Intra-Articular Injection of Adipose-Derived Stromal Vascular Fraction in Osteoarthritic Temporomandibular Joints: Study Design of a Randomized Controlled Clinical Trial
Abstract
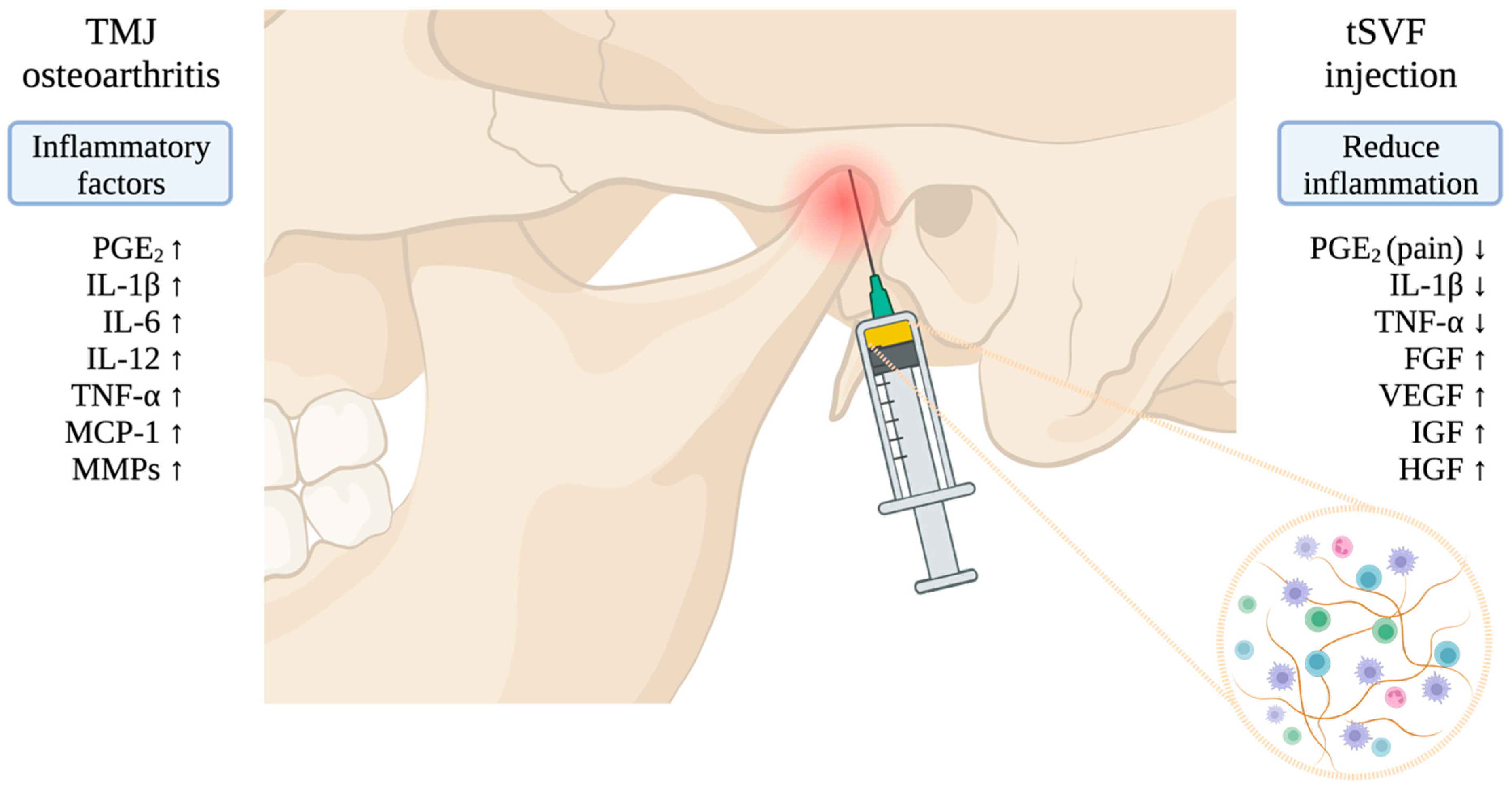
:1. Introduction
2. Methods and Design
2.1. Objectives
2.2. Protocol and Registration
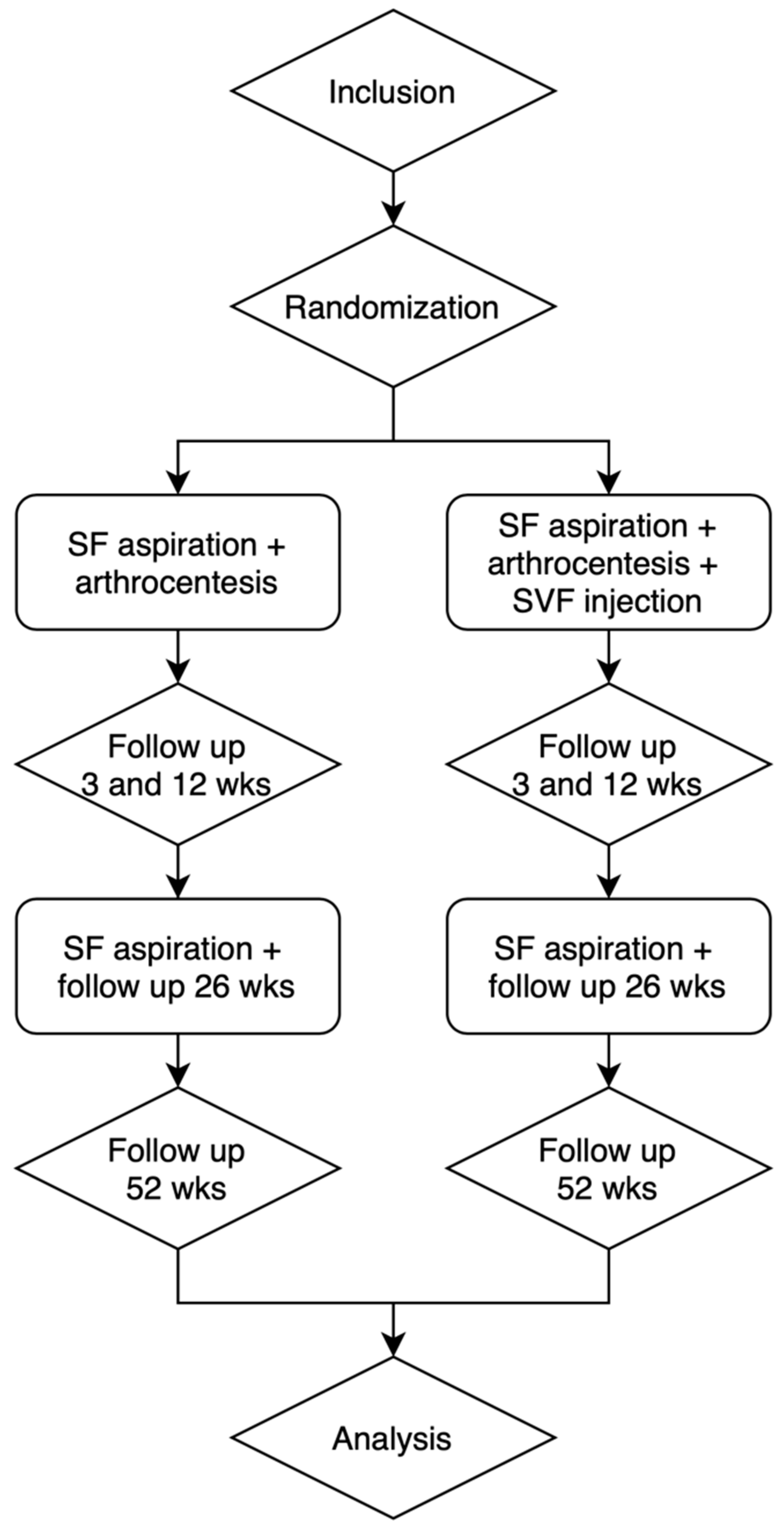
2.3. Trial Design
2.4. Participants
2.5. Surgical Procedure to Obtain and Inject Autologous tSVF
2.6. Outcome Measurements
2.7. Safety
2.8. Sample Size Calculation
2.9. Statistical Analysis
3. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jerjes, W.; Upile, T.; Abbas, S.; Kafas, P.; Vourvachis, M.; Rob, J.; Mc Carthy, E.; Angouridakis, N.; Hopper, C. Muscle Disorders and Dentition-Related Aspects in Temporomandibular Disorders: Controversies in the Most Commonly Used Treatment Modalities. Int. Arch. Med. 2008, 1, 23. [Google Scholar] [CrossRef]
- Murphy, M.K.; MacBarb, R.F.; Wong, M.E.; Athanasiou, K.A. Temporomandibular Disorders: A Review of Etiology, Clinical Management, and Tissue Engineering Strategies. Int. J. Oral Maxillofac. Implant. 2013, 28, e393–e414. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Kramer, P.R.; Mikos, A.G. Emerging Intra-Articular Drug Delivery Systems for the Temporomandibular Joint. Methods 2009, 47, 134–140. [Google Scholar] [CrossRef]
- LeResche, L. Epidemiology of Temporomandibular Disorders: Implications for the Investigation of Etiologic Factors. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 1997, 8, 291–305. [Google Scholar] [CrossRef]
- Farzin, M.; Taghva, M.; Babooie, M. Comparison of Temporomandibular Disorders between Menopausal and Non-Menopausal Women. J. Korean Assoc. Oral Maxillofac. Surg. 2018, 44, 232–236. [Google Scholar] [CrossRef]
- Stegenga, B. Osteoarthritis of the Temporomandibular Joint Organ and Its Relationship to Disc Displacement. J. Orofac. Pain 2001, 15, 193–205. [Google Scholar] [PubMed]
- Stegenga, B.; de Bont, L.G.M. Management of Temporomandibular Joint Degenerative Diseases: Biologic Basis and Treatment Outcome; Birkhäuser: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Milam, S.B. Pathogenesis of Degenerative Temporomandibular Joint Arthritides. Odontology 2005, 93, 7–15. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.F.; Lovato da Silva, C.H.; Nasser, M.; Fedorowicz, Z.; Al-Muharraqi, M.A. Interventions for the Management of Temporomandibular Joint Osteoarthritis. Cochrane Database Syst. Rev. 2012, 2012, CD007261. [Google Scholar] [CrossRef] [PubMed]
- Machon, V.; Hirjak, D.; Lukas, J. Therapy of the Osteoarthritis of the Temporomandibular Joint. J. Cranio-Maxillofac. Surg. 2011, 39, 127–130. [Google Scholar] [CrossRef]
- Vos, L.M.; Huddleston Slater, J.J.R.; Stegenga, B. Lavage Therapy versus Nonsurgical Therapy for the Treatment of Arthralgia of the Temporomandibular Joint: A Systematic Review of Randomized Controlled Trials. J. Orofac. Pain 2013, 27, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Vos, L.M.; Huddleston Slater, J.J.R.; Stegenga, B. Arthrocentesis as Initial Treatment for Temporomandibular Joint Arthropathy: A Randomized Controlled Trial. J. Cranio-Maxillofac. Surg. 2014, 42, e134–e139. [Google Scholar] [CrossRef]
- Tang, Y.H.; Vos, L.M.; Tuin, A.J.; Huddleston Slater, J.J.R.; Gareb, B.; van Bakelen, N.B.; Spijkervet, F.K.L. Arthrocentesis versus Non-Surgical Intervention as Initial Treatment for Temporomandibular Joint Arthralgia: A Randomized Controlled Trial with Long-Term Follow-Up. Int. J. Oral Maxillofac. Surg. 2023, 52, 595–603. [Google Scholar] [CrossRef]
- Scanzello, C.R.; McKeon, B.; Swaim, B.H.; DiCarlo, E.; Asomugha, E.U.; Kanda, V.; Nair, A.; Lee, D.M.; Richmond, J.C.; Katz, J.N.; et al. Synovial Inflammation in Patients Undergoing Arthroscopic Meniscectomy: Molecular Characterization and Relationship to Symptoms. Arthritis Rheum. 2011, 63, 391–400. [Google Scholar] [CrossRef]
- Boada-Pladellorens, A.; Avellanet, M.; Pages-Bolibar, E.; Veiga, A. Stromal Vascular Fraction Therapy for Knee Osteoarthritis: A Systematic Review. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221117879. [Google Scholar] [CrossRef]
- Vernal, R.; Velásquez, E.; Gamonal, J.; Garcia-Sanz, J.A.; Silva, A.; Sanz, M. Expression of Proinflammatory Cytokines in Osteoarthritis of the Temporomandibular Joint. Arch. Oral Biol. 2008, 53, 910–915. [Google Scholar] [CrossRef]
- Cevidanes, L.H.S.; Walker, D.; Schilling, J.; Sugai, J.; Giannobile, W.; Paniagua, B.; Benavides, E.; Zhu, H.; Marron, J.S.; Jung, B.T.; et al. 3D Osteoarthritic Changes in TMJ Condylar Morphology Correlates with Specific Systemic and Local Biomarkers of Disease. Osteoarthr. Cartil. 2014, 22, 1657–1667. [Google Scholar] [CrossRef]
- Vos, L.M.; Kuijer, R.; Huddleston Slater, J.J.R.; Bulstra, S.K.; Stegenga, B. Inflammation Is More Distinct in Temporomandibular Joint Osteoarthritis Compared to the Knee Joint. J. Oral Maxillofac. Surg. 2014, 72, 35–40. [Google Scholar] [CrossRef]
- Kawabata, A. Prostaglandin E2 and Pain—An Update. Biol. Pharm. Bull. 2011, 34, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ellman, M.; Muddasani, P.; Wang, J.H.-C.; Cs-Szabo, G.; van Wijnen, A.J.; Im, H.-J. Prostaglandin E2 and Its Cognate EP Receptors Control Human Adult Articular Cartilage Homeostasis and Are Linked to the Pathophysiology of Osteoarthritis. Arthritis Rheum. 2009, 60, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Lapuente, J.P.; Dos-Anjos, S.; Blázquez-Martínez, A. Intra-Articular Infiltration of Adipose-Derived Stromal Vascular Fraction Cells Slows the Clinical Progression of Moderate-Severe Knee Osteoarthritis: Hypothesis on the Regulatory Role of Intra-Articular Adipose Tissue. J. Orthop. Surg. Res. 2020, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Schipper, J.A.M.; van Laarhoven, C.J.H.C.M.; Schepers, R.H.; Tuin, A.J.; Harmsen, M.C.; Spijkervet, F.K.L.; Jansma, J.; van Dongen, J.A. Mechanical Fractionation of Adipose Tissue—A Scoping Review of Procedures to Obtain Stromal Vascular Fraction. Bioengineering 2023, 10, 1175. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Tran, T.D.-X.; Nguyen, H.T.-N.; Vu, H.T.; Le, P.T.-B.; Phan, N.L.-C.; Vu, N.B.; Phan, N.K.; Van Pham, P. Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis. Stem Cells Transl. Med. 2017, 6, 187–195. [Google Scholar] [CrossRef]
- van Boxtel, J.; Vonk, L.A.; Stevens, H.P.; van Dongen, J.A. Mechanically Derived Tissue Stromal Vascular Fraction Acts Anti-Inflammatory on TNF Alpha-Stimulated Chondrocytes In Vitro. Bioengineering 2022, 9, 345. [Google Scholar] [CrossRef]
- Kim, H.; Lee, B.-K. Anti-Inflammatory Effect of Adipose-Derived Stromal Vascular Fraction on Osteoarthritic Temporomandibular Joint Synoviocytes. Tissue Eng. Regen. Med. 2020, 17, 351–362. [Google Scholar] [CrossRef]
- Mahmmood, V.H.; Shihab, S.M. Assessment of Therapeutic Effect of Intra-Articular Nanofat Injection for Temporomandibular Disorders. J. Craniofac. Surg. 2019, 30, 659–662. [Google Scholar] [CrossRef]
- Carboni, A.; Amodeo, G.; Perugini, M.; Arangio, P.; Orsini, R.; Scopelliti, D. Temporomandibular Disorders Clinical and Anatomical Outcomes After Fat-Derived Stem Cells Injection. J. Craniofac. Surg. 2019, 30, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Sembronio, S.; Tel, A.; Tremolada, C.; Lazzarotto, A.; Isola, M.; Robiony, M. Temporomandibular Joint Arthrocentesis and Microfragmented Adipose Tissue Injection for the Treatment of Internal Derangement and Osteoarthritis: A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2021, 79, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat Grafting: Basic Research and Clinical Applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Cicione, C.; Di Taranto, G.; Barba, M.; Isgrò, M.A.; D’Alessio, A.; Cervelli, D.; Sciarretta, F.V.; Pelo, S.; Michetti, F.; Lattanzi, W. In Vitro Validation of a Closed Device Enabling the Purification of the Fluid Portion of Liposuction Aspirates. Plast. Reconstr. Surg. 2016, 137, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C.; et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-like Elements by Mild Mechanical Forces from Human Lipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Tjakkes, G.-H.E.; Tenvergert, E.M.; de Bont, L.G.M.; Stegenga, B. The Effect of Intra-Articular Injection of Ultracain in the Temporomandibular Joint in Patients with Preauricular Pain: A Randomized Prospective Double-Blind Placebo-Controlled Crossover Study. Clin. J. Pain 2007, 23, 233–236. [Google Scholar] [CrossRef]
- Van Dongen, J.A.; Gostelie, O.F.E.; Vonk, L.A.; De Bruijn, J.J.; Van Der Lei, B.; Harmsen, M.C.; Stevens, H.P. Fractionation of Adipose Tissue Procedure with a Disposable One-Hole Fractionator. Aesthetic Surg. J. 2020, 40, NP194–NP201. [Google Scholar] [CrossRef]
- Shinohara, E.H.; Pardo-Kaba, S.C.; Martini, M.Z.; Horikawa, F.K. Single Puncture for TMJ Arthrocentesis: An Effective Technique for Hydraulic Distention of the Superior Joint Space. Natl. J. Maxillofac. Surg. 2012, 3, 96–97. [Google Scholar] [CrossRef]
- Stegenga, B.; de Bont, L.G.; de Leeuw, R.; Boering, G. Assessment of Mandibular Function Impairment Associated with Temporomandibular Joint Osteoarthrosis and Internal Derangement. J. Orofac. Pain 1993, 7, 183–195. [Google Scholar] [PubMed]
- Slade, G.D. Derivation and Validation of a Short-Form Oral Health Impact Profile. Community Dent. Oral Epidemiol. 1997, 25, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Derogatis, L.R.; Lipman, R.S.; Covi, L. SCL-90: An Outpatient Psychiatric Rating Scale—Preliminary Report. Psychopharmacol. Bull. 1973, 9, 13–28. [Google Scholar] [PubMed]
- Cai, X.-Y.; Yang, C.; Zhang, Z.-Y.; Qiu, W.-L.; Chen, M.-J.; Zhang, S.-Y. Septic Arthritis of the Temporomandibular Joint: A Retrospective Review of 40 Cases. J. Oral Maxillofac. Surg. 2010, 68, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Westesson, P.L.; Eriksson, L.; Liedberg, J. The Risk of Damage to Facial Nerve, Superficial Temporal Vessels, Disk, and Articular Surfaces during Arthroscopic Examination of the Temporomandibular Joint. Oral Surg. Oral Med. Oral Pathol. 1986, 62, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Premaratne, G.U.; Ma, L.-P.; Fujita, M.; Lin, X.; Bollano, E.; Fu, M. Stromal Vascular Fraction Transplantation as an Alternative Therapy for Ischemic Heart Failure: Anti-Inflammatory Role. J. Cardiothorac. Surg. 2011, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Bunnell, B.A.; Chiu, E.S.; Guilak, F. Concise Review: Adipose-Derived Stromal Vascular Fraction Cells and Stem Cells: Let’s Not Get Lost in Translation. Stem Cells 2011, 29, 749–754. [Google Scholar] [CrossRef]
- Yurttutan, M.E.; Öncül, A.T.; Karasu, H.A. Benign Tumors of Temporomandibular Joint; Emes, Y., Aybar, B., Dergin, G., Eds.; IntechOpen: Rijeka, Croatia, 2017; Chapter 5; ISBN 978-953-51-3862-4. [Google Scholar]
- Twisk, J.W.R. (Ed.) Sample Size Calculations. In Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide; Cambridge University Press: Cambridge, UK, 2013; pp. 237–242. ISBN 9781139342834. [Google Scholar]
- De Riu, G.; Vaira, L.A.; Carta, E.; Meloni, S.M.; Sembronio, S.; Robiony, M. Bone Marrow Nucleated Cell Concentrate Autograft in Temporomandibular Joint Degenerative Disorders: 1-Year Results of a Randomized Clinical Trial. J. Cranio-Maxillofac. Surg. 2019, 47, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Scientific Recommendations on Classification of Advanced Therapy Medicinal Products. Available online: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/advanced-therapies/advanced-therapy-classification/scientific-recommendations-classification-advanced-therapy-medicinal-products (accessed on 1 December 2023).
- Comella, K.; Parlo, M.; Daly, R.; Depasquale, V.; Edgerton, E.; Mallory, P.; Schmidt, R.; Drake, W.P. Safety Analysis of Autologous Stem Cell Therapy in a Variety of Degenerative Diseases and Injuries Using the Stromal Vascular Fraction. J. Clin. Med. Res. 2017, 9, 935–942. [Google Scholar] [CrossRef]
- Karina, K.; Rosliana, I.; Rosadi, I.; Schwartz, R.; Sobariah, S.; Afini, I.; Widyastuti, T.; Remelia, M.; Wahyuningsih, K.A.; Pawitan, J.A. Safety of Technique and Procedure of Stromal Vascular Fraction Therapy: From Liposuction to Cell Administration. Scientifica 2020, 2020, 2863624. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Vaish, A.; Chavada, V.; Murrell, W.D.; Vaishya, R. Assessment of Safety and Efficacy of Intra-Articular Injection of Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis—A Systematic Review. Int. Orthop. 2021, 45, 615–625. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.A.; Stevens, H.P.; Parvizi, M.; van der Lei, B.; Harmsen, M.C. The Fractionation of Adipose Tissue Procedure to Obtain Stromal Vascular Fractions for Regenerative Purposes. Wound Repair Regen. 2016, 24, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.A.; Getova, V.; Brouwer, L.A.; Liguori, G.R.; Sharma, P.K.; Stevens, H.P.; van der Lei, B.; Harmsen, M.C. Adipose Tissue-Derived Extracellular Matrix Hydrogels as a Release Platform for Secreted Paracrine Factors. J. Tissue Eng. Regen. Med. 2019, 13, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lan, Z.; Yan, J.; Tang, Z.; Zhou, L.; Jin, D.; Jin, Q. Effect of Intra-Knee Injection of Autologous Adipose Stem Cells or Mesenchymal Vascular Components on Short-Term Outcomes in Patients with Knee Osteoarthritis: An Updated Meta-Analysis of Randomized Controlled Trials. Arthritis Res. Ther. 2023, 25, 147. [Google Scholar] [CrossRef]
- Aletto, C.; Oliva, F.; Maffulli, N. Knee Intra-Articular Administration of Stromal Vascular Fraction Obtained from Adipose Tissue: A Systematic Review. J. Clin. Orthop. Trauma 2022, 25, 101773. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Zeng, Y.; Li, M.; Xie, H.; Shen, B. Intra-Articular Injection of Stromal Vascular Fraction for Knee Degenerative Joint Disease: A Concise Review of Preclinical and Clinical Evidence. Sci. China. Life Sci. 2022, 65, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Köhnke, R.; Ahlers, M.O.; Birkelbach, M.A.; Ewald, F.; Krueger, M.; Fiedler, I.; Busse, B.; Heiland, M.; Vollkommer, T.; Gosau, M.; et al. Temporomandibular Joint Osteoarthritis: Regenerative Treatment by a Stem Cell Containing Advanced Therapy Medicinal Product (ATMP)—An In Vivo Animal Trial. Int. J. Mol. Sci. 2021, 22, 443. [Google Scholar] [CrossRef] [PubMed]
- Siennicka, K.; Zolocinska, A.; Stepien, K.; Lubina-Dabrowska, N.; Maciagowska, M.; Zolocinska, E.; Slysz, A.; Piusinska-Macoch, R.; Mazur, S.; Zdanowicz, U.; et al. Adipose-Derived Cells (Stromal Vascular Fraction) Transplanted for Orthopedical or Neurological Purposes: Are They Safe Enough? Stem Cells Int. 2016, 2016, 5762916. [Google Scholar] [CrossRef] [PubMed]

| Inclusion | Exclusion |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schipper, J.A.M.; Tuin, A.J.; van Dongen, J.A.; van Bakelen, N.B.; Harmsen, M.C.; Spijkervet, F.K.L. Intra-Articular Injection of Adipose-Derived Stromal Vascular Fraction in Osteoarthritic Temporomandibular Joints: Study Design of a Randomized Controlled Clinical Trial. Bioengineering 2024, 11, 171. https://doi.org/10.3390/bioengineering11020171
Schipper JAM, Tuin AJ, van Dongen JA, van Bakelen NB, Harmsen MC, Spijkervet FKL. Intra-Articular Injection of Adipose-Derived Stromal Vascular Fraction in Osteoarthritic Temporomandibular Joints: Study Design of a Randomized Controlled Clinical Trial. Bioengineering. 2024; 11(2):171. https://doi.org/10.3390/bioengineering11020171
Chicago/Turabian StyleSchipper, Jan Aart M., Aartje Jorien Tuin, Joris A. van Dongen, Nico B. van Bakelen, Martin Conrad Harmsen, and Fred K. L. Spijkervet. 2024. "Intra-Articular Injection of Adipose-Derived Stromal Vascular Fraction in Osteoarthritic Temporomandibular Joints: Study Design of a Randomized Controlled Clinical Trial" Bioengineering 11, no. 2: 171. https://doi.org/10.3390/bioengineering11020171
APA StyleSchipper, J. A. M., Tuin, A. J., van Dongen, J. A., van Bakelen, N. B., Harmsen, M. C., & Spijkervet, F. K. L. (2024). Intra-Articular Injection of Adipose-Derived Stromal Vascular Fraction in Osteoarthritic Temporomandibular Joints: Study Design of a Randomized Controlled Clinical Trial. Bioengineering, 11(2), 171. https://doi.org/10.3390/bioengineering11020171








