Scaffolds for Osteochondral Lesions of the Talus: Systematic Review and Meta-Analysis of the Last Ten Years Literature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Searches
2.2. Study Selection
2.3. Data Extraction
2.4. Risk of Bias
2.5. Quantitative Synthesis and Statistical Analysis
3. Results
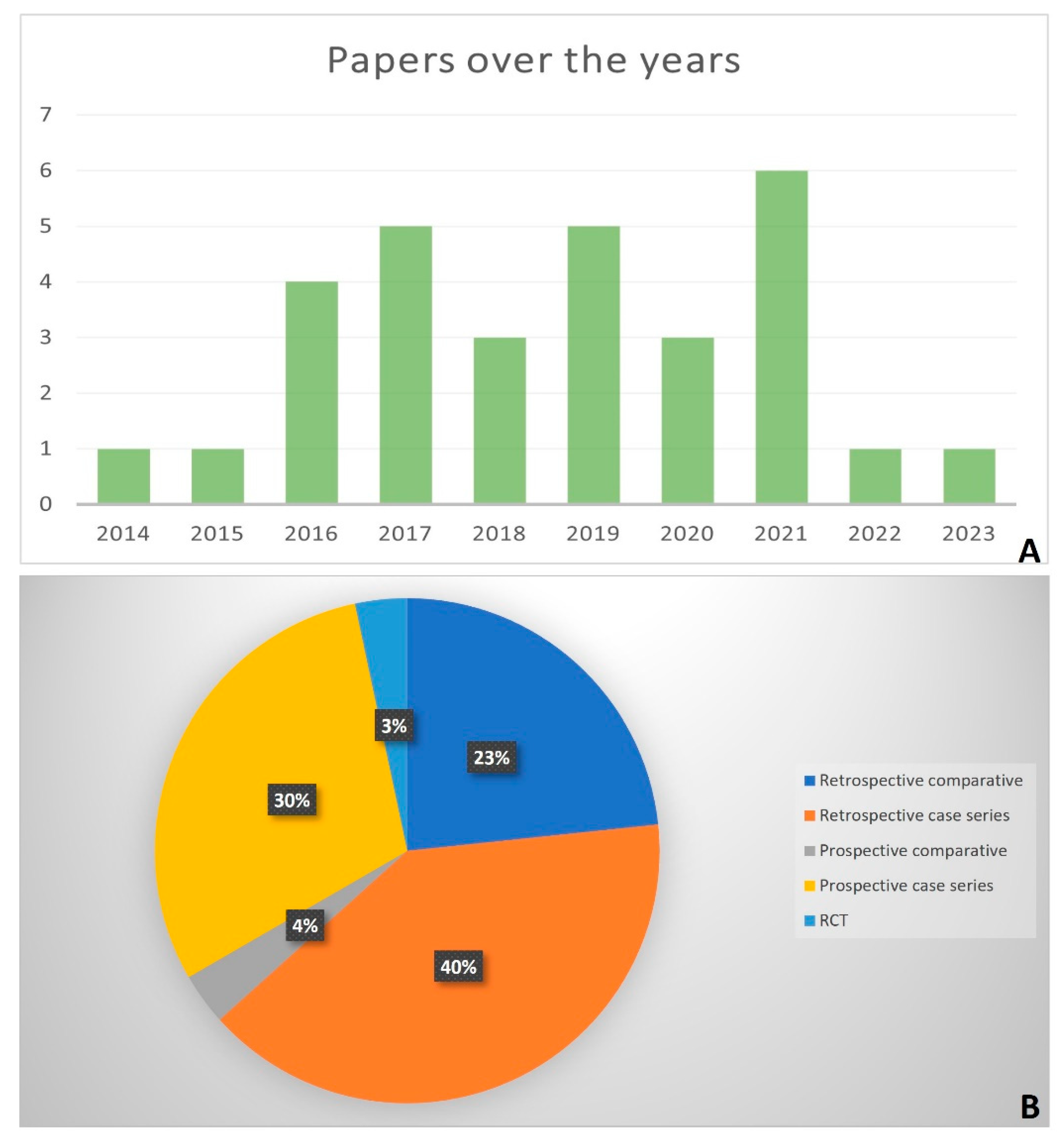
3.1. Study Type
3.2. Risk of Bias
3.3. Patients and Lesion Characteristics
3.4. Surgical Approach
3.5. Qualitative Analysis
3.5.1. Retrospective Case Series
3.5.2. Retrospective Comparative Studies
3.5.3. Prospective Case Series
3.5.4. Prospective Comparative Studies
3.6. Safety and Complications
3.7. Correlations
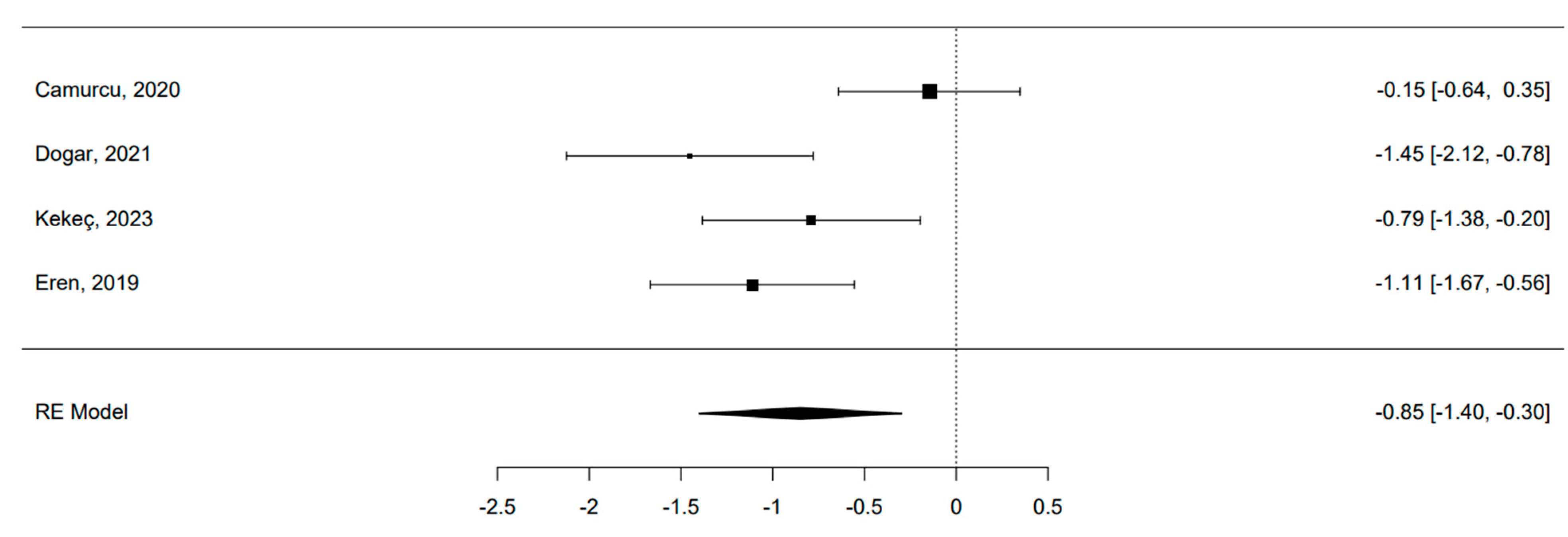
3.8. Quantitative Analysis: MF Alone vs. MF and Scaffold
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ozenci, A.M.; Aydin, A.T. Osteochondral lesions of the talus in adolescents. Acta. Orthop. Traumatol. Turc. 2004, 38 (Suppl. S1), 138–144. [Google Scholar] [PubMed]
- Kappis, M. Weitere beitrage zur traumatisch-mechanischen Entstehung der “spontanen” Knor-pela biosungen. Dtsch. Z. Chir. 1922, 171, 13–29. [Google Scholar] [CrossRef]
- Laffernetre, O. Osteochondral lesions of the talus: Current concept. Orthop. Traumatol. Surg. Res. 2010, 96, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Murawski, C.D.; Kennedy, J.G. Operative treatment of osteochondral lesions of the talus. J. Bone Jt. Surg. Am. 2013, 95, 1045–1054. [Google Scholar] [CrossRef]
- Sandlin, M.I.; Charlton, T.P.; Taghavi, C.E.; Giza, E. Management of osteochondral lesions of the talus. Instr. Course Lect. 2017, 66, 293–299. [Google Scholar]
- Dragoni, M.; Bonasia, D.E.; Amendola, A. Osteochondral talar allograft for large osteochondral defects: Technique tip. Foot Ankle Int. 2011, 32, 910–916. [Google Scholar] [CrossRef]
- Leontaritis, N.; Hinojosa, L.; Panchbhavi, V.K. Arthroscopically detected intra-articular lesions associated with acute ankle fractures. J. Bone Jt. Surg. Am. 2009, 91, 333–339. [Google Scholar] [CrossRef]
- Kılıçaslan, Ö.F.; Levent, A.; Çelik, H.K.; Tokgöz, M.A.; Köse, Ö.; Rennie, A.E.W. Effect of cartilage thickness mismatch in osteochondral grafting from knee to talus on articular contact pressures: A finite element analysis. Jt. Dis. Relat. Surg. 2021, 32, 355–362. [Google Scholar] [CrossRef]
- Looze, C.A.; Capo, J.; Ryan, M.K.; Begly, J.P.; Chapman, C.; Swanson, D.; Singh, B.C.; Strauss, E.J. Evaluation and management of osteochondral lesions of the talus. Cartilage 2017, 8, 19–30. [Google Scholar] [CrossRef]
- Dekker, T.J.; Dekker, P.K.; Tainter, D.M.; Easley, M.E.; Adams, S.B. Treatment of osteochondral lesions of the talus: A critical analysis review. JBJS Rev. 2017, 5, e4. [Google Scholar] [CrossRef]
- Yasui, Y.; Wollstein, A.; Murawski, C.D.; Kennedy, J.G. Operative treatment for osteochondral lesions of the talus: Biologics and scaffold-based therapy. Cartilage 2017, 8, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Şahin, A.A.; Değirmenci, E.; Özturan, K.E.; Fırat, T.; Kükner, A. Effects of adipose tissue-derived stromal vascular fraction on osteochondral defects treated by hyaluronic acid-based scaffold: An experimental study. Jt. Dis. Relat. Surg. 2021, 32, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Goh, G.S.; Bin, A.B.D.; Razak, H.R.; Mitra, A.K. Outcomes are favorable after arthroscopic treatment of osteochondritis dissecans of the talus. J. Foot Ankle Surg. 2015, 54, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Apprich, S.; Trattnig, S.; Welsch, G.H.; Noebauer-Huhmann, I.M.; Sokolowski, M.; Hirschfeld, C.; Stelzeneder, D.; Domayer, S. Assessment of articular cartilage repair tissue after matrix-associated autologous chondrocyte transplantation or the microfracture technique in the ankle joint using diffusion weighted imaging at 3 Tesla. Osteoarthr. Cartil. 2012, 20, 703–711. [Google Scholar] [CrossRef]
- Becher, C.; Malahias, M.A.; Ali, M.M.; Maffulli, N.; Thermann, H. Arthroscopic microfracture vs. arthroscopic autologous matrixinduced chondrogenesis for the treatment of articular cartilage defects of the talus. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2731–2736. [Google Scholar] [CrossRef]
- Van den Borne, M.P.; Raijmakers, N.J.; Vanlauwe, J.; Victor, J.; de Jong, S.N.; Bellemans, J.; Saris, D.B.; International Cartilage Repair Society. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in autologous chondrocyte implantation (ACI) and microfracture. Osteoarthr. Cartil. 2007, 15, 1397–1402. [Google Scholar] [CrossRef]
- Usuelli, F.G.; D’Ambrosi, R.; Maccario, C.; Boga, M.; de Girolamo, L. Allarthroscopic AMIC® (AT-AMIC®) technique with autologous bone graft for talar osteochondral defects: Clinical and radiological results. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 875–881. [Google Scholar] [CrossRef]
- Erickson, B.; Fillingham, Y.; Hellman, M.; Parekh, S.G.; Gross, C.E. Surgical management of large talar osteochondral defects using autologous chondrocyte implantation. Foot Ankle Surg. 2018, 24, 131–136. [Google Scholar] [CrossRef]
- López-Alcorocho, J.M.; Guillén-Vicente, I.; Rodríguez-Iñigo, E.; Navarro, R.; Caballero-Santos, R.; Guillén-Vicente, M.; Casqueiro, M.; Fernández-Jaén, T.M.; Sanz, F.; Arauz, S.; et al. High-density autologous chondrocyte implantation as treatment for ankle osteochondral defects. Cartilage 2021, 12, 307–319. [Google Scholar] [CrossRef]
- Richter, M.; Zech, S. Matrix-associated stem cell transplantation (MAST) in chondral lesions at the ankle as part of a complex surgical approach—5-year-follow-up in 100 patients. Foot Ankle Surg. 2019, 25, 264–271. [Google Scholar] [CrossRef]
- Gillie, J.G.; Moeckel, G.; Bark, S.; Behrens, P. Novel cartilage repair strategies-The AMIC technique. J. Orthop. 2012, 4, 99–104. [Google Scholar]
- Rojo, L. Combination of Polymeric Supports and Drug Delivery Systems for Osteochondral Regeneration. Adv. Exp. Med. Biol. 2018, 1059, 301–303. [Google Scholar] [PubMed]
- Ramzan, F.; Salim, A.; Khan, I. Osteochondral Tissue Engineering Dilemma: Scaffolding Trends in Regenerative Medicine. Stem Cell Rev. Rep. 2023, 19, 1615–1634. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Sun, M.; Leng, X.; Hu, X.; Ao, Y. Recent Progress in 3D Printing of Elastic and High-Strength Hydrogels for the Treatment of Osteochondral and Cartilage Diseases. Front. Bioeng. Biotechnol. 2020, 8, 604814. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Kon, E.; Verdonk, P.; Condello, V.; Delcogliano, M.; Dhollander, A.; Filardo, G.; Pignotti, E.; Marcacci, M. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: Systematic clinical data review and study quality analysis. Am. J. Sports Med. 2009, 37, 156S–166S. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Weigelt, L.; Hartmann, R.; Pfirrmann, C.; Espinosa, N.; Wirth, S.H. Autologous Matrix-Induced Chondrogenesis for Osteochondral Lesions of the Talus: A Clinical and Radiological 2- to 8-Year Follow-up Study. Am. J. Sports Med. 2019, 47, 1679–1686. [Google Scholar] [CrossRef]
- Gottschalk, O.; Baumbach, S.F.; Altenberger, S.; Körner, D.; Aurich, M.; Plaass, C.; Ettinger, S.; Guenther, D.; Becher, C.; Hörterer, H.; et al. Influence of the Medial Malleolus Osteotomy on the Clinical Outcome of M-BMS + I/III Collagen Scaffold in Medial Talar Osteochondral Lesion (German Cartilage Register/Knorpelregister DGOU). Cartilage 2021, 13, 1373S–1379S. [Google Scholar] [CrossRef]
- Ayyaswamy, B.; Salim, M.; Sidaginamale, R.; Elsayed, M.; Karpe, P.; Limaye, R. Early to medium term outcomes of osteochondral lesions of the talus treated by autologous matrix induced chondrogenesis (AMIC). Foot Ankle Surg. 2021, 27, 207–212. [Google Scholar] [CrossRef]
- Yontar, N.S.; Aslan, L.; Can, A.; Ogut, T. One step treatment of talus osteochondral lesions with microfracture and cell free hyaluronic acid based scaffold combination. Acta Orthop. Traumatol. Turc. 2019, 53, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Giannini, S.; Buda, R.; Ruffilli, A.; Cavallo, M.; Pagliazzi, G.; Bulzamini, M.C.; Desando, G.; Luciani, D.; Vannini, F. Arthroscopic autologous chondrocyte implantation in the ankle joint. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 1311–1319. [Google Scholar] [CrossRef]
- Di Cave, E.; Versari, P.; Sciarretta, F.; Luzon, D.; Marcellini, L. Biphasic bioresorbable scaffold (TruFit Plug®) for the treatment of osteochondral lesions of talus: 6- to 8-year follow-up. Foot 2017, 33, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Kanatlı, U.; Eren, A.; Eren, T.K.; Vural, A.; Geylan, D.E.; Öner, A.Y. Single-Step Arthroscopic Repair With Cell-Free Polymer-Based Scaffold in Osteochondral Lesions of the Talus: Clinical and Radiological Results. Arthroscopy 2017, 33, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Martinelli, N.; Bianchi, A.; Messina, C.; Malerba, F.; Sconfienza, L.M. Clinical and imaging outcome of osteochondral lesions of the talus treated using autologous matrix-induced chondrogenesis technique with a biomimetic scaffold. BMC Musculoskelet. Disord. 2017, 18, 306. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, R.; Zhang, J.; Tao, H.; Hua, Y. Outcomes of arthroscopic bone graft transplantation for Hepple stage V osteochondral lesions of the talus. Ann. Transl. Med. 2021, 9, 884. [Google Scholar] [CrossRef]
- Georgiannos, D.; Bisbinas, I.; Badekas, A. Osteochondral transplantation of autologous graft for the treatment of osteochondral lesions of talus: 5- to 7-year follow-up. Knee. Surg. Sports Traumatol. Arthrosc. 2016, 24, 3722–3729. [Google Scholar] [CrossRef]
- Sawa, M.; Nakasa, T.; Ikuta, Y.; Yoshikawa, M.; Tsuyuguchi, Y.; Kanemitsu, M.; Ota, Y.; Adachi, N. Outcome of autologous bone grafting with preservation of articular cartilage to treat osteochondral lesions of the talus with large associated subchondral cysts. Bone Joint. J. 2018, 100-B, 590–595. [Google Scholar] [CrossRef]
- Heida, K.A., Jr.; Tihista, M.C.; Kusnezov, N.A.; Dunn, J.C.; Orr, J.D. Outcomes and Predictors of Postoperative Pain Improvement Following Particulated Juvenile Cartilage Allograft Transplant for Osteochondral Lesions of the Talus. Foot Ankle Int. 2020, 41, 572–581. [Google Scholar] [CrossRef]
- Akmeşe, R.; Ertan, M.B.; Kocaoğlu, H. Comparison of Chitosan-Based Liquid Scaffold and Hyaluronic Acid-Based Soft Scaffold for Treatment of Talus Osteochondral Lesions. Foot Ankle Int. 2020, 41, 1240–1248. [Google Scholar] [CrossRef]
- Camurcu, Y.; Ucpunar, H.; Yapici, F.; Karakose, R.; Ozcan, S.; Cobden, A.; Duman, S.; Sofu, H. Clinical and Magnetic Resonance Imaging Outcomes of Microfracture Plus Chitosan/Blood Implant vs Microfracture for Osteochondral Lesions of the Talus. Foot Ankle Int. 2020, 41, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Doğar, F.; Uzun, E.; Gürbüz, K.; Topak, D.; Akar, M.; Bilal, O.; Güney, A. Comparison of Arthroscopic Treatment Methods in Talar Osteochondral Lesions: A Multicenter, Prospective, Randomized Clinical Trial. J. Am. Podiatr. Med. Assoc. 2021, 111, 5. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, B.; Gamlı, A.; Duran, M.E.; Bayram, B.; Ulku, T.K.; Kocaoglu, B. Collagen Scaffold Application in Arthroscopic Reconstruction of Osteochondral Lesions of the Talus With Autologous Cancellous Bone Grafts. Orthop. J. Sports Med. 2023, 11, 23259671221145733. [Google Scholar] [CrossRef]
- Kekeç, A.F.; Yıldırım, A. Mid-term results of autologous matrix-induced chondrogenesis surgery with or without scaffolds for arthroscopic treatment of deep talus osteochondral lesions: A comparative study. Jt. Dis. Relat. Surg. 2023, 34, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Eren, T.K.; Ataoğlu, M.B.; Eren, A.; Geylan, D.E.; Öner, A.Y.; Kanatlı, U. Comparison of arthroscopic microfracture and cell-free scaffold implantation techniques in the treatment of talar osteochondral lesions. Eklem. Hastalik. Cerrahisi. 2019, 30, 97–105. [Google Scholar] [CrossRef]
- Karnovsky, S.C.; DeSandis, B.; Haleem, A.M.; Sofka, C.M.; O’Malley, M.; Drakos, M.C. Comparison of Juvenile Allogenous Articular Cartilage and Bone Marrow Aspirate Concentrate Versus Microfracture with and Without Bone Marrow Aspirate Concentrate in Arthroscopic Treatment of Talar Osteochondral Lesions. Foot Ankle Int. 2018, 39, 393–405. [Google Scholar] [CrossRef]
- Christensen, B.B.; Foldager, C.B.; Jensen, J.; Jensen, N.C.; Lind, M. Poor osteochondral repair by a biomimetic collagen scaffold: 1- to 3-year clinical and radiological follow-up. Knee. Surg. Sports Traumatol. Arthrosc. 2016, 24, 2380–2387. [Google Scholar] [CrossRef]
- Gottschalk, O.; Altenberger, S.; Baumbach, S.; Kriegelstein, S.; Dreyer, F.; Mehlhorn, A.; Hörterer, H.; Töpfer, A.; Röser, A.; Walther, M. Functional Medium-Term Results After Autologous Matrix-Induced Chondrogenesis for Osteochondral Lesions of the Talus: A 5-Year Prospective Cohort Study. J. Foot Ankle Surg. 2017, 56, 930–936. [Google Scholar] [CrossRef]
- Galla, M.; Duensing, I.; Kahn, T.L.; Barg, A. Open reconstruction with autologous spongiosa grafts and matrix-induced chondrogenesis for osteochondral lesions of the talus can be performed without medial malleolar osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2789–2795. [Google Scholar] [CrossRef]
- Kubosch, E.J.; Erdle, B.; Izadpanah, K.; Kubosch, D.; Uhl, M.; Südkamp, N.P.; Niemeyer, P. Clinical outcome and T2 assessment following autologous matrix-induced chondrogenesis in osteochondral lesions of the talus. Int. Orthop. 2016, 40, 65–71. [Google Scholar] [CrossRef]
- Sadlik, B.; Kolodziej, L.; Blasiak, A.; Szymczak, M.; Warchal, B. Biological reconstruction of large osteochondral lesions of the talar dome with a modified “sandwich” technique-Midterm results. Foot Ankle Surg. 2017, 23, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Drobnič, M.; Kolar, M.; Verdonk, P.; Vannini, F.; Robinson, D.; Altschuler, N.; Shabshin, N.; Kon, E. Complex Osteochondral Lesions of the Talus Treated With a Novel Bi-Phasic Aragonite-based Implant. J. Foot Ankle Surg. 2021, 60, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Younger, A.; Wing, K.; Penner, M.; Cresswell, M. A study to evaluate the safety of platelet-derived growth factor for treatment of osteochondral defects of the talus. Knee. Surg. Sports Traumatol. Arthrosc. 2016, 24, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Sadlik, B.; Kolodziej, L.; Puszkarz, M.; Laprus, H.; Mojzesz, M.; Whyte, G.P. Surgical repair of osteochondral lesions of the talus using biologic inlay osteochondral reconstruction: Clinical outcomes after treatment using a medial malleolar osteotomy approach compared to an arthroscopically-assisted approach. Foot Ankle Surg. 2019, 25, 449–456. [Google Scholar] [CrossRef]
- Guney, A.; Akar, M.; Karaman, I.; Oner, M.; Guney, B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2384–2389. [Google Scholar] [CrossRef]
- Walther, M.; Gottschalk, O.; Madry, H.; Müller, P.E.; Steinwachs, M.; Niemeyer, P.; Niethammer, T.R.; Tischer, T.; Petersen, J.; Feil, R.; et al. Etiology, Classification, Diagnostics, and Conservative Management of Osteochondral Lesions of the Talus. 2023 Recommendations of the Working Group “Clinical Tissue Regeneration” of the German Society of Orthopedics and Traumatology. Cartilage 2023, 14, 292–304. [Google Scholar] [CrossRef]
- Posadzy, M.; Desimpel, J.; Vanhoenacker, F. Staging of Osteochondral Lesions of the Talus: MRI and Cone Beam CT. J. Belg. Soc. Radiol. 2017, 101, 1. [Google Scholar] [CrossRef]
- Mintz, D.N.; Tashjian, G.S.; Connell, D.A.; Deland, J.T.; O’Malley, M.; Potter, H.G. Osteochondral lesions of the talus: A new magnetic resonance grading system with arthroscopic correlation. Arthroscopy. 2003, 19, 353–359. [Google Scholar] [CrossRef]
- Lasanianos, N.G.; Giannoudis, P.V. Osteochondral Lesions of the Talus. In Trauma and Orthopaedic Classifications; Lasanianos, N., Kanakaris, N., Giannoudis, P., Eds.; Springer: London, UK, 2015. [Google Scholar]
- Gowda, B.N.; Kumar, J.M. Outcome of ankle arthrodesis in posttraumatic arthritis. Indian J. Orthop. 2012, 46, 317–320. [Google Scholar] [CrossRef]
- Martin, K.D.; McBride, T.; Wake, J.; Van Buren, J.P.; Dewar, C. Comparison of Visual Analog Pain Score Reported to Physician vs Nurse in Nonoperatively Treated Foot and Ankle Patients. Foot Ankle Int. 2018, 39, 1444–1448. [Google Scholar] [CrossRef]
- Kostogiannis, I.; Ageberg, E.; Neuman, P.; Dahlberg, L.; Fridén, T.; Roos, H. Activity level and subjective knee function 15 years after anterior cruciate ligament injury: A prospective, longitudinal study of nonreconstructed patients. Am. J. Sports Med. 2007, 35, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Picot, B.; Hardy, A.; Terrier, R.; Tassignon, B.; Lopes, R.; Fourchet, F. Which Functional Tests and Self-Reported Questionnaires Can Help Clinicians Make Valid Return to Sport Decisions in Patients With Chronic Ankle Instability? A Narrative Review and Expert Opinion. Front. Sports Act. Living 2022, 4, 902886. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.N.; Carroll, E.A.; Parker, R.J.; Helfet, D.L.; Lorich, D.G. Posterior malleolar stabilization of syndesmotic injuries is equivalent to screw fixation. Clin. Orthop. Relat. Res. 2010, 468, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Driessen, A.; Tingart, M.; Baroncini, A. Reliability of the MOCART score: A systematic review. J. Orthop. Traumatol. 2021, 22, 39. [Google Scholar] [CrossRef]
- Madeley, N.J.; Wing, K.J.; Topliss, C.; Penner, M.J.; Glazebrook, M.A.; Younger, A.S. Responsiveness and validity of the SF-36, Ankle Osteoarthritis Scale, AOFAS Ankle Hindfoot Score, and Foot Function Index in end stage ankle arthritis. Foot Ankle Int. 2012, 33, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Budiman-Mak, E.; Conrad, K.J.; Roach, K.E. The Foot Function Index: A measure of foot pain and disability. J. Clin. Epidemiol. 1991, 44, 561–570. [Google Scholar]
- Tschon, M.; Brogini, S.; Parrilli, A.; Bertoldi, S.; Silini, A.; Parolini, O.; Faré, S.; Martini, L.; Veronesi, F.; Fini, M.; et al. Assessment of the in vivo biofunctionality of a biomimetic hybrid scaffold for osteochondral tissue regeneration. Biotechnol. Bioeng. 2021, 118, 465–480. [Google Scholar] [CrossRef]
- McCarthy, H.S.; Roberts, S. A histological comparison of the repair tissue formed when using either Chondrogide® or periosteum during autologous chondrocyte implantation. Osteoarthr. Cartil. 2013, 21, 2048–2057. [Google Scholar]
- Behrens, P.; Ehlers, E.M.; Kochermann, K.U.; Rohwedel, J.; Russlies, M.; Plotz, W. New therapy procedure for localized cartilage defects. Encouraging results with autologous chondrocyte implantation. MMW Fortschr. Med. 1999, 141, 49–51. [Google Scholar]
- Valderrabano, V.; Miska, M.; Leumann, A.; Wiewiorski, M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am. J. Sports Med. 2013, 41, 519–527. [Google Scholar] [CrossRef]
- Min, K.S.; Ryan, P.M. Arthroscopic allograft cartilage transfer for osteochondral defects of the talus. Arthosc. Tech. 2015, 4, e175–e178. [Google Scholar] [CrossRef] [PubMed]
- Giza, E.; Delman, C.; Coetzee, J.C.; Schon, L.C. Arthroscopic treatment of talus osteochondral lesions with particulated juvenile allograft cartilage. Foot Ankle Int. 2014, 35, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Shimozono, Y.; Yasui, Y.; Ross, A.W.; Miyamoto, W.; Kennedy, J.G. Scaffolds based therapy for osteochondral lesions of the talus: A systematic review. World J. Orthop. 2017, 8, 798–808. [Google Scholar] [CrossRef]
- Zengerink, M.; van Dijk, C.N. Complications in ankle arthroscopy. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1420–1431. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.N.; He, Q.; Panneerselavam, S.; Wang, H.; Hou, H.; Zheng, X.; Pan, J.; Li, J. Open versus arthroscopic ankle arthrodesis: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2020, 15, 187. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef]


| Article | Study Type Blinding | Groups and Scaffolds | Surgical Approach | Pts/Ankle | Age (Mean + SD) | Sex (M/F) | BMI (Mean + SD) | OCL Surface or Volume (cm2 or cm3); Grade | Final f-up (m) | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Weigelt, 2019 [28] | Retrospective case series NO | Group (1): COLL I/III + autologous BG | MMO | 33/33 | Mean 35.1 | 19/14 | 26.8 ± 4.3 | 0.9 ± 0.5 cm2; n.r. | Mean 56.4 | Group (1): ↓ VAS; ↑ AOFAS, Tegner; 79% returned to their previous sports levels. MOCART = 61 ± 21 |
| Gottschalk, 2021 [29] | Retrospective case series NO | Group (1): COLL I/III + M-BMS | MMO | 45/45 | 34 ± 24 | 22/23 | Mean 26 kg/m2 | 1.7 ± 1.3 cm2, 1 ± 1.38 cm3; II–IV grade (ICRS classification); II–V grade (Berndt, Harty, and Loomer classification) | 12 | Group (1): ↑ FAAM-ADL, FAOS-Pain, FAOS-Stiffness, FAOS-ADL, FAOS-Sport, and FAOS-QoL. MOCART = 54 ± 14 |
| Ayyaswamy, 2021 [30] | Retrospective case series NO | Group (1): COLL I/III | AR or MMO | 25/25 | Mean 36 | 14/11 | n.r. | Mean 1.75 cm2; n.r. | Mean 24 | Group (1): ↑ AOFAS; ↓ VAS |
| Yontar, 2019 [31] | Retrospective case series NO | Group (1): HA-based | AR or Open | 20/20 | Mean 32.9 | 14/6 | n.r. | 1 ± 0.5 cm2; n.r. | Mean 20.3 | Group (1): ↑ AOFAS; ↓ VAS |
| Giannini, 2014 [32] | Retrospective case series NO | Group (1): Hyaluronan + Chondrocytes | AR | 46/50 | 31.4 ± 7.6 | 29/17 | n.r. | Mean 1.6 cm2; n.r. | 87.2 ± 14.5 | Group (1): ↑ AOFAS |
| Di Cave, 2017 [33] | Retrospective case series NO | Group (1): Polylactide-co-glycolide, calcium sulphate, polyglycolide fibers | Open or MMO | 12/12 | Mean 38.6 | 9/3 | n.r. | n.r.; 3–5 stages (modified classification based on MRI) | Mean 90 | Group (1): ↑ AOFAS; ↓ VAS. MOCART average 61 |
| Kanatlı, 2017 [34] | Retrospective case Series Single-blind (radiologist assessors) | Group (1): PGA-HA | AR | 32/32 | 38 ± 12 | 21/11 | 28.6 ± 4.3 | 2.5 ± 0.8 cm2; II–IV types (Giannini classification) | 33.8 ± 14.0 | Group (1): ↑ AOFAS. MOCART = 64 ± 12 |
| Albano, 2017 [35] | Retrospective case series NO | Group (1): MaioRegen | MMO | 16/16 | 42.6 ± 18.4 | 8/8 | 26.3 ± 5.2 | >1.5 cm2; II or IIA type (Giannini classification) | 30 ± 16.9 | Group (1): ↓VAS; ↑ AOFAS, MOCART |
| Li, 2021 [36] | Retrospective case series NO | Group (1): Osteochondral autograft | AR | 24/24 | 39.8 ± 12.9 | 15/9 | 25.6 ± 2.4 | n.r.; Stage V (Hepple classification) | 18.9 ± 11.8 | Group (1): ↑ AOFAS, Tegner, KAFS; ↓ VAS. MOCART = 68 ± 15 |
| Georgiannos, 2016 [37] | Retrospective case series NO | Group (1): Autologous BG | MMO | 46/48 | 36.2 ± 8.1 | 37/9 | n.r. | n.r.; Stage III–V (Hepple classification) | Mean 66 | Group (1): ↑ AOFAS; ↓ VAS |
| Sawa, 2018 [38] | Retrospective case series NO | Group (1): Autologous BG | MMO | 12/12 | Mean 35.9 | 7/5 | n.r. | n.r.; n.r. | Mean 25.3 | Group (1): ↑ AOFAS |
| Heida, 2020 [39] | Retrospective case series NO | Group (1): PJCAT allograft | AR | 33/33 | 32.3 ± 6.8 | 26/7 | 28.3 ± 3.8 | 1.3 ± 0.5 cm2; 3–5 stage (modified classification based on MRI) | 41.8 ± 19.4 | Group (1): ↑AOFAS; ↓ VAS |
| Akmeşe, 2020 [40] | Retrospective comparative n.r. | Group (1): HA; Group (2): Chitosan | AR | 81/81 | n.r. | 36/45 | n.r. | 1–4 cm2; III or IV grade (Outerbridge classification) | 24 | Groups (1), (2): ↑ AOFAS; ↓ VAS. Group (1): =AOFAS, VAS, MOCART than group (2) |
| Camurcu, 2020 [41] | Retrospective comparative Single-blind (radiologist assessor) | Group (1): Chitosan-glycerol phosphate/blood; Group (2): no treatment | AR | 63/63 | Mean 40.3 | 29/34 | Mean 30.1 | Group (1): 1.6 ± 0.2 cm2; Group (2): 1.5 ± 0.2 cm2; Stage II–IV (Hepple classification) | 32 ± 13 | Groups (1), (2): ↑ AOFAS; ↓ VAS. Group (1): =AOFAS, MOCART than Group (2); ↑ VAS function than Group (2) |
| Dogar, 2021 [42] | Retrospective comparative NO | Group (1): Chitosan; Group (2): PRP; Group (3): no treatment | AR | 62/76 | 37.72 ± 13.31 | 31/31 | Mean 26.30 | Group (3): Mean 1.5 cm2; Group (2): Mean 1.48 cm2; Group (1): Mean 1.92 cm2; 2–5 stages (Modified Berndt Harty radiologic classification, Mintz classification) | 26.2 ± 18.4 | Groups (1), (2):↑ AOFAS. Group (3): ↓ VAS; ↑ FAAM overall pain, 15-min walking, and running function. Group (1): ↓VAS; ↑AOFAS, FAAM than groups (2), (3) |
| Gorgun, 2022 [43] | Retrospective comparative Single-blind (radiologist assessor) | Group (1): COLL I/III + autologous BG; Group (2): Autologous BG | AR | 94/188 | Mean 32 | 49/45 | n.r. | Mean 1 cm3; n.r. | 69.3 ± 20.7 | Groups (1), (2): ↑AOFAS; ↓VAS. Group (1): =AOFAS and VAS. Group (1): ↓ MOCART than group (2) |
| Kekeç, 2023 [44] | Retrospective comparative NO | Group (1): PGA-HA-based CFS; Group (2): no treatment | AR | 47/47 | 22.8 ± 2.3 | 29/18 | 23.7 ± 4.8 | 2.1 ± 0.3 cm2; 4–5 stage (Bristol) | 36.2 ± 5.6 | Groups (1), (2): ↑ AOFAS. Group (1): ↑ AOFAS, MOCART than Group (2) |
| Eren, 2019 [45] | Retrospective comparative Single-blind (Clinical assessors) | Group (1): Bio-absorbable polyglycolic acid-hyaluronan; Group (2): no treatment | AR | 62/62 | 41 ± 13 | 35/27 | Mean 27.4 | Group (2): Mean 1.65 cm2, Group (1): Mean 1.97 cm2; n.r. | 36.1 ± 14.9 | Groups (1), (2): ↑ AOFAS. Group (1): ↑ AOFAS than group (2) |
| Karnovsky, 2018 [46] | Retrospective comparative NO | Group (1): PJCAT + JACI-BMAC; Group (2): BMAC | AR | 50/50 | Mean 37.2 | 23/27 | n.r. | Group (2): Mean 0.8 cm2, Group (1): Mean 1.2 cm2; n.r. | Mean 30.9 | Groups (1), (2): ↑ FAOS; ↓ VAS; =MOCART; fibrocartilage reparative tissue. |
| Christensen, 2016 [47] | Prospective case series Single-blind (radiologist assessor) | Group (1): MaioRegen | MMO | 8/8 | 27 ± 7 | 5/3 | n.r. | 3.0 ± 1.9 cm2; n.r. | Mean 30 | Group (1): No complete regeneration of the subchondral bone. No improvement in the MOCART and AOFAS |
| Gottschalk, 2017 [48] | Prospective case series NO | Group (1): COLL I/III + autologous BG | Open | 21/37 | 37 ± 15 | 13/8 | 26 ± 5 | 1.4 ± 0.9 cm2; n.r. | 60 | Group (1): ↓ FFI |
| Galla, 2019 [49] | Prospective case series NO | Group (1): COLL I/III + autologous BG | Open | 23/23 | 35.6 ± 13.9 | 15/8 | n.r. | n.r.; Stage II, III, V (Hepple’s classification) | 33.5 ± 10.4 | Group (1): ↓VAS; ↑FFI; = MOCART during time |
| Kubosch, 2016 [50] | Prospective case series NO | Group (1): COLL I/III + autologous BG | MMO | 17/17 | 38.8 ± 15.7 | 9/8 | Mean 27.44 | 2.4 ± 1.6 cm2; 3, 4 stages (Modified Berndt Harty radiologic classification) | 39.5 ± 18.4 | Group (1): ↓ VAS; ↑ AOFAS. MOCART = 53 ± 16 |
| Usuelli, 2018 [17] | Prospective case series NO | Group (1): COLL I/III + autologous BG | AR | 20/20 | 36.1 ± 13.1 | 11/9 | 24.6 ± 2.7 | 1.5 ± 0.9 cm2; 3, 4 stages (Modified Berndt Harty radiologic classification) | 24 | Group (1): ↑ AOFAS, MOCART; ↓ VAS |
| Sadlik, 2017 [51] | Prospective case series NO | Group (1): COLL I/III + autologous BG+BMC | MMO | 10/10 | 37 ± 12.5 | 6/4 | 26.7 ± 3.5 | 1.3 ± 0.6 cm2; n.r. | 46.4 ± 18 | Group (1): ↑ AOFAS; ↓ VAS. MOCART = 70 ± 17 |
| López-Alcorocho, 2021 [19] | Prospective case series Single-blind (Clinical assessor) | Group (1): COLL I/III + high-density chondrocytes | AR or MMO | 24/24 | Mean 31 | 14/10 | n.r. | 2.1 ± 0.6 cm2; 3, 4 grade (ICRS) | 24 | Group (1): ↓ VAS; ↑ AOFAS. MOCART = 72 ± 16 |
| Drobnic, 2021 [52] | Prospective case series NO | Group (1): Aragonite-based bi-phasic | AR or MMO | 4/6 | Mean 42 | 2/2 | 33.6 ± 4.4 | 2.0 ± 0.1 cm2; Stage IV, V (Hepple’s classification) | Mean 26 | Group (1): ↑ FAOS |
| Younger, 2016 [53] | Prospective, case series NO | Group (1): β-TCP matrix + rhPDGF | AR | 5/6 | 52 ± 8.5 | 2/3 | 26.3 ± 5.0 | 1.0 ± 0.4 cm2; n.r. | 6 | Group (1): ↓ VAS; ↑ AOS. MOCART = average 68 |
| Sadlik, 2019 [54] | Prospective comparative NO | Group (1): Autologous bone chips covered with COLL I/III or HA-based + BMC + MMO; Group (2): Autologous bone chips covered with COLL I/III or HA-based+BMC+AR | AR or MMO | 24/24 | Mean 34.1 | 14/10 | Mean 25.2 | Group (1): 1.3 ± 0.6 cm2 Group (2): 1.2 ± 0.4 cm2; n.r. | Mean 22 | Groups (1), (2): ↑ AOFAS; ↓ VAS; =MOCART. No differences between groups |
| Guney, 2015 [55] | RCT Single-blind (radiologist assessors) | Group (1): PRP; Group (2): no treatment | AR | 35/43 | Mean 40.7 | 16/19 | Mean 27.5 | n.r.; n.r. | Mean 16.2 | Group (1): ↓ VAS; ↑ AOFAS, FAAM than group (2) |
| Downs and Black Checklist | Modified Coleman Methodology Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Article | Reporting | External Validity Bias | Internal Validity Bias | Internal Validity Confounding | Power | Total Score | Part A | Part B | Total Score |
| Weigelt, 2019 [28] | 10 | 2 | 5 | 3 | 0 | 20 | 39 | 10 | 49 |
| Gottschalk, 2021 [29] | 9 | 2 | 5 | 3 | 0 | 19 | 34 | 10 | 44 |
| Ayyaswamy, 2021 [30] | 9 | 2 | 5 | 3 | 1 | 20 | 24 | 10 | 34 |
| Yontar, 2019 [31] | 7 | 2 | 5 | 2 | 0 | 16 | 21 | 10 | 31 |
| Giannini, 2014 [32] | 8 | 2 | 5 | 4 | 0 | 19 | 42 | 10 | 52 |
| Di Cave, 2017 [33] | 8 | 2 | 5 | 2 | 0 | 17 | 37 | 10 | 47 |
| Kanatlı, 2017 [34] | 9 | 2 | 6 | 2 | 1 | 20 | 42 | 10 | 52 |
| Albano, 2017 [35] | 9 | 2 | 5 | 2 | 0 | 18 | 33 | 10 | 43 |
| Li, 2021 [36] | 8 | 2 | 5 | 2 | 0 | 17 | 37 | 10 | 47 |
| Georgiannos, 2016 [37] | 8 | 2 | 5 | 2 | 0 | 17 | 37 | 10 | 47 |
| Sawa, 2018 [38] | 10 | 2 | 5 | 3 | 0 | 20 | 25 | 10 | 35 |
| Heida, 2020 [39] | 8 | 2 | 5 | 2 | 0 | 17 | 24 | 10 | 34 |
| Akmeşe, 2020 [40] | 9 | 2 | 5 | 1 | 0 | 17 | 50 | 10 | 60 |
| Camurcu, 2020 [41] | 9 | 2 | 6 | 2 | 1 | 20 | 45 | 10 | 55 |
| Dogar, 2021 [42] | 9 | 2 | 5 | 2 | 0 | 18 | 35 | 10 | 45 |
| Gorgun, 2022 [43] | 9 | 2 | 6 | 3 | 0 | 20 | 50 | 10 | 60 |
| Kekeç, 2023 [44] | 9 | 2 | 5 | 2 | 0 | 18 | 42 | 10 | 52 |
| Eren, 2019 [45] | 8 | 2 | 6 | 2 | 1 | 19 | 33 | 10 | 43 |
| Karnovsky, 2018 [46] | 9 | 2 | 5 | 2 | 0 | 18 | 37 | 10 | 47 |
| Christensen, 2016 [47] | 8 | 2 | 6 | 2 | 0 | 18 | 45 | 10 | 55 |
| Gottschalk, 2017 [48] | 9 | 2 | 5 | 3 | 0 | 19 | 39 | 10 | 49 |
| Galla, 2019 [49] | 10 | 2 | 5 | 2 | 1 | 20 | 49 | 10 | 59 |
| Kubosch, 2016 [50] | 9 | 2 | 5 | 2 | 0 | 18 | 45 | 10 | 55 |
| Usuelli, 2018 [17] | 9 | 2 | 5 | 2 | 1 | 19 | 47 | 10 | 57 |
| Sadlik, 2017 [51] | 8 | 2 | 5 | 2 | 0 | 17 | 45 | 10 | 55 |
| López-Alcorocho, 2021 [19] | 9 | 2 | 6 | 2 | 0 | 19 | 56 | 10 | 66 |
| Drobnic, 2021 [52] | 8 | 2 | 5 | 2 | 0 | 17 | 32 | 10 | 42 |
| Younger, 2016 [53] | 8 | 2 | 5 | 2 | 0 | 17 | 38 | 10 | 48 |
| Sadlik, 2019 [54] | 10 | 2 | 5 | 3 | 0 | 20 | 43 | 10 | 53 |
| Guney, 2015 [55] | 9 | 3 | 6 | 4 | 1 | 23 | 41 | 10 | 51 |
| Surgical Technique | Scaffolds | No Scaffold | Article |
|---|---|---|---|
| AR | Hyaluronan + chondrocytes: Re-operation (6.5%) | Giannini, 2014 [32] | |
| PGA-HA: Ankle swelling (9.4%) | Kanatlı, 2017 [34] | ||
| Osteochondral autograft: NO | Li, 2021 [36] | ||
| PJCAT allograft: Re-operation (6.1%) | Heida, 2020 [39] | ||
| HA-based: Re-operation (2.4%); Chitosan-based: Re-operation (2.6%) | Akmeşe, 2020 [40] | ||
| Chitosan-glycerol phosphate: Hematoma of the ankle (6.3%); erythema and swelling (3.1%) | Hematoma of the ankle (6.5%) | Camurcu, 2020 [41] | |
| Chitosan-based: NO | Transient neurapraxia in the dorsal branch of the superficial peroneal nerve (4.5%); Mosaicplasty due to persistent pain (9.01%) | Dogar, 2021 [42] | |
| COLL I/III + autologous BG: Superficial skin infection of the arthroscopic portal (4.3%); Autologous BG: Superficial skin infection of the arthroscopic portal (2.1%); synovial fistula (2.1%) | Gorgun, 2022 [43] | ||
| Polyglycolic acid-hyaluronan: NO | NO | Eren, 2019 [45] | |
| PJCAT + JACI-BMAC: Re-operation (25%): BMAC: Re-operation (30%) | Karnovsky, 2018 [46] | ||
| COLL I/III + autologous BG: Re-operation (5%) | Usuelli, 2018 [17] | ||
| β-TCP matrix + rhPDGF: NO | Younger, 2016 [53] | ||
| MMO | COLL I/III + autologous BG: Delayed union (3%); Re-operation (57.6%) | Weigelt, 2019 [28] | |
| MaioRegen: Re-operation (25%) | Albano, 2017 [35] | ||
| Autologous BG: Superficial wound infection (6.5%); Numbness at the distribution area of superficial peroneal nerve (2.2%); Occasional ache over the anteromedial aspect of ankle (4.3%) | Georgiannos, 2016 [37] | ||
| Autologous BG: NO | Sawa, 2018 [38] | ||
| COLL I/III + autologous BG: NO | Kubosch, 2016 [50] | ||
| COLL I/III + autologous BG + BMC: Iatrogenic lesion of the posterior tibial tendon (10%) | Sadlik, 2017 [51] | ||
| Open surgery | COLL I/III + autologous BG: Transient p.o. irritation of the deep peroneal nerve (4.3%); painful arthrofibrosis (4.3%); persistent pain (4.3%) | Galla, 2019 [49] |
| Article | Age | BMI | Gender | Duration of Symptoms | P.o. Pain | Lesion Size | Lesion Location |
|---|---|---|---|---|---|---|---|
| Giannini, 2014 [32] | <40 yrs and high AOFAS (p = 0.046 at 12 mo; p = 0.05 at 36 mo; p = 0.008 at final f-up) | / | / | / | / | / | Lateral lesion and high AOFAS (p = 0.007 at 12 mo, p = 0.001 at 36 mo) |
| Gottschalk, 2017 [48] | / | / | / | / | / | Lesion size and FFI pain and function at final f-up (p = 0.012, p = 0.016) | / |
| Kubosch, 2016 [50] | ≥45 yrs and low pain (p = 0.048) | BMI > 30 and low AOFAS Score (p = 0.003); BMI > 30 and high VAS (p = 0.031) | / | / | High p.o. pain and low AOFAS (p = 0.004) | Lesion size ≥ 3 cm3 and low AOFAS (p = 0.041) | / |
| Weigelt, 2019 [28] | NO | NO | / | / | / | NO | / |
| Gottschalk, 2021 [29] | NO | NO | NO | NO | / | / | NO |
| Sadlik, 2017 [51] | / | / | / | / | / | NO | / |
| Ayyaswamy, 2021 [30] | NO | / | / | / | / | NO | NO |
| Sawa, 2018 [38] | / | / | / | / | / | NO | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronesi, F.; Zielli, S.O.; Brogini, S.; Artioli, E.; Arceri, A.; Mazzotti, A.; Faldini, C.; Giavaresi, G. Scaffolds for Osteochondral Lesions of the Talus: Systematic Review and Meta-Analysis of the Last Ten Years Literature. Bioengineering 2024, 11, 970. https://doi.org/10.3390/bioengineering11100970
Veronesi F, Zielli SO, Brogini S, Artioli E, Arceri A, Mazzotti A, Faldini C, Giavaresi G. Scaffolds for Osteochondral Lesions of the Talus: Systematic Review and Meta-Analysis of the Last Ten Years Literature. Bioengineering. 2024; 11(10):970. https://doi.org/10.3390/bioengineering11100970
Chicago/Turabian StyleVeronesi, Francesca, Simone Ottavio Zielli, Silvia Brogini, Elena Artioli, Alberto Arceri, Antonio Mazzotti, Cesare Faldini, and Gianluca Giavaresi. 2024. "Scaffolds for Osteochondral Lesions of the Talus: Systematic Review and Meta-Analysis of the Last Ten Years Literature" Bioengineering 11, no. 10: 970. https://doi.org/10.3390/bioengineering11100970









