A Review on Bioflocculant-Synthesized Copper Nanoparticles: Characterization and Application in Wastewater Treatment
Abstract
:1. Introduction
2. Different Methods for CuNP Synthesis
2.1. Chemical Approaches
2.2. Physical Methods
2.3. Biological Methods for CuNP Synthesis
2.3.1. Green Synthesis of Copper Nanoparticles Using Plants

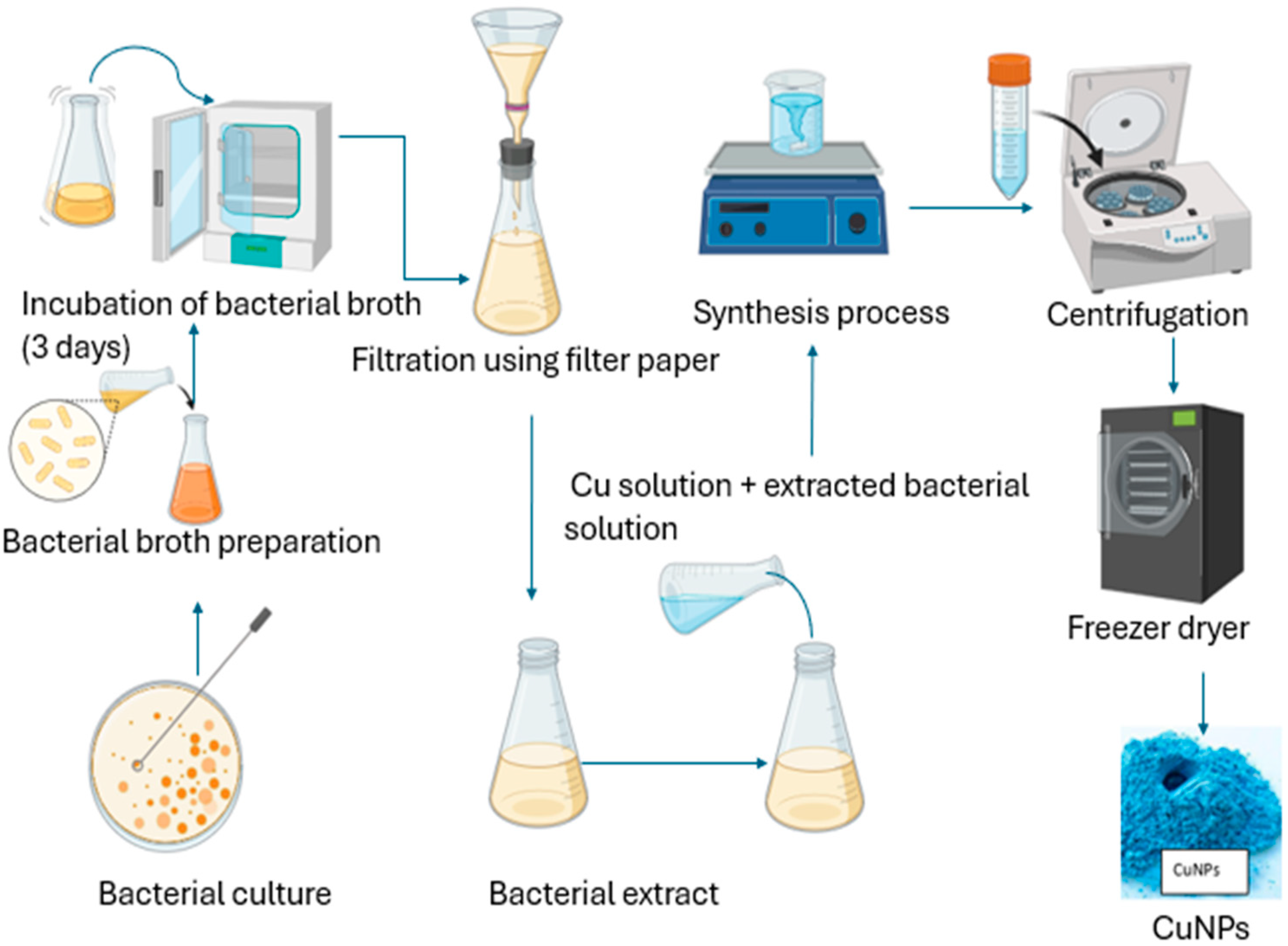
2.3.2. Green Synthesis of Copper NPs Using Bacteria
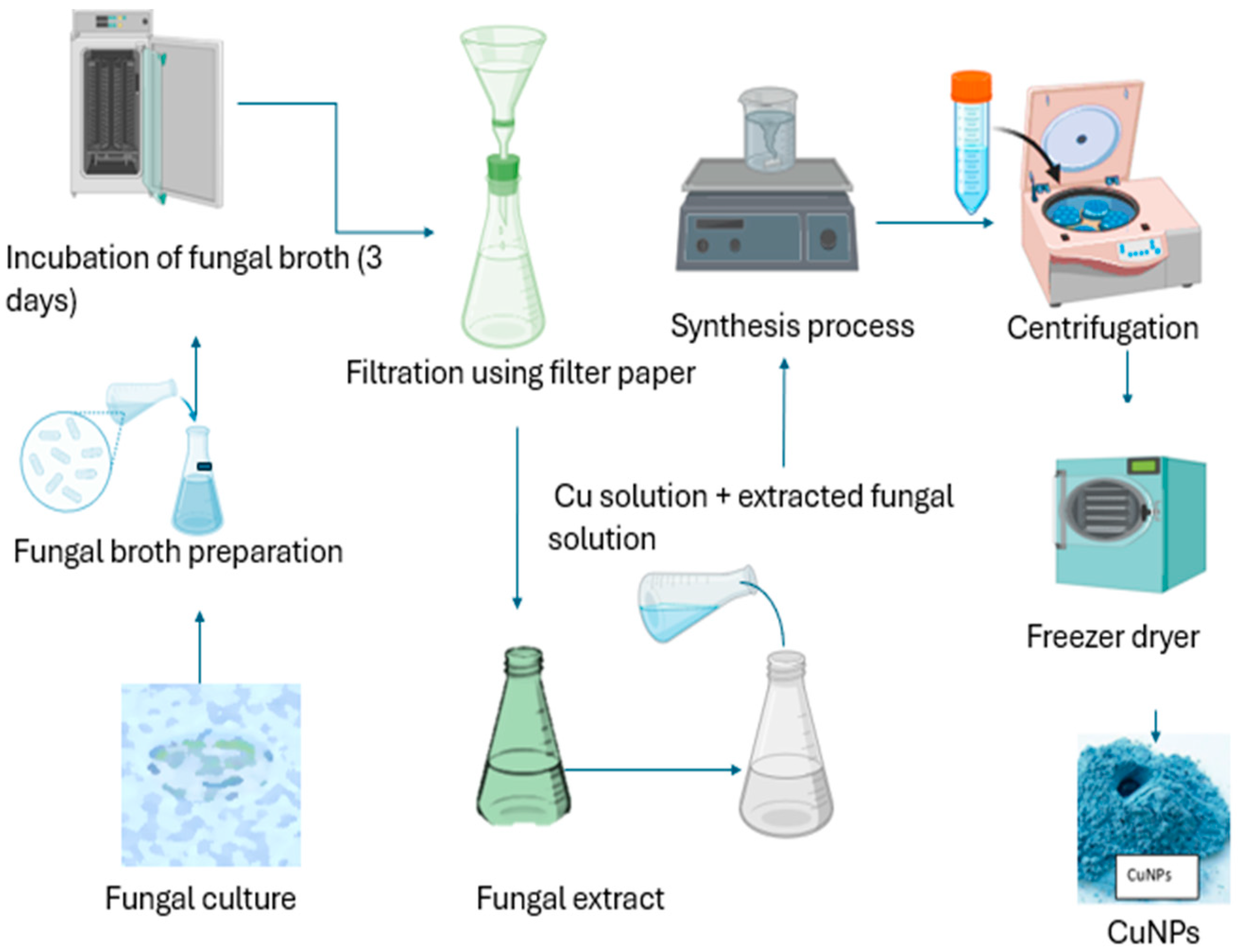
2.3.3. Green Synthesis of Copper Nanoparticles Using Fungi
2.3.4. Green Synthesis of Copper Nanoparticles Using Algae
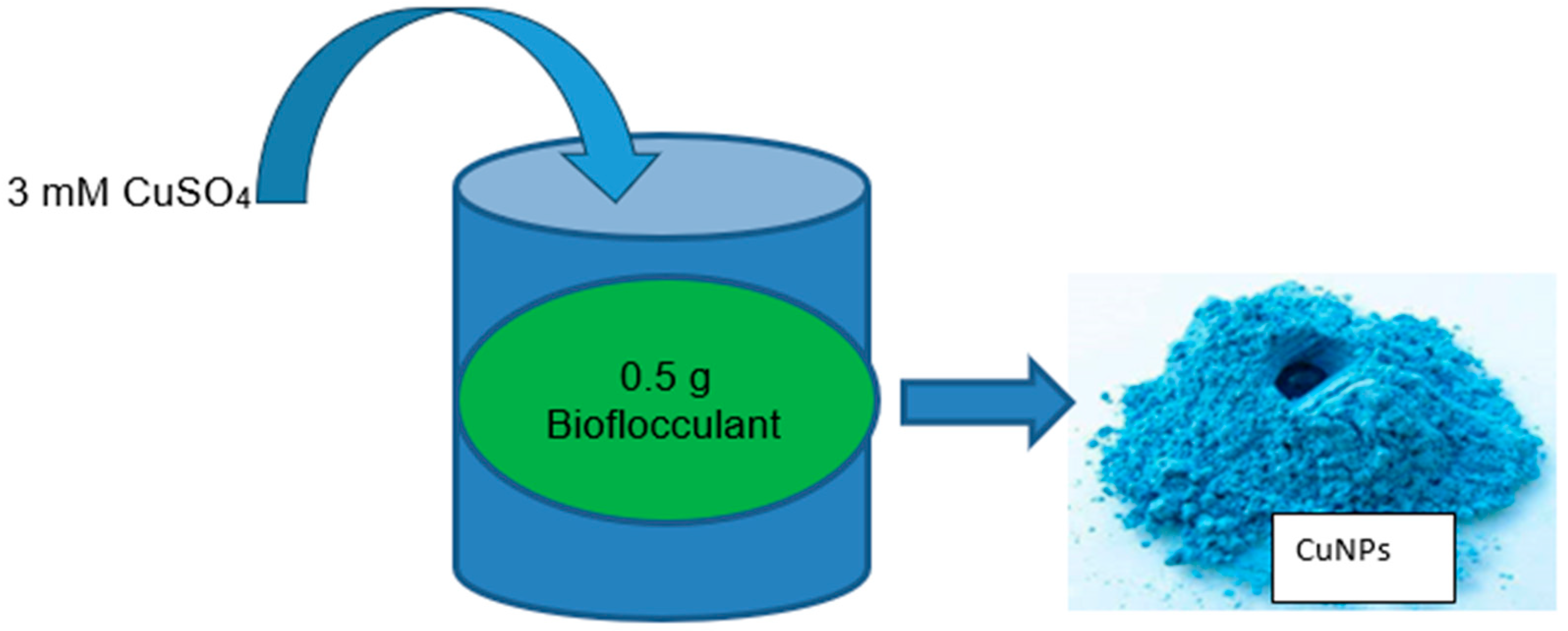
2.3.5. Green Synthesis of Copper Nanoparticles Using Microbial Bioflocculant
3. Factors Affecting CuNP Synthesis
3.1. Effect of Precursor Concentration
3.2. Effect of pH
3.3. Effect of Temperature
3.4. Effect of Reaction Time on Copper Nanoparticle Production
4. The Characterization of Nanoparticles
4.1. X-ray Diffraction (XRD) Analysis
4.2. Transmission Electron Microscopy (TEM) Analysis
4.3. Scanning Electron Microscope (SEM) Analysis
4.4. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
4.5. UV–Vis Spectroscopy Analysis
5. Comparison between the Antibacterial Activity of Copper and Silver Nanoparticles
6. Antimicrobial Activity of Cu Nanoparticles
7. Application of Copper Nanoparticles
7.1. Application of CuNPs for Wastewater Treatment
7.1.1. The Removal of Pollutants by CuNPs Synthesized Using Microbial Bioflocculant and the Greener Method
7.1.2. Heavy Metal Reduction from Water
7.1.3. Removal of Dyes
8. Mechanisms for Wastewater Purification from Heavy Metals and Dyes Using Copper Nanoparticles
9. Toxicity of Copper Nanoparticles
9.1. Toxicity of CuNPs in Animals
9.2. Toxicity of CuNPs in Humans
9.3. Toxicity Mechanisms
10. Methods for Reducing the Toxic Effect of Cu Nanoparticles on the Human Body
10.1. Surface Modification Techniques
10.2. Functionalization with Ligands or Biomolecules
10.3. Controlled Release of Copper Ions
10.4. Targeted Delivery Systems
11. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lichtfouse, E.; Morin-Crini, N.; Fourmentin, M.; Zemmouri, H.; do Carmo Nascimento, I.O.; Queiroz, L.M.; Tadza, M.Y.M.; Picos-Corrales, L.A.; Pei, H.; Wilson, L.D. Chitosan for direct bioflocculation of wastewater. Environ. Chem. Lett. 2019, 17, 1603–1621. [Google Scholar] [CrossRef]
- Peng, Y.; Xiao, J.; Deng, B.; Wang, Z.; Liu, N.; Yang, D.; Ding, W.; Chen, T.; Wu, Q. Study on separation of fine-particle ilmenite and mechanism using flocculation flotation with sodium oleate and polyacrylamide. Physicochem. Probl. Miner. Process. 2020, 56, 162–173. [Google Scholar]
- Pereira, A.G.; Rodrigues, F.H.; Paulino, A.T.; Martins, A.F.; Fajardo, A.R. Recent advances on composite hydrogels designed for the remediation of dye-contaminated water and wastewater: A review. J. Clean. Prod. 2021, 284, 124703. [Google Scholar] [CrossRef]
- Yudaev, P.; Semenova, A.; Chistyakov, E. Gel based on modified chitosan for oil spill cleanup. J. Appl. Polym. Sci. 2024, 141, e54838. [Google Scholar] [CrossRef]
- Zaarour, B.; Liu, W. Recent advances of textile sorbents for oil spills cleanup: A review. J. Ind. Text. 2023, 53, 15280837231186652. [Google Scholar] [CrossRef]
- Suganya, K.; Swathi, K.; Sudha, B.; Sumathi, S. Microbial Flocculants as an Alternate to Chemical Flocculants for Wastewater Treatment. Microb. Flocculants Altern. Chem. Flocculants Wastewater Treat. 2021, 81–99. [Google Scholar]
- Mnif, W.; Ben Rebah, F. Bioflocculants as Alternative to Synthetic Polymers to Enhance Wastewater Sludge Dewaterability: A Review. Energies 2023, 16, 3392. [Google Scholar] [CrossRef]
- Nkosi, N.C.; Basson, A.K.; Ntombela, Z.G.; Maliehe, T.S.; Pullabhotla, R.V. Isolation, identification and characterization of bioflocculant-producing bacteria from activated sludge of Vulindlela Wastewater Treatment Plant. Appl. Microbiol. 2021, 1, 586–606. [Google Scholar] [CrossRef]
- Selepe, T.N.; Maliehe, T.S.; Moganedi, K.; Masoko, P.; Mulaudzi, V. Isolation and Optimisation of Culture Conditions for a Marine Bioflocculant-Producing Bacterium and Application of Its Bioflocculant in Wastewater Treatment. Int. J. Environ. Res. Public Health 2022, 19, 10237. [Google Scholar] [CrossRef]
- Das, N.; Ojha, N.; Mandal, S.K. Wastewater treatment using plant-derived bioflocculants: Green chemistry approach for safe environment. Water Sci. Technol. 2021, 83, 1797–1812. [Google Scholar] [CrossRef]
- Nkosi, N.C.; Basson, A.K.; Ntombela, Z.G.; Dlamini, N.G.; Maliehe, T.S.; Pullabhotla, R.V. Production and characterization of a bioflocculant produced by Proteus mirabilis AB 932526.1 and its application in wastewater treatment and dye removal. Pure Appl. Chem. 2023, 95, 169–180. [Google Scholar] [CrossRef]
- Ngema, S.S.; Basson, A.K.; Maliehe, T.S. Synthesis, characterization and application of polyacrylamide grafted bioflocculant. Phys. Chem. Earth 2020, 115, 102821. [Google Scholar] [CrossRef]
- Maliehe, T.S.; Basson, A.K.; Dlamini, N.G. Removal of pollutants in mine wastewater by a non-cytotoxic polymeric bioflocculant from Alcaligenes faecalis HCB2. Int. J. Environ. Res. Public Health 2019, 16, 4001. [Google Scholar] [CrossRef]
- Heydaryan, K.; Mohammadalizadeh, M.; Montazer, A.H.; Kashi, M.A. Reaction time-induced improvement in hyperthermia properties of cobalt ferrite nanoparticles with different sizes. Mater. Chem. Phys. 2023, 303, 127773. [Google Scholar] [CrossRef]
- Mariappan, N. Recent trends in nanotechnology applications in surgical specialties and orthopedic surgery. Biomed. Pharmacol. J. 2019, 12, 1095–1127. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, M.; Kumar, A. Copper-based nanoparticles in the soil-plant environment: Assessing their applications, interactions, fate and toxicity. Chemosphere 2021, 281, 130940. [Google Scholar] [CrossRef] [PubMed]
- Lashari, A.; Hassan, S.M.; Mughal, S.S. Biosynthesis, Characterization and Biological Applications of BaO Nanoparticles using Linum usitatissimum. Am. J. Appl. Sci. Res. 2022, 8, 58–68. [Google Scholar]
- Jiang, Z.; Li, L.; Huang, H.; He, W.; Ming, W. Progress in Laser Ablation and Biological Synthesis Processes: “Top-Down” and “Bottom-Up” Approaches for the Green Synthesis of Au/Ag Nanoparticles. Int. J. Mol. Sci. 2022, 23, 14658. [Google Scholar] [CrossRef]
- Mughal, S.S.; Hassan, S.M. Comparative study of AgO nanoparticles synthesize via biological, chemical and physical methods: A review. Am. J. Mater. Synth. Process. 2022, 7, 15–28. [Google Scholar]
- Noah, N.M.; Ndangili, P.M. Green synthesis of nanomaterials from sustainable materials for biosensors and drug delivery. Sens. Int. 2022, 3, 100166. [Google Scholar] [CrossRef]
- Jandt, K.D.; Watts, D.C. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Usmani, Z.; Atanasov, A.G.; Singh, V.K.; Singh, N.P.; Abdel-Azeem, A.M.; Prasad, R.; Gupta, G.; Sharma, M.; Bhargava, A. Biological nanofactories: Using living forms for metal nanoparticle synthesis. Mini Rev. Med. Chem. 2021, 21, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, D.; Sasmal, S. A review of green synthesis of metal nanoparticles using algae. Front. Microbiol. 2021, 12, 693899. [Google Scholar] [CrossRef] [PubMed]
- Priya; Naveen; Kaur, K.; Sidhu, A.K. Green synthesis: An eco-friendly route for the synthesis of iron oxide nanoparticles. Front. Nanotechnol. 2021, 3, 655062. [Google Scholar]
- Kumar, B. Green synthesis of gold, silver, and iron nanoparticles for the degradation of organic pollutants in wastewater. J. Compos. Sci. 2021, 5, 219. [Google Scholar] [CrossRef]
- Al-Hakkani, M.F. Biogenic copper nanoparticles and their applications: A review. SN Appl. Sci. 2020, 2, 505. [Google Scholar] [CrossRef]
- Perveen, S.; Zafar, S.; Iqbal, N. Applications of bionanocomposites in agriculture. In Bionanocomposites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 485–504. [Google Scholar]
- Harishchandra, B.D.; Pappuswamy, M.; Antony, P.; Shama, G.; Pragatheesh, A.; Arumugam, V.A.; Periyaswamy, T.; Sundaram, R. Copper nanoparticles: A review on synthesis, characterization and applications. Asian Pac. J. Cancer Biol. 2020, 5, 201–210. [Google Scholar] [CrossRef]
- Manjula, N.; Sarma, G.; Shilpa, B.M.; Suresh Kumar, K. Environmental applications of green engineered copper nanoparticles. In Phytonanotechnology; Springer: Singapore, 2022; pp. 255–276. [Google Scholar]
- Hassanien, R.; Husein, D.Z.; Al-Hakkani, M.F. Biosynthesis of copper nanoparticles using aqueous Tilia extract: Antimicrobial and anticancer activities. Heliyon 2018, 4, e01077. [Google Scholar] [CrossRef]
- Padma, P.N.; Banu, S.T.; Kumari, S.C. Studies on green synthesis of copper nanoparticles using Punica granatum. Annu. Res. Rev. Biol. 2018, 23, 1–10. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Agarwal, H.; Menon, S.; Rajeshkumar, S.; Kumar, S.V. A biological synthesis of copper nanoparticles and its potential applications. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 199–221. [Google Scholar]
- Mali, S.C.; Dhaka, A.; Githala, C.K.; Trivedi, R. Green synthesis of copper nanoparticles using Celastrus paniculatus Willd. leaf extract and their photocatalytic and antifungal properties. Biotechnol. Rep. 2020, 27, e00518. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Rajeshkumar, S.; Madasamy, M.; Mahendran, V. Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1153–1158. [Google Scholar] [CrossRef]
- Varghese, B.; Kurian, M.; Krishna, S.; Athira, T. Biochemical synthesis of copper nanoparticles using Zingiber officinalis and Curcuma longa: Characterization and antibacterial activity study. Mater. Today Proc. 2020, 25, 302–306. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Muniraj, S. Neem flower extract assisted green synthesis of copper nanoparticles–optimisation, characterisation and anti-bacterial study. Mater. Today Proc. 2021, 36, 832–836. [Google Scholar] [CrossRef]
- Sarwar, N.; Humayoun, U.B.; Kumar, M.; Zaidi, S.F.A.; Yoo, J.H.; Ali, N.; Jeong, D.I.; Lee, J.H.; Yoon, D.H. Citric acid mediated green synthesis of copper nanoparticles using cinnamon bark extract and its multifaceted applications. J. Clean. Prod. 2021, 292, 125974. [Google Scholar] [CrossRef]
- Abbas, A.H.; Fairouz, N.Y. Characterization, biosynthesis of copper nanoparticles using ginger roots extract and investigation of its antibacterial activity. Mater. Today Proc. 2022, 61, 908–913. [Google Scholar] [CrossRef]
- Chompunut, L.; Wanaporn, T.; Anupong, W.; Narayanan, M.; Alshiekheid, M.; Sabour, A.; Karuppusamy, I.; Chi, N.T.L.; Shanmuganathan, R. Synthesis of copper nanoparticles from the aqueous extract of Cynodon dactylon and evaluation of its antimicrobial and photocatalytic properties. Food Chem. Toxicol. 2022, 166, 113245. [Google Scholar] [CrossRef]
- Shubhashree, K.; Reddy, R.; Gangula, A.K.; Nagananda, G.; Badiya, P.K.; Ramamurthy, S.S.; Aramwit, P.; Reddy, N. Green synthesis of copper nanoparticles using aqueous extracts from Hyptis suaveolens (L.). Mater. Chem. Phys. 2022, 280, 125795. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, R.; Devi, A.; Thakur, A.; Giri, S.K.; Nagar, S.; Singh, G. Green synthesis of copper nanoparticles and their evaluation for antimicrobial activity and bio-compatibility. Mater. Today Proc. 2023, 70, 154–158. [Google Scholar] [CrossRef]
- Kumar, M.; Kaushik, D.; Kumar, A.; Gupta, P.; Proestos, C.; Oz, E.; Orhan, E.; Kaur, J.; Khan, M.R.; Elobeid, T. Green synthesis of copper nanoparticles from Nigella sativa seed extract and evaluation of their antibacterial and antiobesity activity. Int. J. Food Sci. Technol. 2023, 58, 2883–2892. [Google Scholar] [CrossRef]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, R.V. Wastewater treatment by a polymeric bioflocculant and iron nanoparticles synthesized from a bioflocculant. Polymers 2020, 12, 1618. [Google Scholar] [CrossRef] [PubMed]
- Adebayo-Tayo, B.C.; Adeleke, R.O.; Adekanmbi, A.O. Biogenic Silver and Magnetic Nanoparticles Using Bacillus subtilis B2 Bioflocculants; Production, Properties and Antibacterial Potential in Dairy Wastewater Treatment. Chem. Afr. 2022, 5, 1547–1561. [Google Scholar] [CrossRef]
- Ntombela, Z.G.; Pullabhotla, V.S.R.; Basson, A.K. Biosafety, Optimization, and Application of Bioflocculant-Synthesized Zinc Oxide Nanoparticles. BioNanoScience 2022, 12, 1289–1304. [Google Scholar] [CrossRef]
- Tsilo, P.H.; Basson, A.K.; Ntombela, Z.G.; Dlamini, N.G.; Pullabhotla, R.V. Biosynthesis and Characterization of Copper Nanoparticles Using a Bioflocculant Produced by a Yeast Pichia kudriavzevii Isolated from Kombucha Tea SCOBY. Appl. Nano 2023, 4, 226–239. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.; Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. [Google Scholar]
- Alegria, E.C.; Ribeiro, A.P.; Mendes, M.; Ferraria, A.M.; Rego, A.M.B.d.; Pombeiro, A.J. Effect of phenolic compounds on the synthesis of gold nanoparticles and its catalytic activity in the reduction of nitro compounds. Nanomaterials 2018, 8, 320. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Vu, Q.H.A.; Pham, T.N.N.; Trinh, K.S. Antibacterial filtration using polyethylene terephthalate filters coated with copper nanoparticles. J. Nanomater. 2021, 1–12. [Google Scholar] [CrossRef]
- Alonso, F.; Arroyo, A.; Martín-García, I.; Moglie, Y. Cross-Dehydrogenative Coupling of Tertiary Amines and Terminal Alkynes Catalyzed by Copper Nanoparticles on Zeolite. Adv. Synth. Catal. 2015, 357, 3549–3561. [Google Scholar] [CrossRef]
- Sakono, N.; Ishida, Y.; Ogo, K.; Tsumori, N.; Murayama, H.; Sakono, M. Molar-Fraction-Tunable Synthesis of Ag–Au Alloy Nanoparticles via a Dual Evaporation–Condensation Method as Supported Catalysts for CO Oxidation. ACS Appl. Nano Mater. 2023, 6, 3065–3074. [Google Scholar] [CrossRef]
- Sadeghi-Aghbash, M.; Rahimnejad, M. Zinc phosphate nanoparticles: A review on physical, chemical, and biological synthesis and their applications. Curr. Pharm. Biotechnol. 2022, 23, 1228–1244. [Google Scholar] [PubMed]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper nanoparticles: Synthesis and characterization, physiology, toxicity and antimicrobial applications. Appl. Sci. 2021, 12, 141. [Google Scholar] [CrossRef]
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef] [PubMed]
- Pavithran, S.; Pappuswamy, M.; Annadurai, Y.; Armugam, V.A.; Periyaswamy, T. Green Synthesis of Copper Nanoparticles, Characterization and Their Applications. J. Appl. Life Sci. Int. 2020, 23, 10–24. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-based green synthesis of nanoparticles: Production, characterization and applications. Biomolecules 2021, 12, 31. [Google Scholar] [CrossRef]
- Jayakodi, S.; Senthilnathan, R.; Swaminathan, A.; Shanmugam, V.K.; Shanmugam, R.K.; Krishnan, A.; Ponnusamy, V.K.; Tsai, P.-C.; Lin, Y.-C.; Chen, Y.-H. Bio-inspired nanoparticles mediated from plant extract biomolecules and their therapeutic application in cardiovascular diseases: A review. Int. J. Biol. Macromol. 2023, 242, 125025. [Google Scholar] [CrossRef]
- Alao, I.I.; Oyekunle, I.P.; Iwuozor, K.O.; Emenike, E.C. Green synthesis of copper nanoparticles and investigation of its anti-microbial properties. Adv. J. Chem.-Sect. B 2022, 4, 39–52. [Google Scholar]
- Rajeshkumar, S.; Menon, S.; Kumar, S.V.; Tambuwala, M.M.; Bakshi, H.A.; Mehta, M.; Satija, S.; Gupta, G.; Chellappan, D.K.; Thangavelu, L. Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J. Photochem. Photobiol. B Biol. 2019, 197, 111531. [Google Scholar] [CrossRef]
- Vidovix, T.B.; Quesada, H.B.; Januário, E.F.D.; Bergamasco, R.; Vieira, A.M.S. Green synthesis of copper oxide nanoparticles using Punica granatum leaf extract applied to the removal of methylene blue. Mater. Lett. 2019, 257, 126685. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Chaudhury, A. Biogenesis of copper nanoparticles using peel extract of Punica granatum and their antimicrobial activity against opportunistic pathogens. Green Chem. Lett. Rev. 2016, 9, 33–38. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Ghaneialvar, H.; Akbaribazm, M.; Ghanimatdan, M.; Abbasi, N.; Goorani, S.; Pirabbasi, E.; Zangeneh, A. Novel synthesis of Falcaria vulgaris leaf extract conjugated copper nanoparticles with potent cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing activities under in vitro and in vivo condition. J. Photochem. Photobiol. B Biol. 2019, 197, 111556. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Usman, M.; Liu, Q.-Y.; Shen, Y.-Q.; Yu, B.; Cong, H.-L. Plant mediated synthesis of copper nanoparticles by using Camelia sinensis leaves extract and their applications in dye degradation. Ferroelectrics 2019, 549, 61–69. [Google Scholar] [CrossRef]
- Nieto-Maldonado, A.; Bustos-Guadarrama, S.; Espinoza-Gomez, H.; Flores-López, L.Z.; Ramirez-Acosta, K.; Alonso-Nuñez, G.; Cadena-Nava, R.D. Green synthesis of copper nanoparticles using different plant extracts and their antibacterial activity. J. Environ. Chem. Eng. 2022, 10, 107130. [Google Scholar] [CrossRef]
- Mali, S.C.; Raj, S.; Trivedi, R. Biosynthesis of copper oxide nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem. Biophys. Rep. 2019, 20, 100699. [Google Scholar]
- Mohamed, E.A. Green synthesis of copper & copper oxide nanoparticles using the extract of seedless dates. Heliyon 2020, 6, e03123. [Google Scholar]
- Sasidharan, D.; Namitha, T.; Johnson, S.P.; Jose, V.; Mathew, P. Synthesis of silver and copper oxide nanoparticles using Myristica fragrans fruit extract: Antimicrobial and catalytic applications. Sustain. Chem. Pharm. 2020, 16, 100255. [Google Scholar] [CrossRef]
- Nazar, N.; Bibi, I.; Kamal, S.; Iqbal, M.; Nouren, S.; Jilani, K.; Umair, M.; Ata, S. Cu nanoparticles synthesis using biological molecule of P. granatum seeds extract as reducing and capping agent: Growth mechanism and photo-catalytic activity. Int. J. Biol. Macromol. 2018, 106, 1203–1210. [Google Scholar] [CrossRef]
- Chinnathambi, A.; Awad Alahmadi, T.; Ali Alharbi, S. Biogenesis of copper nanoparticles (Cu-NPs) using leaf extract of Allium noeanum, antioxidant and in-vitro cytotoxicity. Artif. Cells Nanomed. Biotechnol. 2021, 49, 500–510. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Khaksarian, M.; Ebrahimi, K.; Shiravand, S.; Jahanbakhsh, S.; Niazi, M.; Nadri, S. Antinociceptive effects of green synthesized copper nanoparticles alone or in combination with morphine. Ann. Med. Surg. 2020, 51, 31–36. [Google Scholar] [CrossRef]
- Abbas, S.; Nasreen, S.; Haroon, A.; Ashraf, M.A. Synhesis of silver and copper nanoparticles from plants and application as adsorbents for naphthalene decontamination. Saudi J. Biol. Sci. 2020, 27, 1016–1023. [Google Scholar] [CrossRef]
- Amaliyah, S.; Pangesti, D.P.; Masruri, M.; Sabarudin, A.; Sumitro, S.B. Green synthesis and characterization of copper nanoparticles using Piper retrofractum Vahl extract as bioreductor and capping agent. Heliyon 2020, 6, e04636. [Google Scholar] [CrossRef] [PubMed]
- Thakar, M.A.; Jha, S.S.; Phasinam, K.; Manne, R.; Qureshi, Y.; Babu, V.H. X ray diffraction (XRD) analysis and evaluation of antioxidant activity of copper oxide nanoparticles synthesized from leaf extract of Cissus vitiginea. Mater. Today Proc. 2022, 51, 319–324. [Google Scholar] [CrossRef]
- Chakraborty, N.; Banerjee, J.; Chakraborty, P.; Banerjee, A.; Chanda, S.; Ray, K.; Acharya, K.; Sarkar, J. Green synthesis of copper/copper oxide nanoparticles and their applications: A review. Green Chem. Lett. Rev. 2022, 15, 187–215. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 1–24. [Google Scholar] [CrossRef]
- John, M.S.; Nagoth, J.A.; Zannotti, M.; Giovannetti, R.; Mancini, A.; Ramasamy, K.P.; Miceli, C.; Pucciarelli, S. Biogenic synthesis of copper nanoparticles using bacterial strains isolated from an Antarctic consortium associated to a psychrophilic marine ciliate: Characterization and potential application as antimicrobial agents. Mar. Drugs 2021, 19, 263. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, N. Synthesis and biomedical applications of copper oxide nanoparticles: An expanding horizon. ACS Biomater. Sci. Eng. 2019, 5, 1170–1188. [Google Scholar] [CrossRef]
- Yang, Y.; Waterhouse, G.I.; Chen, Y.; Sun-Waterhouse, D.; Li, D. Microbial-enabled green biosynthesis of nanomaterials: Current status and future prospects. Biotechnol. Adv. 2022, 55, 107914. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of metal nanoparticles via microbial enzymes: A mechanistic approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef]
- Sarkar, S.; Ponce, N.T.; Banerjee, A.; Bandopadhyay, R.; Rajendran, S.; Lichtfouse, E. Green polymeric nanomaterials for the photocatalytic degradation of dyes: A review. Environ. Chem. Lett. 2020, 18, 1569–1580. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Olanrewaju, O.S.; Babalola, O.O. Sulfate-reducing bacteria as an effective tool for sustainable acid mine bioremediation. Front. Microbiol. 2018, 9, 1986. [Google Scholar] [CrossRef]
- Ghasemi, N.; Jamali-Sheini, F.; Zekavati, R. CuO and Ag/CuO nanoparticles: Biosynthesis and antibacterial properties. Mater. Lett. 2017, 196, 78–82. [Google Scholar] [CrossRef]
- Kouhkan, M.; Ahangar, P.; Babaganjeh, L.A.; Allahyari-Devin, M. Biosynthesis of copper oxide nanoparticles using Lactobacillus casei subsp. casei and its anticancer and antibacterial activities. Curr. Nanosci. 2020, 16, 101–111. [Google Scholar] [CrossRef]
- Noman, M.; Shahid, M.; Ahmed, T.; Niazi, M.B.K.; Hussain, S.; Song, F.; Manzoor, I. Use of biogenic copper nanoparticles synthesized from a native Escherichia sp. as photocatalysts for azo dye degradation and treatment of textile effluents. Environ. Pollut. 2020, 257, 113514. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, M.; Anand, R.; Sharma, P.; Rajput, P.; Sharma, N.; Singh, K. Multifunctional copper nanoparticles: Synthesis and applications. ECS J. Solid State Sci. Technol. 2021, 10, 063011. [Google Scholar] [CrossRef]
- Bukhari, S.I.; Hamed, M.M.; Al-Agamy, M.H.; Gazwi, H.S.; Radwan, H.H.; Youssif, A.M. Biosynthesis of copper oxide nanoparticles using Streptomyces MHM38 and its biological applications. J. Nanomater. 2021, 2021, 6693302. [Google Scholar] [CrossRef]
- Cuevas, R.; Durán, N.; Diez, M.; Tortella, G.; Rubilar, O. Extracellular biosynthesis of copper and copper oxide nanoparticles by Stereum hirsutum, a native white-rot fungus from chilean forests. J. Nanomater. 2015, 16, 57. [Google Scholar] [CrossRef]
- Mali, S.C.; Dhaka, A.; Sharma, S.; Trivedi, R. Review on biogenic synthesis of copper nanoparticles and its potential applications. Inorg. Chem. Commun. 2023, 149, 110448. [Google Scholar] [CrossRef]
- Qu, Y.; You, S.; Zhang, X.; Pei, X.; Shen, W.; Li, Z.; Li, S.; Zhang, Z. Biosynthesis of gold nanoparticles using cell-free extracts of Magnusiomyces ingens LH-F1 for nitrophenols reduction. Bioprocess Biosyst. Eng. 2018, 41, 359–367. [Google Scholar] [CrossRef]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Biosynthesis of metal and metal oxide nanoparticles. ChemBioEng Rev. 2016, 3, 55–67. [Google Scholar] [CrossRef]
- Kasana, R.C.; Panwar, N.R.; Kaul, R.K.; Kumar, P. Biosynthesis and effects of copper nanoparticles on plants. Environ. Chem. Lett. 2017, 15, 233–240. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Shanmugam, S.; Varukattu, N.B.; MubarakAli, D.; Kathiresan, K.; Wang, M.-H. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J. Photochem. Photobiol. B Biol. 2019, 190, 103–109. [Google Scholar] [CrossRef] [PubMed]
- El-Batal, A.I.; El-Sayyad, G.S.; Mosallam, F.M.; Fathy, R.M. Penicillium chrysogenum-mediated mycogenic synthesis of copper oxide nanoparticles using gamma rays for in vitro antimicrobial activity against some plant pathogens. J. Clust. Sci. 2020, 31, 79–90. [Google Scholar] [CrossRef]
- Noor, S.; Shah, Z.; Javed, A.; Ali, A.; Hussain, S.B.; Zafar, S.; Ali, H.; Muhammad, S.A. A fungal based synthesis method for copper nanoparticles with the determination of anticancer, antidiabetic and antibacterial activities. J. Microbiol. Methods 2020, 174, 105966. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Khanna, P.; Kaur, A.; Goyal, D. Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol. Methods 2019, 163, 105656. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Chen, X.; Chen, F.; Zhou, X.; Parsaee, Z. Ultrasound-assisted biosynthesis of CuO-NPs using brown alga Cystoseira trinodis: Characterization, photocatalytic AOP, DPPH scavenging and antibacterial investigations. Ultrason. Sonochem. 2018, 41, 109–119. [Google Scholar] [CrossRef]
- Arya, A.; Gupta, K.; Chundawat, T.S.; Vaya, D. Biogenic synthesis of copper and silver nanoparticles using green alga Botryococcus braunii and its antimicrobial activity. Bioinorg. Chem. Appl. 2018, 2018, 7879403. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Swarnakar, S.; Ghosh, S.; Majumdar, S.; Banerjee, S. Disinfection of drinking water via algae mediated green synthesized copper oxide nanoparticles and its toxicity evaluation. J. Environ. Chem. Eng. 2019, 7, 102867. [Google Scholar] [CrossRef]
- Waris, A.; Din, M.; Ali, A.; Ali, M.; Afridi, S.; Baset, A.; Khan, A.U. A comprehensive review of green synthesis of copper oxide nanoparticles and their diverse biomedical applications. Inorg. Chem. Commun. 2021, 123, 108369. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Rajalu, A.V.; Kaliaraj, G.S.; Siengchin, S.; Parameswaranpillai, J.; Saraswathi, R. Preparation of cellulose/copper nanoparticles bionanocomposite films using a bioflocculant polymer as reducing agent for antibacterial and anticorrosion applications. Compos. Part B Eng. 2019, 175, 107177. [Google Scholar] [CrossRef]
- Parwani, L.; Bhatt, M.; Singh, J. Potential biotechnological applications of cyanobacterial exopolysaccharides. Braz. Arch. Biol. Technol. 2021, 64, e21200401. [Google Scholar] [CrossRef]
- Manivasagan, P.; Kang, K.-H.; Kim, D.G.; Kim, S.-K. Production of polysaccharide-based bioflocculant for the synthesis of silver nanoparticles by Streptomyces sp. Int. J. Biol. Macromol. 2015, 77, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, V.S.R. Synthesis and Application of FeCu Bimetallic Nanoparticles in Coal Mine Wastewater Treatment. Minerals 2021, 11, 132. [Google Scholar] [CrossRef]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, V.S.R. Biosynthesis of bioflocculant passivated copper nanoparticles, characterization and application. Phys. Chem. Earth Parts A/B/C 2020, 118, 102898. [Google Scholar] [CrossRef]
- Lu, R.; Hao, W.; Kong, L.; Zhao, K.; Bai, H.; Liu, Z. A simple method for the synthesis of copper nanoparticles from metastable intermediates. RSC Adv. 2023, 13, 14361–14369. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Oyebamiji, A.K.; Olugbeko, S.C.; Latona, D.F. Green chemistry approach towards the synthesis of copper nanoparticles and its potential applications as therapeutic agents and environmental control. Curr. Res. Green Sustain. Chem. 2021, 4, 100176. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Abd El-Hack, M.E.; Taha, A.E.; Fouda, M.M.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Elshaer, N. Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials 2020, 10, 587. [Google Scholar] [CrossRef]
- Sadia, B.O.; Cherutoi, J.K.; Achisa, C.M. Optimization, Characterization, and Antibacterial Activity of Copper Nanoparticles Synthesized Using Senna didymobotrya Root Extract. J. Nanotechnol. 2021, 2021, 5611434. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Dlamini, N.G.; Basson, A.K.; Pullabhotla, V.S.R. Optimization and application of bioflocculant passivated copper nanoparticles in the wastewater treatment. Int. J. Environ. Res. Public Health 2019, 16, 2185. [Google Scholar] [CrossRef]
- Din, M.I.; Rehan, R. Synthesis, characterization, and applications of copper nanoparticles. Anal. Lett. 2017, 50, 50–62. [Google Scholar] [CrossRef]
- Marciniak, L.; Nowak, M.; Trojanowska, A.; Tylkowski, B.; Jastrzab, R. The effect of pH on the size of silver nanoparticles obtained in the reduction reaction with citric and malic acids. Materials 2020, 13, 5444. [Google Scholar] [CrossRef] [PubMed]
- Shende, S.; Ingle, A.P.; Gade, A.; Rai, M. Green synthesis of copper nanoparticles by Citrus medica Linn.(Idilimbu) juice and its antimicrobial activity. World J. Microbiol. Biotechnol. 2015, 31, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Din, M.I.; Arshad, F.; Rani, A.; Aihetasham, A.; Mukhtar, M.; Mehmood, H. Single step green synthesis of stable copper oxide nanoparticles as efficient photo catalyst material. Biomed. Mater. 2017, 9, 41–48. [Google Scholar]
- Rajesh, K.; Ajitha, B.; Reddy, Y.A.K.; Suneetha, Y.; Reddy, P.S. Synthesis of copper nanoparticles and role of pH on particle size control. Mater. Today Proc. 2016, 3, 1985–1991. [Google Scholar] [CrossRef]
- Hassabo, A.A.; Ibrahim, E.I.; Ali, B.A.; Emam, H.E. Anticancer effects of biosynthesized Cu2O nanoparticles using marine yeast. Biocatal. Agric. Biotechnol. 2022, 39, 102261. [Google Scholar] [CrossRef]
- Lade, B.D.; Shanware, A.S. Phytonanofabrication: Methodology and factors affecting biosynthesis of nanoparticles. In Smart Nanosystems for Biomedicine, Optoelectronics and Catalysis; IntechOpen: London, UK, 2020. [Google Scholar]
- Vincent, J.; Lau, K.S.; Evyan, Y.C.-Y.; Chin, S.X.; Sillanpää, M.; Chia, C.H. Biogenic Synthesis of Copper-Based Nanomaterials Using Plant Extracts and Their Applications: Current and Future Directions. Nanomaterials 2022, 12, 3312. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Chiang, C.-L.; Lin, K.-S.; Jeng, R.-C. Recycling copper nanoparticles from printed circuit board waste etchants via a microemulsion process. J. Clean. Prod. 2018, 185, 781–796. [Google Scholar] [CrossRef]
- Hong, G.-B.; Wang, J.-F.; Chuang, K.-J.; Cheng, H.-Y.; Chang, K.-C.; Ma, C.-M. Preparing Copper Nanoparticles and Flexible Copper Conductive Sheets. Nanomaterials 2022, 12, 360. [Google Scholar] [CrossRef]
- Usman, M.; Hamid, M.; Haq, R.U.; Wang, W. Heat and fluid flow of water and ethylene-glycol based Cu-nanoparticles between two parallel squeezing porous disks: LSGM approach. Int. J. Heat Mass Transf. 2018, 123, 888–895. [Google Scholar] [CrossRef]
- Kimber, R.L.; Lewis, E.A.; Parmeggiani, F.; Smith, K.; Bagshaw, H.; Starborg, T.; Joshi, N.; Figueroa, A.I.; Van der Laan, G.; Cibin, G. Biosynthesis and characterization of copper nanoparticles using Shewanella oneidensis: Application for click chemistry. Small 2018, 14, 1703145. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, H.R.; Mehr, F.P.; Poor, A.K. Extracellular synthesis of copper nanoparticles using culture supernatants of Salmonella typhimurium. Orient. J. Chem. 2015, 31, 527–529. [Google Scholar] [CrossRef]
- Pham, N.-D.; Duong, M.-M.; Le, M.-V.; Hoang, H.A. Preparation and characterization of antifungal colloidal copper nanoparticles and their antifungal activity against Fusarium oxysporum and Phytophthora capsici. Comptes Rendus Chim. 2019, 22, 786–793. [Google Scholar] [CrossRef]
- Benassai, E.; Del Bubba, M.; Ancillotti, C.; Colzi, I.; Gonnelli, C.; Calisi, N.; Salvatici, M.C.; Casalone, E.; Ristori, S. Green and cost-effective synthesis of copper nanoparticles by extracts of non-edible and waste plant materials from Vaccinium species: Characterization and antimicrobial activity. Mater. Sci. Eng. C 2021, 119, 111453. [Google Scholar] [CrossRef]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle characterization: What to measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef]
- Koo, J.H. Polymer Nanocomposites: Processing, Characterization, and Applications; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Ajose, D.J.; Abolarinwa, T.O.; Oluwarinde, B.O.; Montso, P.K.; Fayemi, O.E.; Aremu, A.O.; Ateba, C.N. Application of Plant-Derived Nanoparticles (PDNP) in Food-Producing Animals as a Bio-Control Agent against Antimicrobial-Resistant Pathogens. Biomedicines 2022, 10, 2426. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Monshi, A.; Nasiri, S.; Vilkauskas, A.; Janusas, G. Comparing methods for calculating nano crystal size of natural hydroxyapatite using X-ray diffraction. Nanomaterials 2020, 10, 1627. [Google Scholar] [CrossRef]
- Whba, F.; Mohamed, F.; Rosli, N.R.A.M.; Rahman, I.A.; Idris, M.I. The crystalline structure of gadolinium oxide nanoparticles (Gd2O3-NPs) synthesized at different temperatures via X-ray diffraction (XRD) technique. Radiat. Phys. Chem. 2021, 179, 109212. [Google Scholar] [CrossRef]
- Duong, N.L.; Nguyen, V.M.; Tran, T.A.N.; Phan, T.D.T.; Tran, T.B.Y.; Do, B.L.; Phung Anh, N.; Nguyen, T.A.T.; Ho, T.G.-T.; Nguyen, T. Durian Shell-Mediated Simple Green Synthesis of Nanocopper against Plant Pathogenic Fungi. ACS Omega 2023, 8, 10968–10979. [Google Scholar] [CrossRef]
- Rónavári, A.; Igaz, N.; Gopisetty, M.K.; Szerencsés, B.; Kovács, D.; Papp, C.; Vágvölgyi, C.; Boros, I.M.; Kónya, Z.; Kiricsi, M. Biosynthesized silver and gold nanoparticles are potent antimycotics against opportunistic pathogenic yeasts and dermatophytes. Int. J. Nanomed. 2018, 13, 695. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.; Saroj, S.K.; Zeeshan, M.; Jadoun, S. Microwave-assisted solid state intercalation of Rhodamine B and polycarbazole in bentonite clay interlayer space: Structural characterization and photophysics of double intercalation. RSC Adv. 2016, 6, 34534–34545. [Google Scholar] [CrossRef]
- Haghighat, M.; Alijani, H.Q.; Ghasemi, M.; Khosravi, S.; Borhani, F.; Sharifi, F.; Iravani, S.; Najafi, K.; Khatami, M. Cytotoxicity properties of plant-mediated synthesized K-doped ZnO nanostructures. Bioprocess Biosyst. Eng. 2022, 45, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Viet, P.V.; Nguyen, H.T.; Cao, T.M.; Hieu, L.V. Fusarium antifungal activities of copper nanoparticles synthesized by a chemical reduction method. J. Nanomater. 2016, 2016, 1957612. [Google Scholar] [CrossRef]
- Rafeeq, C.; Paul, E.; Saagar, E.V.; Ali, P.M. Mycosynthesis of zinc oxide nanoparticles using Pleurotus floridanus and optimization of process parameters. Ceram. Int. 2021, 47, 12375–12380. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; Ur Rehman, M.Z.; Imran, M.; Ahmad, J.; Ahmad, S.; Inam, A.; Razzaq, A.; Rizwan, M.; Ali, S. Synthesis and characterization of titanium dioxide nanoparticles by chemical and green methods and their antifungal activities against wheat rust. Chemosphere 2020, 258, 127352. [Google Scholar] [CrossRef]
- Bezza, F.A.; Tichapondwa, S.M.; Chirwa, E.M. Fabrication of monodispersed copper oxide nanoparticles with potential application as antimicrobial agents. Sci. Rep. 2020, 10, 16680. [Google Scholar] [CrossRef]
- Al-Qasmi, N. Facial eco-friendly synthesis of copper oxide nanoparticles using chia seeds extract and evaluation of its electrochemical activity. Processes 2021, 9, 2027. [Google Scholar] [CrossRef]
- Sagadevan, S.; Koteeswari, P. Analysis of structure, surface morphology, optical and electrical properties of copper nanoparticles. J. Nanomed. Res. 2015, 2, 00040. [Google Scholar] [CrossRef]
- Guo, J. Characteristics and mechanisms of Cu (II) sorption from aqueous solution by using bioflocculant MBFR10543. Appl. Microbiol. Biotechnol. 2015, 99, 229–240. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Sahu, S.; Gupta, I.; Ghorai, T.K. Green synthesis of copper nanoparticles from an extract of Jatropha curcas leaves: Characterization, optical properties, CT-DNA binding and photocatalytic activity. RSC Adv. 2020, 10, 22027–22035. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; Hussain, S.; Niazi, M.B.K.; Shahid, M.; Song, F. Biogenic copper nanoparticles synthesized by using a copper-resistant strain Shigella flexneri SNT22 reduced the translocation of cadmium from soil to wheat plants. J. Hazard. Mater. 2020, 398, 123175. [Google Scholar] [CrossRef] [PubMed]
- Praba, K.L.; Jayashree, M.; Banu, K.S.; Gino, A.; Kurian, A. Green and chemically synthesized Copper oxide nanoparticles-A preliminary research towards its toxic behaviour. Int. J. Pharm. Pharmaceut. Sci. 2015, 7, 156–160. [Google Scholar]
- Haque, N.; Biswas, S.; Basu, P.; Biswas, I.H.; Khatun, R.; Khan, A.; Islam, S.M. Triazinetriamine-derived porous organic polymer-supported copper nanoparticles (Cu-NPs@TzTa-POP): An efficient catalyst for the synthesis of N-methylated products via CO2 fixation and primary carbamates from alcohols and urea. New J. Chem. 2020, 44, 15446–15458. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Li, H.; Matter, I.A. Nano-bioremediation of textile industry wastewater using immobilized CuO-NPs myco-synthesized by a novel Cu-resistant Fusarium oxysporum OSF18. Environ. Sci. Pollut. Res. 2023, 30, 16694–16706. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.I.; Alzuhair, A.Z.; Alqahtani, M.S.; Meshawi, A.A.; Alhifzi, R.Z.; Yahia, I.; Zahran, H.Y.; Alqahtani, F.F.; Reben, M. Optical properties and novelty preparation PVA/PVP doping with Cu as surface plasmonic ions. Optik 2022, 259, 168965. [Google Scholar] [CrossRef]
- Memon, R.; Memon, A.A.; Sherazi, S.T.H.; UDDIN, S.; Balouch, A.; Shah, M.R.; Mahesar, S.A.; Rajar, K.; Agheem, M.H. Application of synthesized copper nanoparticles using aqueous extract of Ziziphus mauritiana L. leaves as a colorimetric sensor for the detection of Ag+. Turk. J. Chem. 2020, 44, 1376–1385. [Google Scholar] [CrossRef]
- Tiwari, M.; Jain, P.; Hariharapura, R.C.; Narayanan, K.; Bhat, U.; Udupa, N.; Rao, J.V. Biosynthesis of copper nanoparticles using copper-resistant Bacillus cereus, a soil isolate. Process Biochem. 2016, 51, 1348–1356. [Google Scholar] [CrossRef]
- Guru Bharathi, B.; Lalitha, K.; Shivakumar, M.S. Biosynthesis of copper nanoparticles using symbiotic bacterium Xenorhabdus sp., isolated from entomopathogenic nematode and its antimicrobial and insecticidal activity against Spodoptera litura. Inorg. Nano-Met. Chem. 2022, 1–13. [Google Scholar] [CrossRef]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 2019, 124, 1132–1136. [Google Scholar] [CrossRef]
- Nair, G.M.; Sajini, T.; Mathew, B. Advanced green approaches for metal and metal oxide nanoparticles synthesis and their environmental applications. Talanta Open 2022, 5, 100080. [Google Scholar] [CrossRef]
- Tan, H.W.; Choong, Y.Y.C.; Kuo, C.N.; Low, H.Y.; Chua, C.K. 3D printed electronics: Processes, materials and future trends. Prog. Mater. Sci. 2022, 127, 100945. [Google Scholar] [CrossRef]
- Nowak, A.; Szade, J.; Talik, E.; Zubko, M.; Wasilkowski, D.; Dulski, M.; Balin, K.; Mrozik, A.; Peszke, J. Physicochemical and antibacterial characterization of ionocity Ag/Cu powder nanoparticles. Mater. Charact. 2016, 117, 9–16. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Anbazhagan, V. Synthesis of copper sulfide nanoparticles and evaluation of in vitro antibacterial activity and in vivo therapeutic effect in bacteria-infected zebrafish. RSC Adv. 2017, 7, 36644–36652. [Google Scholar] [CrossRef]
- Lai, M.-J.; Huang, Y.-W.; Wijaya, J.; Liu, B.R. Programmed Cell Death: The Primary Bactericidal Mechanism Induced by Copper Nanoparticles. In Copper Overview-From Historical Aspects to Applications; IntechOpen: London, UK, 2024. [Google Scholar]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current knowledge on the oxidative-stress-mediated antimicrobial properties of metal-based nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, G.; Kubo, A.-L.; Vija, H.; Kahru, A.; Bondar, D.; Karpichev, Y.; Bondarenko, O. Synergistic antibacterial effect of copper and silver nanoparticles and their mechanism of action. Sci. Rep. 2023, 13, 9202. [Google Scholar] [CrossRef]
- Nabila, M.I.; Kannabiran, K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018, 15, 56–62. [Google Scholar] [CrossRef]
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef]
- Awwad, A.M.; Albiss, B.A.; Salem, N.M. Antibacterial activity of synthesized copper oxide nanoparticles using Malva sylvestris leaf extract. SMU Med. J. 2015, 2, 91–101. [Google Scholar]
- Ranjitham, A.M.; Ranjani, G.S.; Caroling, G. Biosynthesis, characterization, antimicrobial activity of copper nanoparticles using fresh aqueous Ananas comosus L.(Pineapple) extract. Int. J. PharmTech Res. 2015, 8, 750–769. [Google Scholar]
- Jayandran, M.; Haneefa, M.M.; Balasubramanian, V. Green synthesis of copper nanoparticles using natural reducer and stabilizer and an evaluation of antimicrobial activity. J. Chem. Pharm. Res. 2015, 7, 251–259. [Google Scholar]
- Kalpana, V.; Chakraborthy, P.; Palanichamy, V.; Rajeswari, V.D. Synthesis and characterization of copper nanoparticles using Tridax procumbens and its application in degradation of bismarck brown. Analysis 2016, 10, 17. [Google Scholar]
- Hariprasad, S.; Bai, G.S.; Santhoshkumar, J.; Madhu, C.; Sravani, D. Green synthesis of copper nanoparticles by Arevalanata leaves extract and their anti-microbial activites. Int. J. Chem-Tech Res. 2016, 9, 98–105. [Google Scholar]
- Woźniak-Budych, M.J.; Przysiecka, Ł.; Langer, K.; Peplińska, B.; Jarek, M.; Wiesner, M.; Nowaczyk, G.; Jurga, S. Green synthesis of rifampicin-loaded copper nanoparticles with enhanced antimicrobial activity. J. Mater. Sci. Mater. Med. 2017, 28, 42. [Google Scholar] [CrossRef]
- Subbaiya, R.; Selvam, M.M. Green synthesis of copper nanoparticles from Hibicus rosasinensis and their antimicrobial, antioxidant activities. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1183–1190. [Google Scholar]
- Joseph, A.T.; Prakash, P.; Narvi, S. Phytofabrication and Characterization of copper nanoparticles using Allium sativum and its antibacterial activity. Int. J. Sci. Eng. Technol 2016, 4, 463–472. [Google Scholar]
- Caroling, G.; Priyadharshini, M.N.; Vinodhini, E.; Ranjitham, A.M.; Shanthi, P. Biosynthesis of copper nanoparticles using aqueous guava extract-characterisation and study of antibacterial effects. Int. J. Pharm. Biol. Sci. 2015, 5, 25–43. [Google Scholar]
- Caroling, G.; Vinodhini, E.; Ranjitham, A.M.; Shanthi, P. Biosynthesis of copper nanoparticles using aqueous Phyllanthus embilica (Gooseberry) extract-characterisation and study of antimicrobial effects. Int. J. Nano Chem. 2015, 1, 53–63. [Google Scholar]
- Naika, H.R.; Lingaraju, K.; Manjunath, K.; Kumar, D.; Nagaraju, G.; Suresh, D.; Nagabhushana, H. Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J. Taibah Univ. Sci. 2015, 9, 7–12. [Google Scholar] [CrossRef]
- Dashora, A.; Sharma, K. Green synthesis of nanoparticles and their applications. Adv. Sci. Eng. Med. 2018, 10, 523–541. [Google Scholar] [CrossRef]
- Fathima, J.B.; Pugazhendhi, A.; Oves, M.; Venis, R. Synthesis of eco-friendly copper nanoparticles for augmentation of catalytic degradation of organic dyes. J. Mol. Liq. 2018, 260, 1–8. [Google Scholar] [CrossRef]
- Hemdan, B.A.; El Nahrawy, A.M.; Mansour, A.-F.M.; Hammad, A.B.A. Green sol–gel synthesis of novel nanoporous copper aluminosilicate for the eradication of pathogenic microbes in drinking water and wastewater treatment. Environ. Sci. Pollut. Res. 2019, 26, 9508–9523. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, P.; Aravind, A. Antibacterial activity of copper nanoparticles and copper nanocomposites against Escherichia coli bacteria. Int. J. Sci. 2016, 2, 83–90. [Google Scholar] [CrossRef]
- Ahmed, S.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.; Ong, H.C.; Chia, W.Y. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Tsilo, P.H. Green Synthesis and Characterisation of Metal-Based Nanoparticles Using Kombucha Tea SCOBY Yeast-Based Bioflocculant and Their Application in Wastewater Treatment. Ph.D. Thesis, University of Zululand, Richards Bay, South Africa, 2023. [Google Scholar]
- Batool, F.; Shahid, M.; Mahmood, F.; Shahzad, T.; Azeem, F.; Hussain, S.; Algarni, T.S.; Elshikh, M.S.; Onazi, W.A.A.; Mustafa, S. Biosynthesis of copper nanoparticles using Bacillus flexus and estimation of their potential for decolorization of azo dyes and textile wastewater treatment. J. King Saud Univ.-Sci. 2024, 36, 103309. [Google Scholar] [CrossRef]
- Almisbah, S.R.; Mohammed, A.M.; Elgamouz, A.; Bihi, A.; Kawde, A. Green synthesis of CuO nanoparticles using Hibiscus sabdariffa L. extract to treat wastewater in Soba Sewage Treatment Plant, Sudan. Water Sci. Technol. 2023, 87, 3059–3071. [Google Scholar] [CrossRef]
- Nzilu, D.M.; Madivoli, E.S.; Makhanu, D.S.; Wanakai, S.I.; Kiprono, G.K.; Kareru, P.G. Green synthesis of copper oxide nanoparticles and its efficiency in degradation of rifampicin antibiotic. Sci. Rep. 2023, 13, 14030. [Google Scholar] [CrossRef]
- Patel, V.R.; Bhatt, N. Application of stress induces ascorbate peroxidases of S. polyrhiza for green-synthesis Cu nanoparticles. Arab. J. Chem. 2020, 13, 8783–8792. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 227, 643972. [Google Scholar] [CrossRef]
- Gupta, V.K.; Chandra, R.; Tyagi, I.; Verma, M. Removal of hexavalent chromium ions using CuO nanoparticles for water purification applications. J. Colloid Interface Sci. 2016, 478, 54–62. [Google Scholar] [CrossRef]
- Eid, A.M.; Fouda, A.; Hassan, S.E.-D.; Hamza, M.F.; Alharbi, N.K.; Elkelish, A.; Alharthi, A.; Salem, W.M. Plant-Based Copper Oxide Nanoparticles; Biosynthesis, Characterization, Antibacterial Activity, Tanning Wastewater Treatment, and Heavy Metals Sorption. Catalysts 2023, 13, 348. [Google Scholar] [CrossRef]
- Verma, M.; Tyagi, I.; Chandra, R.; Gupta, V.K. Adsorptive removal of Pb (II) ions from aqueous solution using CuO nanoparticles synthesized by sputtering method. J. Mol. Liq. 2017, 225, 936–944. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Al-Qahtani, K.M.; Alflaij, S.O.; Al-Qahtani, S.F.; Alsamhan, F.A. Green copper oxide nanoparticles for lead, nickel, and cadmium removal from contaminated water. Sci. Rep. 2021, 11, 12547. [Google Scholar] [CrossRef] [PubMed]
- Obaideen, K.; Shehata, N.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Olabi, A. The role of wastewater treatment in achieving sustainable development goals (SDGs) and sustainability guideline. Energy Nexus 2022, 7, 100112. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef]
- Thakur, S.; Chauhan, M. Treatment of dye wastewater from textile industry by electrocoagulation and Fenton oxidation: A review. In Water Quality Management: Select Proceedings of the ICWEES-2016, Bhopal, India, 15–18 March 2016; Springer: Singapore, 2018; pp. 117–129. [Google Scholar]
- You, X.; Wang, R.; Zhu, Y.; Sui, W.; Cheng, D. Comparison of adsorption properties of a cellulose-rich modified rice husk for the removal of methylene blue and aluminum (III) from their aqueous solution. Ind. Crops Prod. 2021, 170, 113687. [Google Scholar] [CrossRef]
- Raina, S.; Roy, A.; Bharadvaja, N. Degradation of dyes using biologically synthesized silver and copper nanoparticles. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100278. [Google Scholar] [CrossRef]
- Soomro, R.A.; Nafady, A. Catalytic reductive degradation of methyl orange using air resilient copper nanostructures. J. Nanomater. 2015, 16, 120. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Chen, D.; Qin, Q.; Wu, Y.; Huang, F.; Li, W. Preparation of FeOOH/Cu with high catalytic activity for degradation of organic dyes. Materials 2019, 12, 338. [Google Scholar] [CrossRef]
- Davarnejad, R.; Azizi, A.; Asadi, S.; Mohammadi, M. Green synthesis of copper nanoparticles using Centaurea cyanus plant extract: A cationic dye adsorption application. Iran. J. Chem. Chem. Eng. 2022, 41, 1–14. [Google Scholar]
- Musa, A.; Ahmad, M.B.; Hussein, M.Z.; Saiman, M.I.; Sani, H.A. Effect of gelatin-stabilized copper nanoparticles on catalytic reduction of methylene blue. Nanoscale Res. Lett. 2016, 11, 438. [Google Scholar] [CrossRef]
- Zahmani, A.H.; Kerbadou, R.M.; Benmaati, A.; Hachemaoui, M.; Issam, I.; Iqbal, J.; Hacini, S.; Boukoussa, B.; Zahmani, H.H. CuNPs loaded zeolite 3 Å as an efficient catalyst for the catalytic reduction of hazardous pollutants. Inorg. Chem. Commun. 2023, 156, 111211. [Google Scholar] [CrossRef]
- Hassan, E.; Gahlan, A.A.; Gouda, G.A. Biosynthesis approach of copper nanoparticles, physicochemical characterization, cefixime wastewater treatment, and antibacterial activities. BMC Chem. 2023, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, Y.; Wang, J.; Gao, C.; Zhang, S.; Zhang, P.; Zhang, Z. Interactions of Cu nanoparticles with conventional lubricant additives on tribological performance and some physicochemical properties of an ester base oil. Tribol. Int. 2020, 141, 105941. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, Y.; Sun, B.; Wang, P.; Wang, Z.; Dong, H. Research progress of nano copper lubricant additives on engineering tribology. Metals 2021, 11, 2006. [Google Scholar] [CrossRef]
- Virmani, K.; Deepak, C.; Sharma, S.; Chadha, U.; Selvaraj, S.K. Nanomaterials for automotive outer panel components: A review. Eur. Phys. J. Plus 2021, 136, 921. [Google Scholar] [CrossRef]
- Hou, J.; Wang, X.; Hayat, T.; Wang, X. Ecotoxicological effects and mechanism of CuO nanoparticles to individual organisms. Environ. Pollut. 2017, 221, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Radosová, M.; Tarko, A.; Fabova, Z.; Martín-García, I.; Alonso, F. Abatement of the stimulatory effect of copper nanoparticles supported on titania on ovarian cell functions by some plants and phytochemicals. Nanomaterials 2020, 10, 1859. [Google Scholar] [CrossRef]
- Tang, H.; Xu, M.; Luo, J.; Zhao, L.; Ye, G.; Shi, F.; Lv, C.; Chen, H.; Wang, Y.; Li, Y. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ. Sci. Eur. 2019, 31, 30. [Google Scholar] [CrossRef]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A review on microplastics and nanoplastics in the environment: Their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef]
- Hejazy, M.; Koohi, M.K.; Bassiri Mohamad Pour, A.; Najafi, D. Toxicity of manufactured copper nanoparticles—A review. Nanomed. Res. J. 2018, 3, 1–9. [Google Scholar]
- Rodhe, Y.; Skoglund, S.; Wallinder, I.O.; Potácová, Z.; Möller, L. Copper-based nanoparticles induce high toxicity in leukemic HL60 cells. Toxicol. Vitr. 2015, 29, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-W.; Gao, G.; Jia, H.-R.; Zhang, X.; Zhao, J.; Ma, N.; Liu, J.-B.; Liu, P.; Wu, F.-G. Copper oxide nanoparticles induce enhanced radiosensitizing effect via destructive autophagy. ACS Biomater. Sci. Eng. 2019, 5, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, L.; Ma, H.; Zhang, H.; Guo, L.-H. Quantitative analysis of reactive oxygen species photogenerated on metal oxide nanoparticles and their bacteria toxicity: The role of superoxide radicals. Environ. Sci. Technol. 2017, 51, 10137–10145. [Google Scholar] [CrossRef] [PubMed]


| Method Type | Examples | Key Features | Advantages | Disadvantages | Citation |
|---|---|---|---|---|---|
| Chemical Methods | Liquid-phase reduction, Hydrothermal, Electrochemical | Utilizes reducing agents like sodium borohydride, hydrazine | High yield, controllable particle size | Risk of oxidation requires careful handling | [110] |
| Example: CuNPs synthesized using CuSO₄ and NaBH₄ | Simple process, widely used | Simple equipment requirements | Potential toxicity of chemicals involved | [110] | |
| Example: CuNPs with PVP stabilization | Stable dispersions | Versatile and adaptable to various applications | Environmental concerns with some reagents | [56] | |
| Physical Methods | Mechanical milling, Laser ablation, Physical vapor deposition | In the top-down approach, the bulk material is reduced to the nanoscale | Can produce uniform sizes | Often expensive and complex equipment | [30] |
| Example: Laser ablation targeting bulk copper | High precision in size control | Minimal chemical use | Energy-intensive and may require vacuum conditions | [30] | |
| Biological Methods | Green synthesis using plant extracts | Eco-friendly, utilizes natural reducing agents | Environmentally sustainable | Variability in yield and particle size | [111] |
| Example: Lantana camara extract for CuNP synthesis | Biocompatible materials | Potential for novel properties | Slower synthesis rates compared to chemical methods | [30] |
| SI No | Frequency (cm−1) | Allocated Bond | Citation |
|---|---|---|---|
| 1. | 3406 | -OH widening | [103] |
| 2. | 2857, 2927, and 3562 | C-H and O-H stretch | [149] |
| 3. | 425, 486, 521, 602, 736, 787, 882, 937, 985, 1087, 1116, and 1634 | Cu-O, C-O bond, C=O, and N-H bond | [150] |
| 4 | 521 and 602 | Cu-O bond along (101) direction | [151] |
| Flocculant | Kind of Effluent | Kind of Contaminants in Water | Water Quality Before Treatment (mg/L) | Water Quality after Treatment (mg/L) | Removal Efficiency (%) |
|---|---|---|---|---|---|
| CuNPs | Coal mine water | Phosphate | 2.00 | 0.3 | 85 |
| Sulfate | 0.55 | 0.13 | 76 | ||
| Chemical oxygen demand (COD) | 154 | 11.2 | 93 | ||
| Biological oxygen demand (BOD) | 123.2 | 5.0 | 96 | ||
| Polyamine flocculant | Phosphate | 2.00 | 1.3 | 76 | |
| Sulfate | 0.55 | 0.32 | 63 | ||
| Chemical oxygen demand (COD) | 154 | 32.4 | 89 | ||
| Biological oxygen demand (BOD) | 123.2 | 23.6 | 73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkosi, N.C.; Basson, A.K.; Ntombela, Z.G.; Dlamini, N.G.; Pullabhotla, R.V.S.R. A Review on Bioflocculant-Synthesized Copper Nanoparticles: Characterization and Application in Wastewater Treatment. Bioengineering 2024, 11, 1007. https://doi.org/10.3390/bioengineering11101007
Nkosi NC, Basson AK, Ntombela ZG, Dlamini NG, Pullabhotla RVSR. A Review on Bioflocculant-Synthesized Copper Nanoparticles: Characterization and Application in Wastewater Treatment. Bioengineering. 2024; 11(10):1007. https://doi.org/10.3390/bioengineering11101007
Chicago/Turabian StyleNkosi, Nkanyiso C., Albertus K. Basson, Zuzingcebo G. Ntombela, Nkosinathi G. Dlamini, and Rajasekhar V. S. R. Pullabhotla. 2024. "A Review on Bioflocculant-Synthesized Copper Nanoparticles: Characterization and Application in Wastewater Treatment" Bioengineering 11, no. 10: 1007. https://doi.org/10.3390/bioengineering11101007








