Reliability of Panoramic Ultrasound in Assessing Rectus Femoris Size, Shape, and Brightness: An Inter-Examiner Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Panoramic Ultrasound Imaging Capture
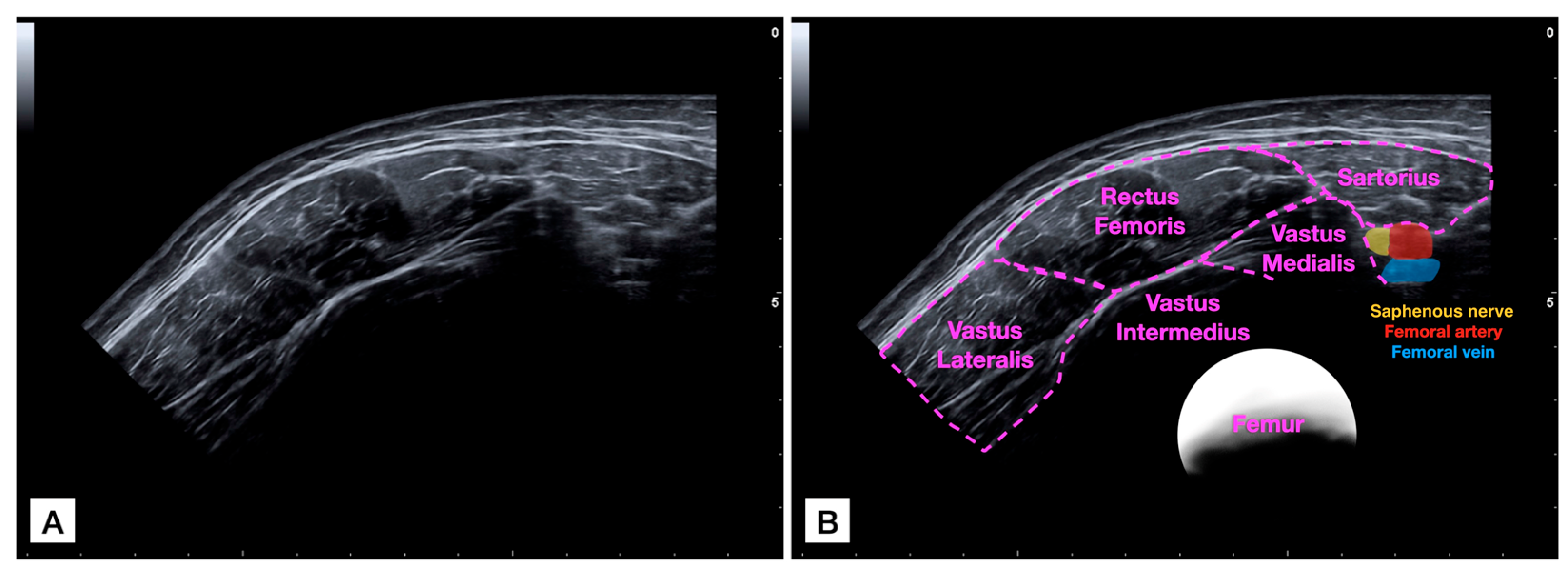
2.4. Imaging Analysis
- -
- Cross-sectional Area: This refers to the two-dimensional size of the muscle, quantified in cm2.
- -
- Perimeter: Measured in cm, this is the total length of the contour of the muscle.
- -
- Circularity: Determined using the formula 4π × Area/Perimeter2. A value of 1 in this metric signifies a perfect circle, indicating how close the muscle’s shape is to being circular.
- -
- Aspect Ratio (AR): This is the quotient obtained by dividing the major axis (longest dimension) by the minor axis (shortest dimension) of the muscle’s shape.
- -
- Roundness: This parameter is calculated using the formula 4 × Area/(π × major axis2), essentially being the inverse of the aspect ratio. It gauges how close the shape of the muscle is to a perfect circle.
- -
- Solidity: This is a geometric measurement used to quantify the “fullness” of an object’s shape. It is calculated as the ratio of the area of the object to the area of its convex hull. The convex hull can be thought of as the smallest convex shape that completely encloses the object. A higher solidity value indicates that the object is closer to being completely convex, with fewer indentations or concavities. For example, a perfectly convex shape such as a circle has a solidity of 1, while shapes with indentations or irregular edges have lower solidity values.
- -
- Mean Echo Intensity: A measure of the average brightness of the selected pixels, ranging on a scale from 0 (darkest) to 255 (brightest), indicating the relative brightness within the muscle image.
2.5. Statistical Analysis
3. Results
4. Discussion
Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whittaker, J.L.; Ellis, R.; Hodges, P.W.; OSullivan, C.; Hides, J.; Fernandez-Carnero, S.; Arias-Buria, J.L.; Teyhen, D.S.; Stokes, M.J. Imaging with ultrasound in physical therapy: What is the PT’s scope of practice? A competency-based educational model and training recommendations. Br. J. Sport. Med. 2019, 53, 1447–1453. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Fernández-de-Las-Peñas, C.; Varol, U.; Ortega-Santiago, R.; Gallego-Sendarrubias, G.M.; Arias-Buría, J.L. Ultrasound Imaging as a Visual Biofeedback Tool in Rehabilitation: An Updated Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7554. [Google Scholar] [CrossRef] [PubMed]
- So, S.; Patel, R.M.; Orebaugh, S.L. Ultrasound imaging in medical student education: Impact on learning anatomy and physical diagnosis. Anat. Sci. Educ. 2017, 10, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Navarro-Santana, M.J.; Fernández-de-Las-Peñas, C.; Varol, U.; López-de-Uralde-Villanueva, I.; Rodríguez-López, E.S.; Plaza-Manzano, G. Inclusion of cross-sectional and radiological images for better understanding of musculoskeletal anatomy and decreasing the risk of adverse events during dry needling in undergraduate physiotherapy students. Anat. Sci. Educ. 2023, 16, 521–530. [Google Scholar] [CrossRef]
- Fitze, D.P.; Franchi, M.V.; Peterhans, L.; Frey, W.O.; Spörri, J. Reliability of panoramic ultrasound imaging and agreement with magnetic resonance imaging for the assessment of lumbar multifidus anatomical cross-sectional area. Sci. Rep. 2023, 13, 19647. [Google Scholar] [CrossRef]
- Fukumoto, Y.; Ikezoe, T.; Yamada, Y.; Tsukagoshi, R.; Nakamura, M.; Mori, N.; Kimura, M.; Ichihashi, N. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur. J. Appl. Physiol. 2012, 112, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Stock, M.S.; Thompson, B.J. Echo intensity as an indicator of skeletal muscle quality: Applications, methodology, and future directions. Eur. J. Appl. Physiol. 2021, 121, 369–380. [Google Scholar] [CrossRef]
- Tanaka, N.I.; Ogawa, M.; Yoshiko, A.; Ando, R.; Akima, H. Reliability of size and echo intensity of abdominal skeletal muscles using extended field-of-view ultrasound imaging. Eur. J. Appl. Physiol. 2017, 117, 2263–2270. [Google Scholar] [CrossRef]
- Kadakia, A.; Zhang, J.; Yao, X.; Zhou, Q.; Heiferman, M.J. Ultrasound in ocular oncology: Technical advances, clinical applications, and limitations. Exp. Biol. Med. 2023, 248, 371–379. [Google Scholar] [CrossRef]
- Zhang, J.; Murgoitio-Esandi, J.; Qian, X.; Li, R.; Gong, C.; Nankali, A.; Zhou, Q. High-Frequency Ultrasound Elastography to Assess the Nonlinear Elastic Properties of the Cornea and Ciliary Body. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2022, 69, 2621–2629. [Google Scholar] [CrossRef]
- Cleary, C.J.; Nabavizadeh, O.; Young, K.L.; Herda, A.A. Skeletal muscle analysis of panoramic ultrasound is reliable across multiple raters. PLoS ONE 2022, 17, e0267641. [Google Scholar] [CrossRef] [PubMed]
- Starkoff, B. Ultrasound physical principles in today’s technology. Australas. J. Ultrasound Med. 2014, 17, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.C.; Lin, J.H.; Xu, J.J.; Guo, Q.; Wang, Y.H.; Jiang, C.; Lu, H.G.; Wu, Y.S. An assessment of morphological and pathological changes in paravertebral muscle degeneration using imaging and histological analysis: A cross-sectional study. BMC Musculoskelet. Disord. 2021, 22, 854. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Martin, D.S.; Ploutz-Snyder, R.; Matz, T.; Caine, T.; Downs, M.; Hackney, K.; Buxton, R.; Ryder, J.W.; Ploutz-Snyder, L. Panoramic ultrasound: A novel and valid tool for monitoring change in muscle mass. J. Cachexia Sarcopenia Muscle 2017, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Valera-Calero, J.A.; Ojedo-Martín, C.; Fernández-de-Las-Peñas, C.; Cleland, J.A.; Arias-Buría, J.L.; Hervás-Pérez, J.P. Reliability and Validity of Panoramic Ultrasound Imaging for Evaluating Muscular Quality and Morphology: A Systematic Review. Ultrasound Med. Biol. 2021, 47, 185–200. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Plaza-Manzano, G.; Ortega-Santiago, R.; Fernández-de-las-Peñas, C.; Varol, U. Panoramic ultrasound imaging does not produce muscle morphology deformation during imaging acquisition: A validity study. Phys. Med. 2023, 106, 102530. [Google Scholar] [CrossRef] [PubMed]
- Sahinis, C.; Kellis, E.; Galanis, N.; Dafkou, K.; Ellinoudis, A. Intra- and inter-muscular differences in the cross-sectional area of the quadriceps muscles assessed by extended field-of-view ultrasonography. Med. Ultrason. 2020, 22, 152–158. [Google Scholar] [CrossRef]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054–1060. [Google Scholar] [CrossRef]
- Ozturk, Y.; Koca, M.; Burkuk, S.; Unsal, P.; Dikmeer, A.; Oytun, M.G.; Bas, A.O.; Kahyaoglu, Z.; Deniz, O.; Coteli, S.; et al. The role of muscle ultrasound to predict sarcopenia. Nutrition 2022, 101, 111692. [Google Scholar] [CrossRef]
- Pons, C.; Borotikar, B.; Garetier, M.; Burdin, V.; Ben Salem, D.; Lempereur, M.; Brochard, S. Quantifying skeletal muscle volume and shape in humans using MRI: A systematic review of validity and reliability. PLoS ONE 2018, 13, e0207847. [Google Scholar] [CrossRef]
- Simera, I.; Moher, D.; Hoey, J.; Schulz, K.F.; Altman, D.G. A catalogue of reporting guidelines for health research. Eur. J. Clin. Investig. 2010, 40, 35–53. [Google Scholar] [CrossRef]
- Kottner, J.; Audige, L.; Brorson, S.; Donner, A.; Gajewski, B.J.; Hróbjartsson, A.; Roberts, C.; Shoukri, M.; Streiner, D.L. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. Int. J. Nurs. Stud. 2011, 48, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.D.; Eliasziw, M.; Donner, A. Sample size and optimal designs for reliability studies. Stat. Med. 1998, 17, 101–110. [Google Scholar] [CrossRef]
- Ando, R.; Nosaka, K.; Inami, T.; Tomita, A.; Watanabe, K.; Blazevich, A.J.; Akima, H. Difference in fascicle behaviors between superficial and deep quadriceps muscles during isometric contractions. Muscle Nerve 2016, 53, 797–802. [Google Scholar] [CrossRef]
- Burton, A.M.; Stock, M.S. Consistency of novel ultrasound equations for estimating percent intramuscular fat. Clin. Physiol. Funct. Imaging 2018, 38, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Furlan, L.; Sterr, A. The Applicability of Standard Error of Measurement and Minimal Detectable Change to Motor Learning Research-A Behavioral Study. Front. Hum. Neurosci. 2018, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Adkins, A.N.; Murray, W.M. Obtaining Quality Extended Field-of-View Ultrasound Images of Skeletal Muscle to Measure Muscle Fascicle Length. J. Vis. Exp. 2020, 166, e61765. [Google Scholar] [CrossRef]
- Rosenberg, J.G.; Ryan, E.D.; Sobolewski, E.J.; Scharville, M.J.; Thompson, B.J.; King, G.E. Reliability of panoramic ultrasound imaging to simultaneously examine muscle size and quality of the medial gastrocnemius. Muscle Nerve 2014, 49, 736–740. [Google Scholar] [CrossRef]
- Jenkins, N.D.; Miller, J.M.; Buckner, S.L.; Cochrane, K.C.; Bergstrom, H.C.; Hill, E.C.; Smith, C.M.; Housh, T.J.; Cramer, J.T. Test-Retest Reliability of Single Transverse versus Panoramic Ultrasound Imaging for Muscle Size and Echo Intensity of the Biceps Brachii. Ultrasound Med. Biol. 2015, 41, 1584–1591. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Gallego-Sendarrubias, G.M.; Fernández-de-Las-Peñas, C.; Cleland, J.A.; Ortega-Santiago, R.; Arias-Buría, J.L. Panoramic Ultrasound Examination of Posterior Neck Extensors in Healthy Subjects: Intra-Examiner Reliability Study. Diagnostics 2020, 10, 740. [Google Scholar] [CrossRef]
- Kennedy, V.L.; Flavell, C.A.; Doma, K. Intra-rater reliability of transversus abdominis measurement by a novice examiner: Comparison of “freehand” to “probe force device” method of real-time ultrasound imaging. Ultrasound 2019, 27, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, S.J.; de Zoete, R.M.J.; Croker, C.; Yerrapothu, M.; Elliott, J.M. Reliability of cervical muscle volume quantification using magnetic resonance imaging. Musculoskelet. Sci. Pract. 2019, 44, 102056. [Google Scholar] [CrossRef]
- Kuzu, Ö.; Aras, B. Sonographic measurement of the neck extensor muscle thickness in patients with fibromyalgia. Musculoskelet. Sci. Pract. 2022, 59, 102541. [Google Scholar] [CrossRef] [PubMed]
- Pedler, A.; McMahon, K.; Galloway, G.; Durbridge, G.; Sterling, M. Intramuscular fat is present in cervical multifidus but not soleus in patients with chronic whiplash associated disorders. PLoS ONE 2018, 13, e0197438. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.; Albin, S.R.; Abbott, R.; Crawford, R.J.; Hoggarth, M.A.; Wasielewski, M.; Elliott, J.M. Confirming the geography of fatty infiltration in the deep cervical extensor muscles in whiplash recovery. Sci. Rep. 2020, 10, 11471. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.M.; Roberts, N.; Whitehouse, G.H. Measurement of the quadriceps femoris muscle using magnetic resonance and ultrasound imaging. Br. J. Sport. Med. 1997, 31, 59–64. [Google Scholar] [CrossRef]
- Ishida, H.; Suehiro, T.; Suzuki, K.; Yoneda, T.; Watanabe, S. Influence of the ultrasound transducer tilt on muscle thickness and echo intensity of the rectus femoris muscle of healthy subjects. J. Phys. Ther. Sci. 2017, 29, 2190–2193. [Google Scholar] [CrossRef]
- Ishida, H.; Suehiro, T.; Watanabe, S. Influence of Inward Pressure of the Transducer on Thickness and Echo Intensity of the Rectus Femoris Muscle During Ultrasonography. Middle East J. Rehabil. Health Stud. 2016, 3, e36059. [Google Scholar] [CrossRef]
- Carr, J.C.; Gerstner, G.R.; Voskuil, C.C.; Harden, J.E.; Dunnick, D.; Badillo, K.M.; Pagan, J.I.; Harmon, K.K.; Girts, R.M.; Beausejour, J.P.; et al. The Influence of Sonographer Experience on Skeletal Muscle Image Acquisition and Analysis. J. Funct. Morphol. Kinesiol. 2021, 6, 91. [Google Scholar] [CrossRef]
- Cavaggion, C.; Navarro-Ledesma, S.; Luque-Suarez, A.; Juul-Kristensen, B.; Voogt, L.; Struyf, F. Subacromial space measured by ultrasound imaging in asymptomatic subjects and patients with subacromial shoulder pain: An inter-rater reliability study. Physiother. Theory Pract. 2023, 39, 2196–2207. [Google Scholar] [CrossRef]
- Fortin, M.; Rosenstein, B.; Levesque, J.; Nandlall, N. Ultrasound Imaging Analysis of the Lumbar Multifidus Muscle Echo Intensity: Intra-Rater and Inter-Rater Reliability of a Novice and an Experienced Rater. Medicina 2021, 57, 512. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Bonfiglio, M.; Castorina, E.G.; Antoci, S.A.M. The primacy of ultrasound in the assessment of muscle architecture: Precision, accuracy, reliability of ultrasonography. Physiatrist, radiologist, general internist, and family practitioner’s experiences. Rev. Assoc. Med. Bras. 2019, 65, 165–170. [Google Scholar] [CrossRef] [PubMed]
| Baseline | Sample (n = 39) | Males (n = 27) | Females (n = 12) | Between-Sex Differences |
|---|---|---|---|---|
| Age (years) | 25.1 ± 9.1 | 27.4 ± 10.3 | 23.2 ± 6.7 | 4.9 (2.6;7.2) p < 0.001 |
| Height (m) | 1.74 ± 0.09 | 1.78 ± 0.08 | 1.66 ± 0.06 | 0.11 (0.01;0.22) p < 0.001 |
| Weight (kg) | 71.9 ± 14.3 | 78.2 ± 12.5 | 57.8 ± 4.0 | 20.4 (6.0;34.8) p < 0.001 |
| Body Mass Index (kg/m2) | 23.3 ± 2.6 | 24.4 ± 2.2 | 20.7 ± 1.2 | 3.6 (1.0;6.3) p < 0.001 |
| Thigh length (cm) | 47.7 ± 2.5 | 48.3 ± 2.3 | 45.7 ± 2.8 | 2.6 (−0.6;5.7) p = 0.101 |
| Thigh girdle (cm) | 57.1 ± 5.3 | 58.6 ± 5.5 | 53.7 ± 2.1 | 4.8 (−1.4;11.2) p = 0.002 |
| Baseline | Mean | Experienced Examiner | Novel Examiner | Absolute Error | ICC2,2 (95% CI) | SEM | MDC | CAE (%) |
|---|---|---|---|---|---|---|---|---|
| Muscle Size | ||||||||
| Area (cm2) * | 10.54 ± 2.61 | 10.52 ± 2.64 | 10.57 ± 2.64 | 0.33 ± 0.26 | 0.993 (0.985;0.997) | 0.02 | 0.06 | 2.46 |
| Perimeter (cm) * | 14.23 ± 2.45 | 14.26 ± 2.49 | 14.19 ± 2.45 | 0.23 ± 0.21 | 0.996 (0.991;0.998) | 0.01 | 0.03 | 1.47 |
| Muscle Shape | ||||||||
| Circularity (0–1) * | 0.62 ± 0.06 | 0.63 ± 0.06 | 0.62 ± 0.05 | 0.02 ± 0.02 | 0.944 (0.874;0.975) | 0.00 | 0.01 | 3.22 |
| Aspect Ratio * | 2.90 ± 0.46 | 2.91 ± 0.48 | 2.89 ± 0.44 | 0.13 ± 0.14 | 0.951 (0.891;0.978) | 0.03 | 0.08 | 4.82 |
| Roundness * | 0.35 ± 0.06 | 0.35 ± 0.06 | 0.35 ± 0.05 | 0.02 ± 0.02 | 0.952 (0.893;0.979) | 0.00 | 0.01 | 5.71 |
| Solidity (0–1) * | 0.98 ± 0.01 | 0.99 ± 0.01 | 0.98 ± 0.01 | 0.01 ± 0.01 | 0.869 (0.709;0.941) | 0.00 | 0.01 | 1.02 |
| Muscle Brightness | ||||||||
| Mean Echo-intensity (0–255) * | 59.1 ± 15.6 | 58.3 ± 15.3 | 59.7 ± 16.1 | 2.82 ± 3.63 | 0.980 (0.956;0.991) | 0.51 | 1.42 | 6.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buffet-García, J.; Plaza-Manzano, G.; Varol, U.; Ríos-León, M.; Díaz-Arribas, M.J.; Álvarez-González, J.; Sánchez-Jorge, S.; Valera-Calero, J.A. Reliability of Panoramic Ultrasound in Assessing Rectus Femoris Size, Shape, and Brightness: An Inter-Examiner Study. Bioengineering 2024, 11, 82. https://doi.org/10.3390/bioengineering11010082
Buffet-García J, Plaza-Manzano G, Varol U, Ríos-León M, Díaz-Arribas MJ, Álvarez-González J, Sánchez-Jorge S, Valera-Calero JA. Reliability of Panoramic Ultrasound in Assessing Rectus Femoris Size, Shape, and Brightness: An Inter-Examiner Study. Bioengineering. 2024; 11(1):82. https://doi.org/10.3390/bioengineering11010082
Chicago/Turabian StyleBuffet-García, Jorge, Gustavo Plaza-Manzano, Umut Varol, Marta Ríos-León, María José Díaz-Arribas, Javier Álvarez-González, Sandra Sánchez-Jorge, and Juan Antonio Valera-Calero. 2024. "Reliability of Panoramic Ultrasound in Assessing Rectus Femoris Size, Shape, and Brightness: An Inter-Examiner Study" Bioengineering 11, no. 1: 82. https://doi.org/10.3390/bioengineering11010082
APA StyleBuffet-García, J., Plaza-Manzano, G., Varol, U., Ríos-León, M., Díaz-Arribas, M. J., Álvarez-González, J., Sánchez-Jorge, S., & Valera-Calero, J. A. (2024). Reliability of Panoramic Ultrasound in Assessing Rectus Femoris Size, Shape, and Brightness: An Inter-Examiner Study. Bioengineering, 11(1), 82. https://doi.org/10.3390/bioengineering11010082






