The Application of Design Thinking in Developing a Deep Learning Algorithm for Hip Fracture Detection
Abstract
1. Introduction
2. Materials and Methods
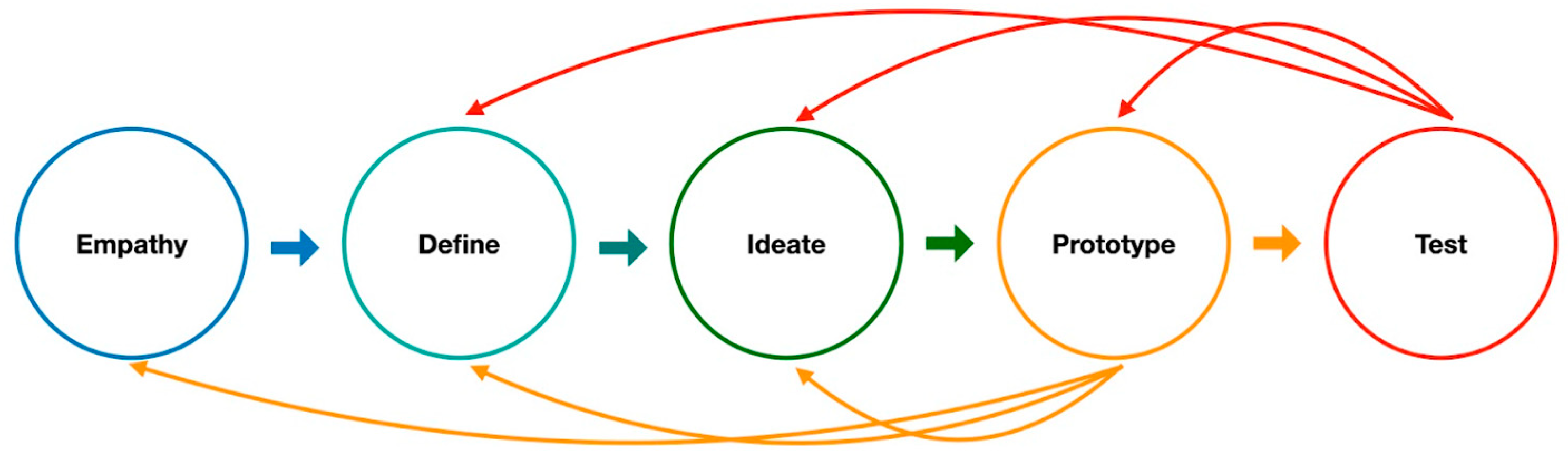
2.1. Empathize the Clinical Physicians
2.2. Defining the Clinical Unmet Needs and Ideating the Solution for the Clinical Unmet Needs
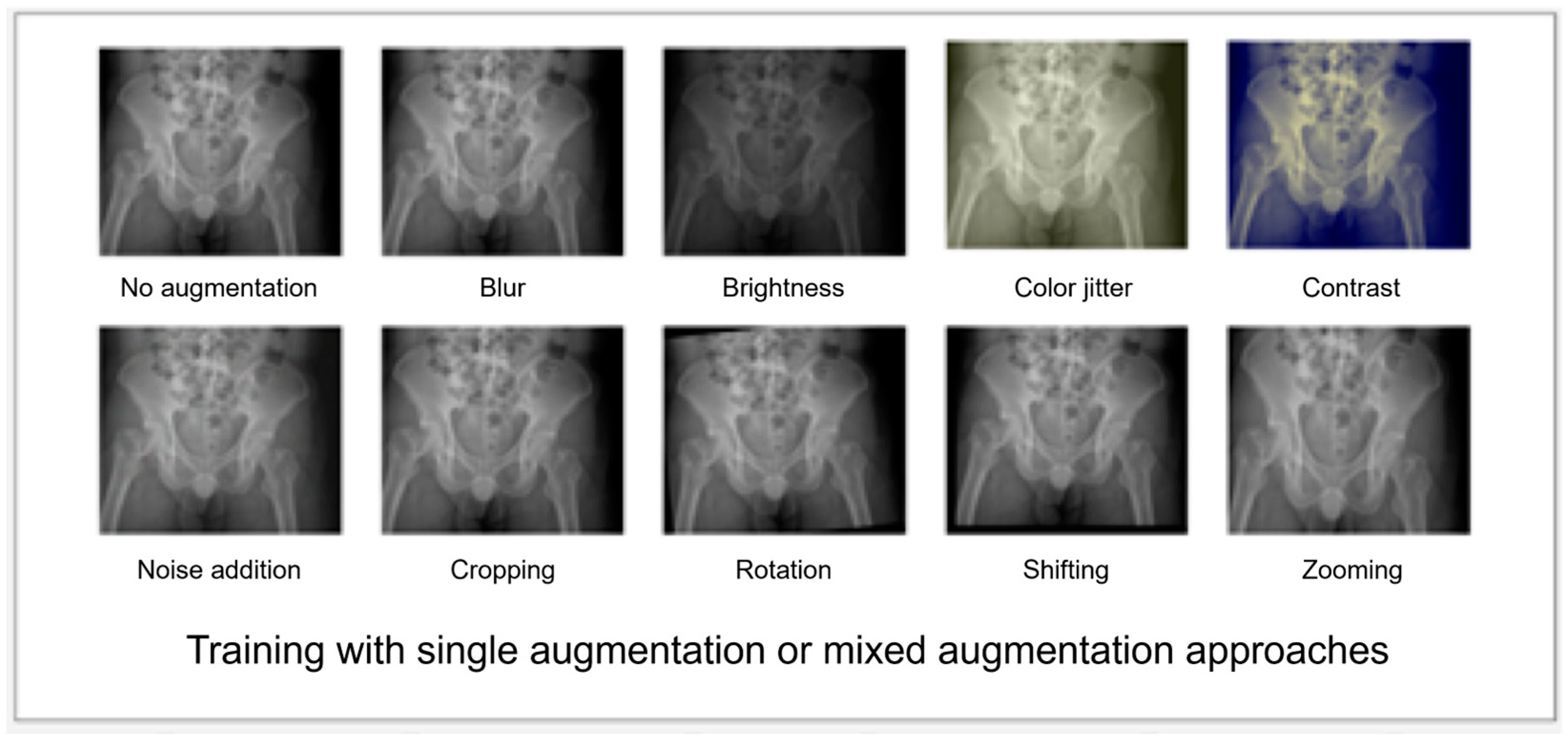
2.3. Prototyping the DL Algorithm
2.4. Testing, Validating, and Remodeling the Algorithm
2.5. Statistical Analysis and Software
3. Results
3.1. Finding Empathy and Definition
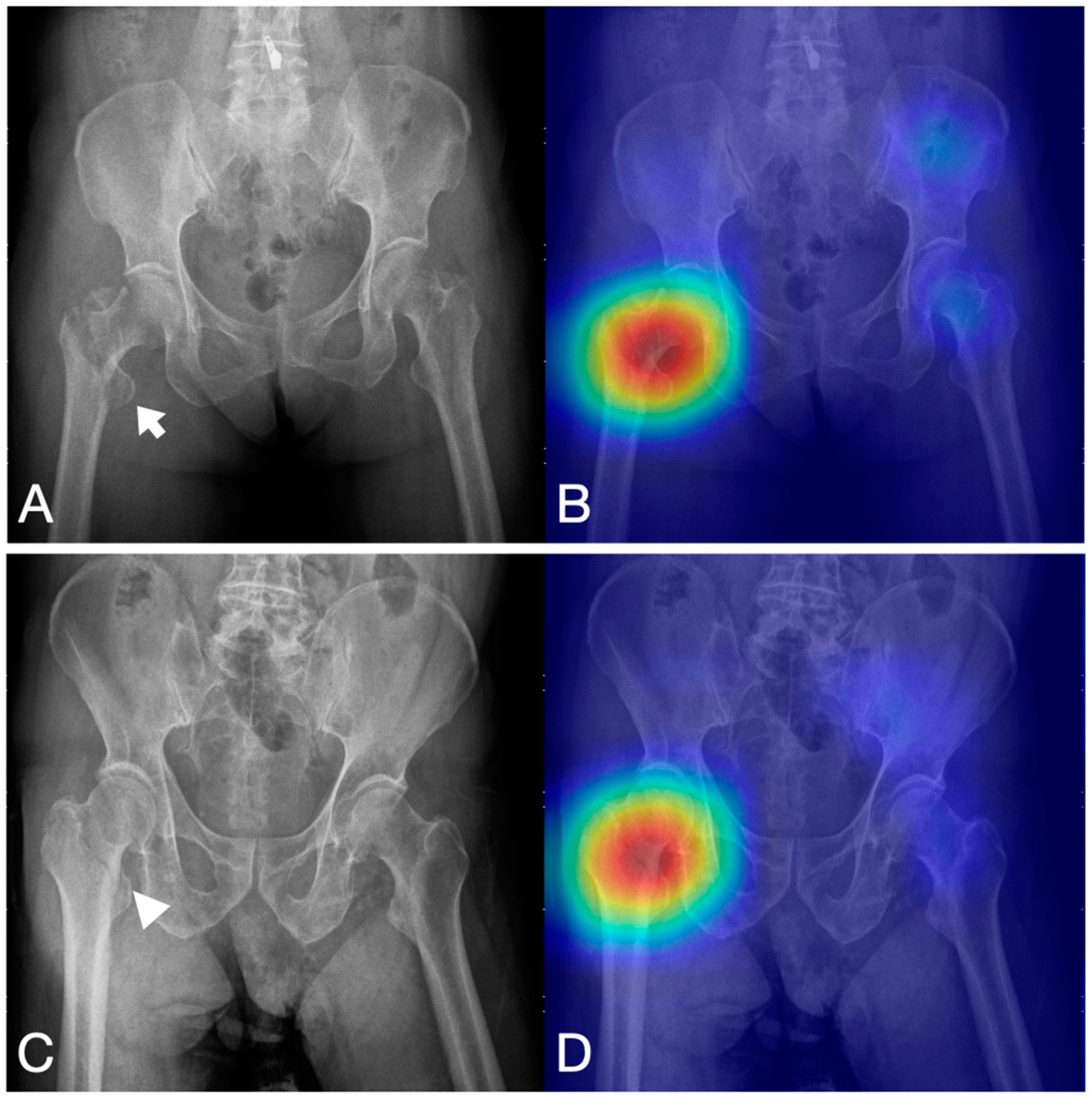
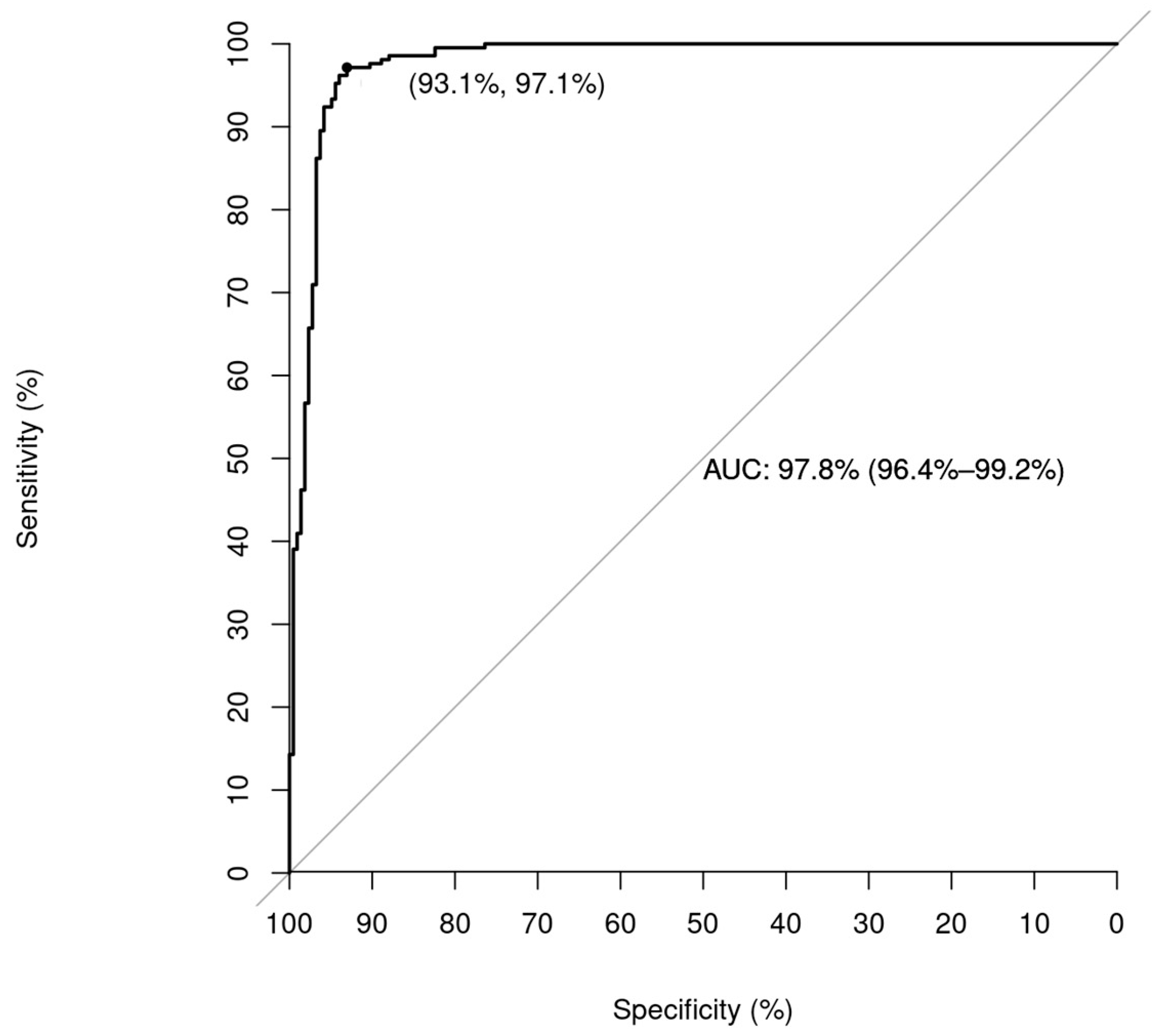
3.2. Prototyping—Testing Cycle to Improve the Performance of the Algorithms
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.; Casalino, L.P.; Khullar, D. Deep Learning in Medicine—Promise, Progress, and Challenges. JAMA Intern. Med. 2019, 179, 293–294. [Google Scholar] [CrossRef]
- Ching, T.; Himmelstein, D.S.; Beaulieu-Jones, B.K.; Kalinin, A.A.; Do, B.T.; Way, G.P.; Ferrero, E.; Agapow, P.-M.; Zietz, M.; Hoffman, M.M.; et al. Opportunities and Obstacles for Deep Learning in Biology and Medicine. J. R. Soc. Interface 2018, 15, 20170387. [Google Scholar] [CrossRef]
- Wang, Z.; Majewicz Fey, A. Deep Learning with Convolutional Neural Network for Objective Skill Evaluation in Robot-Assisted Surgery. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1959–1970. [Google Scholar] [CrossRef]
- Yu, F.; Silva Croso, G.; Kim, T.S.; Song, Z.; Parker, F.; Hager, G.D.; Reiter, A.; Vedula, S.S.; Ali, H.; Sikder, S. Assessment of Automated Identification of Phases in Videos of Cataract Surgery Using Machine Learning and Deep Learning Techniques. JAMA Netw. Open. 2019, 2, e191860. [Google Scholar] [CrossRef]
- Shen, D.; Wu, G.; Suk, H.-I. Deep Learning in Medical Image Analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [CrossRef]
- Cheng, C.-T.; Wang, Y.; Chen, H.-W.; Hsiao, P.-M.; Yeh, C.-N.; Hsieh, C.-H.; Miao, S.; Xiao, J.; Liao, C.-H.; Lu, L. A Scalable Physician-Level Deep Learning Algorithm Detects Universal Trauma on Pelvic Radiographs. Nat. Commun. 2021, 12, 1066. [Google Scholar] [CrossRef]
- Domingues, I.; Pereira, G.; Martins, P.; Duarte, H.; Santos, J.; Abreu, P.H. Using Deep Learning Techniques in Medical Imaging: A Systematic Review of Applications on CT and PET. Artif. Intell. Rev. 2020, 53, 4093–4160. [Google Scholar] [CrossRef]
- Hinton, G. Deep Learning—A Technology with the Potential to Transform Health Care. JAMA 2018, 320, 1101–1102. [Google Scholar] [CrossRef]
- Yu, Y.; Xie, Y.; Thamm, T.; Gong, E.; Ouyang, J.; Huang, C.; Christensen, S.; Marks, M.P.; Lansberg, M.G.; Albers, G.W.; et al. Use of Deep Learning to Predict Final Ischemic Stroke Lesions from Initial Magnetic Resonance Imaging. JAMA Netw. Open. 2020, 3, e200772. [Google Scholar] [CrossRef]
- Lu, M.T.; Ivanov, A.; Mayrhofer, T.; Hosny, A.; Aerts, H.J.W.L.; Hoffmann, U. Deep Learning to Assess Long-Term Mortality from Chest Radiographs. JAMA Netw. Open. 2019, 2, e197416. [Google Scholar] [CrossRef]
- Cheng, C.-T.; Cai, J.; Teng, W.; Zheng, Y.; Huang, Y.-T.; Wang, Y.-C.; Peng, C.-W.; Tang, Y.; Lee, W.-C.; Yeh, T.-S.; et al. A Flexible Three-Dimensional Heterophase Computed Tomography Hepatocellular Carcinoma Detection Algorithm for Generalizable and Practical Screening. Hepatol. Commun. 2022, 6, 2901–2913. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Sharma, A.; Hotchkiss, R.; Sperling, J.W.; Hamburger, J.; Ledig, C.; O’Toole, R.; Gardner, M.; Venkatesh, S.; Roberts, M.M.; et al. Assessment of a Deep-Learning System for Fracture Detection in Musculoskeletal Radiographs. NPJ Digit. Med. 2020, 3, 144. [Google Scholar] [CrossRef]
- Olczak, J.; Fahlberg, N.; Maki, A.; Razavian, A.S.; Jilert, A.; Stark, A.; Sköldenberg, O.; Gordon, M. Artificial Intelligence for Analyzing Orthopedic Trauma Radiographs: Deep Learning Algorithms—Are They on Par with Humans for Diagnosing Fractures? Acta Orthop. 2017, 88, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Newcombe, V.F.J.; Mathieu, F.; Adatia, K.; Kamnitsas, K.; Ferrante, E.; Das, T.; Whitehouse, D.; Rueckert, D.; Menon, D.K.; et al. Multiclass Semantic Segmentation and Quantification of Traumatic Brain Injury Lesions on Head CT Using Deep Learning: An Algorithm Development and Multicentre Validation Study. Lancet Digit. Health 2020, 2, e314–e322. [Google Scholar] [CrossRef]
- Gipson, J.; Tang, V.; Seah, J.; Kavnoudias, H.; Zia, A.; Lee, R.; Mitra, B.; Clements, W. Diagnostic Accuracy of a Commercially Available Deep-Learning Algorithm in Supine Chest Radiographs Following Trauma. Br. J. Radiol. 2022, 95, 20210979. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.-M.; Lin, C.-H.R.; Yau, F.-F.F.; Cheng, F.-J.; Pan, H.-Y.; Lin, X.-H.; Cheng, C.-Y. Use of a Deep-Learning Algorithm to Guide Novices in Performing Focused Assessment with Sonography in Trauma. JAMA Netw. Open. 2023, 6, e235102. [Google Scholar] [CrossRef]
- Regnard, N.-E.; Lanseur, B.; Ventre, J.; Ducarouge, A.; Clovis, L.; Lassalle, L.; Lacave, E.; Grandjean, A.; Lambert, A.; Dallaudière, B.; et al. Assessment of Performances of a Deep Learning Algorithm for the Detection of Limbs and Pelvic Fractures, Dislocations, Focal Bone Lesions, and Elbow Effusions on Trauma X-Rays. Eur. J. Radiol. 2022, 154, 110447. [Google Scholar] [CrossRef]
- Adebayo, O.; Bhuiyan, Z.A.; Ahmed, Z. Exploring the Effectiveness of Artificial Intelligence, Machine Learning and Deep Learning in Trauma Triage: A Systematic Review and Meta-Analysis. Lancet 2022, preprint. [Google Scholar] [CrossRef]
- Haas, B.; Jurkovich, G.J.; Wang, J.; Rivara, F.P.; Mackenzie, E.J.; Nathens, A.B. Survival Advantage in Trauma Centers: Expeditious Intervention or Experience? J. Am. Coll. Surg. 2009, 208, 28–36. [Google Scholar] [CrossRef]
- Haut, E.R.; Chang, D.C.; Efron, D.T.; Cornwell, E.E. 3rd Injured Patients Have Lower Mortality When Treated by “Full-Time” Trauma Surgeons vs. Surgeons Who Cover Trauma “Part-Time”. J. Trauma. 2006, 61, 272–278; discussion 278–279. [Google Scholar] [CrossRef]
- Gay, D.A.T.; Miles, R.M. Use of Imaging in Trauma Decision-Making. J. R. Army Med. Corps 2011, 157, S289–S292. [Google Scholar] [CrossRef]
- Peytel, E.; Menegaux, F.; Cluzel, P.; Langeron, O.; Coriat, P.; Riou, B. Initial Imaging Assessment of Severe Blunt Trauma. Intensive Care Med. 2001, 27, 1756–1761. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; April, M.D.; Summers, S.; Koyfman, A. Whole Body CT versus Selective Radiological Imaging Strategy in Trauma: An Evidence-Based Clinical Review. Am. J. Emerg. Med. 2017, 35, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Alawa, J.; Tennakoon, L.; Forrester, J.D. DeepBackRib: Deep Learning to Understand Factors Associated with Readmissions after Rib Fractures. J. Trauma. Acute Care Surg. 2022, 93, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Muñoz, E.; Couperus, K.; Wachs, J.P. The AI-Medic: An Artificial Intelligent Mentor for Trauma Surgery. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2021, 9, 313–321. [Google Scholar] [CrossRef]
- Rowe, P.G. Design Thinking; MIT Press: Cambridge, MA, USA, 1991; ISBN 9780262680677. [Google Scholar]
- Brown, T. Design Thinking. Harv. Bus. Rev. 2008, 86, 84–92, 141. [Google Scholar]
- Dorst, K. The Core of “design Thinking” and Its Application. Des. Stud. 2011, 32, 521–532. [Google Scholar] [CrossRef]
- Deitte, L.A.; Omary, R.A. The Power of Design Thinking in Medical Education. Acad. Radiol. 2019, 26, 1417–1420. [Google Scholar] [CrossRef]
- Badwan, B.; Bothara, R.; Latijnhouwers, M.; Smithies, A.; Sandars, J. The Importance of Design Thinking in Medical Education. Med. Teach. 2018, 40, 425–426. [Google Scholar] [CrossRef]
- Sandars, J.; Goh, P.-S. Design Thinking in Medical Education: The Key Features and Practical Application. J. Med. Educ. Curric. Dev. 2020, 7, 2382120520926518. [Google Scholar] [CrossRef]
- van de Grift, T.C.; Kroeze, R. Design Thinking as a Tool for Interdisciplinary Education in Health Care. Acad. Med. 2016, 91, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Carroll, N.; Richardson, I. Aligning Healthcare Innovation and Software Requirements through Design Thinking. In Proceedings of the International Workshop on Software Engineering in Healthcare Systems, Austin, TX, USA, 14–22 May 2016; Association for Computing Machinery: New York, NY, USA; pp. 1–7. [Google Scholar]
- Zupan, B.; Pakrac, V.; Nabergoj, A.S. Using Design Thinking to Develop a New Product: Case Study of Developing a Novel Medical Device. In Design Thinking; CRC Press: Boca Raton, FL, USA, 2022; Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003189923-12/using-design-thinking-develop-new-product-bla%C5%BE-zupan-vladimir-pakrac-anja-svetina-nabergoj (accessed on 18 April 2023).
- Brooks, F.P., Jr. The Design of Design: Essays from a Computer Scientist; Pearson Education: London, UK, 2010; ISBN 9780321702067. [Google Scholar]
- Martin, R.; Martin, R.L. The Design of Business: Why Design Thinking Is the Next Competitive Advantage; Harvard Business Press: Cambridge, MA, USA, 2009; ISBN 9781422177808. [Google Scholar]
- Panke, S. Design Thinking in Education: Perspectives, Opportunities and Challenges. Open. Educ. Stud. 2019, 1, 281–306. [Google Scholar] [CrossRef]
- Mintrom, M.; Luetjens, J. Design Thinking in Policymaking Processes: Opportunities and Challenges. Aust. J. Publ. Adm. 2016, 75, 391–402. [Google Scholar] [CrossRef]
- Watson, A.D. Design Thinking for Life. Art. Educ. 2015, 68, 12–18. [Google Scholar] [CrossRef]
- Scholten, H.; Granic, I. Use of the Principles of Design Thinking to Address Limitations of Digital Mental Health Interventions for Youth: Viewpoint. J. Med. Internet Res. 2019, 21, e11528. [Google Scholar] [CrossRef]
- Chollet, F. Xception: Deep Learning with Depthwise Separable Convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–16 July 2017; pp. 1251–1258. [Google Scholar]
- Selvaraju, R.R.; Cogswell, M.; Das, A.; Vedantam, R.; Parikh, D.; Batra, D. Grad-Cam: Visual Explanations from Deep Networks via Gradient-Based Localization. In Proceedings of the IEEE International Conference on Computer Vision, Venice, Italy, 22–29 October 2017; pp. 618–626. [Google Scholar]
- Cheng, C.-T.; Ho, T.-Y.; Lee, T.-Y.; Chang, C.-C.; Chou, C.-C.; Chen, C.-C.; Chung, I.-F.; Liao, C.-H. Application of a Deep Learning Algorithm for Detection and Visualization of Hip Fractures on Plain Pelvic Radiographs. Eur. Radiol. 2019, 29, 5469–5477. [Google Scholar] [CrossRef]
- Shenton, A.; Choudhary, S. The Emergency Radiology of Pelvic Trauma. Traumatology 2014, 16, 279–291. [Google Scholar] [CrossRef]
- Açıcı, K.; Sümer, E.; Beyaz, S. Comparison of Different Machine Learning Approaches to Detect Femoral Neck Fractures in X-Ray Images. Health Technol. 2021, 11, 643–653. [Google Scholar] [CrossRef]
- Olczak, J.; Emilson, F.; Razavian, A.; Antonsson, T.; Stark, A.; Gordon, M. Ankle Fracture Classification Using Deep Learning: Automating Detailed AO Foundation/Orthopedic Trauma Association (AO/OTA) 2018 Malleolar Fracture Identification Reaches a High Degree of Correct Classification. Acta Orthop. 2021, 92, 102–108. [Google Scholar] [CrossRef]
- Pianykh, O.S.; Langs, G.; Dewey, M.; Enzmann, D.R.; Herold, C.J.; Schoenberg, S.O.; Brink, J.A. Continuous Learning AI in Radiology: Implementation Principles and Early Applications. Radiology 2020, 297, 6–14. [Google Scholar] [CrossRef]
- Ngiam, K.Y.; Khor, I.W. Big Data and Machine Learning Algorithms for Health-Care Delivery. Lancet Oncol. 2019, 20, e262–e273. [Google Scholar] [CrossRef] [PubMed]
- Budd, S.; Robinson, E.C.; Kainz, B. A Survey on Active Learning and Human-in-the-Loop Deep Learning for Medical Image Analysis. Med. Image Anal. 2021, 71, 102062. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Han, S.G.; Cho, A.; Shin, H.J.; Baek, S.-E. Effect of Deep Learning-Based Assistive Technology Use on Chest Radiograph Interpretation by Emergency Department Physicians: A Prospective Interventional Simulation-Based Study. BMC Med. Inform. Decis. Mak. 2021, 21, 311. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J.H.; Choi, S.-W.; Lee, H.J.; Hong, J.; Kwon, S.H. Physician Confidence in Artificial Intelligence: An Online Mobile Survey. J. Med. Internet Res. 2019, 21, e12422. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Brown, E.W. Artificial Intelligence in Medical Practice: The Question to the Answer? Am. J. Med. 2018, 131, 129–133. [Google Scholar] [CrossRef]
- Auernhammer, J.; Roth, B. The Origin and Evolution of Stanford University’s Design Thinking: From Product Design to Design Thinking in Innovation Management. J. Prod. Innov. Manag. 2021, 38, 623–644. [Google Scholar] [CrossRef]
- Zenios, S.; Makower, J.; Yock, P.; Denend, L.; Brinton, T.J.; Kumar, U.N.; Krummel, T.M. Biodesign: The Process of Innovating Medical Technologies; Cambridge University Press: Cambridge, MA, USA, 2010; ISBN 9780521517423. [Google Scholar]
- Wall, J.; Hellman, E.; Denend, L.; Rait, D.; Venook, R.; Lucian, L.; Azagury, D.; Yock, P.G.; Brinton, T.J. The Impact of Postgraduate Health Technology Innovation Training: Outcomes of the Stanford Biodesign Fellowship. Ann. Biomed. Eng. 2017, 45, 1163–1171. [Google Scholar] [CrossRef]
- Brinton, T.J.; Kurihara, C.Q.; Camarillo, D.B.; Pietzsch, J.B.; Gorodsky, J.; Zenios, S.A.; Doshi, R.; Shen, C.; Kumar, U.N.; Mairal, A.; et al. Outcomes from a Postgraduate Biomedical Technology Innovation Training Program: The First 12 Years of Stanford Biodesign. Ann. Biomed. Eng. 2013, 41, 1803–1810. [Google Scholar] [CrossRef]
- Wall, J.; Wynne, E.; Krummel, T. Biodesign Process and Culture to Enable Pediatric Medical Technology Innovation. Semin. Pediatr. Surg. 2015, 24, 102–106. [Google Scholar] [CrossRef]
- Dutta, N.; Dhar, D. From Industrial Design to Healthcare Innovation—A Comparative Study on the Role of User-Centered Design and Stanford Biodesign Process. In Design for Tomorrow; Springer: Singapore, 2021; Volume 3, pp. 665–677. [Google Scholar] [CrossRef]
- Gottlieb, M.; Wagner, E.; Wagner, A.; Chan, T. Applying Design Thinking Principles to Curricular Development in Medical Education. AEM Educ. Train. 2017, 1, 21–26. [Google Scholar] [CrossRef]
- Madson, M.J. Making Sense of Design Thinking: A Primer for Medical Teachers. Med. Teach. 2021, 43, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
| Pre DT Model (95%CI) | Post DT Model (95%CI) | |
|---|---|---|
| Accuracy | 0.91 (0.84–0.96) | 0.95 (0.93–0.97) |
| Sensitivity | 0.97 (0.89–1.00) | 0.97 (0.94–0.99) |
| Specificity | 0.84 (0.71–0.93) | 0.93 (0.90–0.97) |
| False negative rate | 0.02 (0.003–0.17) | 0.0286 (0.0095–0.0667) |
| F1 score | 0.916 (0.845–0.956) | 0.951 (0.930–0.973) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, C.-H.; Chen, C.-C.; Tee, Y.-S.; Lin, W.-C.; Kuo, L.-W.; Liao, C.-A.; Cheng, C.-T.; Liao, C.-H. The Application of Design Thinking in Developing a Deep Learning Algorithm for Hip Fracture Detection. Bioengineering 2023, 10, 735. https://doi.org/10.3390/bioengineering10060735
Ouyang C-H, Chen C-C, Tee Y-S, Lin W-C, Kuo L-W, Liao C-A, Cheng C-T, Liao C-H. The Application of Design Thinking in Developing a Deep Learning Algorithm for Hip Fracture Detection. Bioengineering. 2023; 10(6):735. https://doi.org/10.3390/bioengineering10060735
Chicago/Turabian StyleOuyang, Chun-Hsiang, Chih-Chi Chen, Yu-San Tee, Wei-Cheng Lin, Ling-Wei Kuo, Chien-An Liao, Chi-Tung Cheng, and Chien-Hung Liao. 2023. "The Application of Design Thinking in Developing a Deep Learning Algorithm for Hip Fracture Detection" Bioengineering 10, no. 6: 735. https://doi.org/10.3390/bioengineering10060735
APA StyleOuyang, C.-H., Chen, C.-C., Tee, Y.-S., Lin, W.-C., Kuo, L.-W., Liao, C.-A., Cheng, C.-T., & Liao, C.-H. (2023). The Application of Design Thinking in Developing a Deep Learning Algorithm for Hip Fracture Detection. Bioengineering, 10(6), 735. https://doi.org/10.3390/bioengineering10060735









