Neural Plasticity Changes Induced by Motor Robotic Rehabilitation in Stroke Patients: The Contribution of Functional Neuroimaging
Abstract
:1. Robotic Neurorehabilitation in Stroke Patients
2. Determining the Functional Impact of Robotic Neurorehabilitation
3. Methods
4. Results
4.1. Robotic Devices
- (1)
- BCI-guided robot-assisted (exoskeleton) for upper-limb training [40]: This is a rehabilitation system for practicing hand movements. It includes a number of finger assemblies that are operationally connected to a platform: each finger assembly has a motor for a metacarpophalangeal joint having a proximal rail guide operationally connected, and an intermediate assembly for a proximal interphalangeal joint having an intermediate rail. The alignment of the knuckle joint indicators allows motion of the finger to be controlled and maintains rotational axes of the finger about each virtual center when the proximal and intermediate follower assemblies are actuated by the motor. A knuckle joint indicator of the proximal rail guide corresponds to a first virtual center, and a knuckle joint indicator of the intermediate rail guide corresponds to a second virtual center.
- (2)
- Hand-induced robotic device (MR_CHIROD) [41]. This is a redesigned robotic hand device that allows participant grip and release of a handle in response to a variable resistance force while watching an oscillating visual stimulus. The MR_CHIROD v3 (MR-compatible hand-induced robotic device) is a device that displays customizable forces for grasping and releasing motions while simultaneously measuring and recording applied force, grip displacement, and timestamps for each data point [42].
- (3)
- Technology-robot-assisted virtual rehabilitation adaptive training system for upper limbs (NJIT-RAVR) [43,44]. This consists of the Haptic Master, six degrees of freedom, admittance-driven robot, and a collection of rehabilitative simulations that supply the Haptic Master with adaptive algorithms, enabling interaction with detailed virtual worlds. The movement arm can serve as an interface between the participants and the virtual worlds, by measuring the external force applied by the user to the robot, together with end-point location and velocity, in 3D in real time at a rate of up to 1000 Hz. When used as the end effector, the ring gimbal adds the ability to rotate the forearm and counts three extra degrees of freedom. The robot actively generates and records the force that aids or opposes forearm rotation (i.e., roll). Pitch and yaw angles, on the other hand, are passively recorded. The robot can be programmed to provide haptic effects such as springs, dampers, and constant global forces, due to the haptic master application programming interface.
- (4)
- Hand–wrist assistive rehabilitation device (HWARD) [45]. This pneumatically operated, three-degrees-of-freedom tool supports the hand’s grab and release motions. The three angles are the wrist’s flexion and extension, the thumb’s flexion and extension at the MCP joint, and the four fingers together around the MCP joint. The individual is sitting and looking at a computer screen. Three gentle straps hold the hand to the robot’s mechanism, while a padded splint mounted to the platform’s surface holds the forearm in place. The palmar hand is not constrained, allowing genuine things to be placed into a gripping hand. The movement of the robot’s joints and, consequently, the movement of the subject’s limbs when coupled to the robot are measured by joint angle sensors in the robot. This feature allows the operation of a virtual hand on a computer screen, by using the subject’s hand in real-time virtual reality. When the robot is not actively assisting individuals, it can be back-driven, allowing subjects to move freely.
- (5)
- BCI-robot training system of upper limbs (RHB-III) [46]. This consists of a robotic exoskeleton used to help the paretic hand perform the real movement of grasping/opening tasks. Participants were told to view the motions in the video and follow instructions to visualize doing the identical motion with their paretic hand. The calculation of the mu event-related desynchronization score was performed based on the real-time EEG readings. When the score exceeded 60, the robot was activated and helped the paretic hand complete the grasp/open job for the following three seconds. However, if the mu event-related desynchronization score was less than 60, the trial was deemed unsuccessful and the robot was not triggered to move. Motor imagery instruction was promoted until the video screen showed successful or unsuccessful detection. If motor imagery was correctly identified, the robot gave visual and movement feedback by actually moving the paretic hand.
- (6)
- MorningWalk® system for gait recovery [47]. This was created in 2014 by CUREXO Inc. in Korea for the rehabilitation of individuals with gait disorders. It has a saddle that can bear the weight of the sufferer. Virtual reality augmentation software enables interactive training. The gadget allows for complicated treatment protocol designs by allowing users to apply free-walking programs with several parameters for walking speed, stride length, and different walking motions, such as flat ground and stair climbing.
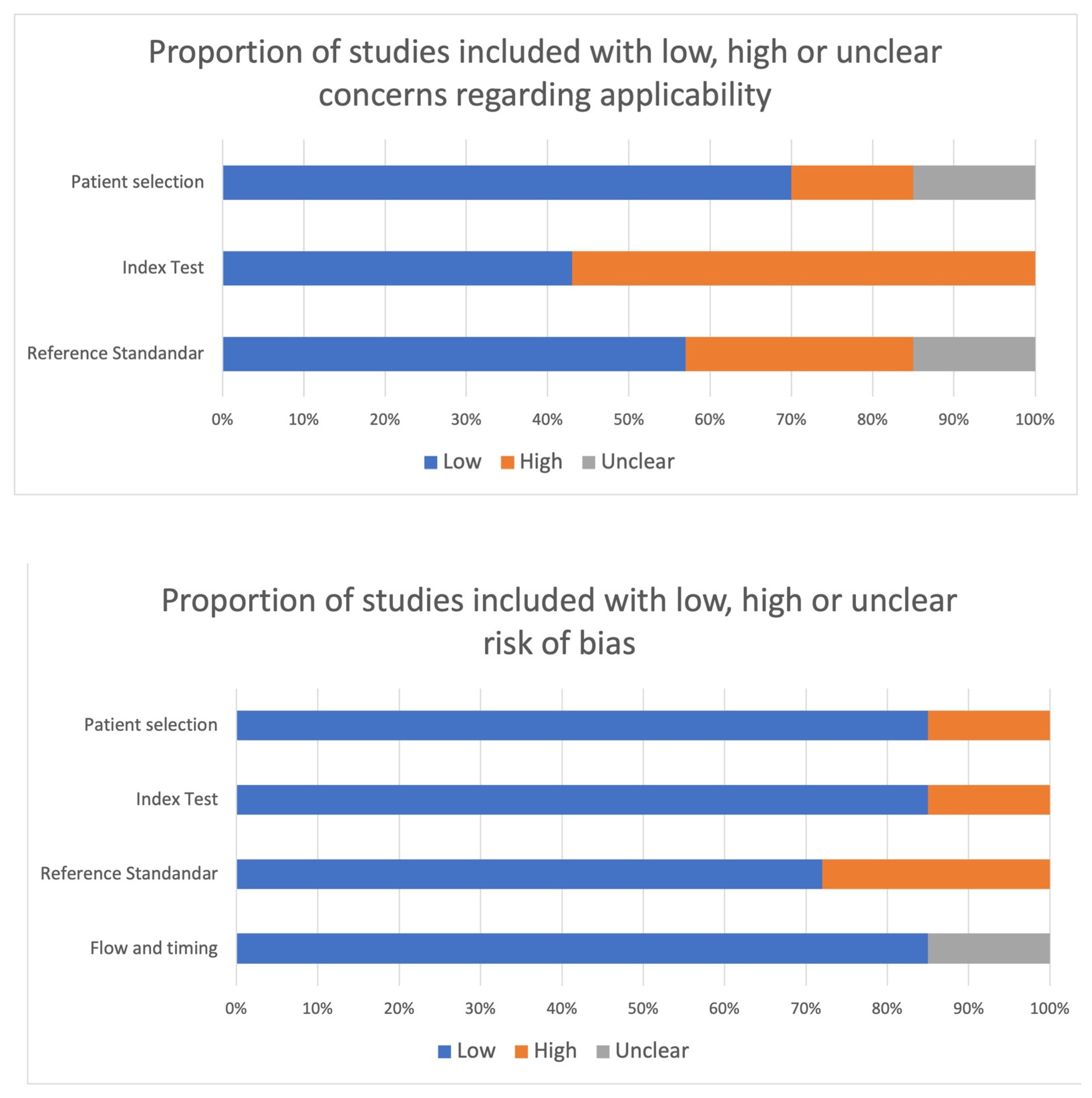
4.2. Risk of Bias within Studies
4.3. fMRI Studies
4.3.1. Work by Yuan et al., 2020 [41]
4.3.2. Astrakas et al., 2021 [42]
4.3.3. Saleh et al., 2017 [43]
4.3.4. Saleh et al., 2012 [44]
4.3.5. Takahashi et al., 2008 [45]
4.4. fNRIS Studies
4.4.1. Liu et al., 2022 [46]
4.4.2. Song et al., 2021 [47]
5. Discussion
Limitations
- (1)
- A bias selection in studies included in this systematic review could be considered. The focus of this review was solely on the existence of stable neural plasticity changes associated with intensive robotic neurorehabilitation therapy. Due to this, we excluded a number of studies that looked at the neurological underpinnings of motor exercises performed with robotic devices and measured during the course of a single neuroimaging session;
- (2)
- Poor employment of the RCT study design. RCT study designs have not been extensively used in many studies in this field. Furthermore, only two of the few trials included in this review used a very active control condition that allowed separation of the impact of robotic interventions from other treatments;
- (3)
- The heterogeneity of the studies is the main weakness of this research. Indeed, individual patients’ data including delay after stroke, lesion site, clinical severity could not be effectively controlled across research. Additionally, neuroimaging task procedures, analysis, and type were also principal sources of variability.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hwang, S.; Song, C.S. Driving Rehabilitation for Stroke Patients: A Systematic Review with Meta-Analysis. Healthcare 2023, 11, 1637. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, M.; Mazzoleni, D.; Campanini, I.; Merlo, A.; Mazzoli, D.; Melegari, C.; Colombo, V.; Cerulli, S.; Piscitelli, D.; Perin, C.; et al. Evidence-based improvement of gait in post-stroke patients following robot-assisted training: A systematic review. NeuroRehabilitation 2022, 51, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Cacciola, A.; Bertè, F.; Manuli, A.; Leo, A.; Bramanti, A.; Naro, A.; Milardi, D.; Bramanti, P. Robotic gait rehabilitation and substitution devices in neurological disorders: Where are we now? Neurol. Sci. 2016, 37, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol. Sci. 2017, 38, 1561–1569. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, H.; Zhang, J.; Yang, S.; Cai, S. Robot-Assisted Therapy for Upper Extremity Motor Impairment After Stroke: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 4, 101. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Werner, C.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2017, 5, CD006185. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, CD006185. [Google Scholar] [CrossRef]
- Morone, G.; Paolucci, S.; Cherubini, A.; De Angelis, D.; Venturiero, V.; Coiro, P.; Iosa, M. Robot-assisted gait training for stroke patients: Current state of the art and perspectives of robotics. Neuropsychiatr. Dis. Treat. 2017, 13, 1303–1311. [Google Scholar] [CrossRef]
- Gramigna, V.; Pellegrino, G.; Cerasa, A.; Cutini, S.; Vasta, R.; Olivadese, G.; Martino, I.; Quattrone, A. Near-Infrared Spectroscopy in gait disorders: Is it time to begin? Neurorehabilit. Neural Repair 2017, 31, 402–412. [Google Scholar] [CrossRef]
- Weingarten, C.P.; Sundman, M.H.; Hickey, P.; Chen, N.K. Neuroimaging of Parkinson’s disease: Expanding views. Neurosci. Biobehav. Rev. 2015, 59, 16–52. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Luppino, G. The cortical motor system. Neuron 2001, 31, 889–901. [Google Scholar] [CrossRef]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [PubMed]
- Cerasa, A.; Novellino, F.; Quattrone, A. Connectivity Changes in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2016, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Fornito, A.; Zalesky, A.; Breakspear, M. The connectomics of brain disorders. Nat. Rev. Neurosci. 2015, 16, 159–172. [Google Scholar] [CrossRef]
- Betzel, R.F. Chapter 2—Network Neuroscience and the Connectomics Revolution. In Connectomic Deep Brain Stimulation; Horn, A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 25–58. ISBN 9780128218617. [Google Scholar] [CrossRef]
- Blumen, H.M.; Holtzer, R.; Brown, L.L.; Gazes, Y.; Verghese, J. Behavioral and neural correlates of imagined walking and walking-while-talking in the elderly. Hum. Brain Mapp. 2014, 35, 4090–4104. [Google Scholar] [CrossRef]
- Guillot, A.; Collet, C.; Nguyen, V.A.; Malouin, F.; Richards, C.; Doyon, J. Brain activity during visual versus kinesthetic imagery: An fMRI study. Hum. Brain Mapp. 2009, 30, 2157–2172. [Google Scholar] [CrossRef]
- La Fougère, C.; Zwergal, A.; Rominger, A.; Förster, S.; Fesl, G.; Dieterich, M.; Brandt, T.; Strupp, M.; Bartenstein, P.; Jahn, K. Real versus imagined locomotion: A [18F]-FDG PET-fMRI comparison. Neuroimage 2010, 50, 1589–1598. [Google Scholar] [CrossRef]
- Dalla Volta, R.; Fasano, F.; Cerasa, A.; Mangone, G.; Quattrone, A.; Buccino, G. Walking indoors, walking outdoors: An fMRI study. Front. Psychol. 2015, 6, 1502. [Google Scholar] [CrossRef]
- Shine, J.M.; Matar, E.; Ward, P.B.; Frank, M.J.; Moustafa, A.A.; Pearson, M.; Naismith, S.L.; Lewis, S.J.G. Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain 2013, 136, 3671–3681. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H.; Firestine, A.; West, M.; Saremi, K.; Woods, R. Ankle dorsiflexion as an fMRI paradigm to assay motor control for walking during rehabilitation. Neuroimage 2004, 23, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Irani, F.; Platek, S.M.; Bunce, S.; Ruocco, A.C.; Chute, D. Functional near infrared spectroscopy (fNIRS): An emerging neuroimaging technology with important applications for the study of brain disorders. Clin. Neuropsychol. 2007, 21, 9–37. [Google Scholar] [CrossRef]
- Boas, D.A.; Elwell, C.E.; Ferrari, M.; Taga, G. Twenty years of functional near-infrared spectroscopy: Introduction for the special issue. Neuroimage 2014, 85, 1–5. [Google Scholar] [CrossRef]
- Cutini, S.; Brigadoi, S. Unleashing the future potential of functional near-infrared spectroscopy in brain sciences. J. Neurosci. Methods 2014, 232, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, G.; Machado, A.; Von Ellenrieder, N.; Watanabe, S.; Hall, J.A.; Lina, J.M.; Kobayashi, E.; Grova, C. Hemodynamic response to interictal epileptiform discharges addressed by personalized EEG-fNIRS recordings. Front. Neurosci. 2016, 10, 102. [Google Scholar] [CrossRef]
- Boas, D.A.; Dale, A.M.; Franceschini, M.A. Diffuse optical imaging of brain activation: Approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage 2004, 23, S275–S288. [Google Scholar] [CrossRef]
- Huppert, T.J.; Hoge, R.D.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage. 2006, 29, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Cope, M.; Delpy, D.T. System for long-term measurement of cerebral blood flow and tissue oxygenation on newborn infants by infrared transillumination pathlength. Med. Biol. Eng. Comput. 1988, 26, 289–294. [Google Scholar] [CrossRef]
- Yücel, M.A.; Selb, J.; Boas, D.A.; Cash, S.S.; Cooper, R.J. Reducing motion artifacts for long-term clinical NIRS monitoring using collodion-fixed prism-based optical fibers. Neuroimage 2014, 85, 192–201. [Google Scholar] [CrossRef]
- Jeppesen, J.; Beniczky, S.; Johansen, P.; Sidenius, P.; Fuglsang-Frederiksen, A. Exploring the capability of wireless near infrared spectroscopy as a portable seizure detection device for epilepsy patients. Seizure 2015, 26, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Holtzer, R.; Mahoney, J.R.; Izzetoglu, M.; Wang, C.; England, S.; Verghese, J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage 2015, 112, 152–159. [Google Scholar] [CrossRef]
- Zhang, Z.; Khatami, R. A biphasic change of regional blood volume in the frontal cortex during non-rapid eye movement sleep: A near-infrared spectroscopy study. Sleep 2015, 38, 1211–1217. [Google Scholar] [CrossRef]
- Minati, L.; Visani, E.; Dowell, N.G.; Medford, N.; Critchley, H.D. Variability comparison of simultaneous brain near-infrared spectroscopy and functional magnetic resonance imaging during visual stimulation. J. Med. Eng. Technol. 2011, 35, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xia, Y.; Uchitel, J.; Collins-Jones, L.; Yang, S.; Loureiro, R.; Cooper, R.J.; Zhao, H. Review of recent advances in frequency-domain near-infrared spectroscopy technologies. Biomed. Opt. Express 2023, 14, 3234–3258. [Google Scholar] [CrossRef] [PubMed]
- Fantini, S. Editorial Special Section on Biomedical Diffuse Optics for the Brain. IEEE Open J. Eng. Med. Biol. 2023, 4, 77–78. [Google Scholar] [CrossRef]
- Hanakawa, T. Neuroimaging of standing and walking: Special emphasis on Parkinsonian gait. Park. Relat. Disord. 2006, 12, S70–S75. [Google Scholar] [CrossRef]
- Raffin, E.; Pellegrino, G.; Di Lazzaro, V.; Thielscher, A.; Siebner, H.R. Bringing transcranial mapping into shape: Sulcus-aligned mapping captures motor somatotopy in human primary motor hand area. Neuroimage 2015, 120, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Dubbioso, R.; Pellegrino, G.; Antenora, A.; De Michele, G.; Filla, A.; Santoro, L.; Manganelli, F. The effect of cerebellar degeneration on human sensori-motor plasticity. Brain Stim. 2015, 8, 1144–1150. [Google Scholar] [CrossRef]
- Favre, I.; Zeffiro, T.A.; Detante, O.; Krainik, A.; Hommel, M.; Jaillard, A. Upper Limb Recovery After Stroke Is Associated with Ipsilesional Primary Motor Cortical Activity. Stroke 2014, 45, 1077–1083. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, X.; Chen, C.; Lau, C.C.; Chu, W.C.; Tong, R.K. Interhemispheric functional reorganization and its structural base after BCI-guided upper-limb training in chronic stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2525–2536. [Google Scholar] [CrossRef]
- Astrakas, L.G.; Li, S.; Ottensmeyer, M.P.; Pusatere, C.; Moskowitz, M.A.; Tzika, A.A. Peak Activation Shifts in the Sensorimotor Cortex of Chronic Stroke Patients Following Robot-assisted Rehabilitation Therapy. Open Neuroimaging J. 2021, 14, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; Fluet, G.; Qiu, Q.; Merians, A.; Adamovich, S.V.; Tunik, E. Neural Patterns of Reorganization after Intensive Robot-Assisted Virtual Reality Therapy and Repetitive Task Practice in Patients with Chronic Stroke. Front. Neurol. 2017, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; Adamovich, S.V.; Tunik, E. Resting state functional connectivity and task- related effective connectivity changes after upper extremity rehabilitation: A pilot study. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 2012, 4559–4562. [Google Scholar] [CrossRef]
- Takahashi, C.D.; Der-Yeghiaian, L.; Le, V.; Motiwala, R.R.; Cramer, S.C. Robot-based hand motor therapy after stroke. Brain 2008, 131, 425–437. [Google Scholar] [CrossRef]
- Liu, L.; Jin, M.; Zhang, L.; Zhang, Q.; Hu, D.; Jin, L.; Nie, Z. Brain–Computer Interface-Robot Training Enhances Upper Extremity Performance and Changes the Cortical Activation in Stroke Patients: A Functional Near-Infrared Spectroscopy Study. Neuroscience 2022, 16, 809657. [Google Scholar] [CrossRef]
- Songa, K.J.; Chuna, M.H.; Leeb, J.; Leec, C. The effect of robot-assisted gait training on cortical activation in stroke patients: A functional near-infrared spectroscopy study. NeuroRehabilitation 2021, 49, 65–73. [Google Scholar] [CrossRef]
- Tong, K.Y.; Pang, P.M.K.; Chen, M.; Ho, S.K.; Zhou, H.; Chan, D.T.W. Wearable Power Assistive Device for Helping a User to Move Their Hand. U.S. Patent US8,574,178B2, 5 November 2013. [Google Scholar]
- Ward, N.S.; Brown, M.M.; Thompson, A.J.; Frackowiak, R.S.J. Neural correlates of motor recovery after stroke: A longitudinal fMRI study. Brain 2003, 126, 2476–2496. [Google Scholar] [CrossRef]
- Qiu, Q.; Ramirez, D.A.; Saleh, S.; Fluet, G.G.; Parikh, H.D.; Kelly, D.; Adamovich, S.V. The New Jersey Institute of Technology Robot-Assisted Virtual Rehabilitation (NJIT-RAVR) system for children with cerebral palsy: A feasibility study. J. Neuroeng. Rehabil. 2009, 6, 40. [Google Scholar] [CrossRef]
- Takahashi, C.; Der-Yeghiaian, L.; Le, V.; Cramer, S.C. A robotic device for hand motor therapy after stroke. In Proceedings of the IEEE 9th International Conference on Rehabilitation Robotics: Frontiers of the Human-Machine Interface, Chicago, IL, USA, 28 June–1 July 2005; pp. 17–20. [Google Scholar] [CrossRef]
- Dobkin, C.; Finkelstein, A.; Kluender, R.; Notowidigdo, M.J. The Economic Consequences of Hospital Admissions. Am. Econ. Rev. 2018, 108, 308–352. [Google Scholar] [CrossRef]
- Baron, J.C. Stroke research in the modern era: Images versus dogmas. Cerebrovasc. Dis. 2005, 20, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Ward, N. Assessment of cortical reorganisation for hand function after stroke. J. Physiol. 2011, 589, 5625–5632. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, R.; Pendlebury, S.; Johansen-Berg, H.; Matthews, P.M. Functional MRIdetects posterior shifts in primary sensorimotor cortex activation after stroke:evidence of local adaptive reorganization? Stroke 2001, 32, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Plow, E.B.; Sankarasubramanian, V.; Cunningham, D.A.; Potter-Baker, K.; Varnerin, N.; Cohen, L.G.; Sterr, A.; Conforto, A.B.; Machado, A.G. Models to tailor brain stimulation therapies in stroke. Neural Plast. 2016, 2016, 4071620. [Google Scholar] [CrossRef]
- Laible, M.; Grieshammer, S.; Seidel, G.; Rijntjes, M.; Weiller, C.; Hamzei, F. Association of activity changes in the primary sensory cortex with successful motor rehabilitation of the hand following stroke. Neurorehabilit. Neural. Repair. 2012, 26, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Schaechter, J.D.; Moore, C.I.; Connell, B.D.; Rosen, B.R.; Dijkhuizen, R.M. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain 2006, 129, 2722–2733. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Wu, Y.; Liu, S.; Jia, J.; Zhang, L. Neurophysiological substrates of stroke patients with motor imagery based brain-computer Interface training. Int. J. Neurosci. 2014, 124, 403–415. [Google Scholar] [CrossRef]
- Loubinoux, I.; Carel, C.; Pariente, J.; Dechaumont, S.; Albucher, J.F.; Marque, P.; Manelfe, C.; Chollet, F. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. NeuroImage 2003, 20, 2166–2180. [Google Scholar] [CrossRef]
- Carey, L.M.; Abbott, D.F.; Egan, G.F.; O’Keefe, G.J.; Jackson, G.D.; Bernhardt, J.; Donnan, G.A. Evolution of brain activation with good and poor motor recovery after stroke. Neurorehabil Neural Repair. 2006, 20, 24–41. [Google Scholar] [CrossRef]
- Park, C.; Chang, W.H.; Ohn, S.H.; Kim, S.T.; Bang, O.Y.; Pascual-Leone, A.; Kim, Y.H. Longitudinal changes of resting state functional connectivity during motor recovery after stroke. Stroke 2011, 42, 1357–1362. [Google Scholar] [CrossRef]
- Doyon, J.; Benali, H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005, 15, 161–167. [Google Scholar] [CrossRef]
- Hatakenaka, M.; Miyai, I.; Mihara, M.; Sakoda, S.; Kubota, K. Frontal regions involved in learning of motor skill—A functional NIRS study. Neuroimage 2007, 34, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, C.; Thach, W.T.; Jones, E.G. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res. 1983, 286, 237–265. [Google Scholar] [CrossRef] [PubMed]


| Reference | Neuroimaging Modality | Patients | Robotic Rehabilitation Procedure | Experimental Procedures | Clinical Outcome | Main Results |
|---|---|---|---|---|---|---|
| Yuan K. et al., 2020 [41] | fMRI |
|
|
|
| Robotic rehabilitation induced an increased functional connectivity between primary motor cortex and controlesional SMA and premotor cortex. |
| Astrakas L.G. et al., 2021 [42] | fMRI |
|
|
|
| In comparison with HC, patients moving the affected hand showed correspondingly higher peak activations in the primary motor area and lower peak activations in the somatosensory cortex. Additionally, they demonstrated an average 5.3 mm anterior shift in peak activity. |
| Saleh S. et al., 2017 [43] | fMRI |
|
|
|
| A significant difference in lateralization index between groups was observed after training. The shift toward greater ipsilesional hemisphere dominance was more pronounced in the RAVR group than RTP group. A strong association between bilateral primary sensory regions, ventral premotor area, and ipsilateral primary motor cortex was found in the RAVR but not in the RTP group. |
| Saleh S. et al., 2012 [44] | fMRI |
|
|
|
| Reorganization in functional connectivity between primary motor cortex and sensorimotor cortex and decreased connectivity between the contralesional sensorimotor cortex and primary motor cortex, while secondary sensorimotor cortex had substantial increase in connectivity between bilateral sensorimotor/premotor areas and primary motor cortex. |
| Takahashi et al., 2008 [45] | fMRI |
|
|
|
| Increased activity within the left (stroke-affected) primary sensorimotor cortex after treatment. |
| Liu L. et al., 2022 [46] | fNIRS |
|
|
|
| Changes in neural functional connectivity were observed after BCI-robot training in both motor and sensory areas in the ipsilesional brain. |
| Song K.J. et al., 2021 [47] | fNIRS |
|
|
|
| After robot-assisted rehabilitation, motor cortical activation was increased significantly in both hemispheres, and the degree of increased activation was greater in the affected hemisphere than in the unaffected hemisphere. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanno, L.; Cannuli, A.; Pignolo, L.; Marino, S.; Quartarone, A.; Calabrò, R.S.; Cerasa, A. Neural Plasticity Changes Induced by Motor Robotic Rehabilitation in Stroke Patients: The Contribution of Functional Neuroimaging. Bioengineering 2023, 10, 990. https://doi.org/10.3390/bioengineering10080990
Bonanno L, Cannuli A, Pignolo L, Marino S, Quartarone A, Calabrò RS, Cerasa A. Neural Plasticity Changes Induced by Motor Robotic Rehabilitation in Stroke Patients: The Contribution of Functional Neuroimaging. Bioengineering. 2023; 10(8):990. https://doi.org/10.3390/bioengineering10080990
Chicago/Turabian StyleBonanno, Lilla, Antonio Cannuli, Loris Pignolo, Silvia Marino, Angelo Quartarone, Rocco Salvatore Calabrò, and Antonio Cerasa. 2023. "Neural Plasticity Changes Induced by Motor Robotic Rehabilitation in Stroke Patients: The Contribution of Functional Neuroimaging" Bioengineering 10, no. 8: 990. https://doi.org/10.3390/bioengineering10080990








