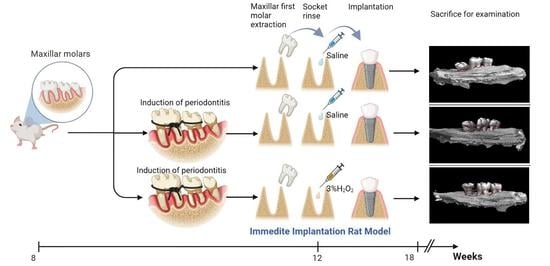
Initial Development of an Immediate Implantation Model in Rats and Assessing the Prognostic Impact of Periodontitis on Immediate Implantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Group Assignment
3. Induction of Periodontitis
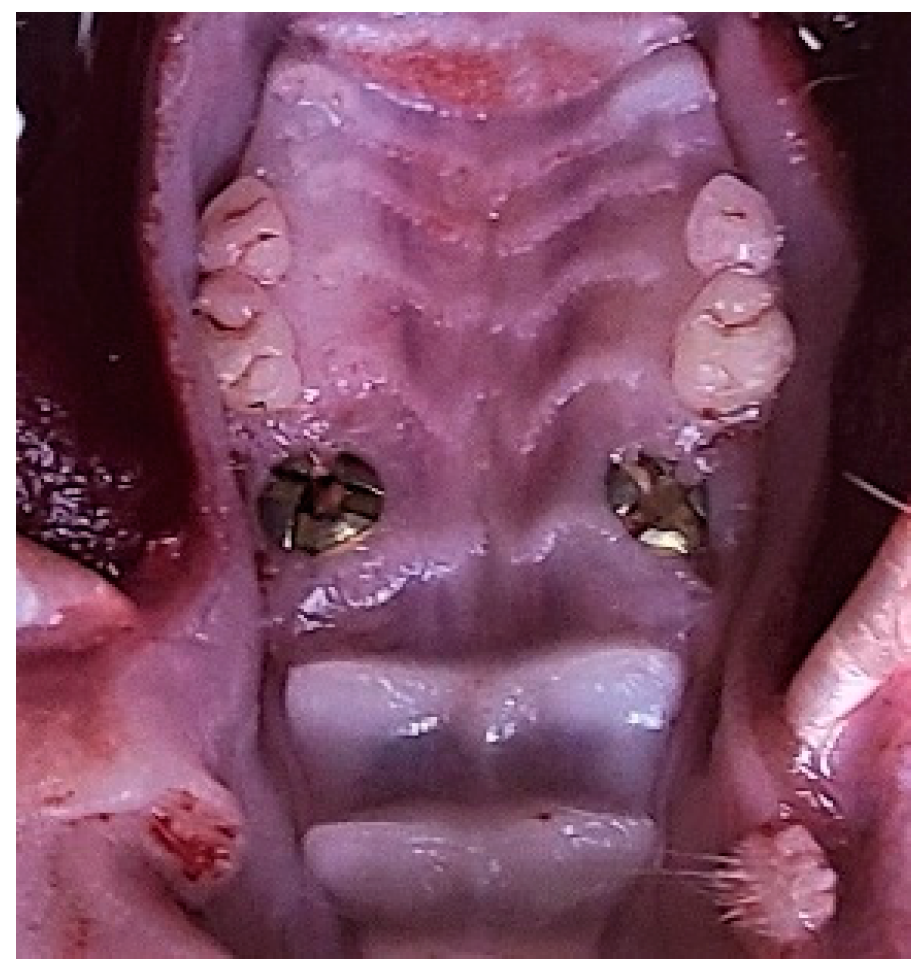
4. Tooth Extraction and Immediate Implantation
5. Insertion Torque and Removal Torque Test
6. Clinical Examination
7. Micro-CT Analysis
8. Histological Analysis
9. Statistical Analysis
10. Results
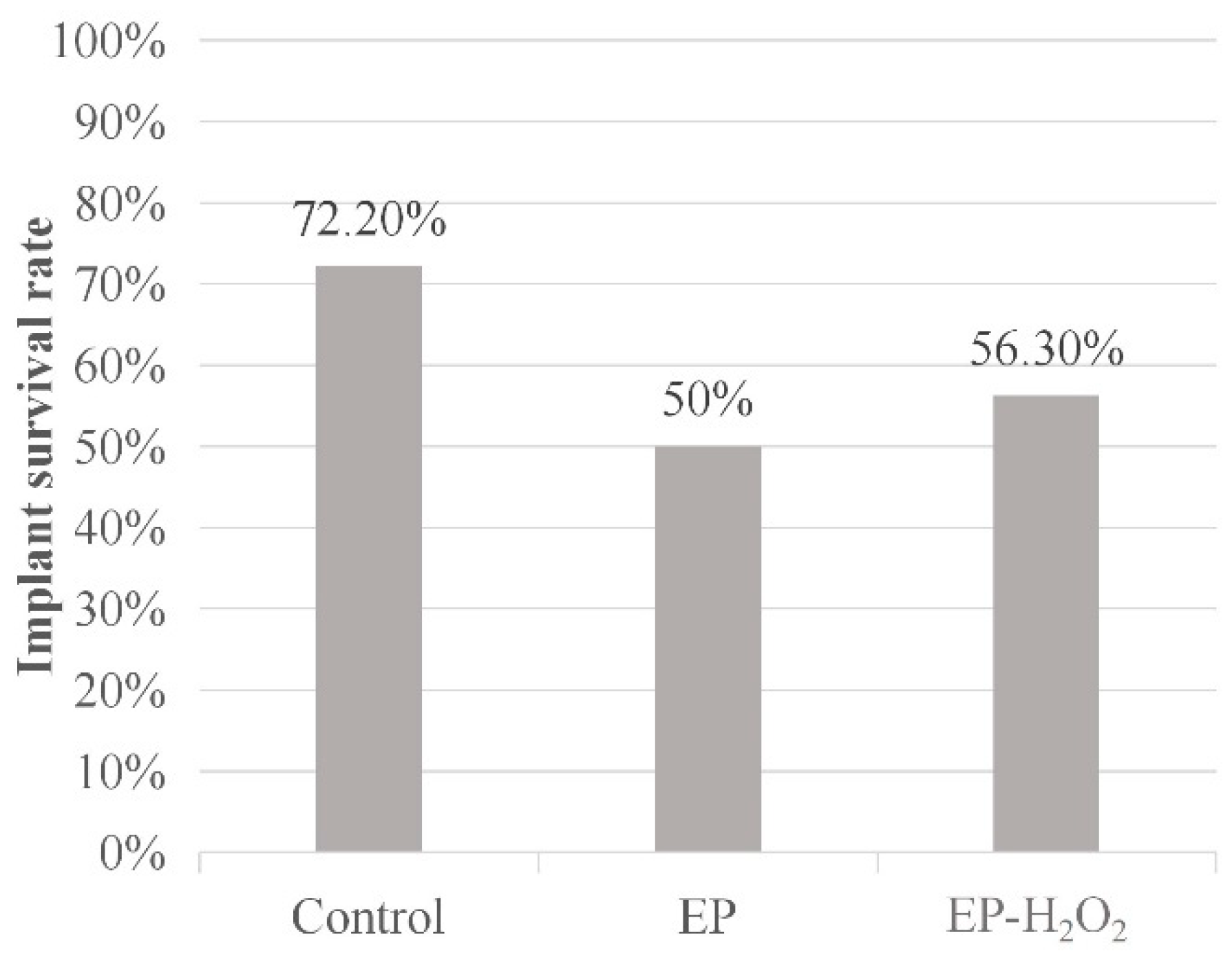
10.1. Survival Rate
10.2. The Effect of IT on Dental Implant Failure
10.3. Clinical Examination
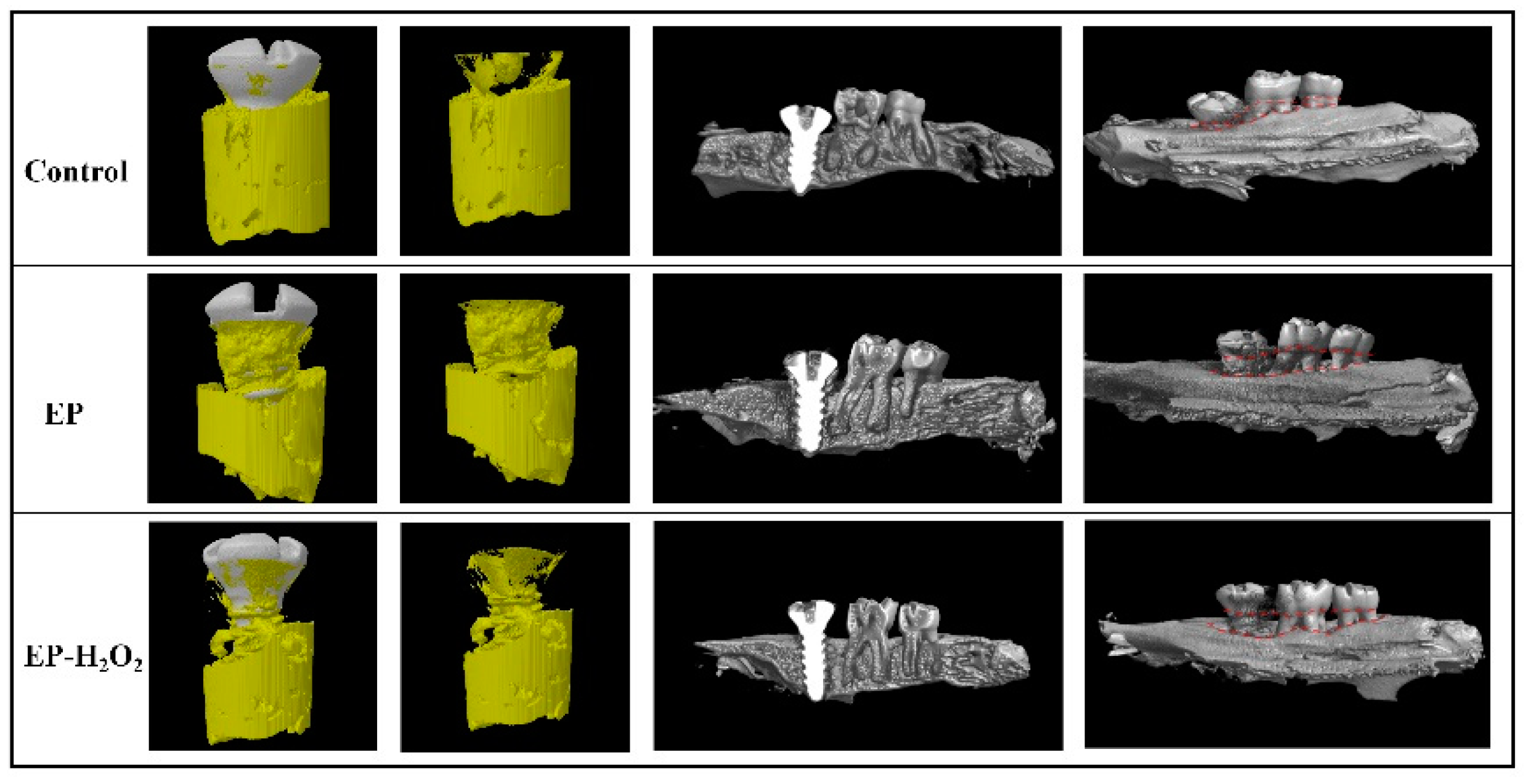
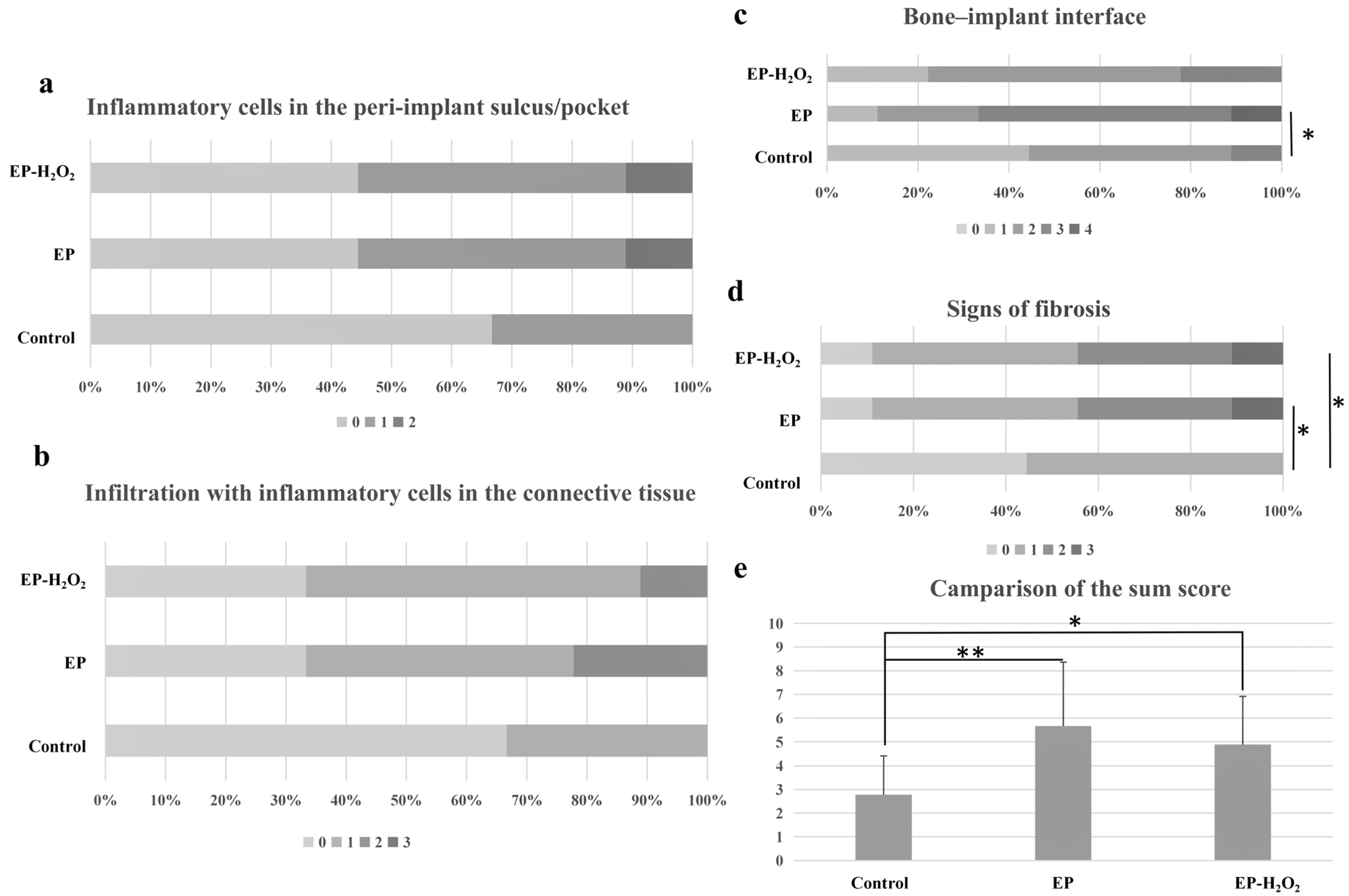
10.4. Characterization of the Peri-Implant Osteogenesis
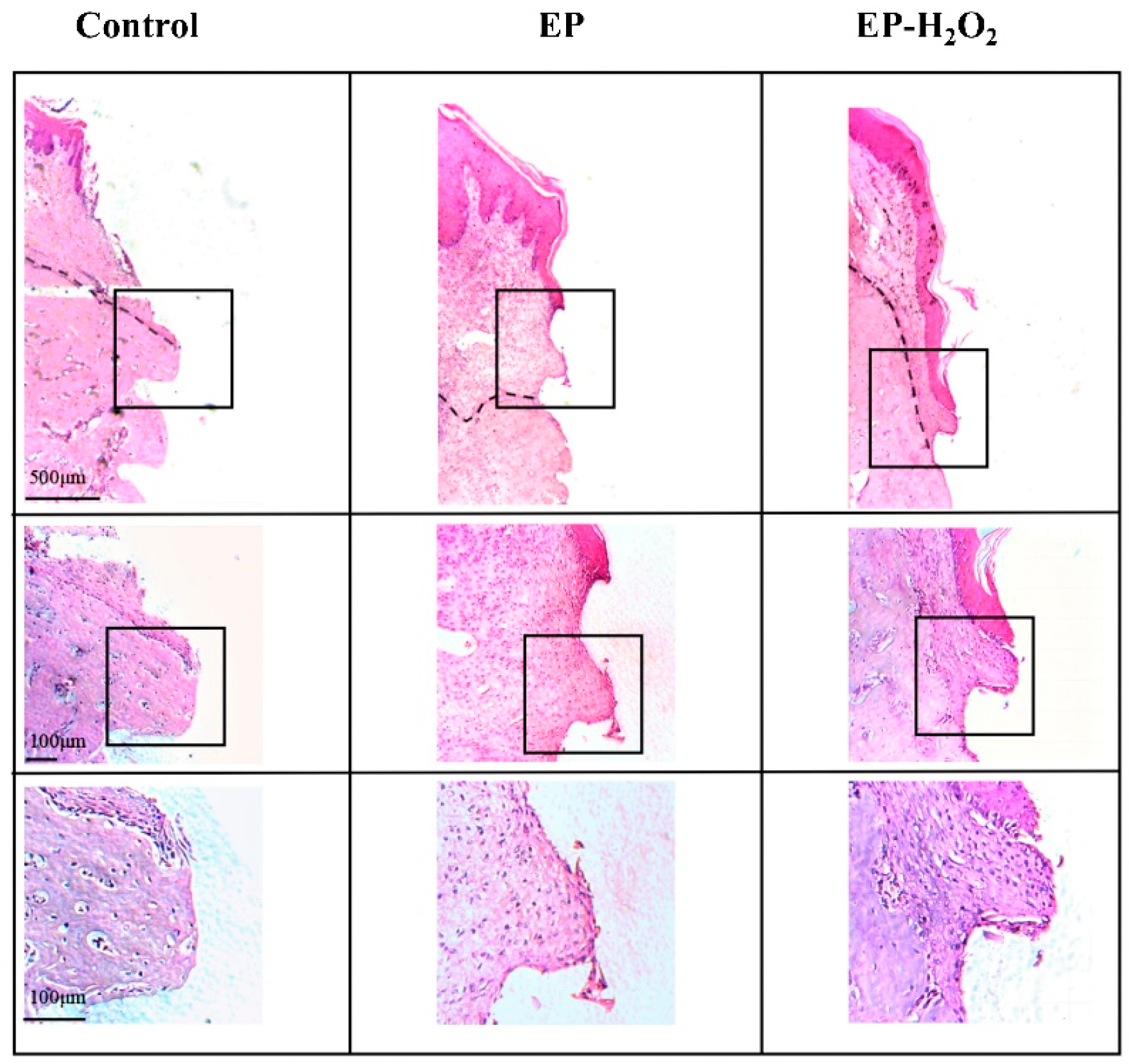
10.5. Characterization of the Peri-Implant H&E Staining
11. Discussion
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Sprague-Dawley | (SD) |
| The group with experimentally induced periodontitis | (EP) |
| The group with induced periodontitis and with extraction sockets rinsed with three percent H2O2 | (EP-H2O2) |
| Insertion torque | (IT) |
| Bone volume fraction | (BV/TV) |
| Trabecular thickness | (Tb.Th) |
| Trabecular number | (Tb.N) |
| Trabecular separation | (Tb.Sp) |
| Bone mineral density | (BMD) |
| Bone–implant contact ratio | (BIC) |
| Hematoxylin and eosin | (H&E) |
References
- Clark, D.; Levin, L. In the dental implant era, why do we still bother saving teeth? Dent. Traumatol. 2019, 35, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adell, R. Tissue integrated prostheses in clinical dentistry. Int. Dent. J. 1985, 35, 259–265. [Google Scholar] [PubMed]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Sukekava, F.; Wennström, J.L.; Lindhe, J. Ridge alterations following implant placement in fresh extraction sockets: An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.; Chen, S.T.; Wilson, T.G., Jr. Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. Int. J. Oral. Maxillofac. Implants 2004, 19, 26–28. [Google Scholar] [PubMed]
- Schulte, W.; Heimke, G. The Tübinger immediate implant. Quintessenz 1976, 27, 17–23. [Google Scholar]
- Andrade, C.A.S.; Paz, J.L.C.; de Melo, G.S.; Mahrouseh, N.; Januário, A.L.; Capeletti, L.R. Survival rate and peri-implant evaluation of immediately loaded dental implants in individuals with type 2 diabetes mellitus: A systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 1797–1810. [Google Scholar] [CrossRef]
- Sugimura, Y.; Schmidt, A.K.; Lichtenberg, A.; Assmann, A.; Akhyari, P. A Rat Model for the In Vivo Assessment of Biological and Tissue-Engineered Valvular and Vascular Grafts. Tissue Eng. Part. C Methods 2017, 23, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Mangraviti, A.; Gorelick, N.L.; Shapira-Furman, T.; Alomari, S.; Cecia, A.; Darjee, N.; Brem, H.; Rottenberg, Y.; Domb, A.J.; et al. Combined intracranial Acriflavine, temozolomide and radiation extends survival in a rat glioma model. Eur. J. Pharm. Biopharm. 2022, 170, 179–186. [Google Scholar] [CrossRef]
- Viera-Negrón, Y.E.; Ruan, W.H.; Winger, J.N.; Hou, X.; Sharawy, M.M.; Borke, J.L. Effect of ovariectomy and alendronate on implant osseointegration in rat maxillary bone. J. Oral Implantol. 2008, 34, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Pei, X.; Tulu, U.S.; Aghvami, M.; Chen, C.-T.; Gaudillière, D.; Arioka, M.; Moghim, M.M.; Bahat, O.; Kolinski, M.; et al. A Comparative Assessment of Implant Site Viability in Humans and Rats. J. Dent. Res. 2018, 97, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Shobara, K.; Ogawa, T.; Shibamoto, A.; Miyashita, M.; Ito, A.; Sitalaksmi, R.M. Osteogenic effect of low-intensity pulsed ultrasound and whole-body vibration on peri-implant bone. An experimental in vivo study. Clin. Oral Implants Res. 2021, 32, 641–650. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, Y.; Zhu, Z.; Xiao, W.; Xu, Q.; Li, L.; Li, X.; Chen, W. Effects of Low-Intensity Pulsed Ultrasound on Implant Osseointegration in Ovariectomized Rats. J. Ultrasound Med. 2016, 35, 747–754. [Google Scholar] [CrossRef]
- Miura, K.; Motoyoshi, M.; Inaba, M.; Iwai, H.; Karasawa, Y.; Shimizu, N. A preliminary study of the effects of low-intensity pulsed ultrasound exposure on the stability of orthodontic miniscrews in growing rats. Eur. J. Orthod. 2014, 36, 419–424. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Mu, Z.; Shrestha, A.; Wang, C.; Wang, S.; Tang, H.; Li, Y.; Song, J.; Ji, P.; Huang, Y.; et al. Development of a rat model for type 2 diabetes mellitus peri-implantitis: A preliminary study. Oral Dis. 2022, 28, 1936–1946. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Eberhard, J.; Glage, S.; Held, N.; Voigt, H.; Schwabe, K.; Winkel, A.; Stiesch, M. Development of a peri-implantitis model in the rat. Clin. Oral Implants Res. 2020, 31, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Son, C.; Choi, M.S.; Park, J.C. Different Responsiveness of Alveolar Bone and Long Bone to Epithelial-Mesenchymal Interaction-Related Factor. JBMR Plus 2020, 4, e10382. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Sohn, B.; Kim, K.H.; Kim, S.; Koo, K.-T.; Kim, T.-I.; Seol, Y.-J.; Lee, Y.-M.; Rhyu, I.-C.; Ku, Y. Effects of Untreated Periodontitis on Osseointegration of Dental Implants in a Beagle Dog Model. J. Periodontol. 2016, 87, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhang, H.; Zhang, H.; Shao, H.; He, Q.; Zhang, P. A comparison of clinical outcomes for implants placed in fresh extraction sockets versus healed sites in periodontally compromised patients: A 1-year follow-up report. Int. J. Oral Maxillofac. Implants 2010, 25, 1036–1040. [Google Scholar] [PubMed]
- Miron, R.J.; Pinto, N.R.; Quirynen, M.; Ghanaati, S. Standardization of relative centrifugal forces in studies related to platelet-rich fibrin. J. Periodontol. 2019, 90, 817–820. [Google Scholar] [CrossRef]
- Annunziata, M.; Guida, L.; Nastri, L.; Piccirillo, A.; Sommese, L.; Napoli, C. The Role of Autologous Platelet Concentrates in Alveolar Socket Preservation: A Systematic Review. Transfus. Med. Hemother. 2018, 45, 195–203. [Google Scholar] [CrossRef]
- Menchini-Fabris, G.B.; Cosola, S.; Toti, P.; Hwan Hwang, M.; Crespi, R.; Covani, U. Immediate Implant and Customized Healing Abutment for a Periodontally Compromised Socket: 1-Year Follow-Up Retrospective Evaluation. J. Clin. Med. 2023, 12, 2783. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Martins, M.D.; Wennerberg, A. Immediate placement of implants into infected sites: A systematic review. Clin. Implant Dent. Relat. Res. 2015, 17, e1–e16. [Google Scholar] [CrossRef]
- Marconcini, S.; Barone, A.; Gelpi, F.; Briguglio, F.; Covani, U. Immediate implant placement in infected sites: A case series. J. Periodontol. 2013, 84, 196–202. [Google Scholar] [CrossRef]
- Manor, Y.; Alkasem, A.; Mardinger, O.; Chaushu, G.; Greenstein, R.B. Levels of bacterial contamination in fresh extraction sites after a saline rinse. Int. J. Oral Maxillofac Implants 2015, 30, 1362–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stähli, A.; Lanzrein, C.; Milia, E.; Sculean, A.; Eick, S. In Vitro Effect of Instrumentation Using Ultrasonication with and without Hydrogen Peroxide on the Removal of Biofilms and Spread of Viable Microorganisms in Aerosols. Oral Health Prev. Dent. 2022, 20, 11–17. [Google Scholar]
- Fan, X.; Zhao, B.; Zhang, W.; Li, N.; Mi, K.; Wang, B. Coevolution of furA-Regulated Hyper-Inflammation and Mycobacterial Resistance to Oxidative Killing through Adaptation to Hydrogen Peroxide. Microbiol. Spectr. 2023, 26, e0536722. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J. Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health. Antioxidants 2020, 9, 797. [Google Scholar] [CrossRef]
- Kirkwood, J.; Robert, H. The design of animal experiments. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th ed.; Kirkwood, J., Robert, H., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Volume 3, pp. 23–36. [Google Scholar]
- Becker, S.T.; Föge, M.; Beck-Broichsitter, B.E.; Gavrilova, O.; Bolte, H.; Rosenstiel, P.; Wiltfang, J. Induction of periimplantitis in dental implants. J. Craniofac. Surg. 2013, 24, e15–e18. [Google Scholar] [CrossRef]
- Koutouzis, T.; Eastman, C.; Chukkapalli, S.; Larjava, H.; Kesavalu, L. A Novel Rat Model of Polymicrobial Peri-Implantitis: A Preliminary Study. J. Periodontol. 2017, 88, e32–e41. [Google Scholar] [CrossRef]
- Maekawa, S.; Katagiri, S.; Takeuchi, Y.; Komazaki, R.; Ohtsu, A.; Udagawa, S.; Izumi, Y. Bone metabolic microarray analysis of ligature-induced periodontitis in streptozotocin-induced diabetic mice. J. Periodontal. Res. 2017, 52, 233–245. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, Q.; Xu, Y.; Zeng, F.; Liu, X.; Zhao, D.; Zhang, L.; Bai, G. Effect of Eucommia water extract on gingivitis and periodontitis in experimental rats. BMC Oral Health. 2022, 22, 326. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Bleich, A.; Mähler, M.; Most, C.; Leiter, E.H.; Liebler-Tenorio, E.; Elson, C.O.; Hedrich, H.J.; Schlegelberger, B.; Sundberg, J.P. Refined histopathologic scoring system improves power to detect colitis QTL in mice. Mamm. Genome 2004, 15, 865–871. [Google Scholar] [CrossRef]
- Lazzara, R.J. Immediate implant placement into extraction sites: Surgical and restorative advantages. Int. J. Periodontics Restorative Dent. 1989, 9, 332–343. [Google Scholar] [PubMed]
- Villa, R.; Rangert, B. Early loading of interforaminal implants immediately installed after extraction of teeth presenting endodontic and periodontal lesions. Clin. Implant Dent. Relat. Res. 2005, 7, S28–S35. [Google Scholar] [CrossRef] [PubMed]
- Casap, N.; Zeltser, C.; Wexler, A.; Tarazi, E.; Zeltser, R. Immediate placement of dental implants into debrided infected dentoalveolar sockets. J. Oral Maxillofac. Surg. 2007, 65, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Lindeboom, J.A.; Tjiook, Y.; Kroon, F.H. Immediate placement of implants in periapical infected sites: A prospective randomized study in 50 patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 705–710. [Google Scholar] [CrossRef]
- Horwitz, J.; Machtei, E.E. Immediate and delayed restoration of dental implants in patients with a history of periodontitis: A prospective evaluation up to 5 years. Int. J. Oral Maxillofac. Implants 2012, 27, 1137–1143. [Google Scholar]
- Tonetti, M.S.; Cortellini, P.; Graziani, F.; Cairo, F.; Lang, N.P.; Abundo, R.; Conforti, G.P.; Marquardt, S.; Rasperini, G.; Silvestri, M.; et al. Immediate versus delayed implant placement after anterior single tooth extraction: The timing randomized controlled clinical trial. J. Clin. Periodontol. 2017, 44, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Falco, A.; Berardini, M.; Trisi, P. Correlation Between Implant Geometry, Implant Surface, Insertion Torque, and Primary Stability: In Vitro Biomechanical Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Szmukler-Moncler, S.; Piattelli, A.; Favero, G.A.; Dubruille, J.H. Considerations preliminary to the application of early and immediate loading protocols in dental implantology. Clin. Oral Implants Res. 2000, 11, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Brunski, J.B. In vivo bone response to biomechanical loading at the bone/dental-implant interface. Adv. Dent. Res. 1999, 13, 99–119. [Google Scholar] [CrossRef]
- Calandriello, R.; Tomatis, M.; Rangert, B. Immediate functional loading of Brånemark System implants with enhanced initial stability: A prospective 1- to 2-year clinical and radiographic study. Clin. Implant Dent. Relat. Res. 2003, 5, 10–20. [Google Scholar] [CrossRef]
- Carr, B.R.; Jeon-Slaughter, H.; Neal, T.W.; Gulko, J.A.; Kolar, N.C.; Finn, R.A. Low Insertional Torque and Early Dental Implant Failure. J. Oral Maxillofac. Surg. 2022, 80, 1069–1077. [Google Scholar] [CrossRef]
- Roca-Millan, E.; González-Navarro, B.; Domínguez-Mínger, J.; Marí-Roig, A.; Jané-Salas, E.; López-López, J. Implant insertion torque and marginal bone loss: A systematic review and meta-analysis. Int. J. Oral Implantol. 2020, 13, 345–353. [Google Scholar]
- Schwitalla, A.D.; Zimmermann, T.; Spintig, T.; Abou-Emara, M.; Lackmann, J.; Müller, W.D.; Houshmand, A. Maximum insertion torque of a novel implant-abutment-interface design for PEEK dental implants. J. Mech. Behav. Biomed. Mater. 2018, 77, 85–89. [Google Scholar] [CrossRef]
- Lemos, C.A.; Verri, F.R.; de Oliveira Neto, O.B.; Cruz, R.S.; Gomes JM, L.; da Silva Casado, B.G.; Pellizzer, E.P. Clinical effect of the high insertion torque on dental implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2021, 126, 490–496. [Google Scholar] [CrossRef]
- Quirynen, M.; Gijbels, F.; Jacobs, R. An infected jawbone site compromising successful osseointegration. Periodontology 2000 2003, 33, 129–144. [Google Scholar] [CrossRef]
- Quirynen, M.; Vogels, R.; Alsaadi, G.; Naert, I.; Jacobs, R.; van Steenberghe, D. Predisposing conditions for retrograde peri-implantitis, and treatment suggestions. Clin. Oral Implants Res. 2005, 16, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Kissa, J.; El Kholti, W.; Chemlali, S.; Kawtari, H.; Laalou, Y.; Albandar, J.M. Prevalence and risk indicators of peri-implant diseases in a group of Moroccan patients. J. Periodontol. 2021, 92, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Evian, C.I.; Emling, R.; Rosenberg, E.S.; Waasdorp, J.A.; Halpern, W.; Shah, S.; Garcia, M. Retrospective analysis of implant survival and the influence of periodontal disease and immediate placement on long-term results. Int. J. Oral Maxillofac. Implants 2004, 19, 393–398. [Google Scholar] [PubMed]
- Kakar, A.; Kakar, K.; Leventis, M.D.; Jain, G. Immediate Implant Placement in Infected Sockets: A Consecutive Cohort Study. J. Lasers Med. Sci. 2020, 11, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Novaes, A.B., Jr.; Marcaccini, A.M.; Souza, S.L.; Taba, M., Jr.; Grisi, M.F. Immediate placement of implants into periodontally infected sites in dogs: A histomorphometric study of bone-implant contact. Int. J. Oral Maxillofac. Implants 2003, 18, 391–398. [Google Scholar]
- Marcaccini, A.M.; Novaes, A.B., Jr.; Souza, S.L.; Taba, M., Jr.; Grisi, M.F. Immediate placement of implants into periodontally infected sites in dogs. Part 2: A fluorescence microscopy study. Int. J. Oral Maxillofac. Implants 2003, 18, 812–819. [Google Scholar]
- Tehemar, S.; Hanes, P.; Sharawy, M. Enhancement of osseointegration of implants placed into extraction sockets of healthy and periodontally diseased teeth by using graft material, an ePTFE membrane, or a combination. Clin. Implant Dent. Relat. Res. 2003, 5, 193–211. [Google Scholar] [CrossRef]
- Papalexiou, V.; Novaes, A.B., Jr.; Grisi, M.F.; Souza, S.S.; Taba, M., Jr.; Kajiwara, J.K. Influence of implant microstructure on the dynamics of bone healing around immediate implants placed into periodontally infected sites. A confocal laser scanning microscopic study. Clin. Oral Implants Res. 2004, 15, 44–53. [Google Scholar] [CrossRef]
- Brancaccio, Y.; Antonelli, A.; Barone, S.; Bennardo, F.; Fortunato, L.; Giudice, A. Evaluation of local hemostatic efficacy after dental extractions in patients taking antiplatelet drugs: A randomized clinical trial. Clin. Oral Investig. 2021, 25, 1159–1167. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; León-Cano, A.; Ortega-Oller, I.; Monje, A.; Valle, F.O.; Catena, A. Marginal bone loss as success criterion in implant dentistry: Beyond 2 mm. Clin. Oral Implants Res. 2015, 26, e28–e34. [Google Scholar] [CrossRef] [Green Version]
- Qu, Q.; Yang, F.; Zhao, C.; Liu, X.; Yang, P.; Li, Z.; Shi, X. Effects of fermented ginseng on the gut microbiota and immunity of rats with antibiotic-associated diarrhea. J. Ethnopharmacol. 2021, 267, 113594. [Google Scholar] [CrossRef]
- Chen, Y.C.; Greenbaum, J.; Shen, H.; Deng, H.W. Association Between Gut Microbiota and Bone Health: Potential Mechanisms and Prospective. J. Clin. Endocrinol. Metab. 2017, 102, 3635–3646. [Google Scholar] [CrossRef] [Green Version]
- Durand, R.; Kersheh, I.; Marcotte, S.; Boudrias, P.; Schmittbuhl, M.; Cresson, T.; Rei, N.; Rompré, P.H.; Voyer, R. Do postoperative antibiotics influence one-year peri-implant crestal bone remodelling and morbidity? A double-blinded randomized clinical trial. Clin. Oral Implants Res. 2021, 32, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Nakahara, K.; Haga-Tsujimura, M.; Fujioka-Kobayashi, M.; Iizuka, T.; Miron, R.J. Effect of Irrigation Time of Antiseptic Solutions on Bone Cell Viability and Growth Factor Release. J. Craniofac. Surg. 2018, 29, 376–381. [Google Scholar] [CrossRef]
- Sawada, K.; Fujioka-Kobayashi, M.; Kobayashi, E.; Schaller, B.; Miron, R.J. Effects of Antiseptic Solutions Commonly Used in Dentistry on Bone Viability, Bone Morphology, and Release of Growth Factors. J. Oral Maxillofac. Surg. 2016, 74, 247–254. [Google Scholar] [CrossRef]
- Novaes, A.B., Jr.; Papalexiou, V.; Grisi, M.F.; Souza, S.S.; Taba, M., Jr.; Kajiwara, J.K. Influence of implant microstructure on the osseointegration of immediate implants placed in periodontally infected sites. A histomorphometric study in dogs. Clin. Oral Implants Res. 2004, 15, 34–43. [Google Scholar] [CrossRef]
- Siegenthaler, D.W.; Jung, R.E.; Holderegger, C.; Roos, M.; Hämmerle, C.H. Replacement of teeth exhibiting periapical pathology by immediate implants: A prospective, controlled clinical trial. Clin. Oral Implants Res. 2007, 18, 727–737. [Google Scholar] [CrossRef]
- Villa, R.; Rangert, B. Immediate and early function of implants placed in extraction sockets of maxillary infected teeth: A pilot study. J. Prosthet. Dent. 2007, 97, S96–S108. [Google Scholar] [CrossRef]
- Kusek, E.R. Immediate implant placement into infected sites: Bacterial studies of the Hydroacoustic effects of the YSGG laser. J. Oral Implantol. 2011, 37, 205–211. [Google Scholar] [CrossRef]
- Fugazzotto, P. A retrospective analysis of immediately placed implants in 418 sites exhibiting periapical pathology: Results and clinical considerations. Int. J. Oral Maxillofac. Implants 2012, 27, 194–202. [Google Scholar]
- Bell, C.L.; Diehl, D.; Bell, B.M.; Bell, R.E. The immediate placement of dental implants into extraction sites with periapical lesions: A retrospective chart review. J. Oral Maxillofac. Surg. 2011, 69, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Boggian, C.; Taschieri, S. Immediate implant placement into fresh extraction sites with chronic periapical pathologic features combined with plasma rich in growth factors: Preliminary results of single-cohort study. J. Oral Maxillofac. Surg. 2009, 67, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- Jofre, J.; Valenzuela, D.; Quintana, P.; Asenjo-Lobos, C. Protocol for immediate implant replacement of infected teeth. Implant Dent. 2012, 21, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Mazza Bleeding Index | |||
|---|---|---|---|
| Score | Gingiva Looking | BOP | Edema |
| 0 | healthy | - | - |
| 1 | healthy | + | - |
| 2 | unhealthy | + | - |
| 3 | unhealthy | + | slight |
| 4 | unhealthy | + | obvious |
| 5 | unhealthy | + | marked |
| Histological Semi-Quantitative Grading | ||
|---|---|---|
| Parameter | Score | Historical Appearance |
| Infiltration with inflammatory cells in the connective tissue | 0 | No neutrophils/macrophages per field of view |
| 1 | Mild, some neutrophils/macrophages per field of view | |
| 2 | Moderate, up to 50 neutrophils/macrophages per field of view | |
| 3 | Severe, more than 50 neutrophils/macrophages per field of view | |
| Inflammatory cells in the peri-implant sulcus/pocket | 0 | No neutrophils |
| 1 | Some neutrophils | |
| 2 | Many neutrophils | |
| Signs of fibrosis | 0 | No |
| 1 | Mild | |
| 2 | Moderate | |
| 3 | Severe, with prominent neoformation of fibrotic tissue | |
| Bone–implant interface | 0 | Fully healed (or beginning of healing without signs of bone destruction), mostly osteoblasts |
| 1 | Some osteoclasts/osteoclast activity | |
| 2 | Mild osteoclast activity | |
| 3 | Moderate osteoclast activity | |
| 4 | Severe osteoclast activity (loss of implant) | |
| Control | EP | EP-H2O2 | |
|---|---|---|---|
| Number of rats | 10 | 10 | 10 |
| Number of placed implants | 18 | 18 | 16 |
| Number of final residual implants | 13 | 9 | 9 |
| Implant survival rate | 72.20% | 50% | 56.30% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cao, X.; Shen, Y.; Zhong, Q.; Huang, Y.; Zhang, Y.; Wang, S.; Xu, C. Initial Development of an Immediate Implantation Model in Rats and Assessing the Prognostic Impact of Periodontitis on Immediate Implantation. Bioengineering 2023, 10, 896. https://doi.org/10.3390/bioengineering10080896
Wang Y, Cao X, Shen Y, Zhong Q, Huang Y, Zhang Y, Wang S, Xu C. Initial Development of an Immediate Implantation Model in Rats and Assessing the Prognostic Impact of Periodontitis on Immediate Implantation. Bioengineering. 2023; 10(8):896. https://doi.org/10.3390/bioengineering10080896
Chicago/Turabian StyleWang, Yingying, Ximeng Cao, Yingyi Shen, Qi Zhong, Yujie Huang, Yifan Zhang, Shaohai Wang, and Chun Xu. 2023. "Initial Development of an Immediate Implantation Model in Rats and Assessing the Prognostic Impact of Periodontitis on Immediate Implantation" Bioengineering 10, no. 8: 896. https://doi.org/10.3390/bioengineering10080896
APA StyleWang, Y., Cao, X., Shen, Y., Zhong, Q., Huang, Y., Zhang, Y., Wang, S., & Xu, C. (2023). Initial Development of an Immediate Implantation Model in Rats and Assessing the Prognostic Impact of Periodontitis on Immediate Implantation. Bioengineering, 10(8), 896. https://doi.org/10.3390/bioengineering10080896