A Wearable Extracorporeal CO2 Removal System with a Closed-Loop Feedback
Abstract
:1. Introduction
2. Materials and Methods
2.1. Engineering Design
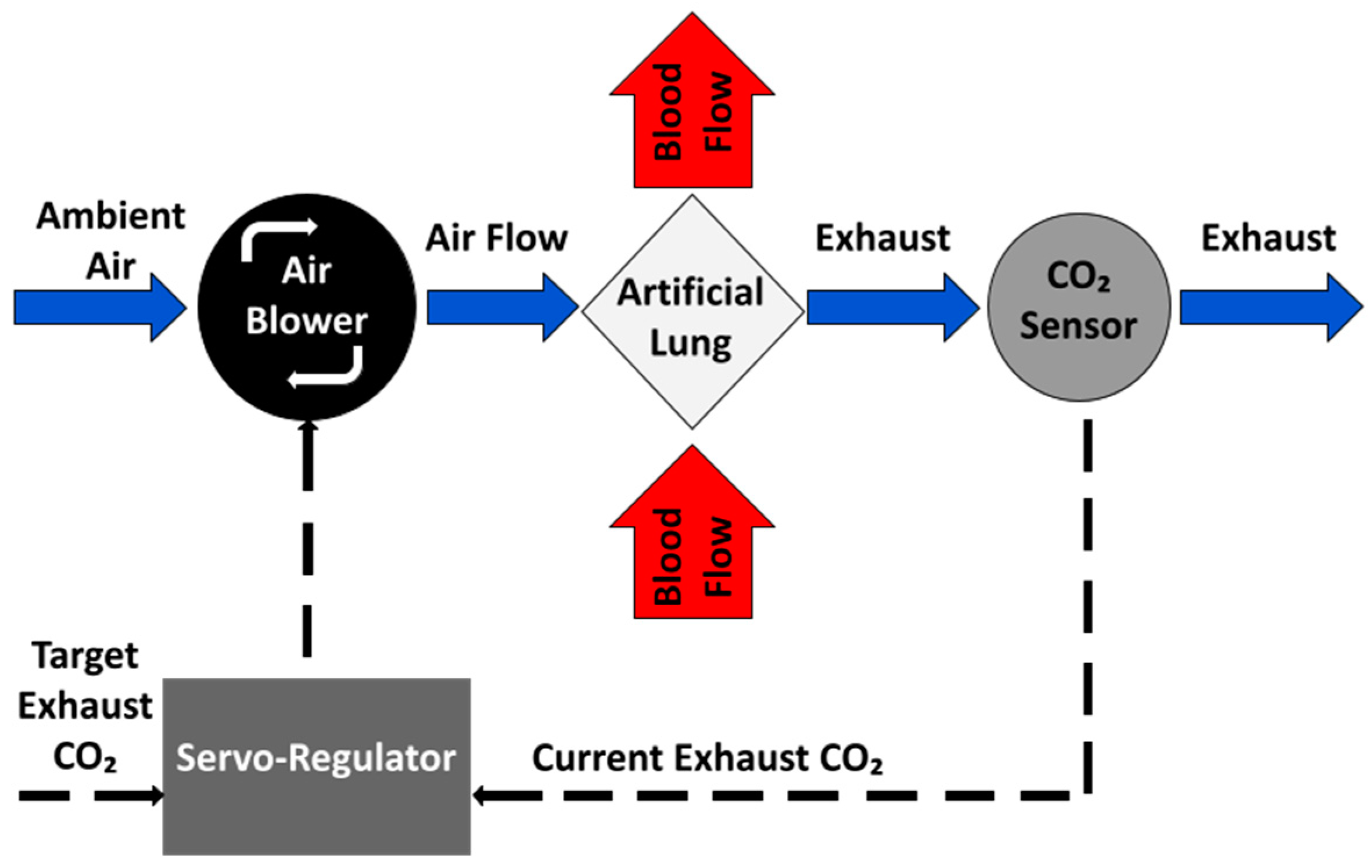
2.1.1. Negative Feedback Control of EGCO2
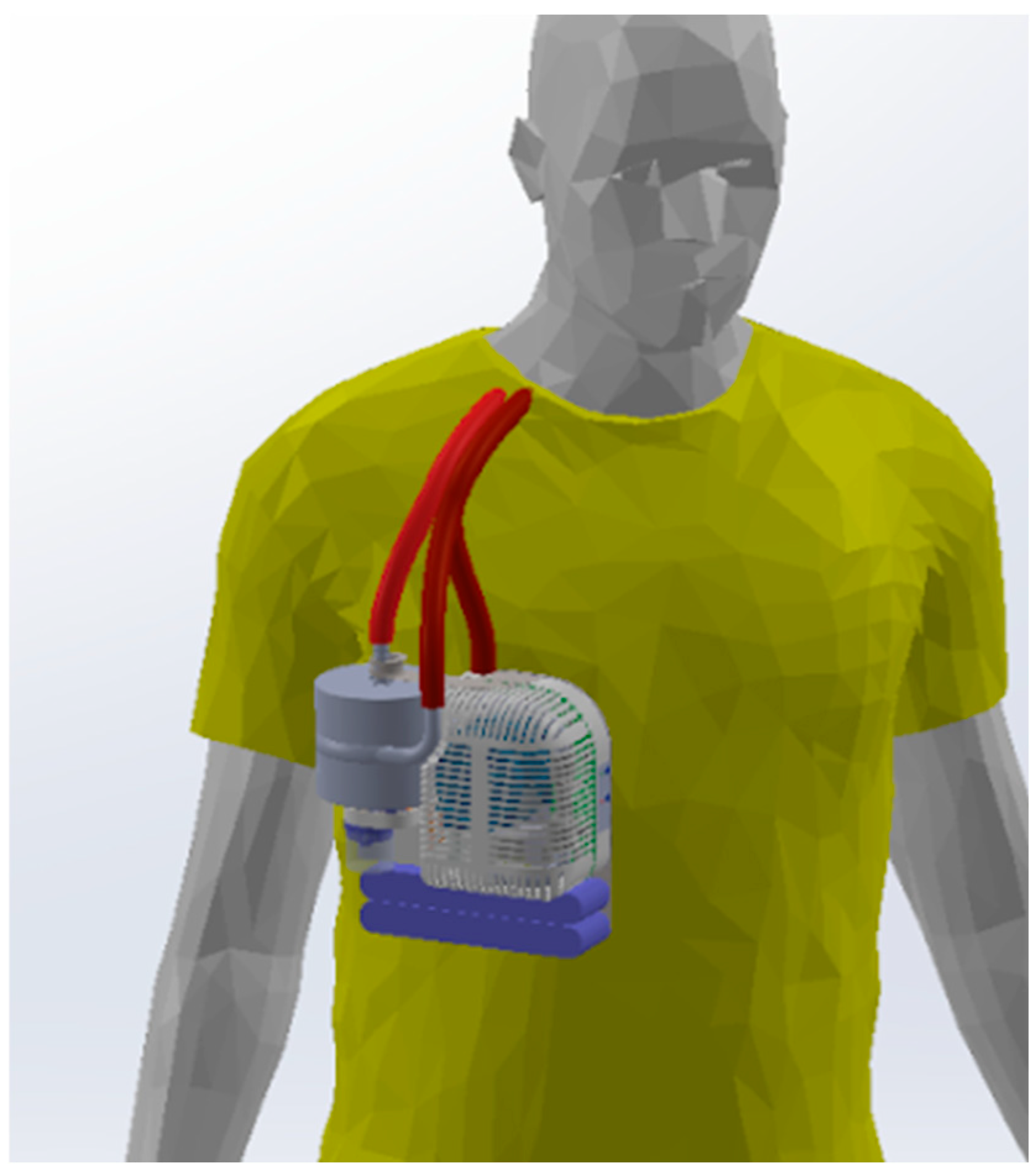
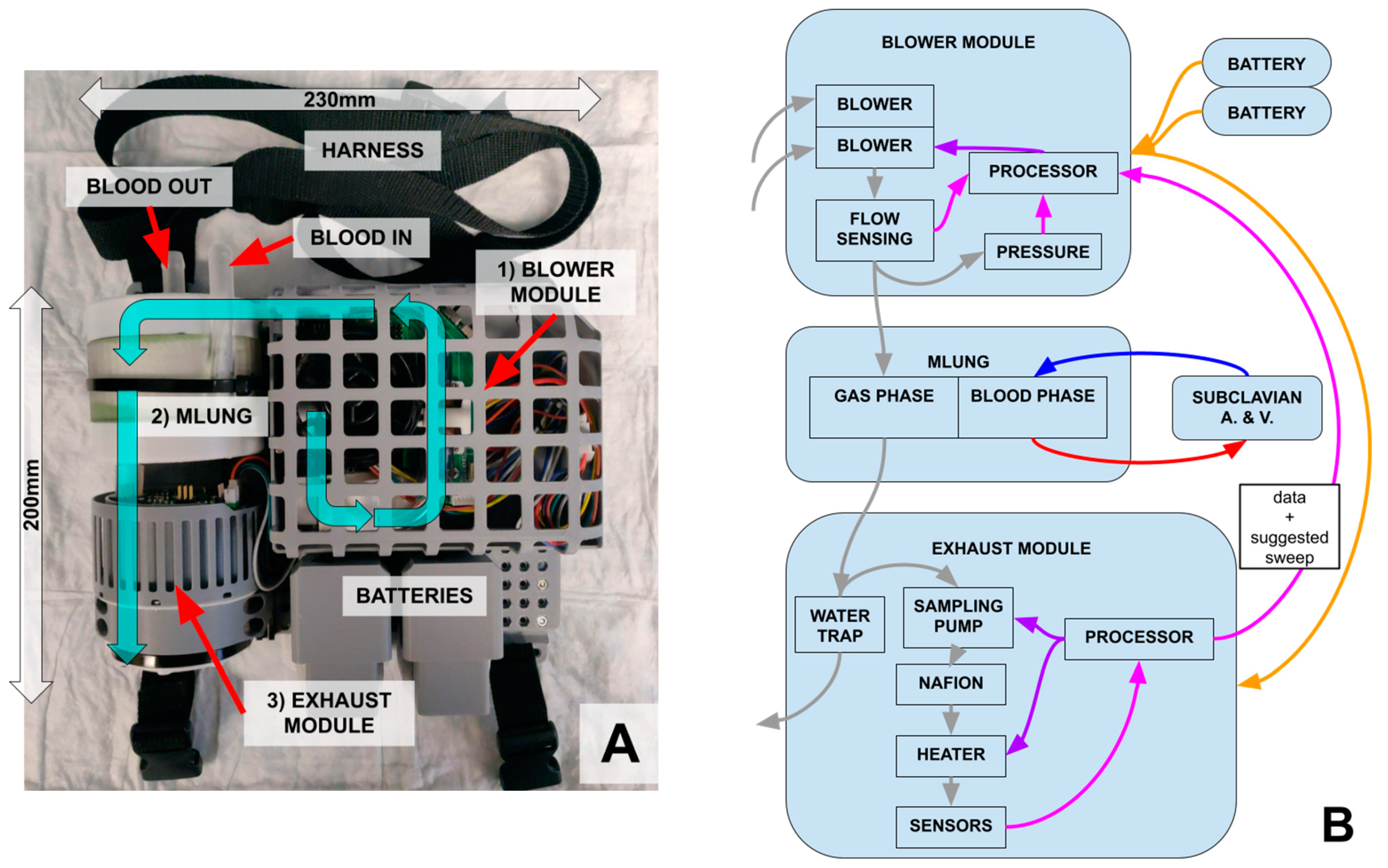
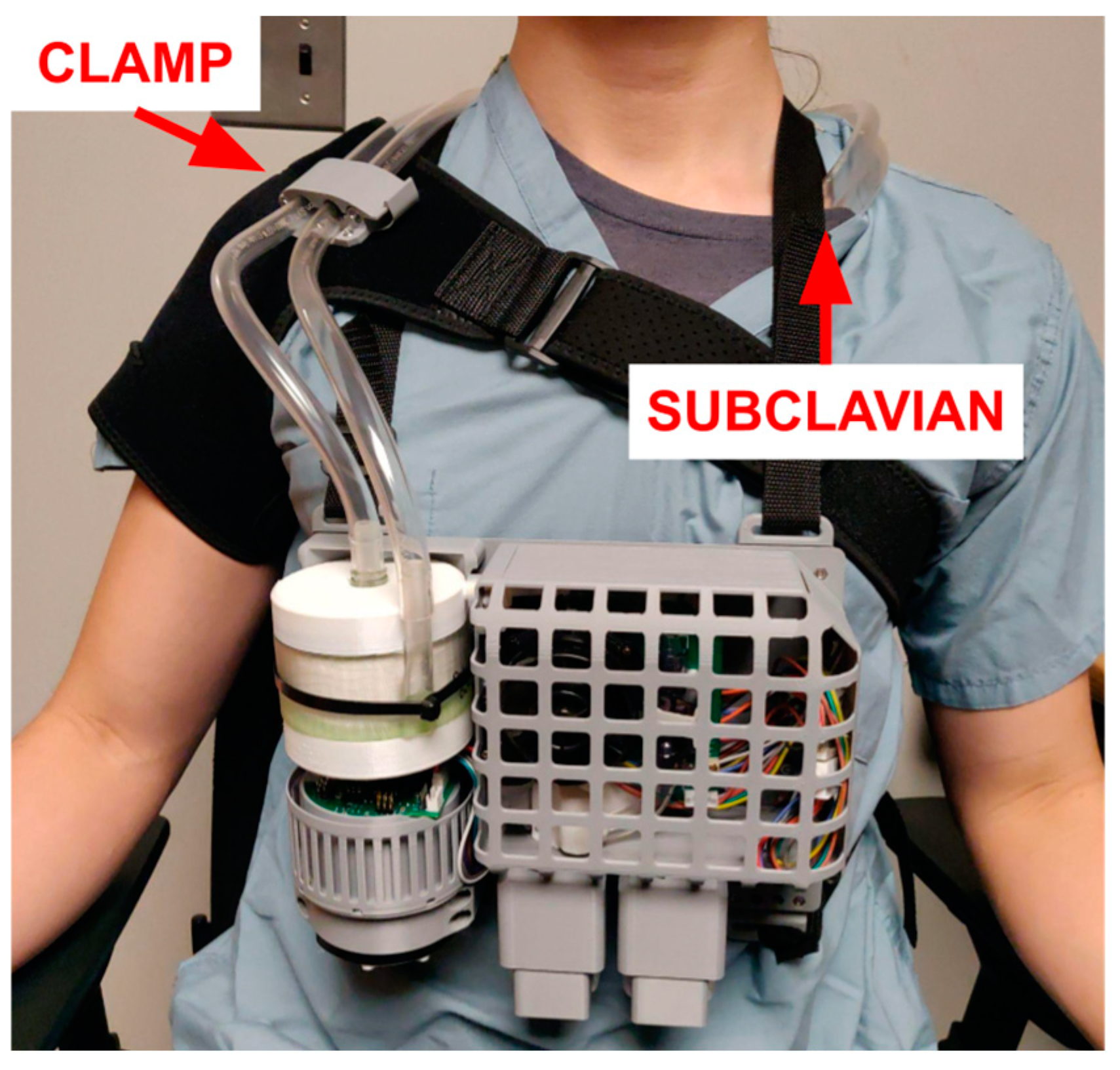
2.1.2. Miniaturization and Overall Design
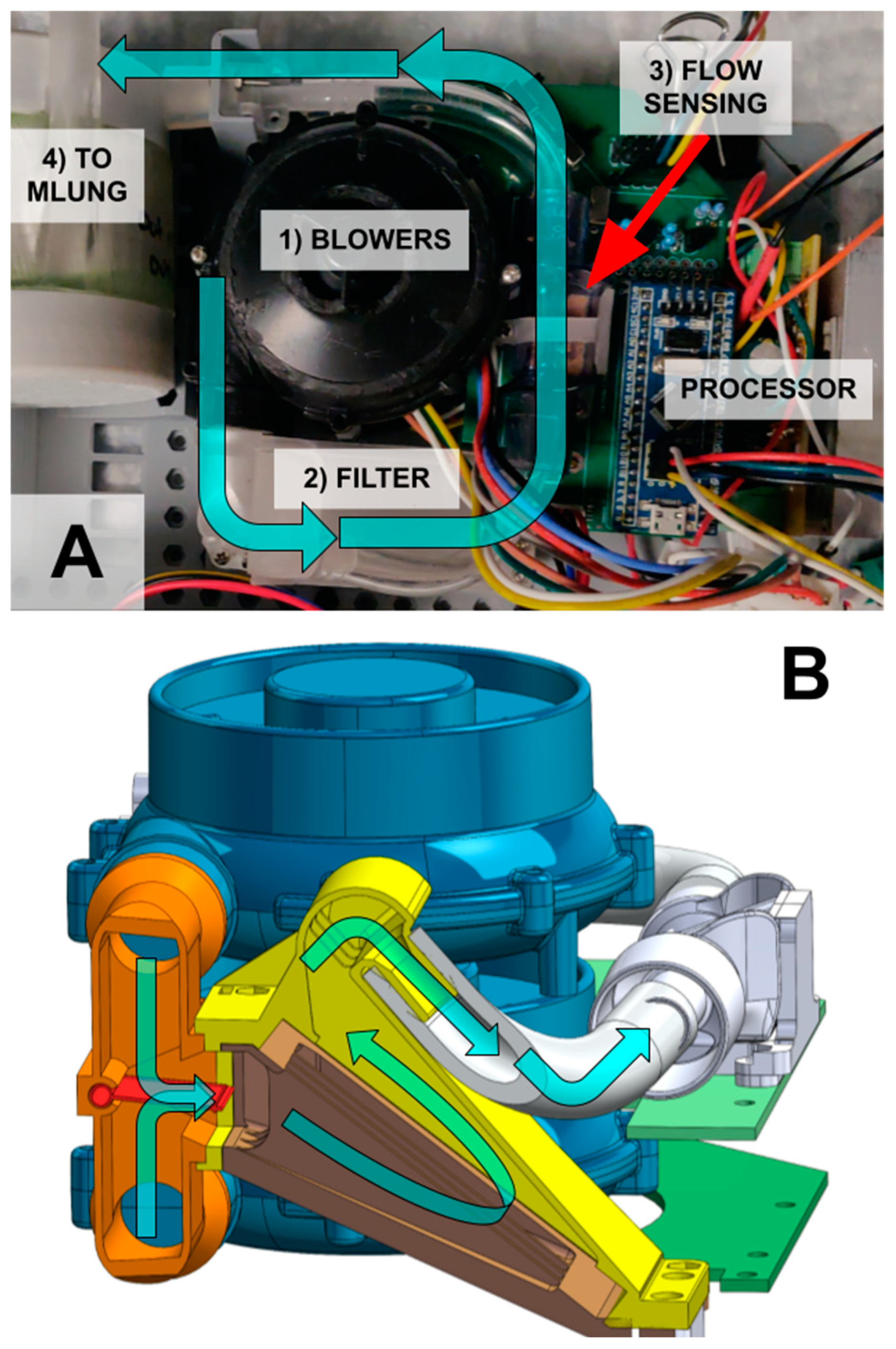
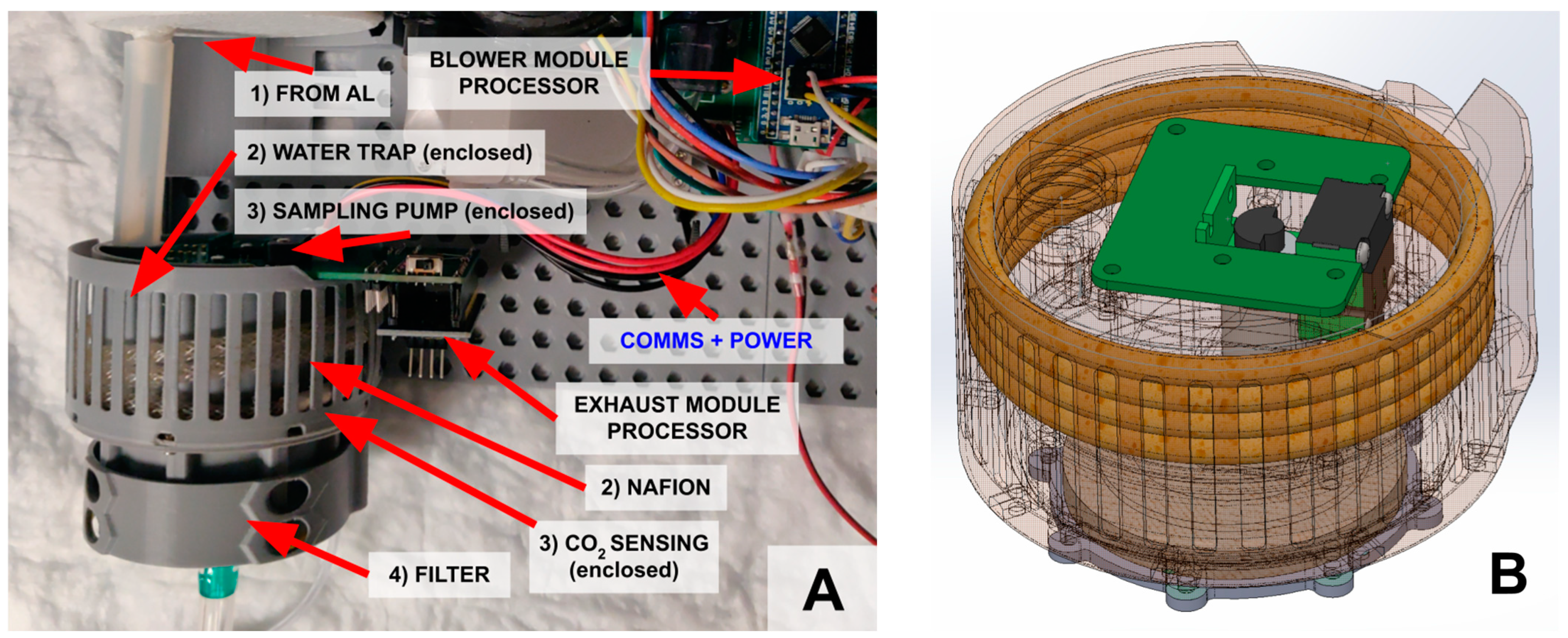
2.1.3. Blower Module
2.1.4. Exhaust (Smart) Module
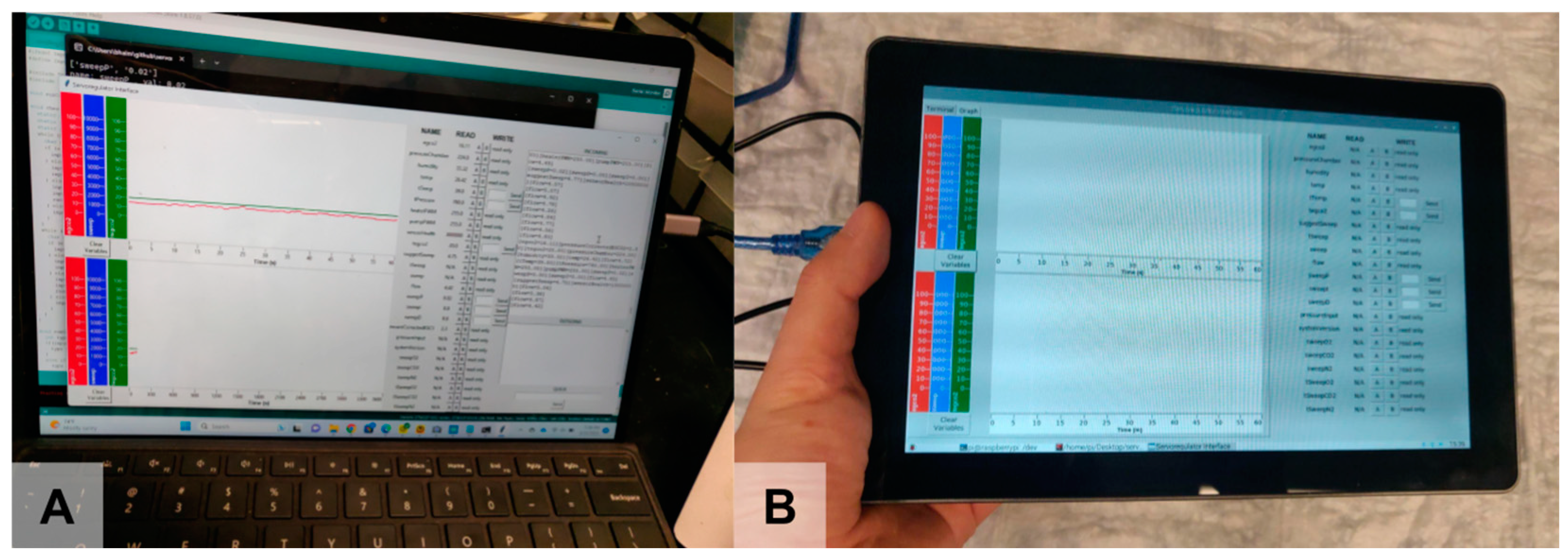
2.1.5. Usability Features
2.2. Subsystem Testing
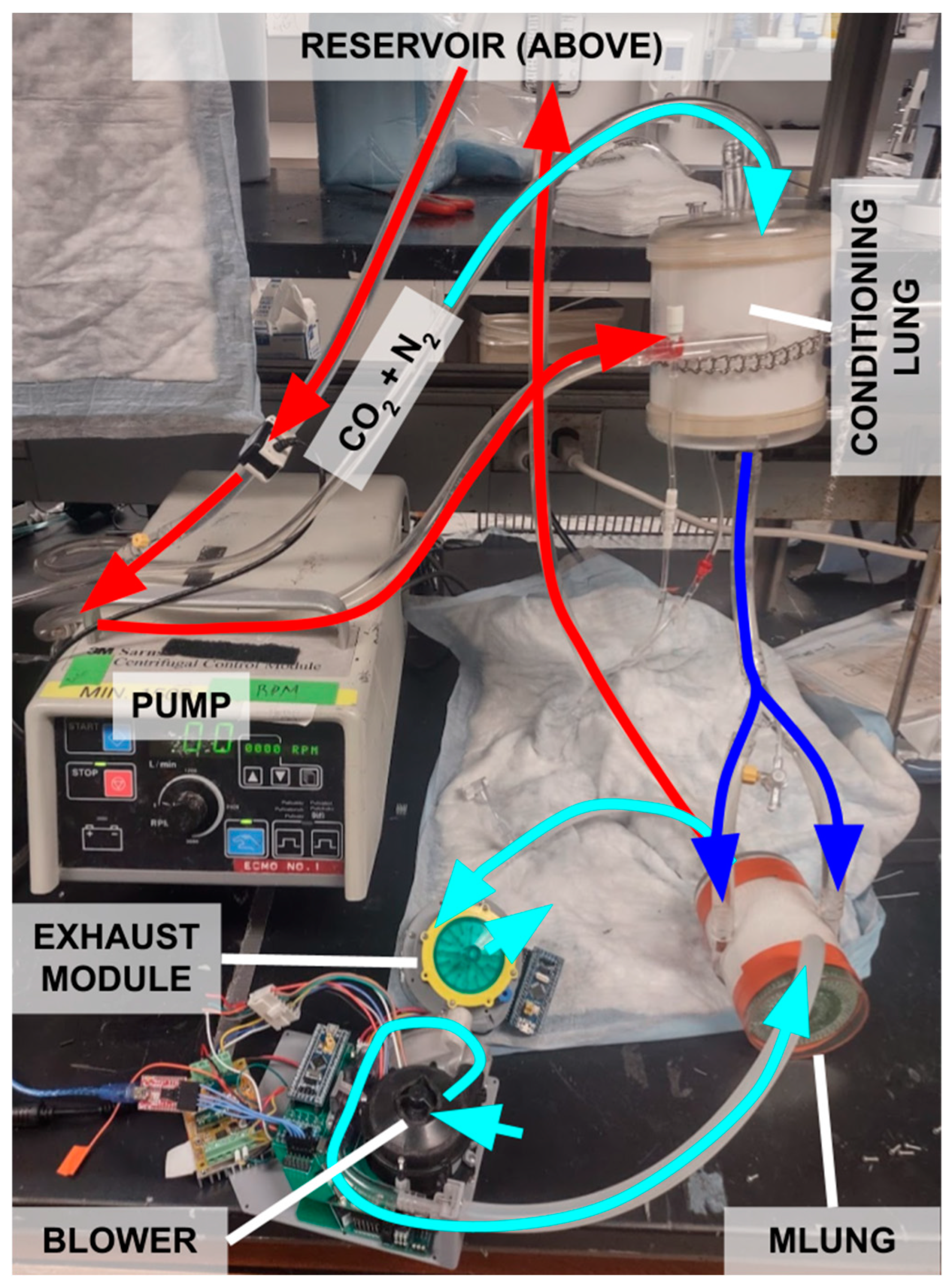
2.3. In Vitro Benchtop Testing with Water
2.4. Blood Testing Method
3. Results and Analysis
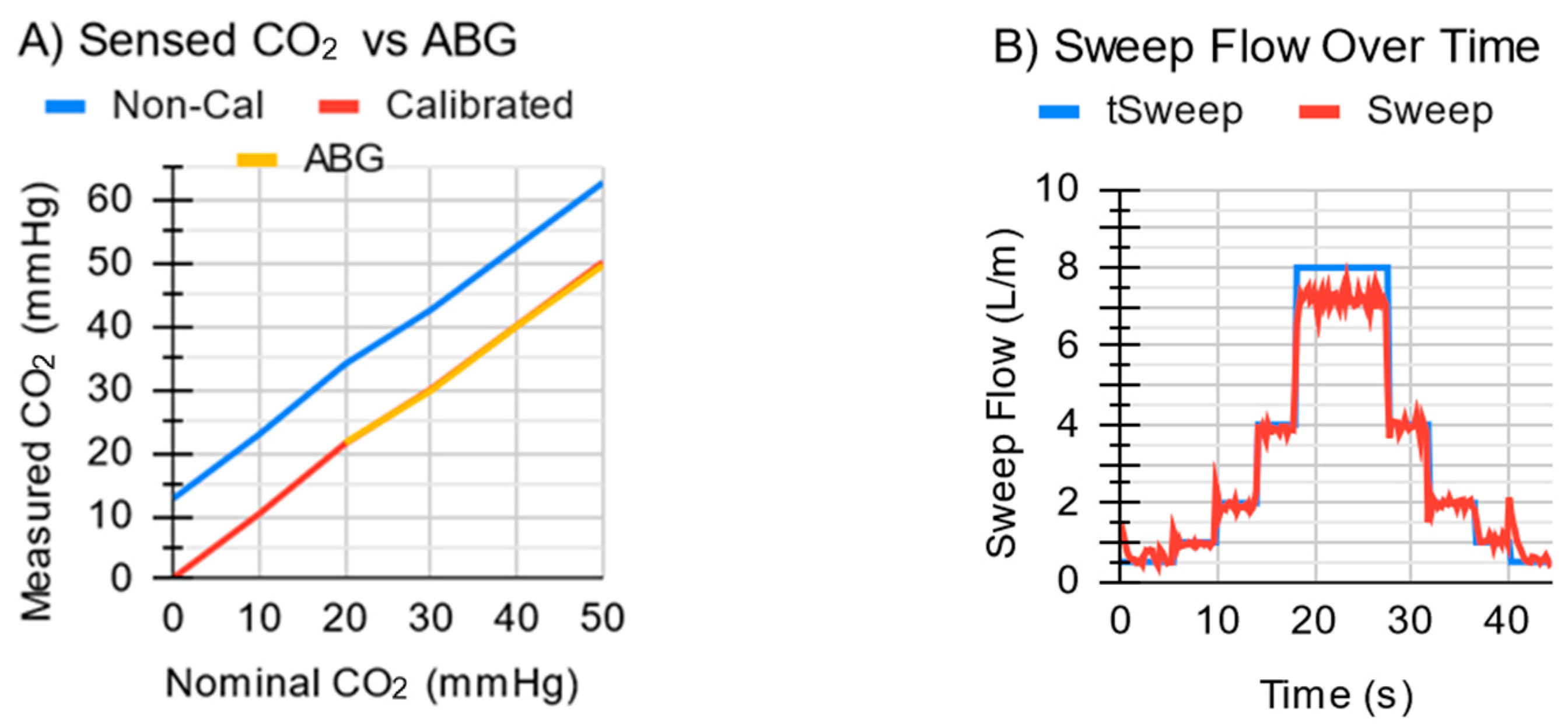
3.1. Subsystem Testing Results
3.2. In Vitro Benchtop Testing with Water Results
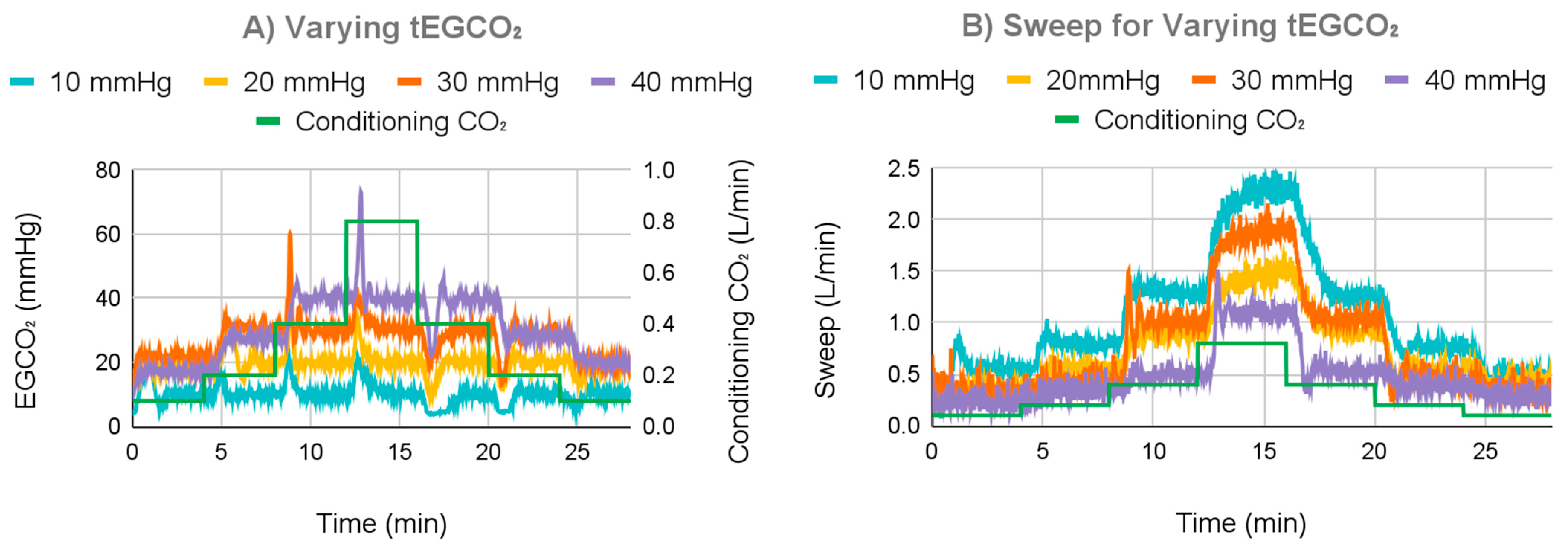
3.2.1. Varying tEGCO2 Results
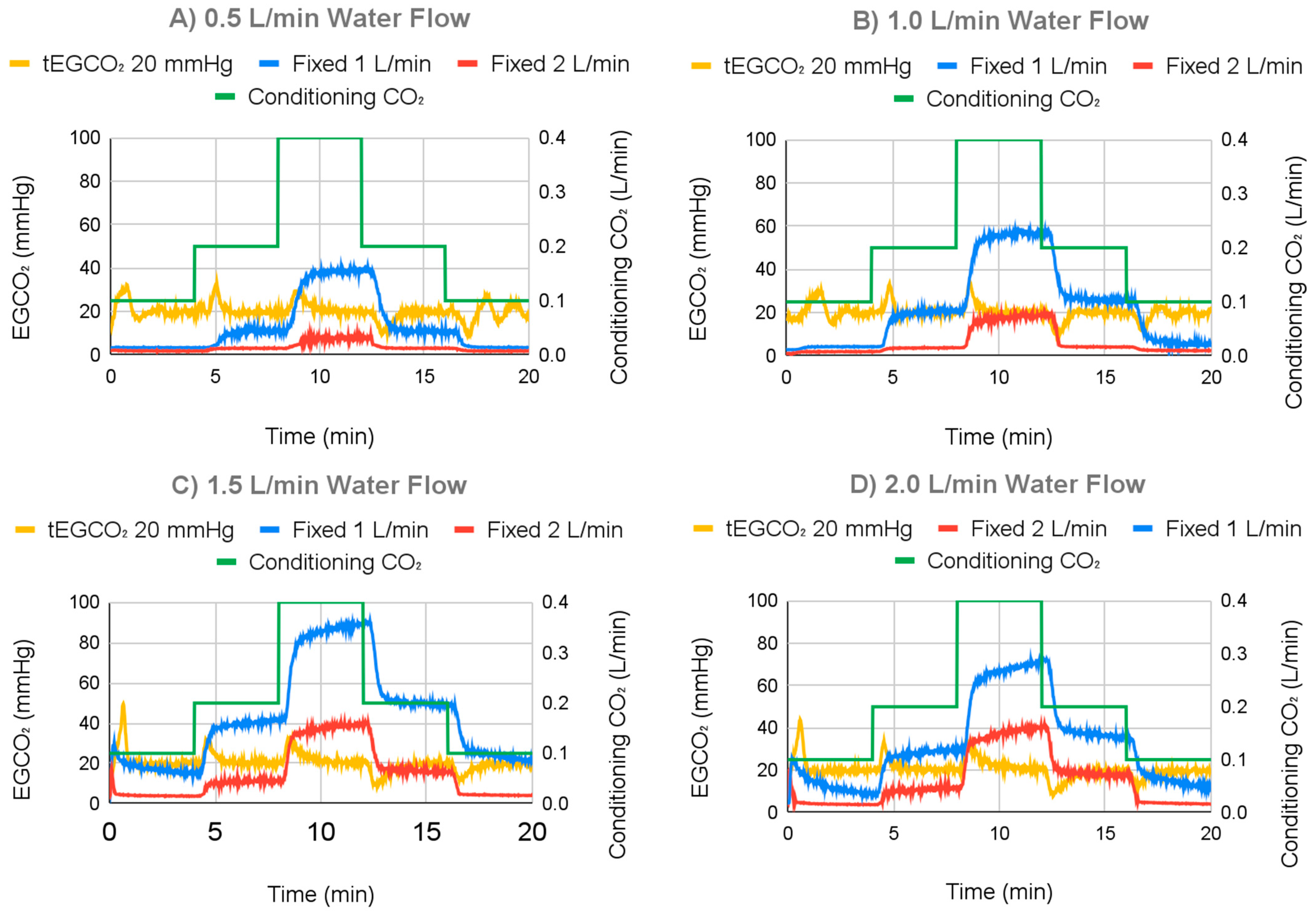
3.2.2. Varying Water Flow Rate Results
3.3. In Vitro Benchtop Testing with Blood Results
4. Discussion
4.1. Analysis of Performance
4.2. Mechanism of Action
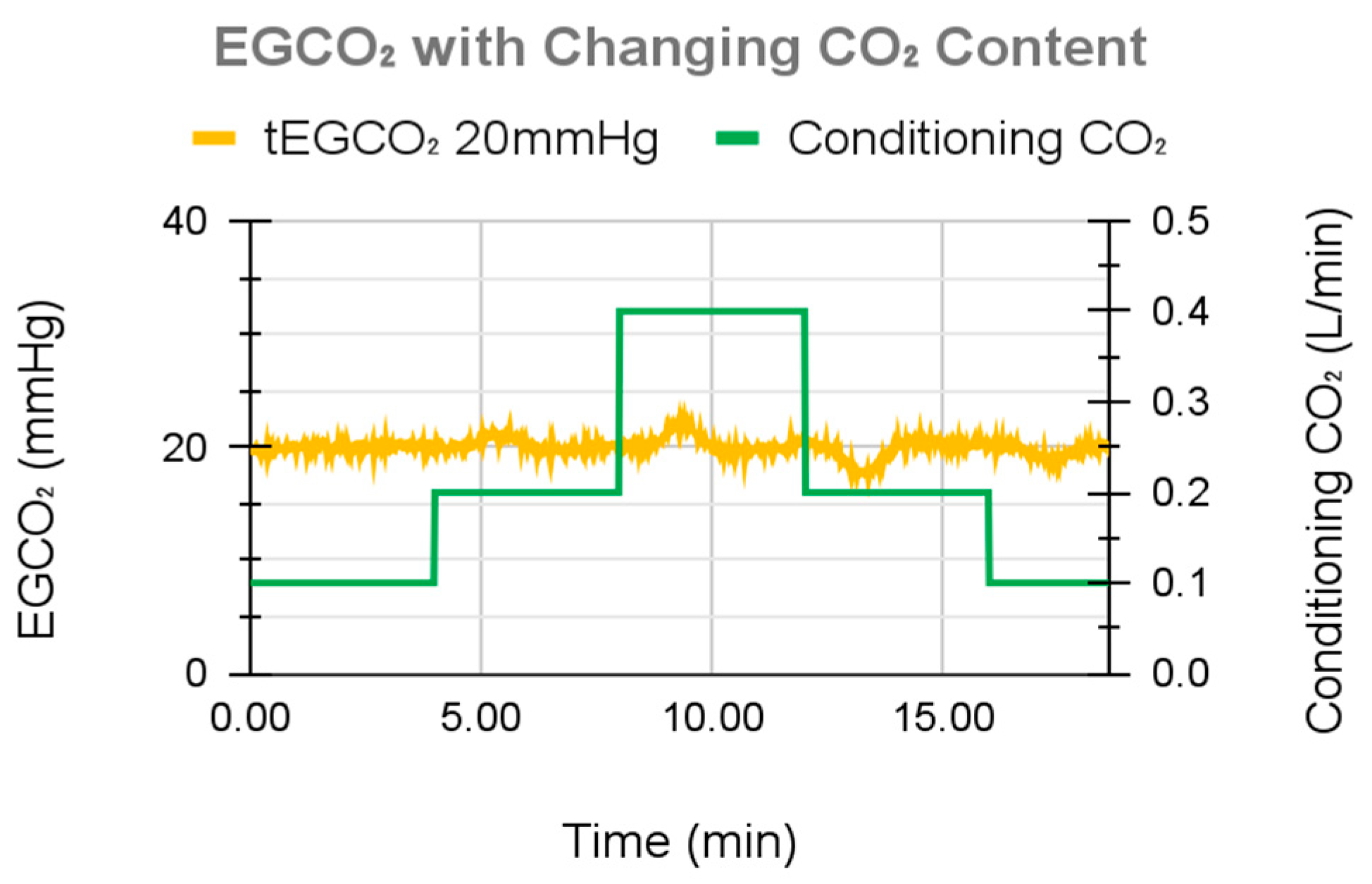
4.3. EGCO2 Overshoot during Simulated Metabolic Changes
4.4. Inability to Perform Low CO2 Evacuation
4.5. Regulation of EGCO2 Rather than Blood CO2
4.6. Tradeoffs in Physical Size
4.7. Planned Future Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makdisi, G.; Wang, I. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J. Thorac. Dis. 2015, 7, E166–E176. [Google Scholar] [PubMed]
- Smith, B.R.; Rinder, H.M. Chapter 52—Cardiopulmonary Bypass. In Platelets, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 1075–1096. [Google Scholar]
- Hillis, L.D.; Smith, P.K.; Anderson, J.L.; Bittl, J.A.; Bridges, C.R.; Byrne, J.G.; Cigarroa, J.E.; DiSesa, V.J.; Hiratzka, L.F.; Hutter, A.M.; et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011, 124, 2610–2642. [Google Scholar] [PubMed]
- Friedman, D.F.; Montenegro, L.M. Chapter 17—Extracorporeal Membrane Oxygenation and Cardiopulmonary Bypass. In Handbook of Pediatric Transfusion Medicine; Academic Press: Cambridge, MA, USA, 2004; pp. 181–189. [Google Scholar]
- Potkay, J.A.; Magnetta, M.; Vinson, A.; Cmolik, B. Bio-inspired, efficient, artificial lung employing air as the ventilating gas. Lab Chip 2011, 11, 2901–2909. [Google Scholar] [CrossRef]
- Matheis, G. New technologies for respiratory assist. Perfusion 2003, 18, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Hoganson, D.M.; Ii, H.I.P.; Bassett, E.K.; Spool, I.D.; Vacanti, J.P. Lung assist device technology with physiologic blood flow developed on a tissue engineered scaffold platform. Lab Chip 2011, 11, 700–707. [Google Scholar] [CrossRef]
- Kniazeva, T.; Hsiao, J.C.; Charest, J.L.; Borenstein, J.T. A microfluidic respiratory assist device with high gas permeance for artificial lung applications. Biomed. Microdevices 2011, 13, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zwischenberger, J.B.; Conrad, S.A.; Alpard, S.K.; Grier, L.R.; Bidani, A. Percutaneous extracorporeal arteriovenous CO2 removal for severe respiratory failure. Ann. Thorac. Surg. 1999, 68, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Guzman, E.; Hoopes, C.W.; Zwischenberger, J.B. The evolution of extracorporeal life support as a bridge to lung transplantation. ASAIO J. Am. Soc. Artif. Intern. Organs 2013, 59, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, C.W.; Kukreja, J.; Golden, J.; Davenport, D.L.; Diaz-Guzman, E.; Zwischenberger, J.B. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J. Thorac. Cardiovasc. Surg. 2013, 145, 862–867; discussion 867–868. [Google Scholar] [CrossRef]
- Bartlett, R.H. Physiology of Extracorporeal Gas Exchange. In Comprehensive Physiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 879–891. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/cphy.c190006 (accessed on 23 December 2022).
- Bartlett, R.H.; Gattinoni, L. Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva Anestesiol. 2010, 76, 534–540. [Google Scholar]
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators; Davies, A.; Jones, D.; Bailey, M.; Beca, J.; Bellomo, R.; Blackwell, N.; Forrest, P.; Gattas, D.; Granger, E.; et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009, 302, 1888–1895. [Google Scholar] [PubMed]
- Mangi, A.A.; Mason, D.P.; Yun, J.J.; Murthy, S.C.; Pettersson, G.B. Bridge to lung transplantation using short-term ambulatory extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2010, 140, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.P.; Kon, Z.N.; Evans, C.; Wu, Z.; Iacono, A.T.; McCormick, B.; Griffith, B.P. Ambulatory veno-venous extracorporeal membrane oxygenation: Innovation and pitfalls. J. Thorac. Cardiovasc. Surg. 2011, 142, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Bharat, A.; Pham, D.T.; Prasad, S.M. Ambulatory extracorporeal membrane oxygenation: A surgical innovation for adult respiratory distress syndrome. JAMA Surg. 2016, 151, 478–479. [Google Scholar] [CrossRef]
- Javidfar, J. The Challenges Faced with Early Mobilization of Patients on Extracorporeal Membrane Oxygenation. Crit. Care Med. 2018, 46, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Rehder, K.J.; Turner, D.A.; Hartwig, M.G.; Williford, W.L.; Bonadonna, D.; Walczak, R.J.; Davis, R.D.; Zaas, D.; Cheifetz, I.M. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir. Care 2013, 58, 1291–1298. [Google Scholar] [CrossRef]
- Olsson, K.M.; Simon, A.; Strueber, M.; Hadem, J.; Wiesner, O.; Gottlieb, J.; Fuehner, T.; Fischer, S.; Warnecke, G.; Kühn, C.; et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am. J. Transpl. Transplant. Off. J. Am. Soc. Transpl. Transplant. Am. Soc. Transpl. Surg. 2010, 10, 2173–2178. [Google Scholar] [CrossRef]
- Bain, J.C.; Turner, D.A.; Rehder, K.J.; Eisenstein, E.L.; Davis, R.D.; Cheifetz, I.M.; Zaas, D.W. Economic Outcomes of Extracorporeal Membrane Oxygenation with and Without Ambulation as a Bridge to Lung Transplantation. Respir. Care. 2016, 61, 1–7. [Google Scholar] [CrossRef]
- Mathies, G.; Novosel, E.; Hammer, T. Final Report Summary—AMBULUNG (Ambulatory Bio-Artificial Lung) [Internet]. 2016. Available online: https://cordis.europa.eu/project/id/304932/reporting/pl (accessed on 25 December 2022).
- Wu, Z.J.; Zhang, T.; Bianchi, G.; Wei, X.; Son, H.S.; Zhou, K.; Sanchez, P.G.; Garcia, J.; Griffith, B.P. Thirty-day in-vivo performance of a wearable artificial pump-lung for ambulatory respiratory support. Ann. Thorac. Surg. 2012, 93, 274–281. [Google Scholar] [CrossRef]
- Madhani, S.P.; Frankowski, B.J.; Burgreen, G.W.; Antaki, J.F.; Kormos, R.; D’Cunha, J.; Federspiel, W.J. In Vitro and In Vivo Evaluation of a Novel Integrated Wearable Artificial Lung. J. Heart Lung Transpl. Transplant. Off. Publ. Int. Soc. Heart Transplant. 2017, 36, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Zhang, A.; Jenter, J.; Nikpreljevic, B.; Toomasian, J.; Lynch, W.; Rojas-Peña, A.; Bartlett, R.H.; Potkay, J.A. A Portable Servoregulation Controller to Automate CO2 Removal in Artificial Lungs. Bioeng. Basel Switz. 2022, 9, 593. [Google Scholar] [CrossRef] [PubMed]
- Griffith, B.P.; Wu, Z.J.; Zhang, J. Pasta for all: Abiomed Breethe extracorporeal membrane oxygenation system. JTCVS Open 2021, 8, 108–113. [Google Scholar] [CrossRef]
- Orizondo, R.A.; Omecinski, K.S.; May, A.G.; Dhamotharan, V.; Frankowski, B.J.; Burgreen, G.W.; Ye, S.-H.; Kocyildirim, E.; Sanchez, P.G.; D’cUnha, J.; et al. Month-long Respiratory Support by a Wearable Pumping Artificial Lung in an Ovine Model. Transplantation 2021, 105, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Fisher, A.C.; Gaylor, J.D.; Razieh, A.R. Development of a digital adaptive control system for PO2 regulation in a membrane oxygenator. J. Biomed. Eng. 1992, 14, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, D.; Philipp, A.; Kaluza, M.; Detterbeck, M. On the way to an automatic heart-lung machine: A control system for oxygen tension in the oxygenator. Biomed. Tech. 1997, 42, 313–314. [Google Scholar] [CrossRef]
- Chauveau, N.; Lautier, A.; Frikha, M.R.; Barthelemy, R. Closed Loop Control of PaCO2 During ECC. Int. J. Artif. Organs. 1995, 18, 81–85. [Google Scholar] [CrossRef]
- Metz, S.I.; Bartlett, R.H.; Jenkins, J.M.; Kabamba, P.T. Controller design for extracorporeal life support. In Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Amsterdam, The Netherlands, 31 October–3 November 1996; Volume 4, pp. 1733–1735. [Google Scholar]
- Tobi, T. A practical application of fuzzy theory to an auto-regulation system for extra-corporeal circulation(ecc). In Industrial Applications of Fuzzy Technology in the World; Advances in Fuzzy Systems? Applications and Theory; World Scientific: Singapore, 1995; Volume 2, pp. 61–113. Available online: https://www.worldscientific.com/doi/10.1142/9789812830708_0004 (accessed on 25 December 2022).
- Hexamer, M.; Misgeld, B.; Prenger-Berninghoff, A.; Schütt, U.; Knobl, H.J.; Körfer, R.; Werner, J. Automatic control of the extra-corporal bypass: System analysis, modelling and evaluation of different control modes. Biomed. Tech. 2004, 49, 316–321. [Google Scholar] [CrossRef]
- Misgeld, B.J.E. Automatic Control of the Heart-Lung Machine. Ph.D. Thesis, Ruhr-University Bochum, Bochum, Germany, 2007. [Google Scholar]
- Wartzek, T.; Walter, M.; Schmitz-Rode, T.; Kowalewski, S.; Rossaint, R.; Leonhardt, S. In Vivo Validation of an Automatic Controlled Extracorporeal Membrane Oxygenator. In World Congress on Medical Physics and Biomedical Engineering; IFMBE Proceedings; Dössel, O., Schlegel, W.C., Eds.; Springer: Munich, Germany; Berlin/Heidelberg, Germany, 2009; pp. 118–121. [Google Scholar]
- Mendoza García, A.; Krane, M.; Baumgartner, B.; Sprunk, N.; Schreiber, U.; Eichhorn, S.; Lange, R.; Knoll, A. Automation of a portable extracorporeal circulatory support system with adaptive fuzzy controllers. Med. Eng. Phys. 2014, 36, 981–990. [Google Scholar] [CrossRef]
- Conway, R.G.; Berk, Z.B.; Zhang, J.; Li, T.; Tran, D.; Wu, Z.J.; Griffith, B.P. Evaluation of an autoregulatory ECMO system for total respiratory support in an acute ovine model. Artif. Organs. 2020, 44, 478–487. [Google Scholar] [CrossRef]
- Witer, L.; Howard, R.; Trahanas, J.; Bryner, B.S.; Alghanem, F.; Hoffman, H.R.; Cornell, M.S.; Bartlett, R.H.; Rojas-Peña, A. Large Animal Model of Pumpless Arteriovenous Extracorporeal CO2 Removal Using Room Air Via Subclavian Vessels. ASAIO J. Am. Soc. Artif. Intern. Organs 2016, 62, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Potkay, J.A.; Thompson, A.J.; Toomasian, J.; Lynch, W.; Bartlett, R.H.; Rojas-Peña, A. Toward a Servoregulation Controller to Automate CO2 Removal in Wearable Artificial Lungs. ASAIO J. Am. Soc. Artif. Intern. Organs 2022, 68, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Spencer, B.; Gudex, L.; Dann, T.; Langley, M.; Longan, K.; Matich, H.J.; Toomasian, J.; Lynch, W.; Bartlett, R.; et al. BIO2: Acute In Vivo Testing of a Portable Controller to Automate CO2 Removal in Artificial Lungs. ASAIO J. 2022, 68, 7–8. [Google Scholar] [CrossRef]
- Spencer, B.; Shaikh, N.; Gudex, L.; Dann, T.; Langley, M.; Matich, H.; Bartlett, R.H.; Rojas-Peña, A.; Potkay, J.A. In Vivo Testing of an Ambient Air Based, Portable, and Automated CO2 Removal Controller for Artificial Lungs. ASAIO J. Am. Soc. Artif. Intern. Organs, 2023; ahead of print. [Google Scholar]
- Zhang, A.; Hamowitz, B.J.; Shaikh, N.K.; Matich, H.; Rojas Pena, A.; Bartlett, R.H.; Rojas-Peña, A.; Potkay, J.A. BIO12: Toward a Wearable Extracorporeal CO2 Removal System with Integrated Exhaust Gas CO2 Sensing. ASAIO J. 2023, 69 (Suppl. S2), 21. [Google Scholar] [CrossRef]
- Biscotti, M.; Bacchetta, M. The “sport model”: Extracorporeal membrane oxygenation using the subclavian artery. Ann. Thorac. Surg. 2014, 98, 1487–1489. [Google Scholar] [CrossRef]
- Chen, J.M.; Richmond, M.E.; Charette, K.; Takayama, H.; Williams, M.; Gilmore, L.; Garcia, A.; Addonizio, L.J. A decade of pediatric mechanical circulatory support before and after cardiac transplantation. J. Thorac. Cardiovasc. Surg. 2012, 143, 344–351. [Google Scholar] [CrossRef]
- Fraser, C.D.; Jaquiss, R.D.B.; Rosenthal, D.N.; Humpl, T.; Canter, C.E.; Blackstone, E.H.; Naftel, D.C.; Ichord, R.N.; Bomgaars, L.; Tweddell, J.S.; et al. Prospective trial of a pediatric ventricular assist device. N. Engl. J. Med. 2012, 367, 532–541. [Google Scholar] [CrossRef]
- Fernando, U.P.; Thompson, A.J.; Potkay, J.; Cheriyan, H.; Toomasian, J.; Kaesler, A.; Schlanstein, P.; Arens, J.; Hirschl, R.B.; Bull, J.L.; et al. A Membrane Lung Design Based on Circular Blood Flow Paths. ASAIO J. Am. Soc. Artif. Intern. Organs 2017, 63, 637–643. [Google Scholar] [CrossRef]
- Lund, L.W.; Federspiel, W.J. Removing extra CO2 in COPD patients. Curr. Respir. Care Rep. 2013, 2, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Buchan, S.; Carr, B.; Poling, C.; Hayes, M.; Fernando, U.P.; Kaesler, A.; Schlanstein, P.; Hesselmann, F.; Arens, J.; et al. Low-Resistance, Concentric-Gated Pediatric Artificial Lung for End-Stage Lung Failure. ASAIO J. Am. Soc. Artif. Intern. Organs 2020, 66, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.L.; Johnson, M.D.; Wilhelm, S.K.; Lautner-Csorba, O.D.; Matich, H.; Lautner, G.; Fallon, B.P.; Dann, T.; Gudex, L.; Langley, M.; et al. A Pumpless Pediatric Artificial Lung Maintains Function for 72 h Without Systemic Anticoagulation Using the Nitric Oxide Surface Anticoagulation System. J. Pediatr. Surg. 2024, 59, 103–108. [Google Scholar] [CrossRef]
- Galen, D.; Meinders, Q.; Halfwerk, F.; Arens, J. ECMOve: A Mobilization Device for Extracorporeal Membrane Oxygenation Patients. ASAIO J. Am. Soc. Artif. Intern. Organs 2024, 70, 377–386. [Google Scholar] [CrossRef]
- Galen, D.J.; Meinders, Q.; Halfwerk, F.; Arens, J. P69: ECMO mobilizing device. ASAIO J. 2023, 69 (Suppl. S2), 148. [Google Scholar] [CrossRef]
- Lukitsch, B.; Koller, R.; Ecker, P.; Elenkov, M.; Janeczek, C.; Pekovits, M.; Haddadi, B.; Jordan, C.; Gfoehler, M.; Harasek, M. Water as a Blood Model for Determination of CO2 Removal Performance of Membrane Oxygenators. Membranes 2021, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology, 13th ed.; W B Saunders: London, UK, 2024. [Google Scholar]
- Chen, Y.; Li, D.; Liu, Z.; Liu, Y.; Fan, H.; Hou, S. Research progress of portable extracorporeal membrane oxygenation. Expert Rev. Med. Devices 2023, 20, 221–232. [Google Scholar] [CrossRef] [PubMed]
- May, A.G.; Orizondo, R.A.; Frankowski, B.J.; Wearden, P.D.; Federspiel, W.J. Acute In Vivo Evaluation of the Pittsburgh Pediatric Ambulatory Lung. ASAIO J. Am. Soc. Artif. Intern. Organs 2019, 65, 395–400. [Google Scholar] [CrossRef]
- Schmack, B.; Hanke, J.S.; Schmitto, J.D.; Kühn, C.; Ruhparwar, A. ECMO-TO-GO: Application of a portable on the body veno-arterial ECMO device in a bridge-to-transplantation setting. JHLT Open 2024, 4, 100069. [Google Scholar] [CrossRef]
- Flynn, A. What is the Smallest Portable Oxygen Concentrator [2023] [Internet]. SpryLyfe. 600. Available online: https://sprylyfe.com/blogs/oxygen-blog/smallest-portable-oxygen-concentrator (accessed on 25 January 2024).
- M6 Cylinder [Internet]. Clinical 1. Available online: https://clinical1.com/product/m6-cylinder/ (accessed on 25 January 2024).

| Sweep Control | Avg EGCO2 (mmHg) | Stdev EGCO2 (mmHg) | Max EGCO2 (mmHg) | Min EGCO2 (mmHg) |
|---|---|---|---|---|
| 10 mmHg | 10.09 | 3.05 | 23.15 | 3.92 |
| 20 mmHg | 19.43 | 3.15 | 34.38 | 9.24 |
| 30 mmHg | 27.23 | 5.49 | 60.09 | 9.80 |
| 40 mmHg | 30.22 | 9.80 | 73.02 | 9.80 |
| 1.0 L/min | 16.92 | 17.80 | 57.85 | 2.73 |
| 2.0 L/min | 4.72 | 5.40 | 20.20 | 0.85 |
| Sweep Control | Settling Time (min) | Avg EGCO2 (mmHg) | Stdev EGCO2 (mmHg) | Max EGCO2 (mmHg) | Min EGCO2 (mmHg) |
|---|---|---|---|---|---|
| 10 mmHg | 0.63 | 9.92 | 0.70 | 12.61 | 6.99 |
| 20 mmHg | 0.51 | 19.30 | 1.27 | 22.16 | 14.72 |
| 30 mmHg | 0.58 | 29.97 | 0.97 | 32.42 | 26.52 |
| 40 mmHg | 0.53 | 39.92 | 0.90 | 42.53 | 37.48 |
| Feature | Wearable Smart ECCO2R | Portable Smart ECCO2R [25] | Breathe [26] | Wearable Pumping AL [27] |
|---|---|---|---|---|
| High FiO2 | No | No | Yes | Yes |
| Compatible with multiple ALs | No | Yes | No | No |
| Wearable | Yes | No | With separate gas supply and controller | With separate gas supply and controller |
| Active negative feedback | Yes | Yes | No | No |
| Blood pump (for VA or VV operation) | No | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Haimowitz, B.J.; Tharwani, K.; Rojas-Peña, A.; Bartlett, R.H.; Potkay, J.A. A Wearable Extracorporeal CO2 Removal System with a Closed-Loop Feedback. Bioengineering 2024, 11, 969. https://doi.org/10.3390/bioengineering11100969
Zhang A, Haimowitz BJ, Tharwani K, Rojas-Peña A, Bartlett RH, Potkay JA. A Wearable Extracorporeal CO2 Removal System with a Closed-Loop Feedback. Bioengineering. 2024; 11(10):969. https://doi.org/10.3390/bioengineering11100969
Chicago/Turabian StyleZhang, Andrew, Brian J. Haimowitz, Kartik Tharwani, Alvaro Rojas-Peña, Robert H. Bartlett, and Joseph A. Potkay. 2024. "A Wearable Extracorporeal CO2 Removal System with a Closed-Loop Feedback" Bioengineering 11, no. 10: 969. https://doi.org/10.3390/bioengineering11100969
APA StyleZhang, A., Haimowitz, B. J., Tharwani, K., Rojas-Peña, A., Bartlett, R. H., & Potkay, J. A. (2024). A Wearable Extracorporeal CO2 Removal System with a Closed-Loop Feedback. Bioengineering, 11(10), 969. https://doi.org/10.3390/bioengineering11100969








