- Matyka, M.; Khalili, A.; Koza, Z. Tortuosity-Porosity Relation in Porous Media Flow. Phys. Rev. E 2008, 78, 026306. [Google Scholar] [CrossRef]
- Yun, M.; Yu, B.; Xu, P.; Wu, J. Geometrical Models for Tortuosity of Streamlines in Three-Dimensional Porous Media. Can. J. Chem. Eng. 2006, 84, 301–309. [Google Scholar] [CrossRef]
- Wu, Y.S.; van Vliet, L.J.; Frijlink, H.W.; van der Voort Maarschalk, K. The Determination of Relative Path Length as a Measure for Tortuosity in Compacts Using Image Analysis. Eur. J. Pharm. Sci. 2006, 28, 433–440. [Google Scholar] [CrossRef]
- Jin, S.; Verkman, A.S. Single Particle Tracking of Complex Diffusion in Membranes: Simulation and Detection of Barrier, Raft, and Interaction Phenomena. J. Phys. Chem. B 2007, 111, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Momot, K.I. Diffusion Tensor of Water in Model Articular Cartilage. Eur. Biophys. J. 2011, 40, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Stylianopoulos, T.; Poh, M.Z.; Insin, N.; Bawendi, M.G.; Fukumura, D.; Munn, L.L.; Jain, R.K. Diffusion of Particles in the Extracellular Matrix: The Effect of Repulsive Electrostatic Interactions. Biophys. J. 2010, 99, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.C. Random Walks in Biology; Princeton University Press: Princeton, NJ, USA, 1983. [Google Scholar]
- Einstein, A.; Furth, R. Investigations on the Theory of the Brownian Movement; Dover Publications: New York, NY, USA, 1956. [Google Scholar]
- Codling, E.A.; Plank, M.J.; Benhamou, S. Random Walk Models in Biology. J. R. Soc. Interface 2008, 5, 813–834. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. 3D Random Walk Model of Diffusion in Human Hypo- and Hyper- Mineralized Collagen Fibrils. J. Biomech. 2021, 125, 110586. [Google Scholar] [CrossRef] [PubMed]
- Petruska, J.A.; Hodge, A.J. A Subunit Model for the Tropocollagen Macromolecule. Proc. Natl. Acad. Sci. USA 1964, 51, 871–876. [Google Scholar] [CrossRef]
- Jäger, I.; Fratzl, P. Mineralized Collagen Fibrils: A Mechanical Model with a Staggered Arrangement of Mineral Particles. Biophys. J. 2000, 79, 1737–1746. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. Percolation Networks inside 3D Model of the Mineralized Collagen Fibril. Sci. Rep. 2021, 11, 11398. [Google Scholar] [CrossRef]
- Buehler, M.J. Nature Designs Tough Collagen: Explaining the Nanostructure of Collagen Fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar] [CrossRef]
- Lees, S. Considerations Regarding the Structure of the Mammalian Mineralized Osteoid from Viewpoint of the Generalized Packing Model. Connect. Tissue Res. 1987, 16, 281–303. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S.; Paschalis, E.P.; Roschger, P. Structure and Mechanical Quality of the Collagen-Mineral Nano-Composite in Bone. J. Mater. Chem. 2004, 14, 2115–2123. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone Structure and Formation: A New Perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Landis, W.J.; Song, M.J.; Leith, A.; McEwen, L.; McEwen, B.F. Mineral and Organic Matrix Interaction in Normally Calcifying Tendon Visualized in Three Dimensions by High-Voltage Electron Microscopic Tomography and Graphic Image Reconstruction. J. Struct. Biol. 1993, 110, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Vercher-Martínez, A.; Giner, E.; Arango, C.; Javier Fuenmayor, F. Influence of the Mineral Staggering on the Elastic Properties of the Mineralized Collagen Fibril in Lamellar Bone. J. Mech. Behav. Biomed. Mater. 2015, 42, 243–256. [Google Scholar] [CrossRef]
- Depalle, B.; Qin, Z.; Shefelbine, S.J.; Buehler, M.J. Large Deformation Mechanisms, Plasticity, and Failure of an Individual Collagen Fibril with Different Mineral Content. J. Bone Miner. Res. 2016, 31, 380–390. [Google Scholar] [CrossRef]
- Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. 3D Diffusion Model within the Collagen Apatite Porosity: An Insight to the Nanostructure of Human Trabecular Bone. PLoS ONE 2017, 12, e0189041. [Google Scholar] [CrossRef]
- Xu, Y.F.; Nudelman, F.; Eren, E.D.; Wirix, M.J.M.; Cantaert, B.; Nijhuis, W.H.; Hermida-Merino, D.; Portale, G.; Bomans, P.H.H.; Ottmann, C.; et al. Intermolecular Channels Direct Crystal Orientation in Mineralized Collagen. Nat. Commun. 2020, 11, 5068. [Google Scholar] [CrossRef]
- Chiavazzo, E.; Fasano, M.; Asinari, P.; Decuzzi, P. Scaling Behaviour for the Water Transport in Nanoconfined Geometries. Nat. Commun. 2014, 5, 3565. [Google Scholar] [CrossRef]
- Saxton, M.J. Single-Particle Tracking: The Distribution of Diffusion Coefficients. Biophys. J. 1997, 72, 1744–1753. [Google Scholar] [CrossRef]
- Weimann, L.; Ganzinger, K.A.; McColl, J.; Irvine, K.L.; Davis, S.J.; Gay, N.J.; Bryant, C.E.; Klenerman, D. A Quantitative Comparison of Single-Dye Tracking Analysis Tools Using Monte Carlo Simulations. PLoS ONE 2013, 8, e64287. [Google Scholar] [CrossRef]
- Monnier, N.; Guo, S.M.; Mori, M.; He, J.; Lénárt, P.; Bathe, M. Bayesian Approach to MSD-Based Analysis of Particle Motion in Live Cells. Biophys. J. 2012, 103, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Watson, E.; Dempsey, C.; Suh, J. Real-Time Particle Tracking for Studying Intracellular Trafficking of Pharmaceutical Nanocarriers. Methods Mol. Biol. 2013, 991, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Gul-E-Noor, F.; Singh, C.; Papaioannou, A.; Sinha, N.; Boutis, G.S. Behavior of Water in Collagen and Hydroxyapatite Sites of Cortical Bone: Fracture, Mechanical Wear, and Load Bearing Studies. J. Phys. Chem. C 2015, 119, 21528–21537. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Han, Y.; Henderson, S.C.; Majeska, R.J.; Weinbaum, S.; Schaffler, M.B. In Situ Measurement of Solute Transport in the Bone Lacunar-Canalicular System. Proc. Natl. Acad. Sci. USA 2005, 102, 11911–11916. [Google Scholar] [CrossRef]
- Oosterlaken, B.M.; Vena, M.P.; de With, G. In Vitro Mineralization of Collagen. Adv. Mater. 2021, 33, 2004418. [Google Scholar] [CrossRef]
- Araneo, R.; Rinaldi, A.; Notargiacomo, A.; Bini, F.; Marinozzi, F.; Pea, M.; Lovat, G.; Celozzi, S. Effect of the Scaling of the Mechanical Properties on the Performances of ZnO Piezo-Semiconductive Nanowires. AIP Conf. Proc. 2014, 1603, 14–22. [Google Scholar] [CrossRef]
- Othman, N.S.; Jaafar, M.S.; Rahman, A.A.; Othman, E.S.; Rozlan, A.A. Ultrasound Speed of Polymer Gel Mimicked Human Soft Tissue within Three Weeks. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 223–225. [Google Scholar] [CrossRef]
- Blyakhman, F.A.; Makarova, E.B.; Fadeyev, F.A.; Lugovets, D.V.; Safronov, A.P.; Shabadrov, P.A.; Shklyar, T.F.; Melnikov, G.Y.; Orue, I.; Kurlyandskaya, G.V. The Contribution of Magnetic Nanoparticles to Ferrogel Biophysical Properties. Nanomaterials 2019, 9, 232. [Google Scholar] [CrossRef]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic Hydrogels and Their Potential Biomedical Applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
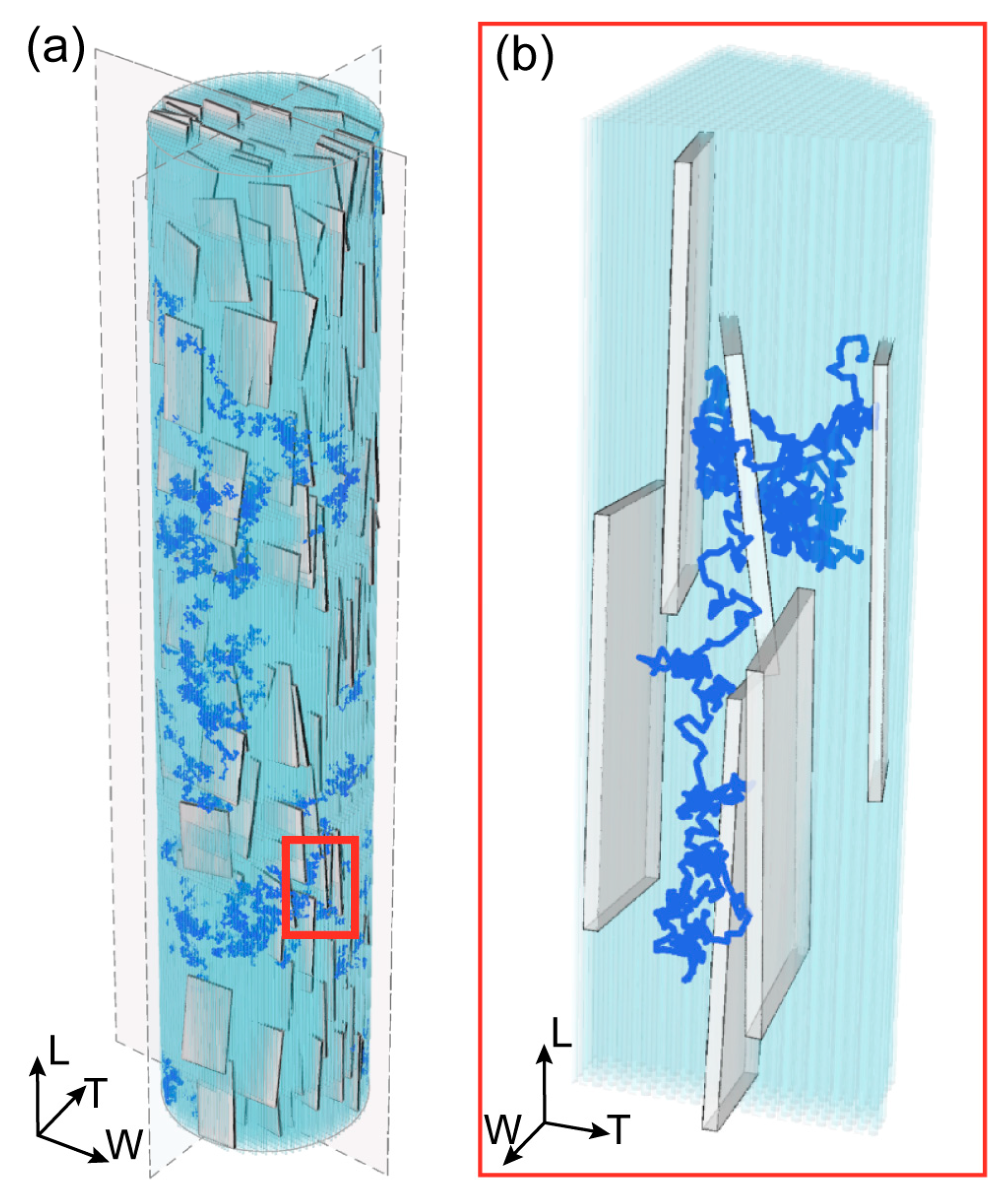
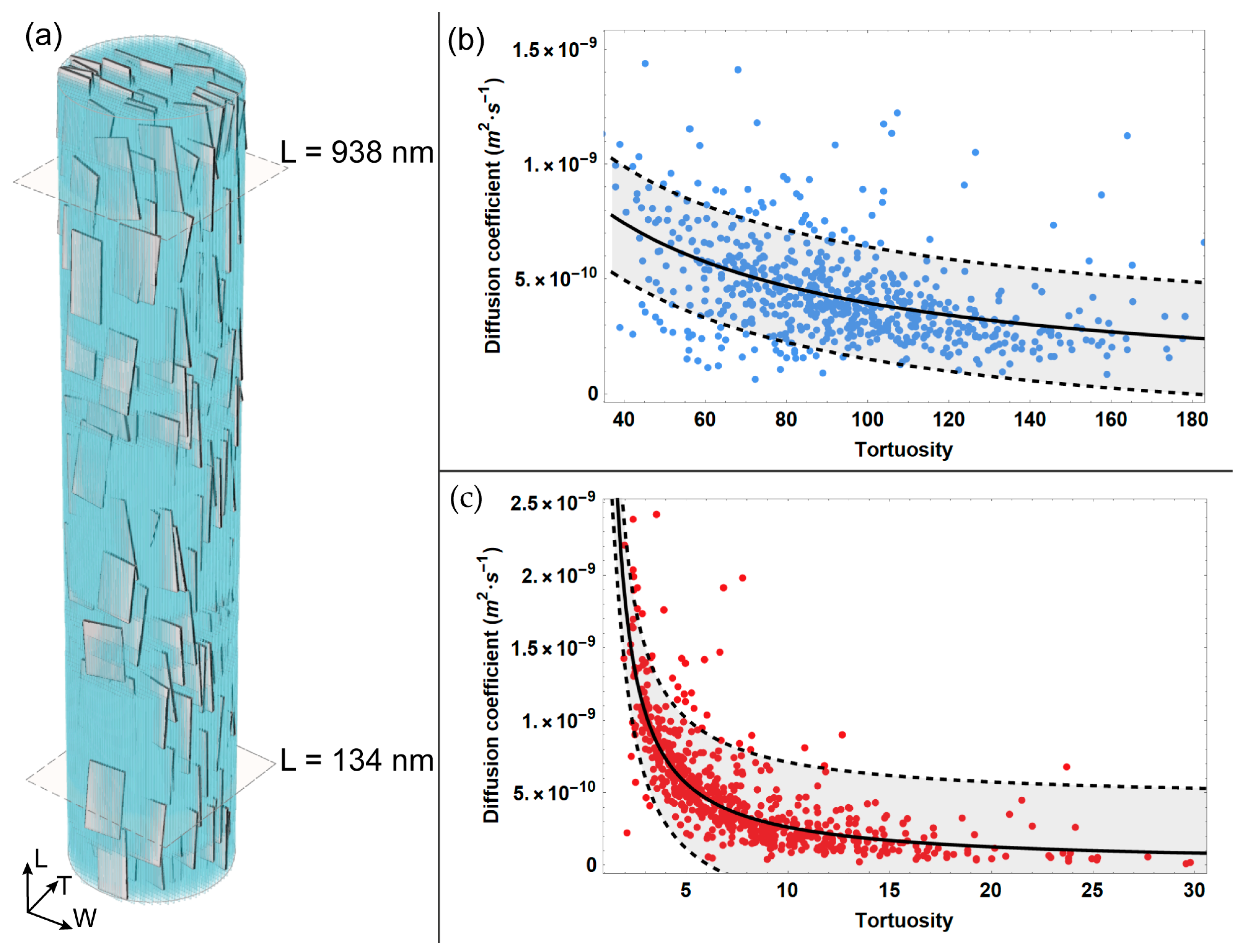
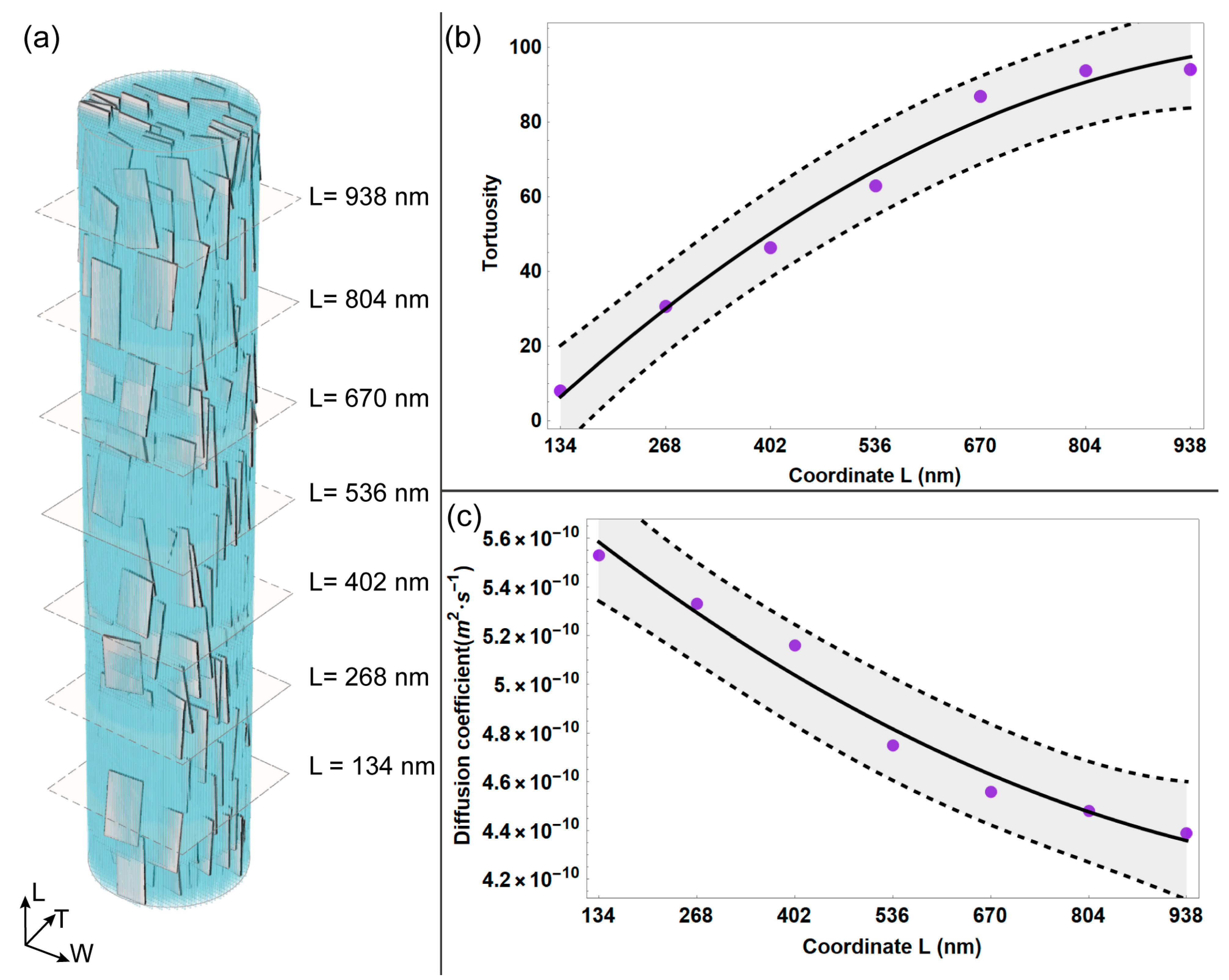
| Parameter | Mean Value |
|---|---|
| Width | 41.80 nm |
| Thickness | 3.55 nm |
| Length | 94.51 nm |
| aW | 13.19 nm |
| aT | 2.35 nm |
| aL | 39.49 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bini, F.; Pica, A.; Marinozzi, A.; Marinozzi, F. 3D Tortuosity and Diffusion Characterization in the Human Mineralized Collagen Fibril Using a Random Walk Model. Bioengineering 2023, 10, 558. https://doi.org/10.3390/bioengineering10050558
Bini F, Pica A, Marinozzi A, Marinozzi F. 3D Tortuosity and Diffusion Characterization in the Human Mineralized Collagen Fibril Using a Random Walk Model. Bioengineering. 2023; 10(5):558. https://doi.org/10.3390/bioengineering10050558
Chicago/Turabian StyleBini, Fabiano, Andrada Pica, Andrea Marinozzi, and Franco Marinozzi. 2023. "3D Tortuosity and Diffusion Characterization in the Human Mineralized Collagen Fibril Using a Random Walk Model" Bioengineering 10, no. 5: 558. https://doi.org/10.3390/bioengineering10050558








