Effect of Pressure Conditions in Uterine Decellularization Using Hydrostatic Pressure on Structural Protein Preservation
Abstract
:1. Introduction
2. Materials and Methods
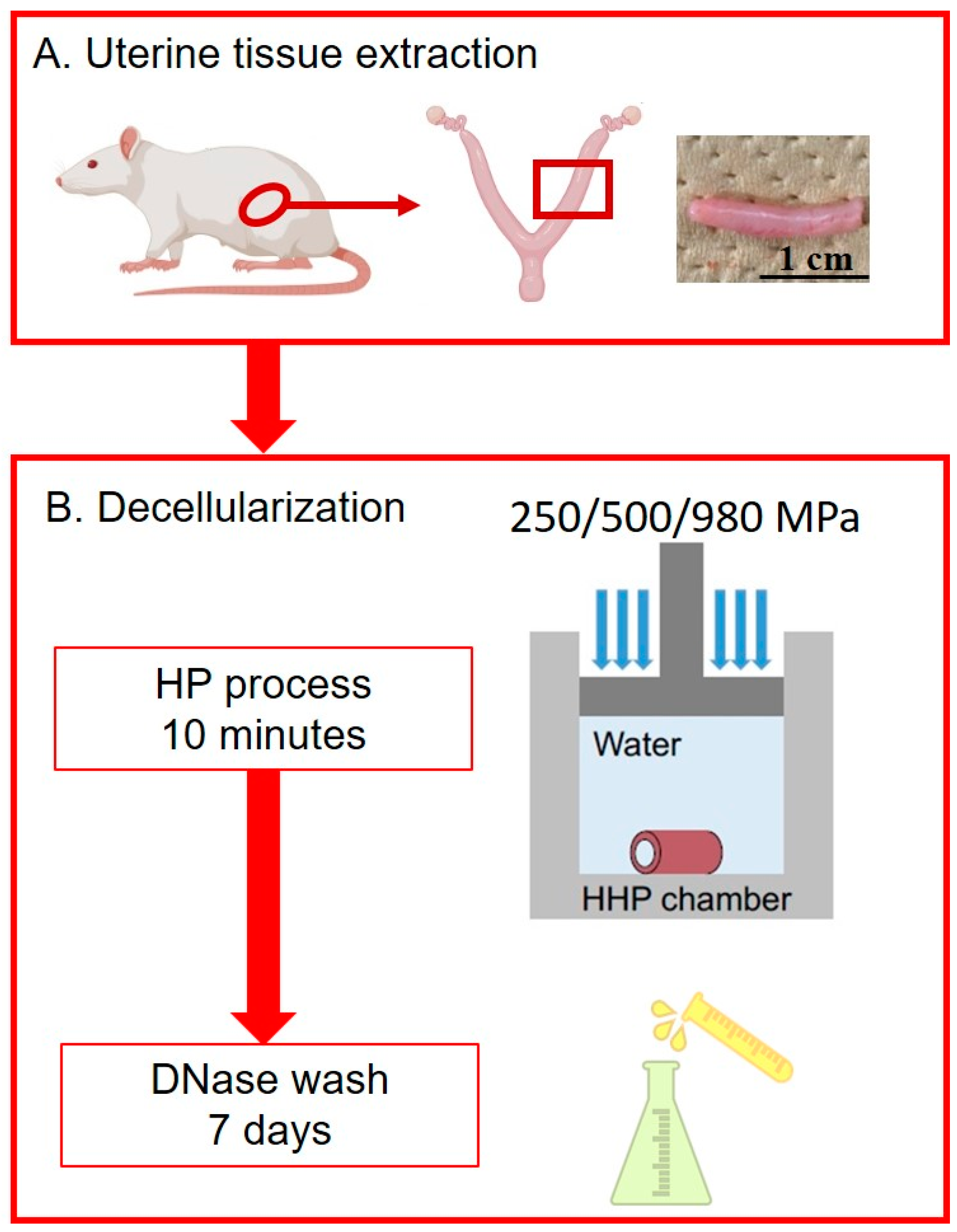
2.1. Uterine Tissue Extraction
2.2. Decellularization
2.3. Protein Extraction and Biochemical Analysis of DUS
2.4. Histological Analysis
2.5. Transmission Electron Microscopy (TEM)
2.6. Mechanical Test
2.7. Statistical Analysis
3. Results
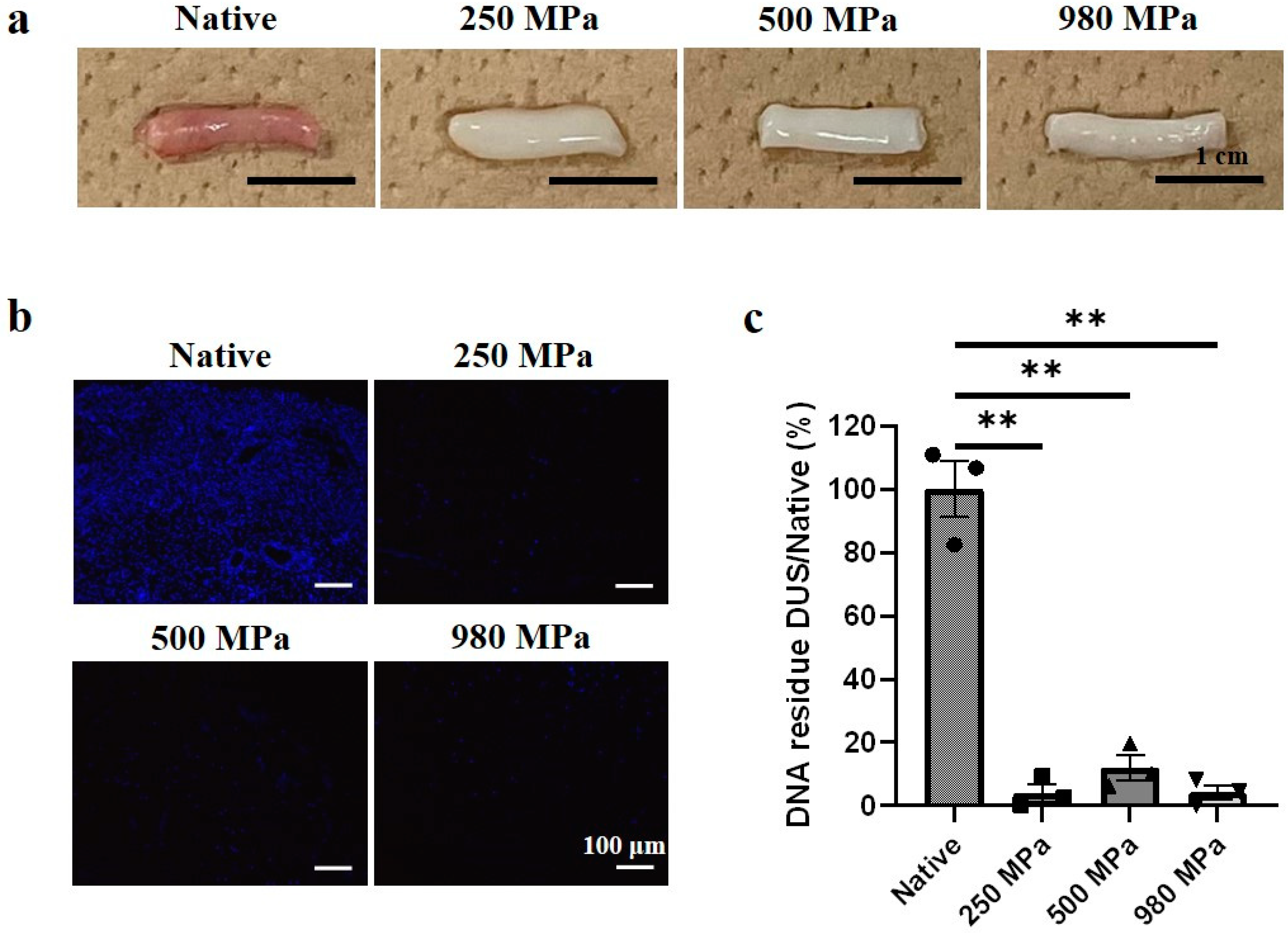
3.1. Decellularized Uterine Scaffold (DUS) Fabrication and Characterization
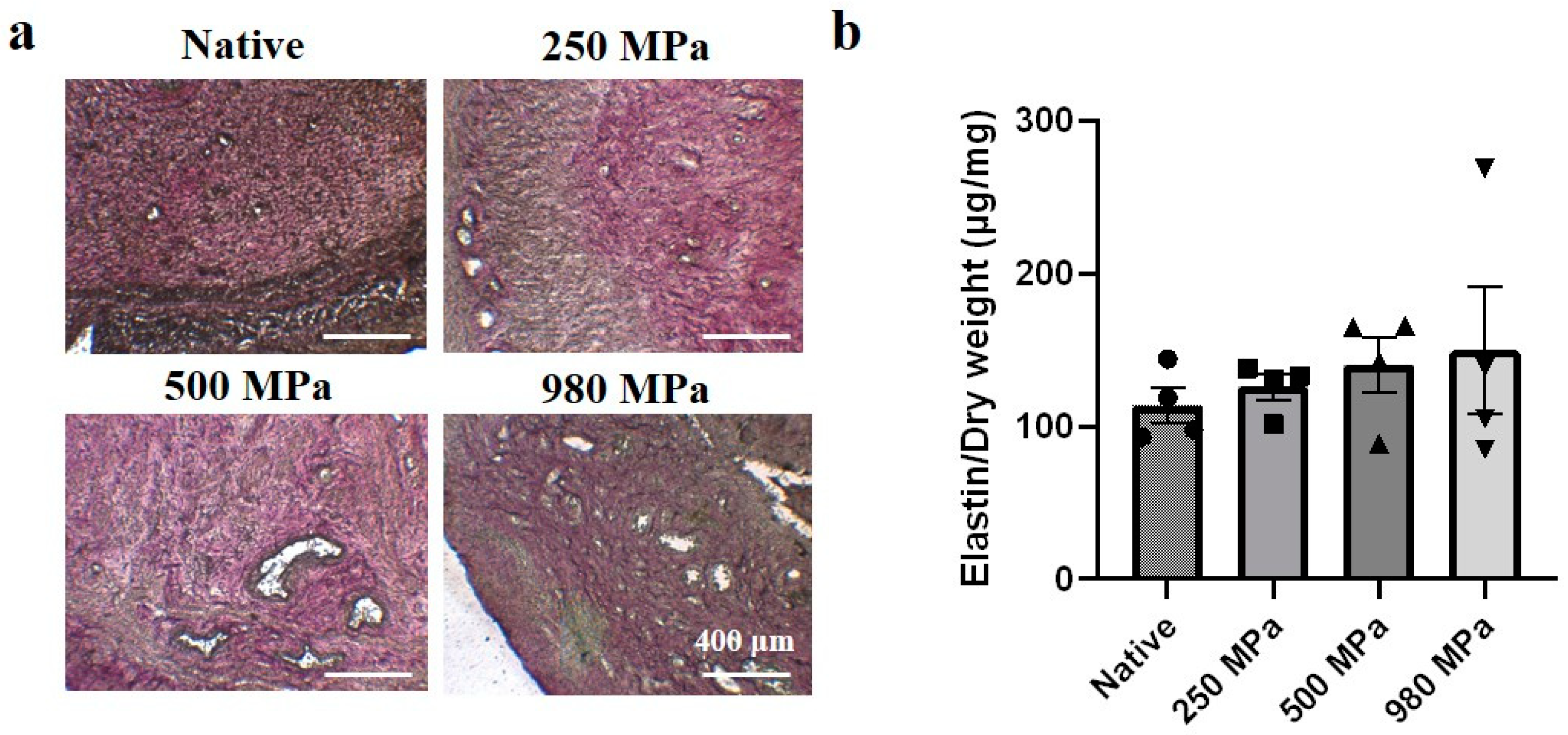
3.2. Effects of Decellularization Pressure on Structural Protein Contents in DUS
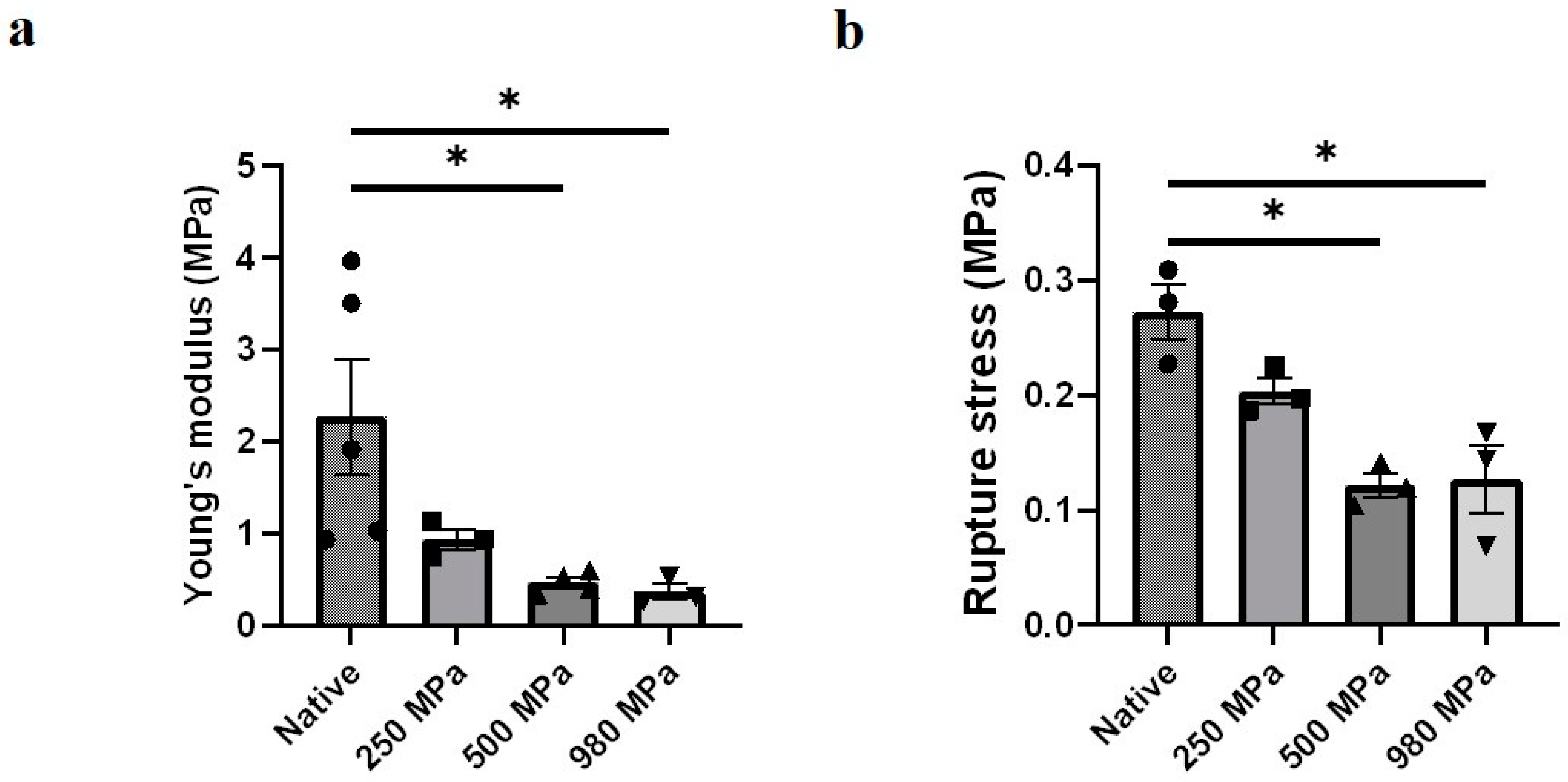
3.3. Effects of Decellularization Pressure on Mechanical Properties in DUS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, W. Mother or Nothing: The Agony of Infertility. Bull. World Health Organ. 2010, 88, 881. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Baker, H.; Fries, M.H.; Yoo, J.J.; Kim, P.C.W.; Fisher, J.P. Bioengineering Strategies to Treat Female Infertility. Tissue Eng.-Part B Rev. 2017, 23, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Brännström, M.; Johannesson, L.; Bokström, H.; Kvarnström, N.; Mölne, J.; Dahm-Kähler, P.; Enskog, A.; Milenkovic, M.; Ekberg, J.; Diaz-Garcia, C.; et al. Livebirth after Uterus Transplantation. Lancet 2015, 385, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fageeh, W.; Raffa, H.; Jabbad, H.; Marzouki, A. Transplantation of the Human Uterus. Int. J. Gynecol. Obstet. 2002, 76, 245–251. [Google Scholar] [CrossRef]
- Santoso, E.G.; Yoshida, K.; Hirota, Y.; Aizawa, M.; Yoshino, O.; Kishida, A.; Osuga, Y.; Saito, S.; Ushida, T.; Furukawa, K.S. Application of Detergents or High Hydrostatic Pressure as Decellularization Processes in Uterine Tissues and Their Subsequent Effects on in Vivo Uterine Regeneration in Murine Models. PLoS ONE 2014, 9, e103201. [Google Scholar] [CrossRef]
- Miyazaki, K.; Maruyama, T. Partial Regeneration and Reconstruction of the Rat Uterus through Recellularization of a Decellularized Uterine Matrix. Biomaterials 2014, 35, 8791–8800. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, Y.W.; Lin, H.L.; Lan, Q.H.; Huang, Z.W.; Wang, L.F.; Chen, R.; Xiao, J.; Kou, L.; Xu, H.L.; et al. Exploiting Crosslinked Decellularized Matrix to Achieve Uterus Regeneration and Construction. Artif. Cells Nanomed. Biotechnol. 2020, 48, 218–229. [Google Scholar] [CrossRef]
- Campo, H.; García-Domínguez, X.; López-Martínez, S.; Faus, A.; Vicente Antón, J.S.; Marco-Jiménez, F.; Cervelló, I. Tissue-Specific Decellularized Endometrial Substratum Mimicking Different Physiological Conditions Influences In Vitro Embryo Development in a Rabbit Model. Acta Biomater. 2019, 89, 126–138. [Google Scholar] [CrossRef]
- Campo, H.; Baptista, P.M.; López-Pérez, N.; Faus, A.; Cervelló, I.; Simón, C. De- and Recellularization of the Pig Uterus: A Bioengineering Pilot Study. Biol. Reprod. 2017, 96, 34–45. [Google Scholar] [CrossRef]
- Daryabari, S.S.; Kajbafzadeh, A.M.; Fendereski, K.; Ghorbani, F.; Dehnavi, M.; Rostami, M.; Garajegayeh, B.A.; Tavangar, S.M. Development of an Efficient Perfusion-Based Protocol for Whole-Organ Decellularization of the Ovine Uterus as a Human-Sized Model and In Vivo Application of the Bioscaffolds. J. Assist. Reprod. Genet. 2019, 36, 1211–1223. [Google Scholar] [CrossRef]
- Daryabari, S.S.; Fendereski, K.; Ghorbani, F.; Dehnavi, M.; Shafikhani, Y.; Omranipour, A.; Zeraatian-Nejad Davani, S.; Majidi Zolbin, M.; Tavangar, S.M.; Kajbafzadeh, A.M. Whole-Organ Decellularization of the Human Uterus and In Vivo Application of the Bio-Scaffolds in Animal Models. J. Assist. Reprod. Genet. 2022, 39, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muiznieks, L.D.; Keeley, F.W. Molecular Assembly and Mechanical Properties of the Extracellular Matrix: A Fibrous Protein Perspective. Biochim. Biophys. Acta-Mol. Basis Dis. 2013, 1832, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Londono, R.; Badylak, S.F. Biologic Scaffolds for Regenerative Medicine: Mechanisms of In Vivo Remodeling. Ann. Biomed. Eng. 2014, 43, 577–592. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miki, F.; Maruyama, T.; Miyazaki, K.; Takao, T.; Yoshimasa, Y.; Katakura, S.; Hihara, H.; Uchida, S.; Masuda, H.; Uchida, H.; et al. The Orientation of a Decellularized Uterine Scaffold Determines the Tissue Topology and Architecture of the Regenerated Uterus in Rats. Biol. Reprod. 2019, 100, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Hellström, M.; Moreno-Moya, J.M.; Bandstein, S.; Bom, E.; Akouri, R.R.; Miyazaki, K.; Maruyama, T.; Brännström, M. Bioengineered Uterine Tissue Supports Pregnancy in a Rat Model. Fertil. Steril. 2016, 106, 487–496.e1. [Google Scholar] [CrossRef] [Green Version]
- Yoshimasa, Y.; Takao, T.; Katakura, S.; Tomisato, S.; Masuda, H.; Tanaka, M.; Maruyama, T. A Decellularized Uterine Endometrial Scaffold Enhances Regeneration of the Endometrium in Rats. Int. J. Mol. Sci. 2023, 24, 7605. [Google Scholar] [CrossRef]
- Masoomikarimi, M.; Salehi, M.; Noorbakhsh, F.; Rajaei, S. A Combination of Physical and Chemical Treatments Is More Effective in The Preparation of Acellular Uterine Scaffolds. Cell J. 2023, 25, 25–34. [Google Scholar] [CrossRef]
- Fujisato, T.; Minatoya, K.; Yamazaki, S.; Meng, Y.; Niwaya, K.; Kishida, A.; Nakatani, T.; Kitamura, S. Preparation and Recellularization of Tissue Engineered Bioscaffold for Heart Valve Replacement. In Cardiovascular Regeneration Therapies Using Tissue Engineering Approaches; Springer: Berlin/Heidelberg, Germany, 2005; pp. 83–94. ISBN 4431239251. [Google Scholar]
- Sasaki, S.; Funamoto, S.; Hashimoto, Y.; Kimura, T.; Honda, T.; Hattori, S.; Kobayashi, H.; Kishida, A.; Mochizuki, M. In Vivo Evaluation of a Novel Scaffold for Artificial Corneas Prepared by Using Ultrahigh Hydrostatic Pressure to Decellularize Porcine Corneas. Mol. Vis. 2009, 15, 2022–2028. [Google Scholar]
- Suzuki, A.; Watanabe, M.; Ikeuchi, Y.; Saito, M.; Takahashi, K. Effects of High-Pressure Treatment on the Ultrastructure and Thermal Behaviour of Beef Intramuscular Collagen. Meat Sci. 1993, 35, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ichinoseki, S.; Nishiumi, T.; Suzuki, A. Tenderizing Effect of High Hydrostatic Pressure on Bovine Intramuscular Connective Tissue. J. Food Sci. 2006, 71, E276–E281. [Google Scholar] [CrossRef]
- Kim, J.; Takeda, S.; Charoensmbut, N.; Kawabata, K.; Kishimoto, Y.; Kimura, T.; Kishida, A.; Ushida, T.; Furukawa, K.S. Fabrication of Uterine Decellularized Matrix Using High Hydrostatic Pressure through Depolymerization of Actin Filaments. J. Biomech. Sci. Eng. 2019, 14, 19-00097. [Google Scholar] [CrossRef] [Green Version]
- Mahara, A.; Morimoto, N.; Sakuma, T.; Fujisato, T.; Yamaoka, T. Complete Cell Killing by Applying High Hydrostatic Pressure for Acellular Vascular Graft Preparation. Biomed Res. Int. 2014, 2014, 379607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemmyo, D.; Yamamoto, M.; Miyata, S. Fundamental Study of Decellularization Method Using Cyclic Application of High Hydrostatic Pressure. Micromachines 2020, 11, 1008. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef]
- Potekhin, S.A.; Senin, A.A.; Abdurakhmanov, N.N.; Tiktopulo, E.I. High Pressure Stabilization of Collagen Structure. Biochim. Biophys. Acta-Proteins Proteom. 2009, 1794, 1151–1158. [Google Scholar] [CrossRef]
- Diehl, P.; Steinhauser, E.; Gollwitzer, H.; Heister, C.; Schauwecker, J.; Milz, S.; Mittelmeier, W.S. Biomechanical and Immunohistochemical Analysis of High Hydrostatic Pressure-Treated Achilles Tendons. J. Orthop. Sci. 2006, 11, 380–385. [Google Scholar] [CrossRef]
- Peitschl, M.C.; Polzar, B.; Stephan, H.; Crompton, T.; Robson Macdonald, H.; Mannherz, H.G.; Tschopp’, J. Characterization of the Endogenous Deoxyribonuclease Involved in Nuclear DNA Degradation during Apoptosis (Programmed Cell Death). EMBO J. 1993, 12, 371–377. [Google Scholar] [CrossRef]
- Napirei, M.; Wulf, S.; Mannherz, H.G. Chromatin Breakdown during Necrosis by Serum Dnase1 and the Plasminogen System. Arthritis Rheum. 2004, 50, 1873–1883. [Google Scholar] [CrossRef]
- Birch, H.L.; Thorpe, C.T.; Rumian, A.P. Specialisation of Extracellular Matrix for Function in Tendons and Ligaments. Muscles. Ligaments Tendons J. 2013, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Dutov, P.; Antipova, O.; Varma, S.; Orgel, J.P.R.O.; Schieber, J.D. Measurement of Elastic Modulus of Collagen Type i Single Fiber. PLoS ONE 2016, 11, e0145711. [Google Scholar] [CrossRef] [PubMed]
- Pan-Castillo, B.; Gazze, S.A.; Thomas, S.; Lucas, C.; Margarit, L.; Gonzalez, D.; Francis, L.W.; Conlan, R.S. Morphophysical Dynamics of Human Endometrial Cells during Decidualization. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, H.; Zhou, C.; Gu, L. Mechanical Contribution of Vascular Smooth Muscle Cells in the Tunica Media of Artery. Nanotechnol. Rev. 2019, 8, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Pajic-Lijakovic, I.; Milivojevic, M.; Clark, A.G. Collective Cell Migration on Collagen-I Networks: The Impact of Matrix Viscoelasticity. Front. Cell Dev. Biol. 2022, 10, 901026. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Charoensombut, N.; Kawabata, K.; Kimura, T.; Kishida, A.; Ushida, T.; Furukawa, K.S. Effect of Pressure Conditions in Uterine Decellularization Using Hydrostatic Pressure on Structural Protein Preservation. Bioengineering 2023, 10, 814. https://doi.org/10.3390/bioengineering10070814
Wang D, Charoensombut N, Kawabata K, Kimura T, Kishida A, Ushida T, Furukawa KS. Effect of Pressure Conditions in Uterine Decellularization Using Hydrostatic Pressure on Structural Protein Preservation. Bioengineering. 2023; 10(7):814. https://doi.org/10.3390/bioengineering10070814
Chicago/Turabian StyleWang, Dongzhe, Narintadeach Charoensombut, Kinyoshi Kawabata, Tsuyoshi Kimura, Akio Kishida, Takashi Ushida, and Katsuko S. Furukawa. 2023. "Effect of Pressure Conditions in Uterine Decellularization Using Hydrostatic Pressure on Structural Protein Preservation" Bioengineering 10, no. 7: 814. https://doi.org/10.3390/bioengineering10070814





