Towards Novel Biomimetic In Vitro Models of the Blood–Brain Barrier for Drug Permeability Evaluation
Abstract
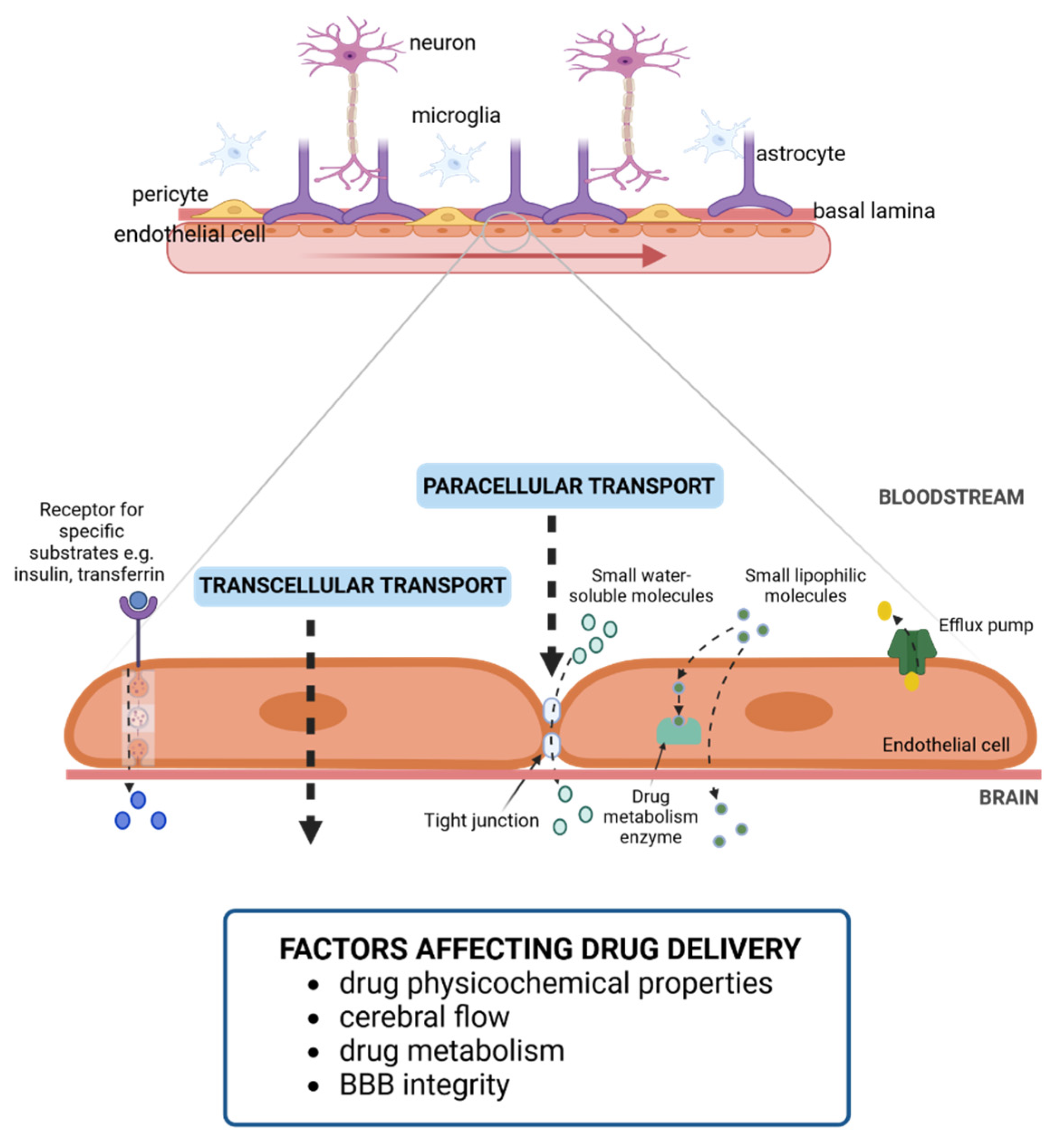
:1. Blood–Brain Barrier
2. Challenges in Drug Delivery to the CNS
3. Towards Biomimetic Models of the BBB
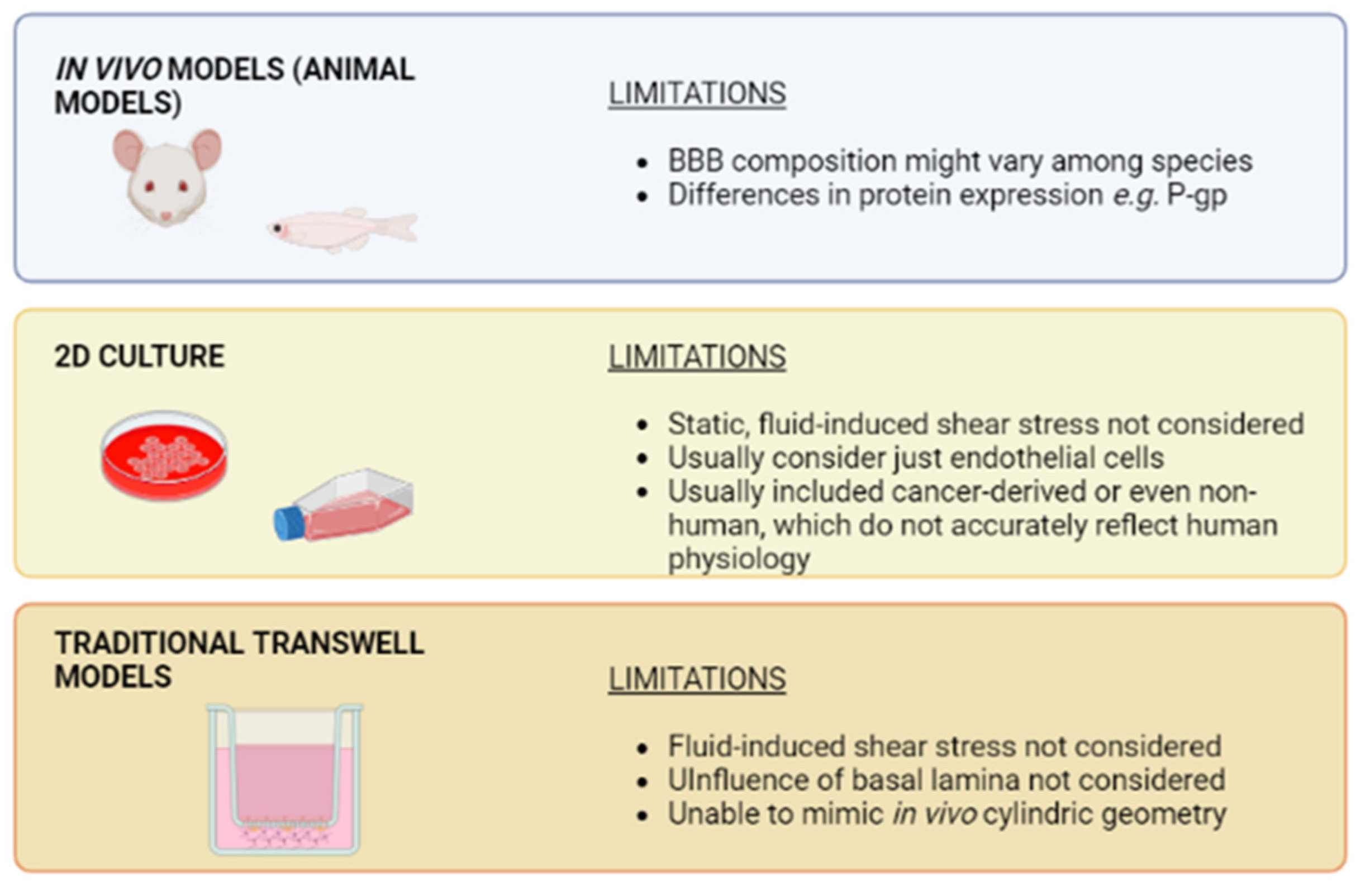
4. Advantages of Organ-on-Chip Devices versus Traditional Cell Culture Systems
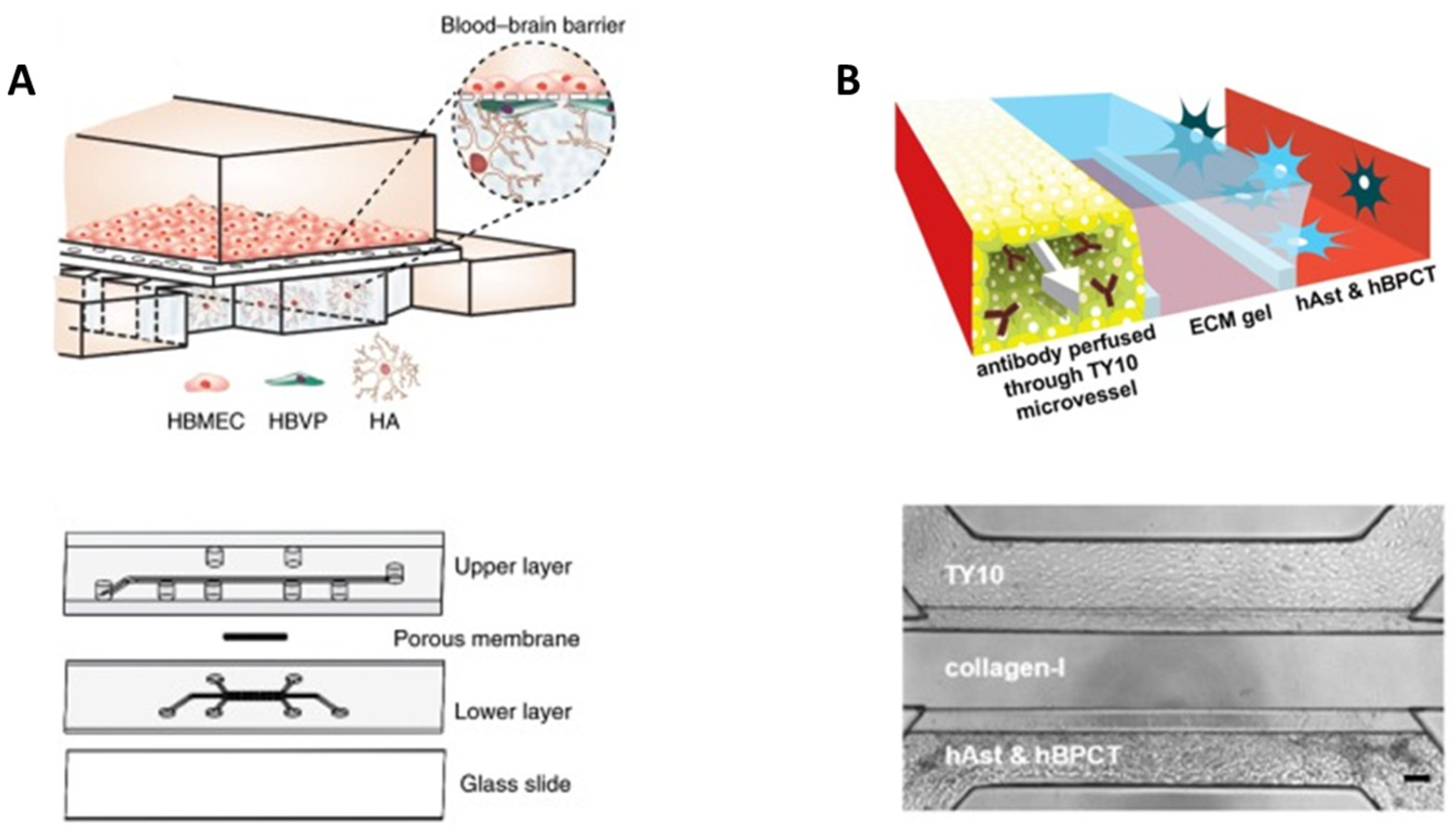
5. BBB-on-Chip: Mimicking Tissue Microarchitecture
5.1. Porous Membranes
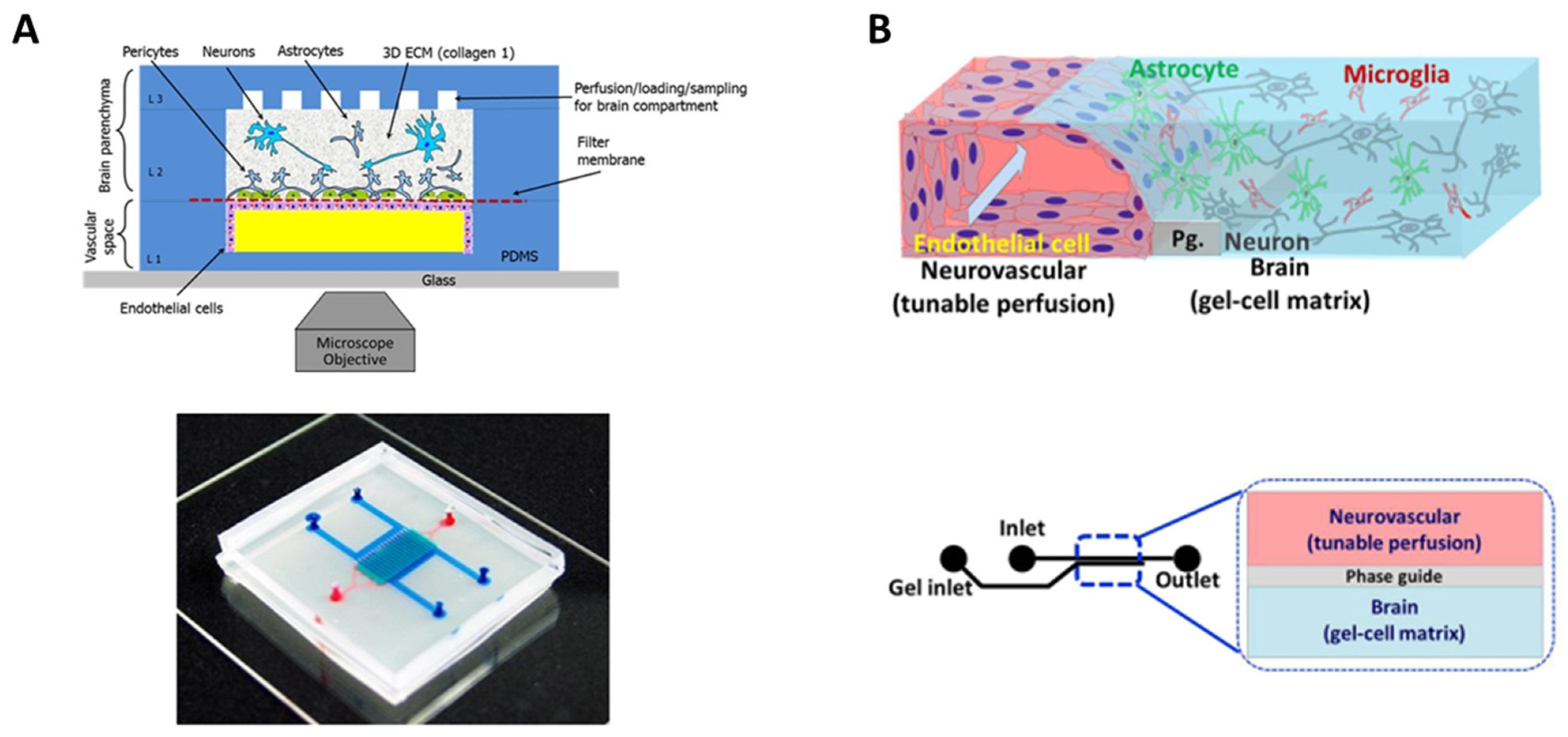
5.2. Hydrogels
6. BBB-on-Chip for Drug Evaluation
7. Mimicking Brain Pathology to Evaluate Potential Treatments
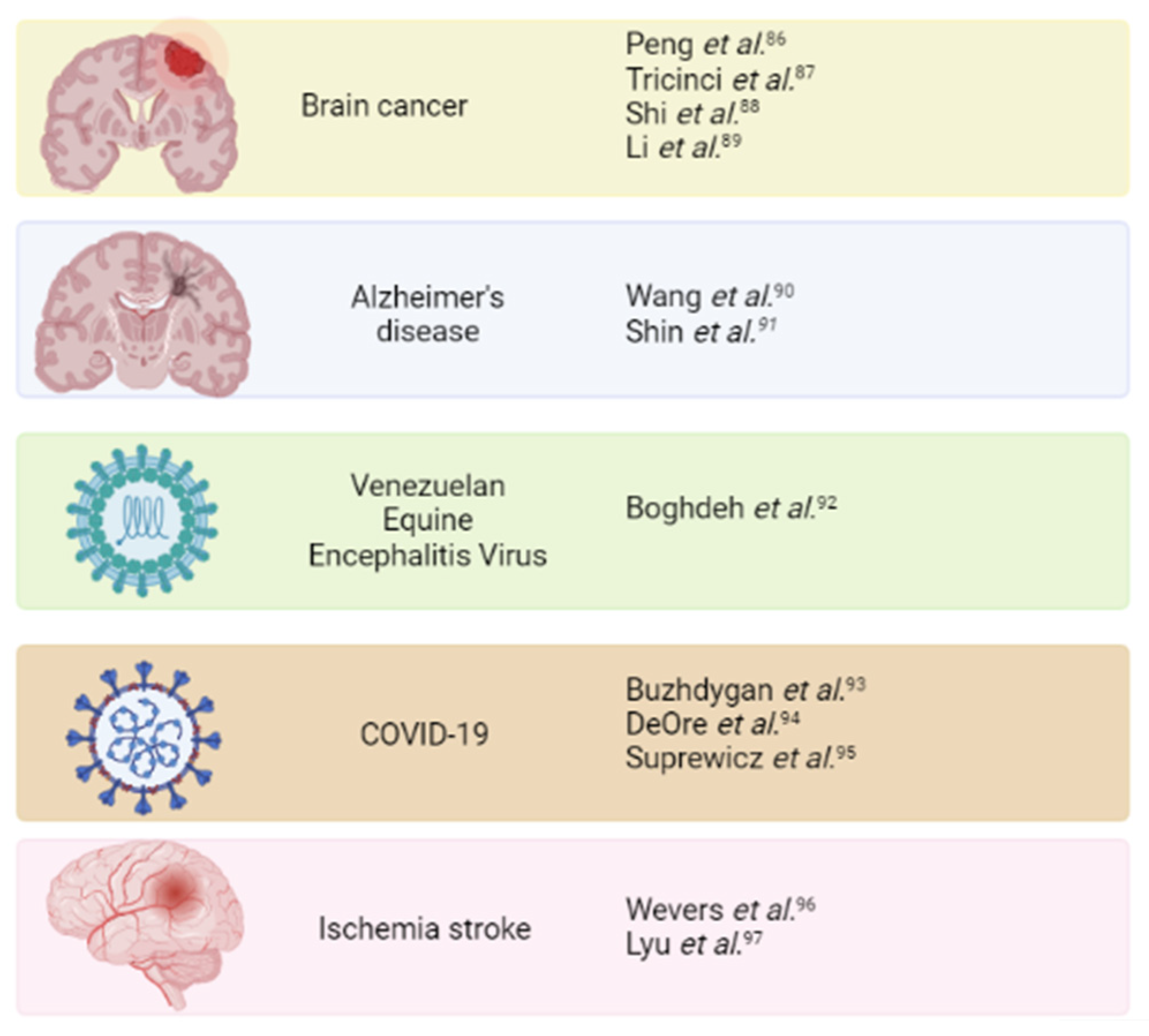
7.1. Brain Cancer
7.2. Alzheimer’s Disease
7.3. Virus Infection
7.4. Ischemia Stroke
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Gan, L.; Cao, F.; Wang, H.; Gong, P.; Ma, C.; Ren, L.; Lin, Y.; Lin, X. The Barrier and Interface Mechanisms of the Brain Barrier, and Brain Drug Delivery. Brain Res. Bull. 2022, 190, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.; Nascimento, M.; Liz, M.A.; Cardoso, I. Key Brain Cell Interactions and Contributions to the Pathogenesis of Alzheimer’s Disease. Front. Cell Dev. Biol. 2022, 10, 1036123. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Elabi, O.F. Microvascular Changes in Parkinson’s Disease- Focus on the Neurovascular Unit. Front. Aging Neurosci. 2022, 14, 853372. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Kagawa, Y.; Sun, J.; Turner, B.J.; Huang, C.; Shah, A.D.; Schittenhelm, R.B.; Nicolazzo, J.A. Altered Blood–Brain Barrier Dynamics in the C9orf72 Hexanucleotide Repeat Expansion Mouse Model of Amyotrophic Lateral Sclerosis. Pharmaceutics 2022, 14, 2803. [Google Scholar] [CrossRef]
- Bonetto, V.; Pasetto, L.; Lisi, I.; Carbonara, M.; Zangari, R.; Ferrari, E.; Punzi, V.; Luotti, S.; Bottino, N.; Biagianti, B.; et al. Markers of Blood-Brain Barrier Disruption Increase Early and Persistently in COVID-19 Patients with Neurological Manifestations. Front. Immunol. 2022, 13, 1070379. [Google Scholar] [CrossRef]
- Monje, M.; Iwasaki, A. The Neurobiology of Long COVID. Neuron 2022, 110, 3484–3496. [Google Scholar] [CrossRef]
- Smith, B.C.; Tinkey, R.A.; Shaw, B.C.; Williams, J.L. Targetability of the Neurovascular Unit in Inflammatory Diseases of the Central Nervous System. Immunol. Rev. 2022, 311, 39–49. [Google Scholar] [CrossRef]
- Mizutani, K.-I.; Kidoya, H.; Nakayama, K.; Hattori, Y. The Multiple Roles of Pericytes in Vascular Formation and Microglial Functions in the Brain. Life 2022, 12, 1835. [Google Scholar] [CrossRef]
- Zhang, S.; Shang, D.; Shi, H.; Teng, W.; Tian, L. Function of Astrocytes in Neuroprotection and Repair after Ischemic Stroke. Eur. Neurol. 2021, 84, 426–434. [Google Scholar] [CrossRef]
- Lee, K.Y. Common Immunopathogenesis of Central Nervous System Diseases: The Protein-Homeostasis-System Hypothesis. Cell Biosci. 2022, 12, 184. [Google Scholar] [CrossRef]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period. Front. Neurosci. 2020, 13, 1452. [Google Scholar] [CrossRef]
- McConnell, H.L.; Kersch, C.N.; Woltjer, R.L.; Neuwelt, E.A. The Translational Significance of the Neurovascular Unit. J. Biol. Chem. 2017, 292, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Sapkota, A.; Milner, R. The Impact of Genetic Manipulation of Laminin and Integrins at the Blood–Brain Barrier. Fluids Barriers CNS 2022, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Bix, G.; Yao, Y. Basal Lamina Changes in Neurodegenerative Disorders. Mol. Neurodegener. 2021, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nirwane, A.; Yao, Y. Basement Membrane and Blood-Brain Barrier. Stroke Vasc. Neurol. 2019, 4, 78–82. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, P.; Peng, Y.; Sun, Y.; Chen, X.; Zhao, Z.; Han, B. Recent Advances in Drug Delivery Systems for Targeting Brain Tumors. Drug Deliv. 2023, 30, 1–18. [Google Scholar] [CrossRef]
- Pawar, B.; Vasdev, N.; Gupta, T.; Mhatre, M.; More, A.; Anup, N.; Tekade, R.K. Current Update on Transcellular Brain Drug Delivery. Pharmaceutics 2022, 14, 2719. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Van Haver, V.; Vandenbroucke, R.E.; Decrock, E.; Wang, N.; Leybaert, L. Into Rather Unexplored Terrain-Transcellular Transport across the Blood-Brain Barrier. Glia 2016, 64, 1097–1123. [Google Scholar] [CrossRef]
- Kaya, M.; Ahishali, B. Basic Physiology of the Blood-Brain Barrier in Health and Disease: A Brief Overview. Tissue Barriers 2021, 9, 1840913. [Google Scholar] [CrossRef]
- Liu, H.J.; Xu, P. Strategies to Overcome/Penetrate the BBB for Systemic Nanoparticle Delivery to the Brain/Brain Tumor. Adv. Drug Deliv. Rev. 2022, 191, 114619. [Google Scholar] [CrossRef]
- Bellettato, C.M.; Scarpa, M. Possible Strategies to Cross the Blood-Brain Barrier. Ital. J. Pediatr. 2018, 44, 131. [Google Scholar] [CrossRef] [PubMed]
- Tjakra, M.; Wang, Y.; Vania, V.; Hou, Z.; Durkan, C.; Wang, N.; Wang, G. Overview of Crosstalk Between Multiple Factor of Transcytosis in Blood Brain Barrier. Front. Neurosci. 2020, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; Davis, T.P. Transport Mechanisms at the Blood–Brain Barrier and in Cellular Compartments of the Neurovascular Unit: Focus on CNS Delivery of Small Molecule Drugs. Pharmaceutics 2022, 14, 1501. [Google Scholar] [CrossRef]
- Kutuzov, N.; Flyvbjerg, H.; Lauritzen, M. Contributions of the Glycocalyx, Endothelium, and Extravascular Compartment to the Blood–Brain Barrier. Proc. Natl. Acad. Sci. USA 2018, 115, E9429–E9438. [Google Scholar] [CrossRef]
- Lynch, M.J.; Gobbo, O.L. Advances in Non-Animal Testing Approaches towards Accelerated Clinical Translation of Novel Nanotheranostic Therapeutics for Central Nervous System Disorders. Nanomaterials 2021, 11, 2632. [Google Scholar] [CrossRef] [PubMed]
- O’brown, N.M.; Pfau, S.J.; Gu, C. Bridging Barriers: A Comparative Look at the Blood-Brain Barrier across Organisms. Genes Dev. 2018, 32, 466–478. [Google Scholar] [CrossRef]
- Syvänen, S.; Lindhe, Ö.; Palner, M.; Kornum, B.R.; Rahman, O.; Långström, B.; Knudsen, G.M.; Hammarlund-Udenaes, M. Species Differences in Blood-Brain Barrier Transport of Three Positron Emission Tomography Radioligands with Emphasis on P-Glycoprotein Transport. Drug Metab. Dispos. 2009, 37, 635–643. [Google Scholar] [CrossRef]
- Verscheijden, L.F.M.; Koenderink, J.B.; de Wildt, S.N.; Russel, F.G.M. Differences in P-Glycoprotein Activity in Human and Rodent Blood–Brain Barrier Assessed by Mechanistic Modelling. Arch. Toxicol. 2021, 95, 3015–3029. [Google Scholar] [CrossRef]
- Cui, B.; Cho, S.W. Blood-Brain Barrier-on-a-Chip for Brain Disease Modeling and Drug Testing. BMB Rep. 2022, 55, 213–219. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Creangă-Murariu, I.; Tamba, B.I.; Lucanu, N.; Popescu, B.O. In Vitro Modeling of the Blood–Brain Barrier for the Study of Physiological Conditions and Alzheimer’s Disease. Biomolecules 2022, 12, 1136. [Google Scholar] [CrossRef]
- De Stefano, J.G.; Xu, Z.S.; Williams, A.J.; Yimam, N.; Searson, P.C. Effect of Shear Stress on IPSC-Derived Human Brain Microvascular Endothelial Cells (DhBMECs). Fluids Barriers CNS 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Choublier, N.; Taghi, M.; Menet, M.C.; Le Gall, M.; Bruce, J.; Chafey, P.; Guillonneau, F.; Moreau, A.; Denizot, C.; Parmentier, Y.; et al. Exposure of Human Cerebral Microvascular Endothelial Cells HCMEC/D3 to Laminar Shear Stress Induces Vascular Protective Responses. Fluids Barriers CNS 2022, 19, 41. [Google Scholar] [CrossRef]
- Jamieson, J.J.; Searson, P.C.; Gerecht, S. Engineering the Human Blood-Brain Barrier in Vitro. J. Biol. Eng. 2017, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A Novel Transwell Blood Brain Barrier Model Using Primary Human Cells. Front. Cell. Neurosci. 2019, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Vigh, J.P.; Kincses, A.; Ozgür, B.; Walter, F.R.; Santa-Maria, A.R.; Valkai, S.; Vastag, M.; Neuhaus, W.; Brodin, B.; Dér, A.; et al. Transendothelial Electrical Resistance Measurement across the Blood–Brain Barrier: A Critical Review of Methods. Micromachines 2021, 12, 685. [Google Scholar] [CrossRef]
- Harding, I.C.; O’Hare, N.R.; Vigliotti, M.; Caraballo, A.; Lee, C.I.; Millican, K.; Herman, I.M.; Ebong, E.E. Developing a Transwell Millifluidic Device for Studying Blood–Brain Barrier Endothelium. Lab. Chip. 2022, 22, 4603–4620. [Google Scholar] [CrossRef]
- Katt, M.E.; Linville, R.M.; Mayo, L.N.; Xu, Z.S.; Searson, P.C. Functional Brain-Specific Microvessels from IPSC-Derived Human Brain Microvascular Endothelial Cells: The Role of Matrix Composition on Monolayer Formation. Fluids Barriers CNS 2018, 15, 7. [Google Scholar] [CrossRef]
- Bischel, L.L.; Sung, K.E.; Jiménez-Torres, J.A.; Mader, B.; Keely, P.J.; Beebe, D.J. The Importance of Being a Lumen. FASEB J. 2014, 28, 4583–4590. [Google Scholar] [CrossRef]
- Destefano, J.G.; Jamieson, J.J.; Linville, R.M.; Searson, P.C. Benchmarking in Vitro Tissue-Engineered Blood-Brain Barrier Models. Fluids Barriers CNS 2018, 15, 32. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Virumbrales-Muñoz, M.; Lang, J.M.; Beebe, D.J. A Role for Microfluidic Systems in Precision Medicine. Nat. Commun. 2022, 13, 3086. [Google Scholar] [CrossRef]
- Bernardin, A.A.; Colombani, S.; Rousselot, A.; Andry, V.; Goumon, Y.; Delanoë-Ayari, H.; Pasqualin, C.; Brugg, B.; Jacotot, E.D.; Pasquié, J.-L.; et al. Impact of Neurons on Patient-Derived Cardiomyocytes Using Organ-On-A-Chip and IPSC Biotechnologies. Cells 2022, 11, 3764. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.S.; Kammala, A.K.; Costantine, M.M.; Fortunato, S.J.; Radnaa, E.; Kim, S.; Taylor, R.N.; Han, A.; Menon, R. Testing of Drugs Using Human Feto-Maternal Interface Organ-on-Chips Provide Insights into Pharmacokinetics and Efficacy. Lab. Chip. 2022, 22, 4574–4592. [Google Scholar] [CrossRef]
- Ferreira, D.A.; Conde, J.P.; Rothbauer, M.; Ertl, P.; Granja, P.L.; Oliveira, C. Bioinspired Human Stomach-on-a-Chip with in Vivo like Function and Architecture. Lab. Chip. 2023, 23, 495–510. [Google Scholar] [CrossRef]
- Liu, X.; Su, Q.; Zhang, X.; Yang, W.; Ning, J.; Jia, K.; Xin, J.; Li, H.; Yu, L.; Liao, Y.; et al. Recent Advances of Organ-on-a-Chip in Cancer Modeling Research. Biosensors 2022, 12, 1045. [Google Scholar] [CrossRef] [PubMed]
- Li, S.R.; Gulieva, R.E.; Helms, L.; Cruz, N.M.; Vincent, T.; Fu, H.; Himmelfarb, J.; Freedman, B.S. Glucose Absorption Drives Cystogenesis in a Human Organoid-on-Chip Model of Polycystic Kidney Disease. Nat. Commun. 2022, 13, 7918. [Google Scholar] [CrossRef]
- Cai, H.; Ao, Z.; Tian, C.; Wu, Z.; Kaurich, C.; Chen, Z.; Gu, M.; Hohmann, A.G.; Mackie, K.; Guo, F. Engineering Human Spinal Microphysiological Systems to Model Opioid-Induced Tolerance. Bioact. Mater. 2023, 22, 482–490. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Z.; Gao, W.; Gao, W.; He, R.; Li, Y.; Crawford, R.; Zhou, Y.; Xiao, L.; Xiao, Y. Current Advances in 3D Dynamic Cell Culture Systems. Gels 2022, 8, 829. [Google Scholar] [CrossRef]
- Marzagalli, M.; Pelizzoni, G.; Fedi, A.; Vitale, C.; Fontana, F.; Bruno, S.; Poggi, A.; Dondero, A.; Aiello, M.; Castriconi, R.; et al. A Multi-Organ-on-Chip to Recapitulate the Infiltration and the Cytotoxic Activity of Circulating NK Cells in 3D Matrix-Based Tumor Model. Front. Bioeng. Biotechnol. 2022, 10, 945149. [Google Scholar] [CrossRef]
- Grassart, A.; Malardé, V.; Gobba, S.; Sartori-Rupp, A.; Kerns, J.; Karalis, K.; Marteyn, B.; Sansonetti, P.; Sauvonnet, N. Bioengineered Human Organ-on-Chip Reveals Intestinal Microenvironment and Mechanical Forces Impacting Shigella Infection. Cell Host. Microbe. 2019, 26, 435–444.e4. [Google Scholar] [CrossRef]
- Fois, C.A.M.; Schindeler, A.; Valtchev, P.; Dehghani, F. Dynamic Flow and Shear Stress as Key Parameters for Intestinal Cells Morphology and Polarization in an Organ-on-a-Chip Model. Biomed. Microdevices 2021, 23, 55. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, H.; Cheng, S.; Kourmatzis, A.; Traini, D.; Young, P.; Sheikh, Z.; Ong, H.X. In Vitro Interactions of Aerosol Formulations with Human Nasal Epithelium Using Real-Time Monitoring of Drug Transport in a Nasal Mucosa-on-a-Chip. Biosens. Bioelectron. 2023, 223, 115010. [Google Scholar] [CrossRef]
- Nashimoto, Y.; Mukomoto, R.; Imaizumi, T.; Terai, T.; Shishido, S.; Ino, K.; Yokokawa, R.; Miura, T.; Onuma, K.; Inoue, M.; et al. Electrochemical Sensing of Oxygen Metabolism for a Three-Dimensional Cultured Model with Biomimetic Vascular Flow. Biosens. Bioelectron. 2023, 219, 114808. [Google Scholar] [CrossRef] [PubMed]
- Zoio, P.; Lopes-Ventura, S.; Oliva, A. Barrier-on-a-Chip with a Modular Architecture and Integrated Sensors for Real-Time Measurement of Biological Barrier Function. Micromachines 2021, 12, 816. [Google Scholar] [CrossRef]
- Heidari, H.; Taylor, H. Review Article: Capturing the Physiological Complexity of the Brain’s Neuro-Vascular Unit in Vitro. Biomicrofluidics 2018, 12, 051502. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, S.; Buonocore, J.; Park, J.; Welsh, C.J.; Li, J.; Han, A. A Three-Dimensional Arrayed Microfluidic Blood-Brain Barrier Model with Integrated Electrical Sensor Array. IEEE Trans. Biomed. Eng. 2018, 65, 431–439. [Google Scholar] [CrossRef]
- Tu, K.H.; Yu, L.S.; Sie, Z.H.; Hsu, H.Y.; Al-Jamal, K.T.; Wang, J.T.W.; Chiang, Y.Y. Development of Real-Time Transendothelial Electrical Resistance Monitoring for an in Vitro Blood-Brain Barrier System. Micromachines 2021, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Palma-Florez, S.; Lagunas, A.; López-Martínez, M.J.; Samitier, J. Biosensors Integration in Blood-Brain Barrier-on-a-Chip: Emerging Platform for Monitoring Neurodegenerative Diseases. ACS Sens. 2022, 7, 1237–1247. [Google Scholar] [CrossRef]
- Santa-Maria, A.R.; Walter, F.R.; Figueiredo, R.; Kincses, A.; Vigh, J.P.; Heymans, M.; Culot, M.; Winter, P.; Gosselet, F.; Dér, A.; et al. Flow Induces Barrier and Glycocalyx-Related Genes and Negative Surface Charge in a Lab-on-a-Chip Human Blood-Brain Barrier Model. J. Cereb. Blood Flow Metab. 2021, 41, 2201–2215. [Google Scholar] [CrossRef]
- Herland, A.; Van Der Meer, A.D.; FitzGerald, E.A.; Park, T.E.; Sleeboom, J.J.F.; Ingber, D.E. Distinct Contributions of Astrocytes and Pericytes to Neuroinflammation Identified in a 3D Human Blood-Brain Barrier on a Chip. PLoS ONE 2016, 11, 0150360. [Google Scholar] [CrossRef]
- Ahn, S.I.; Sei, Y.J.; Park, H.J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.J.; MacDonald, T.J.; Levey, A.I.; Kim, Y.T. Microengineered Human Blood–Brain Barrier Platform for Understanding Nanoparticle Transport Mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.; Kim, H. Characterization of a Microfluidic in Vitro Model of the Blood-Brain Barrier (ΜBBB). Lab. Chip. 2012, 12, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Pensabene, V.; Markov, D.A.; Allwardt, V.; Diana Neely, M.; Shi, M.; Britt, C.M.; Hoilett, O.S.; Yang, Q.; Brewer, B.M.; et al. Recreating Blood-Brain Barrier Physiology and Structure on Chip: A Novel Neurovascular Microfluidic Bioreactor. Biomicrofluidics 2015, 9, 054124. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Gao, D.; Chen, Y.; Jin, F.; Hu, G.; Jiang, Y.; Liu, H. Development of a Blood-Brain Barrier Model in a Membrane-Based Microchip for Characterization of Drug Permeability and Cytotoxicity for Drug Screening. Anal. Chim. Acta 2016, 934, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic Blood–Brain Barrier Model Provides in Vivo-like Barrier Properties for Drug Permeability Screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef]
- Bonakdar, M.; Graybill, P.M.; Davalos, R.V. A Microfluidic Model of the Blood-Brain Barrier to Study Permeabilization by Pulsed Electric Fields. RSC Adv. 2017, 7, 42811–42818. [Google Scholar] [CrossRef]
- Choi, B.; Choi, J.W.; Jin, H.; Sim, H.R.; Park, J.H.; Park, T.E.; Kang, J.H. Condensed ECM-Based Nanofilms on Highly Permeable PET Membranes for Robust Cell-to-Cell Communications with Improved Optical Clarity. Biofabrication 2021, 13, 045020. [Google Scholar] [CrossRef]
- Falanga, A.P.; Pitingolo, G.; Celentano, M.; Cosentino, A.; Melone, P.; Vecchione, R.; Guarnieri, D.; Netti, P.A. Shuttle-Mediated Nanoparticle Transport across an in Vitro Brain Endothelium under Flow Conditions. Biotechnol. Bioeng. 2017, 114, 1087–1095. [Google Scholar] [CrossRef]
- Mancinelli, E.; Takuma, M.; Fujie, T.; Pensabene, V. Recreating Cellular Barriers in Human Microphysiological Systems In-Vitro. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2022, 2022, 3923–3926. [Google Scholar] [CrossRef]
- Meena, M.; Vandormael, R.; De Laere, M.; Pintelon, I.; Berneman, Z.; Watts, R.; Cools, N. A Microfluidic In Vitro Three-Dimensional Dynamic Model of the Blood–Brain Barrier to Study the Transmigration of Immune Cells. Brain Sci. 2022, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Motallebnejad, P.; Thomas, A.; Swisher, S.L.; Azarin, S.M. An Isogenic HiPSC-Derived BBB-on-a-Chip. Biomicrofluidics 2019, 13, 064119. [Google Scholar] [CrossRef]
- Hudecz, D.; Khire, T.; Chung, H.L.; Adumeau, L.; Glavin, D.; Luke, E.; Nielsen, M.S.; Dawson, K.A.; McGrath, J.L.; Yan, Y. Ultrathin Silicon Membranes for in Situ Optical Analysis of Nanoparticle Translocation across a Human Blood-Brain Barrier Model. ACS Nano 2020, 14, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Mossu, A.; Rosito, M.; Khire, T.; Li Chung, H.; Nishihara, H.; Gruber, I.; Luke, E.; Dehouck, L.; Sallusto, F.; Gosselet, F.; et al. A Silicon Nanomembrane Platform for the Visualization of Immune Cell Trafficking across the Human Blood–Brain Barrier under Flow. J. Cereb. Blood Flow Metab. 2019, 39, 395–410. [Google Scholar] [CrossRef]
- Jiang, L.; Li, S.; Zheng, J.; Li, Y.; Huang, H. Recent Progress in Microfluidic Models of the Blood-Brain Barrier. Micromachines 2019, 10, 375. [Google Scholar] [CrossRef]
- Chung, H.H.; Mireles, M.; Kwarta, B.J.; Gaborski, T.R. Use of Porous Membranes in Tissue Barrier and Co-Culture Models. Lab. Chip. 2018, 18, 1671–1689. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Chandrasekar, V.; Laux, P.; Luch, A.; Dakua, S.P.; Zamboni, P.; Shelar, A.; Yang, Y.; Pandit, V.; Tisato, V.; et al. Micropatterned Neurovascular Interface to Mimic the Blood–Brain Barrier’s Neurophysiology and Micromechanical Function: A BBB-on-CHIP Model. Cells 2022, 11, 2801. [Google Scholar] [CrossRef]
- Koo, Y.; Hawkins, B.T.; Yun, Y. Three-Dimensional (3D) Tetra-Culture Brain on Chip Platform for Organophosphate Toxicity Screening. Sci. Rep. 2018, 8, 2841. [Google Scholar] [CrossRef]
- Liu, L.; Koo, Y.; Akwitti, C.; Russell, T.; Gay, E.; Laskowitz, D.T.; Yun, Y. Three-Dimensional (3D) Brain Microphysiological System for Organophosphates and Neurochemical Agent Toxicity Screening. PLoS ONE 2019, 14, e0224657. [Google Scholar] [CrossRef]
- Yu, F.; Kumar, N.D.S.; Foo, L.C.; Ng, S.H.; Hunziker, W.; Choudhury, D. A Pump-Free Tricellular Blood-Brain Barrier on-a-Chip Model to Understand Barrier Property and Evaluate Drug Response. Biotechnol. Bioeng. 2020, 117, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.L.; Campisi, M.; Osaki, T.; Possenti, L.; Mattu, C.; Adriani, G.; Kamm, R.D.; Chiono, V. Modeling Nanocarrier Transport across a 3D In Vitro Human Blood-Brain–Barrier Microvasculature. Adv. Healthc. Mater. 2020, 9, e1901486. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D Self-Organized Microvascular Model of the Human Blood-Brain Barrier with Endothelial Cells, Pericytes and Astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef]
- Winkelman, M.A.; Kim, D.Y.; Kakarla, S.; Grath, A.; Silvia, N.; Dai, G. Interstitial Flow Enhances the Formation, Connectivity, and Function of 3D Brain Microvascular Networks Generated within a Microfluidic Device. Lab. Chip. 2022, 22, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, K.T.; Lee, J.S.; Shin, J.; Cui, B.; Yang, K.; Choi, Y.S.; Choi, N.; Lee, S.H.; Lee, J.H.; et al. Fungal Brain Infection Modelled in a Human-Neurovascular-Unit-on-a-Chip with a Functional Blood–Brain Barrier. Nat. Biomed. Eng. 2021, 5, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Kumar, A.; Dev, A.; Gupta, V.K.; Han, S.S. Advances in Hydrogel-Based Microfluidic Blood–Brain-Barrier Models in Oncology Research. Pharmaceutics 2022, 14, 993. [Google Scholar] [CrossRef]
- Wevers, N.R.; Kasi, D.G.; Gray, T.; Wilschut, K.J.; Smith, B.; Vught, R.; Shimizu, F.; Sano, Y.; Kanda, T.; Marsh, G.; et al. A Perfused Human Blood-Brain Barrier on-a-Chip for High-Throughput Assessment of Barrier Function and Antibody Transport. Fluids Barriers CNS 2018, 15, 23. [Google Scholar] [CrossRef]
- Noorani, B.; Bhalerao, A.; Raut, S.; Nozohouri, E.; Bickel, U.; Cucullo, L. A Quasi-Physiological Microfluidic Blood-Brain Barrier Model for Brain Permeability Studies. Pharmaceutics 2021, 13, 1474. [Google Scholar] [CrossRef]
- Sahtoe, D.D.; Coscia, A.; Mustafaoglu, N.; Miller, L.M.; Olal, D.; Vulovic, I.; Yu, T.Y.; Goreshnik, I.; Lin, Y.R.; Clark, L.; et al. Transferrin Receptor Targeting by de Novo Sheet Extension. Proc. Natl. Acad. Sci. USA 2021, 118, 2021569118. [Google Scholar] [CrossRef] [PubMed]
- Fengler, S.; Kurkowsky, B.; Kaushalya, S.K.; Roth, W.; Fava, E.; Denner, P. Human IPSC-Derived Brain Endothelial Microvessels in a Multi-Well Format Enable Permeability Screens of Anti-Inflammatory Drugs. Biomaterials 2022, 286, 121525. [Google Scholar] [CrossRef]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human IPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 2019, 24, 995–1005.e6. [Google Scholar] [CrossRef] [PubMed]
- Maoz, B.M.; Herland, A.; Fitzgerald, E.A.; Grevesse, T.; Vidoudez, C.; Pacheco, A.R.; Sheehy, S.P.; Park, T.E.; Dauth, S.; Mannix, R.; et al. A Linked Organ-on-Chip Model of the Human Neurovascular Unit Reveals the Metabolic Coupling of Endothelial and Neuronal Cells. Nat. Biotechnol. 2018, 36, 865–877. [Google Scholar] [CrossRef]
- Brown, J.A.; Faley, S.L.; Faley, S.L.; Shi, Y.; Hillgren, K.M.; Sawada, G.A.; Baker, T.K.; Wikswo, J.P.; Wikswo, J.P.; Wikswo, J.P.; et al. Advances in Blood-Brain Barrier Modeling in Microphysiological Systems Highlight Critical Differences in Opioid Transport Due to Cortisol Exposure. Fluids Barriers CNS 2020, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Su, S.H.; Song, Y.; Stephens, A.; Situ, M.; McCloskey, M.C.; McGrath, J.L.; Andjelkovic, A.V.; Singer, B.H.; Kurabayashi, K. A Tissue Chip with Integrated Digital Immunosensors: In Situ Brain Endothelial Barrier Cytokine Secretion Monitoring. Biosens. Bioelectron. 2023, 224, 115030. [Google Scholar] [CrossRef]
- Matthiesen, I.; Voulgaris, D.; Nikolakopoulou, P.; Winkler, T.E.; Herland, A. Continuous Monitoring Reveals Protective Effects of N-Acetylcysteine Amide on an Isogenic Microphysiological Model of the Neurovascular Unit. Small 2021, 17, e2101785. [Google Scholar] [CrossRef]
- Koenig, L.; Ramme, A.P.; Faust, D.; Mayer, M.; Flötke, T.; Gerhartl, A.; Brachner, A.; Neuhaus, W.; Appelt-Menzel, A.; Metzger, M.; et al. A Human Stem Cell-Derived Brain-Liver Chip for Assessing Blood-Brain-Barrier Permeation of Pharmaceutical Drugs. Cells 2022, 11, 3295. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Tong, Z.; Tong, W.Y.; Pasic, P.J.; Oddo, A.; Dai, Y.; Luo, M.; Frescene, J.; Welch, N.G.; Easton, C.D.; et al. In Situ Surface Modification of Microfluidic Blood-Brain-Barriers for Improved Screening of Small Molecules and Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 56753–56766. [Google Scholar] [CrossRef]
- Shi, Y.; He, X.; Wang, H.; Dai, J.; Fang, J.; He, Y.; Chen, X.; Hong, Z.; Chai, Y. Construction of a Novel Blood Brain Barrier-Glioma Microfluidic Chip Model: Applications in the Evaluation of Permeability and Anti-Glioma Activity of Traditional Chinese Medicine Components. Talanta 2023, 253, 123971. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, D.; Guo, Y.; Wang, Y.; Su, W. Evaluation of Hepatic Drug-Metabolism for Glioblastoma Using Liver-Brain Chip. Biotechnol. Lett. 2021, 43, 383–392. [Google Scholar] [CrossRef]
- Wang, J.; Gu, Y.; Liu, X.; Fan, Y.; Zhang, Y.; Yi, C.; Cheng, C.; Yang, M. Near-Infrared Photothermally Enhanced Photo-Oxygenation for Inhibition of Amyloid-β Aggregation Based on RVG-Conjugated Porphyrinic Metal–Organic Framework and Indocyanine Green Nanoplatform. Int. J. Mol. Sci. 2022, 23, 10885. [Google Scholar] [CrossRef]
- Shin, Y.; Choi, S.H.; Kim, E.; Bylykbashi, E.; Kim, J.A.; Chung, S.; Kim, D.Y.; Kamm, R.D.; Tanzi, R.E. Blood–Brain Barrier Dysfunction in a 3D In Vitro Model of Alzheimer’s Disease. Adv. Sci. 2019, 6, 1900962. [Google Scholar] [CrossRef] [PubMed]
- Wevers, N.R.; Nair, A.L.; Fowke, T.M.; Pontier, M.; Kasi, D.G.; Spijkers, X.M.; Hallard, C.; Rabussier, G.; van Vught, R.; Vulto, P.; et al. Modeling Ischemic Stroke in a Triculture Neurovascular Unit On-a-Chip. Fluids Barriers CNS 2021, 18, 59. [Google Scholar] [CrossRef]
- Boghdeh, N.A.; Risner, K.H.; Barrera, M.D.; Britt, C.M.; Schaffer, D.K.; Alem, F.; Brown, J.A.; Wikswo, J.P.; Narayanan, A. Application of a Human Blood Brain Barrier Organ-on-a-Chip Model to Evaluate Small Molecule Effectiveness against Venezuelan Equine Encephalitis Virus. Viruses 2022, 14, 2799. [Google Scholar] [CrossRef]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 Spike Protein Alters Barrier Function in 2D Static and 3D Microfluidic in-Vitro Models of the Human Blood–Brain Barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef]
- DeOre, B.J.; Tran, K.A.; Andrews, A.M.; Ramirez, S.H.; Galie, P.A. SARS-CoV-2 Spike Protein Disrupts Blood–Brain Barrier Integrity via RhoA Activation. J. Neuroimmune Pharmacol. 2021, 16, 722–728. [Google Scholar] [CrossRef]
- Suprewicz, Ł.; Tran, K.A.; Piktel, E.; Fiedoruk, K.; Janmey, P.A.; Galie, P.A.; Bucki, R. Recombinant Human Plasma Gelsolin Reverses Increased Permeability of the Blood–Brain Barrier Induced by the Spike Protein of the SARS-CoV-2 Virus. J. Neuroinflammation 2022, 19, 282. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Park, J.; Kim, K.M.; Jin, H.J.; Wu, H.; Rajadas, J.; Kim, D.H.; Steinberg, G.K.; Lee, W. A Neurovascular-Unit-on-a-Chip for the Evaluation of the Restorative Potential of Stem Cell Therapies for Ischaemic Stroke. Nat. Biomed. Eng. 2021, 5, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A Guide to the Organ-on-a-Chip. Nat. Rev. Methods Prim. 2022, 2, 3232. [Google Scholar] [CrossRef]

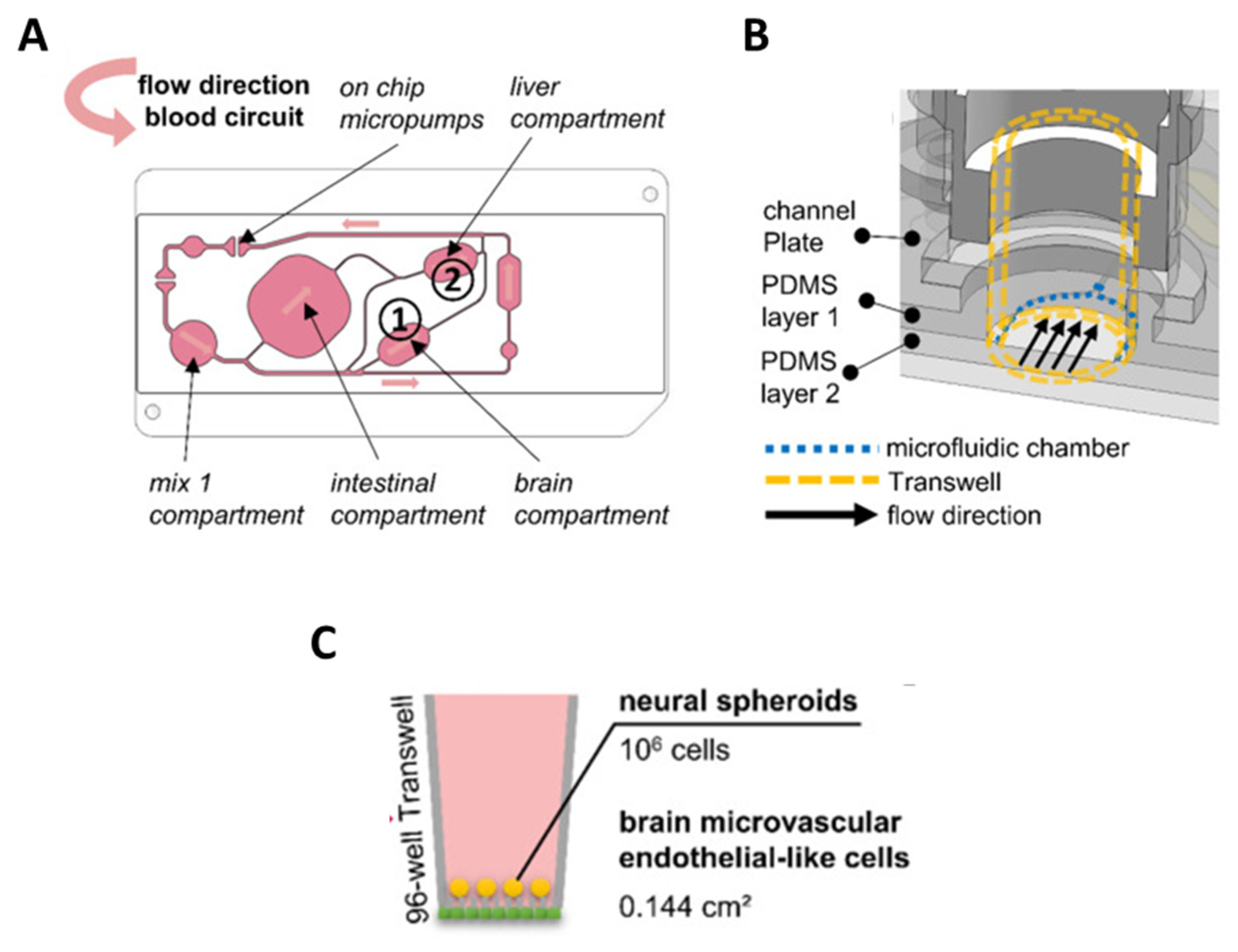


| Cell Type | Function |
|---|---|
| Endothelial cell | Responsible for high selective permeability of the BBB |
| Production of BL components | |
| Pericytes | Maintenance of tight junctions and barrier function |
| Production of BL components | |
| Regulation of vesicle endothelial transcytosis | |
| Regulation of immune cells infiltration | |
| Astrocytes | Endothelial cells maintenance |
| Connection of neurons with endothelial cells | |
| Regulation of immune cells infiltration | |
| Microglia | Phagocytosis Immune response activation Maintenance of BBB integrity by close connection with pericytes |
| Neurons | Indirect control of blood flow |
| Promotion of angiogenesis during the development |
| Semipermeable Porous Membrane | Hydrogel | References |
|---|---|---|
| Polycarbonate | Collagen and hyaluronic acid | Ahn et al. [60] Boot et al. [61] Brown et al. [62] Jeong et al. [55] Shao et al. [63] Wang et al. [64] |
| Polyester | Collagen | Bonakdar et al. [65] Choi et al. [66] Falanga et al. [67] Santa-Maria et al. [58] |
| Poly(D-L-lactic acid) | Fibrin | Mancinelli et al. [68] |
| Polyethylene terephthalate | - | Meena et al. [69] Motallebnejad et al. [70] Tu et al. [56] |
| Silicon | - | Hudecz et al. [71] Mossu et al. [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mármol, I.; Abizanda-Campo, S.; Ayuso, J.M.; Ochoa, I.; Oliván, S. Towards Novel Biomimetic In Vitro Models of the Blood–Brain Barrier for Drug Permeability Evaluation. Bioengineering 2023, 10, 572. https://doi.org/10.3390/bioengineering10050572
Mármol I, Abizanda-Campo S, Ayuso JM, Ochoa I, Oliván S. Towards Novel Biomimetic In Vitro Models of the Blood–Brain Barrier for Drug Permeability Evaluation. Bioengineering. 2023; 10(5):572. https://doi.org/10.3390/bioengineering10050572
Chicago/Turabian StyleMármol, Inés, Sara Abizanda-Campo, Jose M. Ayuso, Ignacio Ochoa, and Sara Oliván. 2023. "Towards Novel Biomimetic In Vitro Models of the Blood–Brain Barrier for Drug Permeability Evaluation" Bioengineering 10, no. 5: 572. https://doi.org/10.3390/bioengineering10050572
APA StyleMármol, I., Abizanda-Campo, S., Ayuso, J. M., Ochoa, I., & Oliván, S. (2023). Towards Novel Biomimetic In Vitro Models of the Blood–Brain Barrier for Drug Permeability Evaluation. Bioengineering, 10(5), 572. https://doi.org/10.3390/bioengineering10050572





