Seeing the Future: A Review of Ocular Therapy
Abstract
:1. Background
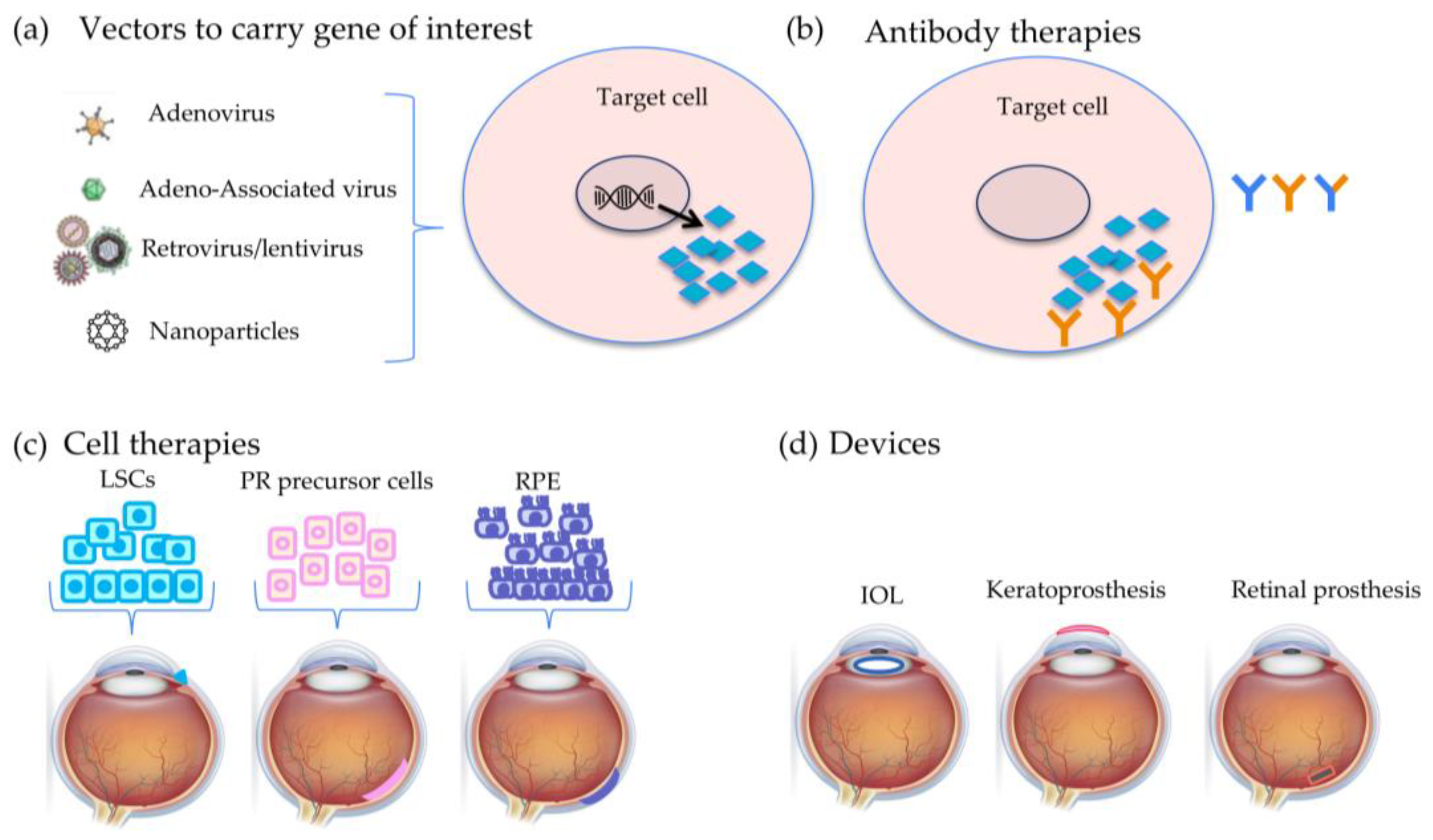
2. Types of Therapy
2.1. Gene Therapy
2.1.1. Gene Augmentation
2.1.2. Modifier Gene Therapy
2.1.3. Optogenetics
2.1.4. Gene Editing
2.1.5. Gene Silencing
2.1.6. Future Directions
2.2. Antibody Therapy
2.2.1. Single-Target Therapies
2.2.2. Dual-Target Therapies
2.2.3. Future Directions
2.3. Cell Therapy
2.3.1. Limbal Stem Cells (LSCs)
2.3.2. Mesenchymal Stem Cells (MSCs)
2.3.3. Human Embryonic-like Stem Cells (hESCs)
2.3.4. Induced Pluripotent Stem Cells (iPSCs)
2.3.5. Neural Stem Cells (NSCs)
2.3.6. Future Directions
2.4. Prostheses
2.4.1. Retinal Prosthesis
2.4.2. Lens Prosthesis
2.4.3. Keratoprosthesis
2.4.4. Future Directions
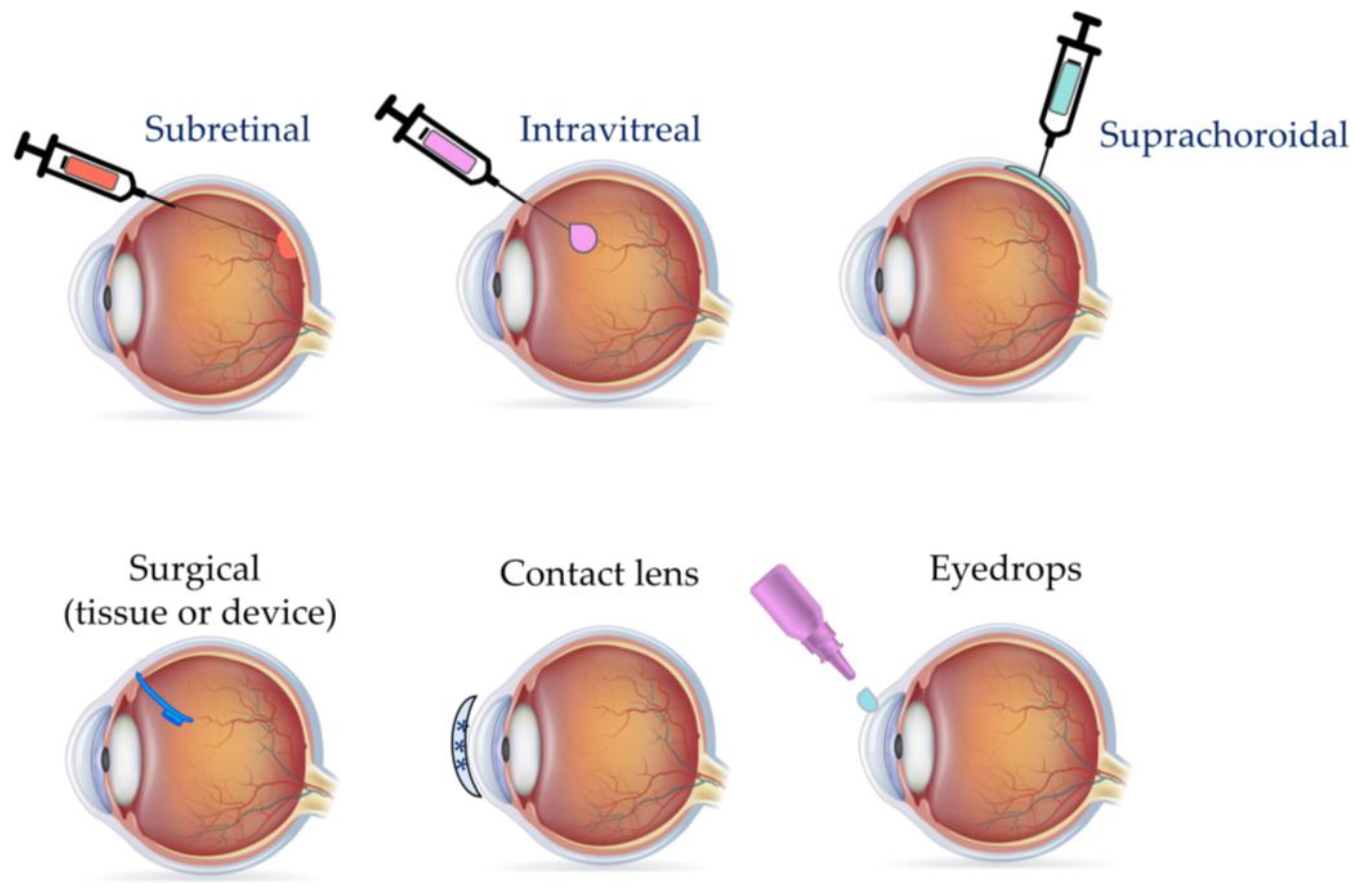
3. Mode of Delivery
3.1. Injection Routes
3.1.1. Intravitreal
3.1.2. Subretinal
3.1.3. Suprachoroidal
3.1.4. Future Directions
3.2. Vectors
3.2.1. Adenoviral (Ad) Vectors
3.2.2. Adeno-Associated Viral Vectors (AAVs)
3.2.3. Lentiviral Vectors
3.2.4. Naked Plasmid
3.2.5. Future Directions
3.3. Eye Drops
Future Directions
3.4. Nanoparticles
3.4.1. Nanomaterials
3.4.2. Nanomicelles
3.4.3. Liposomes
3.4.4. Dendrimers
3.4.5. Peptides
3.4.6. Future Directions
3.5. Contact Lenses
Future Directions
3.6. Ocular Implants
Future Directions
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sotiropulos, K.; Kourkoutas, D.; Almaliotis, D.; Ploumidou, K.; Karampatakis, V. Ocular Stem Cells: A Narrative Review of Current Clinical Trials. Int. J. Ophthalmol. 2022, 15, 1529–1537. [Google Scholar] [CrossRef]
- Vision Impairment and Blindness. Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 5 December 2023).
- Cross, N.; van Steen, C.; Zegaoui, Y.; Satherley, A.; Angelillo, L. Retinitis Pigmentosa: Burden of Disease and Current Unmet Needs. Clin. Ophthalmol. 2022, 16, 1993–2010. [Google Scholar] [CrossRef]
- Liu, M.M.; Tuo, J.; Chan, C.-C. Gene Therapy for Ocular Diseases. Br. J. Ophthalmol. 2011, 95, 604–612. [Google Scholar] [CrossRef]
- Zhou, R.; Caspi, R.R. Ocular Immune Privilege. F1000 Biol. Rep. 2010, 2, 3. [Google Scholar] [CrossRef]
- Caspi, R.R. Ocular Autoimmunity: The Price of Privilege? Immunol. Rev. 2006, 213, 23–35. [Google Scholar] [CrossRef]
- Ghoraba, H.H.; Akhavanrezayat, A.; Karaca, I.; Yavari, N.; Lajevardi, S.; Hwang, J.; Regenold, J.; Matsumiya, W.; Pham, B.; Zaidi, M.; et al. Ocular Gene Therapy: A Literature Review with Special Focus on Immune and Inflammatory Responses. Clin. Ophthalmol. 2022, 16, 1753–1771. [Google Scholar] [CrossRef]
- Leclercq, B.; Mejlachowicz, D.; Behar-Cohen, F. Ocular Barriers and Their Influence on Gene Therapy Products Delivery. Pharmaceutics 2022, 14, 998. [Google Scholar] [CrossRef]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene Therapy for Retinal Degenerative Diseases: Progress, Challenges, and Future Directions. Investig. Ophthalmol. Vis. Sci. 2023, 64, 39. [Google Scholar] [CrossRef]
- Nanegrungsunk, O.; Au, A.; Sarraf, D.; Sadda, S.R. New Frontiers of Retinal Therapeutic Intervention: A Critical Analysis of Novel Approaches. Ann. Med. 2022, 54, 1067–1080. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular Drug Delivery Systems: An Overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Wu, K.Y.; Mina, M.; Sahyoun, J.-Y.; Kalevar, A.; Tran, S.D. Retinal Prostheses: Engineering and Clinical Perspectives for Vision Restoration. Sensors 2023, 23, 5782. [Google Scholar] [CrossRef]
- RetNet: Summaries. Available online: https://web.sph.uth.edu/RetNet/sum-dis.htm?csrt=145452747213119187#A-genes (accessed on 9 December 2023).
- Marrone, L.; Marchi, P.M.; Azzouz, M. Circumventing the Packaging Limit of AAV-Mediated Gene Replacement Therapy for Neurological Disorders. Expert Opin. Biol. Ther. 2022, 22, 1163–1176. [Google Scholar] [CrossRef]
- Wong, C.H.; Li, D.; Wang, N.; Gruber, J.; Lo, A.W.; Conti, R.M. The Estimated Annual Financial Impact of Gene Therapy in the United States. Gene Ther. 2023, 30, 761–773. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and Safety of Voretigene Neparvovec (AAV2-hRPE65v2) in Patients with RPE65-Mediated Inherited Retinal Dystrophy: A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Parks, R.J.; Chen, L.; Anton, M.; Sankar, U.; Rudnicki, M.A.; Graham, F.L. A Helper-Dependent Adenovirus Vector System: Removal of Helper Virus by Cre-Mediated Excision of the Viral Packaging Signal. Proc. Natl. Acad. Sci. USA 1996, 93, 13565–13570. [Google Scholar] [CrossRef]
- Kong, J.; Kim, S.-R.; Binley, K.; Pata, I.; Doi, K.; Mannik, J.; Zernant-Rajang, J.; Kan, O.; Iqball, S.; Naylor, S.; et al. Correction of the Disease Phenotype in the Mouse Model of Stargardt Disease by Lentiviral Gene Therapy. Gene Ther. 2008, 15, 1311–1320. [Google Scholar] [CrossRef]
- Parker, M.A.; Erker, L.R.; Audo, I.; Choi, D.; Mohand-Said, S.; Sestakauskas, K.; Benoit, P.; Appelqvist, T.; Krahmer, M.; Ségaut-Prévost, C.; et al. Three-Year Safety Results of SAR422459 (EIAV-ABCA4) Gene Therapy in Patients with ABCA4-Associated Stargardt Disease: An Open-Label Dose-Escalation Phase I/IIa Clinical Trial, Cohorts 1-5. Am. J. Ophthalmol. 2022, 240, 285–301. [Google Scholar] [CrossRef]
- Aleman, T.S.; Huckfeldt, R.M.; Serrano, L.W.; Pearson, D.J.; Vergilio, G.K.; McCague, S.; Marshall, K.A.; Ashtari, M.; Doan, T.M.; Weigel-DiFranco, C.A.; et al. Adeno-Associated Virus Serotype 2-hCHM Subretinal Delivery to the Macula in Choroideremia: Two-Year Interim Results of an Ongoing Phase I/II Gene Therapy Trial. Ophthalmology 2022, 129, 1177–1191. [Google Scholar] [CrossRef]
- Sakai, D.; Tomita, H.; Maeda, A. Optogenetic Therapy for Visual Restoration. Int. J. Mol. Sci. 2022, 23, 15041. [Google Scholar] [CrossRef]
- Simon, C.-J.; Sahel, J.-A.; Duebel, J.; Herlitze, S.; Dalkara, D. Opsins for Vision Restoration. Biochem. Biophys. Res. Commun. 2020, 527, 325–330. [Google Scholar] [CrossRef]
- Deisseroth, K. Optogenetics: 10 Years of Microbial Opsins in Neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef]
- Tomita, H.; Sugano, E.; Fukazawa, Y.; Isago, H.; Sugiyama, Y.; Hiroi, T.; Ishizuka, T.; Mushiake, H.; Kato, M.; Hirabayashi, M.; et al. Visual Properties of Transgenic Rats Harboring the Channelrhodopsin-2 Gene Regulated by the Thy-1.2 Promoter. PLoS ONE 2009, 4, e7679. [Google Scholar] [CrossRef]
- Lagali, P.S.; Balya, D.; Awatramani, G.B.; Münch, T.A.; Kim, D.S.; Busskamp, V.; Cepko, C.L.; Roska, B. Light-Activated Channels Targeted to ON Bipolar Cells Restore Visual Function in Retinal Degeneration. Nat. Neurosci. 2008, 11, 667–675. [Google Scholar] [CrossRef]
- Ahmad, I. CRISPR/Cas9—A Promising Therapeutic Tool to Cure Blindness: Current Scenario and Future Prospects. Int. J. Mol. Sci. 2022, 23, 11482. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, C.; Hu, Z.; Li, M.; Wei, J.; Wang, Y.; Liu, H. Characterization of Brevibacillus Laterosporus Cas9 (BlatCas9) for Mammalian Genome Editing. Front. Cell Dev. Biol. 2020, 8, 583164. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Tee, L.Y.; Wang, X.-G.; Huang, Q.-S.; Yang, S.-H. Off-Target Effects in CRISPR/Cas9-Mediated Genome Engineering. Mol. Ther. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef]
- Uddin, F.; Rudin, C.M.; Sen, T. CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future. Front. Oncol. 2020, 10, 1387. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Lin, C.-Y.; Gootenberg, J.S.; Konermann, S.; Trevino, A.E.; Scott, D.A.; Inoue, A.; Matoba, S.; Zhang, Y.; et al. Double Nicking by RNA-Guided CRISPR Cas9 for Enhanced Genome Editing Specificity. Cell 2013, 154, 1380–1389. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-Fidelity CRISPR-Cas9 Nucleases with No Detectable Genome-Wide off-Target Effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, L.; Zhu, D.; Ding, W.; Wang, X.; Zhang, C.; Wang, L.; Jiang, X.; Shen, H.; He, D.; et al. Disruption of HPV16-E7 by CRISPR/Cas System Induces Apoptosis and Growth Inhibition in HPV16 Positive Human Cervical Cancer Cells. BioMed Res. Int. 2014, 2014, 612823. [Google Scholar] [CrossRef]
- Watts, J.K.; Corey, D.R. Gene Silencing by siRNAs and Antisense Oligonucleotides in the Laboratory and the Clinic. J. Pathol. 2012, 226, 365–379. [Google Scholar] [CrossRef]
- Gao, S.; Dagnaes-Hansen, F.; Nielsen, E.J.B.; Wengel, J.; Besenbacher, F.; Howard, K.A.; Kjems, J. The Effect of Chemical Modification and Nanoparticle Formulation on Stability and Biodistribution of siRNA in Mice. Mol. Ther. 2009, 17, 1225–1233. [Google Scholar] [CrossRef]
- Friedrich, M.; Aigner, A. Therapeutic siRNA: State-of-the-Art and Future Perspectives. BioDrugs 2022, 36, 549–571. [Google Scholar] [CrossRef]
- Russell, S.R.; Drack, A.V.; Cideciyan, A.V.; Jacobson, S.G.; Leroy, B.P.; Van Cauwenbergh, C.; Ho, A.C.; Dumitrescu, A.V.; Han, I.C.; Martin, M.; et al. Intravitreal Antisense Oligonucleotide Sepofarsen in Leber Congenital Amaurosis Type 10: A Phase 1b/2 Trial. Nat. Med. 2022, 28, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, D.V.; Lockridge, J.A.; Shaw, L.; Blanchard, K.; Jensen, K.; Breen, W.; Hartsough, K.; Machemer, L.; Radka, S.; Jadhav, V.; et al. Potent and Persistent in Vivo Anti-HBV Activity of Chemically Modified siRNAs. Nat. Biotechnol. 2005, 23, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Seet, L.F.; Tan, Y.F.; Toh, L.Z.; Chu, S.W.; Lee, Y.S.; Venkatraman, S.S.; Wong, T.T. Targeted Therapy for the Post-Operative Conjunctiva: SPARC Silencing Reduces Collagen Deposition. Br. J. Ophthalmol. 2018, 102, 1460–1470. [Google Scholar] [CrossRef]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- McClements, M.E.; MacLaren, R.E. Adeno-Associated Virus (AAV) Dual Vector Strategies for Gene Therapy Encoding Large Transgenes. Yale J. Biol. Med. 2017, 90, 611–623. [Google Scholar]
- Li, S.; Datta, S.; Brabbit, E.; Love, Z.; Woytowicz, V.; Flattery, K.; Capri, J.; Yao, K.; Wu, S.; Imboden, M.; et al. Nr2e3 Is a Genetic Modifier That Rescues Retinal Degeneration and Promotes Homeostasis in Multiple Models of Retinitis Pigmentosa. Gene Ther. 2021, 28, 223–241. [Google Scholar] [CrossRef]
- Pickard, G.E.; Sollars, P.J. Intrinsically Photosensitive Retinal Ganglion Cells. Rev. Physiol. Biochem. Pharmacol. 2012, 162, 59–90. [Google Scholar] [CrossRef]
- Rodrigues, E.B.; Farah, M.E.; Maia, M.; Penha, F.M.; Regatieri, C.; Melo, G.B.; Pinheiro, M.M.; Zanetti, C.R. Therapeutic Monoclonal Antibodies in Ophthalmology. Prog. Retin. Eye Res. 2009, 28, 117–144. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M.; Chang, T.S.; Fine, J.T.; Dolan, C.M.; Ward, J. Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration (ANCHOR) Research Group Improved Vision-Related Function after Ranibizumab vs Photodynamic Therapy: A Randomized Clinical Trial. Arch. Ophthalmol. 2009, 127, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Michels, M.; Kaiser, P.K.; Heier, J.S.; Sy, J.P.; Ianchulev, T. ANCHOR Study Group Ranibizumab versus Verteporfin Photodynamic Therapy for Neovascular Age-Related Macular Degeneration: Two-Year Results of the ANCHOR Study. Ophthalmology 2009, 116, 57–65.e5. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, M.; Nagpal, K.; Nagpal, P. A Comparative Debate on the Various Anti-Vascular Endothelial Growth Factor Drugs: Pegaptanib Sodium (Macugen), Ranibizumab (Lucentis) and Bevacizumab (Avastin). Indian J. Ophthalmol. 2007, 55, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M. Targeting the Complement System for the Management of Retinal Inflammatory and Degenerative Diseases. Eur. J. Pharmacol. 2016, 787, 94–104. [Google Scholar] [CrossRef]
- Anderson, D.H.; Radeke, M.J.; Gallo, N.B.; Chapin, E.A.; Johnson, P.T.; Curletti, C.R.; Hancox, L.S.; Hu, J.; Ebright, J.N.; Malek, G.; et al. The Pivotal Role of the Complement System in Aging and Age-Related Macular Degeneration: Hypothesis Re-Visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef]
- Muramatsu, D.; Wakabayashi, Y.; Usui, Y.; Okunuki, Y.; Kezuka, T.; Goto, H. Correlation of Complement Fragment C5a with Inflammatory Cytokines in the Vitreous of Patients with Proliferative Diabetic Retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 15–17. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Westby, K.; Csaky, K.G.; Monés, J.; Pearlman, J.A.; Patel, S.S.; Joondeph, B.C.; Randolph, J.; Masonson, H.; Rezaei, K.A. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology 2021, 128, 576–586. [Google Scholar] [CrossRef]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef]
- Garg, A.; Nanji, K.; Tai, F.; Phillips, M.; Zeraatkar, D.; Garg, S.J.; Sadda, S.R.; Kaiser, P.K.; Guymer, R.H.; Sivaprasad, S.; et al. The Effect of Complement C3 or C5 Inhibition on Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Living Systematic Review and Meta-Analysis. Surv. Ophthalmol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Khanani, A.M.; Patel, S.S.; Staurenghi, G.; Tadayoni, R.; Danzig, C.J.; Eichenbaum, D.A.; Hsu, J.; Wykoff, C.C.; Heier, J.S.; Lally, D.R.; et al. Efficacy and Safety of Avacincaptad Pegol in Patients with Geographic Atrophy (GATHER2): 12-Month Results from a Randomised, Double-Masked, Phase 3 Trial. Lancet 2023, 402, 1449–1458. [Google Scholar] [CrossRef]
- Rafael, D.; Guerrero, M.; Marican, A.; Arango, D.; Sarmento, B.; Ferrer, R.; Durán-Lara, E.F.; Clark, S.J.; Schwartz, S. Delivery Systems in Ocular Retinopathies: The Promising Future of Intravitreal Hydrogels as Sustained-Release Scaffolds. Pharmaceutics 2023, 15, 1484. [Google Scholar] [CrossRef] [PubMed]
- Shughoury, A.; Sevgi, D.D.; Ciulla, T.A. The Complement System: A Novel Therapeutic Target for Age-Related Macular Degeneration. Expert. Opin. Pharmacother. 2023, 24, 1887–1899. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Faricimab: First Approval. Drugs 2022, 82, 825–830. [Google Scholar] [CrossRef]
- Tomczak, W.; Winkler-Lach, W.; Tomczyk-Socha, M.; Misiuk-Hojło, M. Advancements in Ocular Regenerative Therapies. Biology 2023, 12, 737. [Google Scholar] [CrossRef]
- Daya, S.M.; Chan, C.C.; Holland, E.J. Members of The Cornea Society Ocular Surface Procedures Nomenclature Committee Cornea Society Nomenclature for Ocular Surface Rehabilitative Procedures. Cornea 2011, 30, 1115–1119. [Google Scholar] [CrossRef]
- Daya, S.M. Conjunctival-Limbal Autograft. Curr. Opin. Ophthalmol. 2017, 28, 370–376. [Google Scholar] [CrossRef]
- Fernandez-Buenaga, R.; Aiello, F.; Zaher, S.S.; Grixti, A.; Ahmad, S. Twenty Years of Limbal Epithelial Therapy: An Update on Managing Limbal Stem Cell Deficiency. BMJ Open Ophthalmol. 2018, 3, e000164. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Inatomi, T.; Suzuki, T.; Sotozono, C.; Kinoshita, S. Cultivated Corneal Epithelial Stem Cell Transplantation in Ocular Surface Disorders. Ophthalmology 2001, 108, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Sepsakos, L.; Cheung, A.Y.; Nerad, J.A.; Mogilishetty, G.; Holland, E.J. Donor-Derived Conjunctival-Limbal Melanoma After a Keratolimbal Allograft. Cornea 2017, 36, 1415–1418. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef]
- Jurkunas, U.V.; Yin, J.; Johns, L.K.; Li, S.; Negre, H.; Shaw, K.L.; Samarakoon, L.; Ayala, A.R.; Kheirkhah, A.; Katikireddy, K.; et al. Cultivated Autologous Limbal Epithelial Cell (CALEC) Transplantation: Development of Manufacturing Process and Clinical Evaluation of Feasibility and Safety. Sci. Adv. 2023, 9, eadg6470. [Google Scholar] [CrossRef]
- Calonge, M.; Pérez, I.; Galindo, S.; Nieto-Miguel, T.; López-Paniagua, M.; Fernández, I.; Alberca, M.; García-Sancho, J.; Sánchez, A.; Herreras, J.M. A Proof-of-Concept Clinical Trial Using Mesenchymal Stem Cells for the Treatment of Corneal Epithelial Stem Cell Deficiency. Transl. Res. 2019, 206, 18–40. [Google Scholar] [CrossRef]
- Jiang, T.-S.; Cai, L.; Ji, W.-Y.; Hui, Y.-N.; Wang, Y.-S.; Hu, D.; Zhu, J. Reconstruction of the Corneal Epithelium with Induced Marrow Mesenchymal Stem Cells in Rats. Mol. Vis. 2010, 16, 1304–1316. [Google Scholar]
- Nieto-Nicolau, N.; Martínez-Conesa, E.M.; Fuentes-Julián, S.; Arnalich-Montiel, F.; García-Tuñón, I.; De Miguel, M.P.; Casaroli-Marano, R.P. Priming Human Adipose-Derived Mesenchymal Stem Cells for Corneal Surface Regeneration. J. Cell Mol. Med. 2021, 25, 5124–5137. [Google Scholar] [CrossRef]
- Coulson-Thomas, V.J.; Coulson-Thomas, Y.M.; Gesteira, T.F.; Kao, W.W.-Y. Extrinsic and Intrinsic Mechanisms by Which Mesenchymal Stem Cells Suppress the Immune System. Ocul. Surf. 2016, 14, 121–134. [Google Scholar] [CrossRef]
- Nieto-Miguel, T.; Galindo, S.; Reinoso, R.; Corell, A.; Martino, M.; Pérez-Simón, J.A.; Calonge, M. In Vitro Simulation of Corneal Epithelium Microenvironment Induces a Corneal Epithelial-like Cell Phenotype from Human Adipose Tissue Mesenchymal Stem Cells. Curr. Eye Res. 2013, 38, 933–944. [Google Scholar] [CrossRef]
- Uyama, H.; Mandai, M.; Takahashi, M. Stem-Cell-Based Therapies for Retinal Degenerative Diseases: Current Challenges in the Establishment of New Treatment Strategies. Dev. Growth Differ. 2021, 63, 59–71. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Chen, W.; Jiang, Z.; Luo, Y.; Wang, D.; Zheng, Y.; Liu, Y. Acellularized Uvea Hydrogel as Novel Injectable Platform for Cell-Based Delivering Treatment of Retinal Degeneration and Optimizing Retinal Organoids Inducible System. Adv. Healthc. Mater. 2022, 11, e2202114. [Google Scholar] [CrossRef]
- Brant Fernandes, R.A.; Lojudice, F.H.; Zago Ribeiro, L.; Santos da Cruz, N.F.; Polizelli, M.U.; Cristovam, P.C.; Innocenti, F.; Morimoto, L.; Magalhães, O.; Ferraz Sallum, J.M.; et al. Transplantation of subretinal stem cell-derived retinal pigment epithelium for stargardt disease: A Phase I Clinical Trial. Retina 2023, 43, 263–274. [Google Scholar] [CrossRef]
- Li, S.-Y.; Liu, Y.; Wang, L.; Wang, F.; Zhao, T.-T.; Li, Q.-Y.; Xu, H.-W.; Meng, X.-H.; Hao, J.; Zhou, Q.; et al. A Phase I Clinical Trial of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells for Early-Stage Stargardt Macular Degeneration: 5-Years’ Follow-Up. Cell Prolif. 2021, 54, e13100. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Aguzzi, E.A.; Johnson, T.V. Retinal Ganglion Cell Transplantation: Approaches for Overcoming Challenges to Functional Integration. Cells 2021, 10, 1426. [Google Scholar] [CrossRef]
- Moore, D.L.; Blackmore, M.G.; Hu, Y.; Kaestner, K.H.; Bixby, J.L.; Lemmon, V.P.; Goldberg, J.L. KLF Family Members Regulate Intrinsic Axon Regeneration Ability. Science 2009, 326, 298–301. [Google Scholar] [CrossRef]
- Trakhtenberg, E.F.; Li, Y.; Feng, Q.; Tso, J.; Rosenberg, P.A.; Goldberg, J.L.; Benowitz, L.I. Zinc Chelation and Klf9 Knockdown Cooperatively Promote Axon Regeneration after Optic Nerve Injury. Exp. Neurol. 2018, 300, 22–29. [Google Scholar] [CrossRef]
- de Lima, S.; Koriyama, Y.; Kurimoto, T.; Oliveira, J.T.; Yin, Y.; Li, Y.; Gilbert, H.-Y.; Fagiolini, M.; Martinez, A.M.B.; Benowitz, L. Full-Length Axon Regeneration in the Adult Mouse Optic Nerve and Partial Recovery of Simple Visual Behaviors. Proc. Natl. Acad. Sci. USA 2012, 109, 9149–9154. [Google Scholar] [CrossRef]
- Ripolles-Garcia, A.; Dolgova, N.; Phillips, M.J.; Savina, S.; Ludwig, A.L.; Stuedemann, S.A.; Nlebedum, U.; Wolfe, J.H.; Garden, O.A.; Maminishkis, A.; et al. Systemic Immunosuppression Promotes Survival and Integration of Subretinally Implanted Human ESC-Derived Photoreceptor Precursors in Dogs. Stem Cell Rep. 2022, 17, 1824–1841. [Google Scholar] [CrossRef]
- Singh, M.S.; Balmer, J.; Barnard, A.R.; Aslam, S.A.; Moralli, D.; Green, C.M.; Barnea-Cramer, A.; Duncan, I.; MacLaren, R.E. Transplanted Photoreceptor Precursors Transfer Proteins to Host Photoreceptors by a Mechanism of Cytoplasmic Fusion. Nat. Commun. 2016, 7, 13537. [Google Scholar] [CrossRef]
- Tay, H.G.; Andre, H.; Chrysostomou, V.; Adusumalli, S.; Guo, J.; Ren, X.; Tan, W.S.; Tor, J.E.; Moreno-Moral, A.; Plastino, F.; et al. Photoreceptor Laminin Drives Differentiation of Human Pluripotent Stem Cells to Photoreceptor Progenitors That Partially Restore Retina Function. Mol. Ther. 2023, 31, 825–846. [Google Scholar] [CrossRef]
- Mamalis, N.; Davis, B.; Nilson, C.D.; Hickman, M.S.; Leboyer, R.M. Complications of Foldable Intraocular Lenses Requiring Explantation or Secondary Intervention--2003 Survey Update. J. Cataract. Refract. Surg. 2004, 30, 2209–2218. [Google Scholar] [CrossRef]
- Raj, S.M.; Vasavada, A.R.; Johar, S.R.K.; Vasavada, V.A.; Vasavada, V.A. Post-Operative Capsular Opacification: A Review. Int. J. Biomed. Sci. 2007, 3, 237–250. [Google Scholar]
- Lin, H.; Ouyang, H.; Zhu, J.; Huang, S.; Liu, Z.; Chen, S.; Cao, G.; Li, G.; Signer, R.A.J.; Xu, Y.; et al. Lens Regeneration Using Endogenous Stem Cells with Gain of Visual Function. Nature 2016, 531, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef]
- Mellough, C.B.; Cui, Q.; Spalding, K.L.; Symons, N.A.; Pollett, M.A.; Snyder, E.Y.; Macklis, J.D.; Harvey, A.R. Fate of Multipotent Neural Precursor Cells Transplanted into Mouse Retina Selectively Depleted of Retinal Ganglion Cells. Exp. Neurol. 2004, 186, 6–19. [Google Scholar] [CrossRef]
- Grozdanic, S.D.; Ast, A.M.; Lazic, T.; Kwon, Y.H.; Kardon, R.H.; Sonea, I.M.; Sakaguchi, D.S. Morphological Integration and Functional Assessment of Transplanted Neural Progenitor Cells in Healthy and Acute Ischemic Rat Eyes. Exp. Eye Res. 2006, 82, 597–607. [Google Scholar] [CrossRef]
- McGill, T.J.; Cottam, B.; Lu, B.; Wang, S.; Girman, S.; Tian, C.; Huhn, S.L.; Lund, R.D.; Capela, A. Transplantation of Human Central Nervous System Stem Cells - Neuroprotection in Retinal Degeneration. Eur. J. Neurosci. 2012, 35, 468–477. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernández-Sánchez, L.; McGill, T.J.; Lu, B.; Wang, S.; Lund, R.; Huhn, S.; Capela, A. Phagocytosis of Photoreceptor Outer Segments by Transplanted Human Neural Stem Cells as a Neuroprotective Mechanism in Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6745–6756. [Google Scholar] [CrossRef]
- Nishida, A.; Takahashi, M.; Tanihara, H.; Nakano, I.; Takahashi, J.B.; Mizoguchi, A.; Ide, C.; Honda, Y. Incorporation and Differentiation of Hippocampus-Derived Neural Stem Cells Transplanted in Injured Adult Rat Retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4268–4274. [Google Scholar]
- Loewenstein, J.I.; Montezuma, S.R.; Rizzo, J.F. Outer Retinal Degeneration: An Electronic Retinal Prosthesis as a Treatment Strategy. Arch. Ophthalmol. 2004, 122, 587–596. [Google Scholar] [CrossRef]
- Humayun, M.S.; de Juan, E.; Dagnelie, G.; Greenberg, R.J.; Propst, R.H.; Phillips, D.H. Visual Perception Elicited by Electrical Stimulation of Retina in Blind Humans. Arch. Ophthalmol. 1996, 114, 40–46. [Google Scholar] [CrossRef]
- Rizzo, J.F.; Wyatt, J.; Loewenstein, J.; Kelly, S.; Shire, D. Perceptual Efficacy of Electrical Stimulation of Human Retina with a Microelectrode Array during Short-Term Surgical Trials. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5362–5369. [Google Scholar] [CrossRef]
- Finn, A.P.; Grewal, D.S.; Vajzovic, L. Argus II Retinal Prosthesis System: A Review of Patient Selection Criteria, Surgical Considerations, and Post-Operative Outcomes. Clin. Ophthalmol. 2018, 12, 1089–1097. [Google Scholar] [CrossRef]
- Ramirez, K.A.; Drew-Bear, L.E.; Vega-Garces, M.; Betancourt-Belandria, H.; Arevalo, J.F. An Update on Visual Prosthesis. Int. J. Retin. Vitr. 2023, 9, 73. [Google Scholar] [CrossRef]
- Werner, L. Intraocular Lenses: Overview of Designs, Materials, and Pathophysiologic Features. Ophthalmology 2021, 128, e74–e93. [Google Scholar] [CrossRef]
- Luo, C.; Wang, H.; Chen, X.; Xu, J.; Yin, H.; Yao, K. Recent Advances of Intraocular Lens Materials and Surface Modification in Cataract Surgery. Front. Bioeng. Biotechnol. 2022, 10, 913383. [Google Scholar] [CrossRef]
- Javitt, J.C.; Wang, F.; West, S.K. Blindness Due to Cataract: Epidemiology and Prevention. Annu. Rev. Public Health 1996, 17, 159–177. [Google Scholar] [CrossRef]
- Zvorničanin, J.; Zvorničanin, E. Premium Intraocular Lenses: The Past, Present and Future. J. Curr. Ophthalmol. 2018, 30, 287–296. [Google Scholar] [CrossRef]
- Kwon, Y.R.; Hwang, Y.N.; Kim, S.M. Posterior Capsule Opacification after Cataract Surgery via Implantation with Hydrophobic Acrylic Lens Compared with Silicone Intraocular Lens: A Systematic Review and Meta-Analysis. J. Ophthalmol. 2022, 2022, 3570399. [Google Scholar] [CrossRef]
- Riau, A.K.; Venkatraman, S.S.; Dohlman, C.H.; Mehta, J.S. Surface Modifications of the PMMA Optic of a Keratoprosthesis to Improve Biointegration. Cornea 2017, 36 (Suppl. S1), S15–S25. [Google Scholar] [CrossRef]
- Riau, A.K.; Mondal, D.; Yam, G.H.F.; Setiawan, M.; Liedberg, B.; Venkatraman, S.S.; Mehta, J.S. Surface Modification of PMMA to Improve Adhesion to Corneal Substitutes in a Synthetic Core–Skirt Keratoprosthesis. ACS Appl. Mater. Interfaces 2015, 7, 21690–21702. [Google Scholar] [CrossRef]
- Salvador-Culla, B.; Kolovou, P.E. Keratoprosthesis: A Review of Recent Advances in the Field. J. Funct. Biomater. 2016, 7, 13. [Google Scholar] [CrossRef]
- Akpek, E.K.; Cassard, S.D.; Dunlap, K.; Hahn, S.; Ramulu, P.Y. Donor Corneal Transplantation vs Boston Type 1 Keratoprosthesis in Patients with Previous Graft Failures: A Retrospective Single Center Study (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2015, 113, T3. [Google Scholar]
- Prabhasawat, P.; Chotikavanich, S.; Ngowyutagon, P.; Pinitpuwadol, W. Long-Term Outcomes of Boston Type I Keratoprosthesis, and Efficacy of Amphotericin B and Povidone-Iodine in Infection Prophylaxis. Am. J. Ophthalmol. 2021, 232, 40–48. [Google Scholar] [CrossRef]
- Dugel, P.U.; Singh, N.; Francom, S.; Cantrell, R.A.; Grzeschik, S.M.; Fung, A.E. The Systemic Safety of Ranibizumab in Patients 85 Years and Older with Neovascular Age-Related Macular Degeneration. Ophthalmol. Retin. 2018, 2, 667–675. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef]
- Veritti, D.; Sarao, V.; Chhablani, J.; Loewenstein, A.; Lanzetta, P.; Bandello, F.; Midena, E.; Nicolò, M.; Parravano, M.; Pilotto, E.; et al. The Ideal Intravitreal Injection Setting: Office, Ambulatory Surgery Room or Operating Theatre? A Narrative Review and International Survey. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 3299–3306. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Le Goff, M.M.; Allen, A.; Lucas, R.J.; Bishop, P.N. Glycosidic Enzymes Enhance Retinal Transduction Following Intravitreal Delivery of AAV2. Mol. Vis. 2011, 17, 1771–1783. [Google Scholar]
- Ross, M.; Obolensky, A.; Averbukh, E.; Ezra-Elia, R.; Yamin, E.; Honig, H.; Dvir, H.; Rosov, A.; Hauswirth, W.W.; Gootwine, E.; et al. Evaluation of Photoreceptor Transduction Efficacy of Capsid-Modified Adeno-Associated Viral Vectors Following Intravitreal and Subretinal Delivery in Sheep. Hum. Gene Ther. 2020, 31, 719–729. [Google Scholar] [CrossRef]
- Irigoyen, C.; Amenabar Alonso, A.; Sanchez-Molina, J.; Rodríguez-Hidalgo, M.; Lara-López, A.; Ruiz-Ederra, J. Subretinal Injection Techniques for Retinal Disease: A Review. J. Clin. Med. 2022, 11, 4717. [Google Scholar] [CrossRef]
- Qi, Y.; Dai, X.; Zhang, H.; He, Y.; Zhang, Y.; Han, J.; Zhu, P.; Zhang, Y.; Zheng, Q.; Li, X.; et al. Trans-Corneal Subretinal Injection in Mice and Its Effect on the Function and Morphology of the Retina. PLoS ONE 2015, 10, e0136523. [Google Scholar] [CrossRef]
- Ladha, R.; Caspers, L.E.; Willermain, F.; de Smet, M.D. Subretinal Therapy: Technological Solutions to Surgical and Immunological Challenges. Front. Med. 2022, 9, 846782. [Google Scholar] [CrossRef]
- Mühlfriedel, R.; Michalakis, S.; Garcia Garrido, M.; Biel, M.; Seeliger, M.W. Optimized Technique for Subretinal Injections in Mice. Methods Mol. Biol. 2013, 935, 343–349. [Google Scholar] [CrossRef]
- Schlichtenbrede, F.C.; da Cruz, L.; Stephens, C.; Smith, A.J.; Georgiadis, A.; Thrasher, A.J.; Bainbridge, J.W.B.; Seeliger, M.W.; Ali, R.R. Long-Term Evaluation of Retinal Function in Prph2Rd2/Rd2 Mice Following AAV-Mediated Gene Replacement Therapy. J. Gene Med. 2003, 5, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Tang, L.; Zhou, Y. Subretinal Injection: A Review on the Novel Route of Therapeutic Delivery for Vitreoretinal Diseases. Ophthalmic Res. 2017, 58, 217–226. [Google Scholar] [CrossRef]
- Wert, K.J.; Skeie, J.M.; Davis, R.J.; Tsang, S.H.; Mahajan, V.B. Subretinal Injection of Gene Therapy Vectors and Stem Cells in the Perinatal Mouse Eye. J. Vis. Exp. 2012, 4286. [Google Scholar] [CrossRef]
- Simunovic, M.P.; Xue, K.; Jolly, J.K.; MacLaren, R.E. Structural and Functional Recovery Following Limited Iatrogenic Macular Detachment for Retinal Gene Therapy. JAMA Ophthalmol. 2017, 135, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Emi, K.; Pederson, J.E.; Toris, C.B. Hydrostatic Pressure of the Suprachoroidal Space. Investig. Ophthalmol. Vis. Sci. 1989, 30, 233–238. [Google Scholar]
- Wu, K.Y.; Fujioka, J.K.; Gholamian, T.; Zaharia, M.; Tran, S.D. Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery. Pharmaceuticals 2023, 16, 1241. [Google Scholar] [CrossRef]
- Kim, Y.C.; Edelhauser, H.F.; Prausnitz, M.R. Targeted Delivery of Antiglaucoma Drugs to the Supraciliary Space Using Microneedles. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7387–7397. [Google Scholar] [CrossRef]
- Yeh, S.; Kurup, S.K.; Wang, R.C.; Foster, C.S.; Noronha, G.; Nguyen, Q.D.; Do, D.V.; DOGWOOD Study Team. Suprachoroidal injection of triamcinolone acetonide, CLS-TA, for macular edema due to noninfectious uveitis: A Randomized, Phase 2 Study (DOGWOOD). Retina 2019, 39, 1880–1888. [Google Scholar] [CrossRef]
- Kansara, V.; Muya, L.; Wan, C.-R.; Ciulla, T.A. Suprachoroidal Delivery of Viral and Nonviral Gene Therapy for Retinal Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 384–392. [Google Scholar] [CrossRef]
- Han, I.C.; Burnight, E.R.; Ulferts, M.J.; Worthington, K.S.; Russell, S.R.; Sohn, E.H.; Mullins, R.F.; Stone, E.M.; Tucker, B.A.; Wiley, L.A. Helper-Dependent Adenovirus Transduces the Human and Rat Retina but Elicits an Inflammatory Reaction When Delivered Subretinally in Rats. Hum. Gene Ther. 2019, 30, 1371–1384. [Google Scholar] [CrossRef]
- Follenzi, A.; Santambrogio, L.; Annoni, A. Immune Responses to Lentiviral Vectors. Curr. Gene Ther. 2007, 7. [Google Scholar] [CrossRef] [PubMed]
- Lebherz, C.; Maguire, A.; Tang, W.; Bennett, J.; Wilson, J.M. Novel AAV Serotypes for Improved Ocular Gene Transfer. J. Gene Med. 2008, 10, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Wiley, L.A.; Burnight, E.R.; Kaalberg, E.E.; Jiao, C.; Riker, M.J.; Halder, J.A.; Luse, M.A.; Han, I.C.; Russell, S.R.; Sohn, E.H.; et al. Assessment of Adeno-Associated Virus Serotype Tropism in Human Retinal Explants. Hum. Gene Ther. 2018, 29, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Wiley, L.A.; Boyce, T.M.; Meyering, E.E.; Ochoa, D.; Sheehan, K.M.; Stone, E.M.; Mullins, R.F.; Tucker, B.A.; Han, I.C. The Degree of Adeno-Associated Virus-Induced Retinal Inflammation Varies Based on Serotype and Route of Delivery: Intravitreal, Subretinal, or Suprachoroidal. Hum. Gene Ther. 2023, 34, 530–539. [Google Scholar] [CrossRef]
- Auricchio, A.; Kobinger, G.; Anand, V.; Hildinger, M.; O’Connor, E.; Maguire, A.M.; Wilson, J.M.; Bennett, J. Exchange of Surface Proteins Impacts on Viral Vector Cellular Specificity and Transduction Characteristics: The Retina as a Model. Hum. Mol. Genet. 2001, 10, 3075–3081. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.S.; Schmidt, M.; Yan, Z.; Lindbloom, J.D.; Harding, T.C.; Donahue, B.A.; Engelhardt, J.F.; Kotin, R.; Davidson, B.L. Virus-Mediated Transduction of Murine Retina with Adeno-Associated Virus: Effects of Viral Capsid and Genome Size. J. Virol. 2002, 76, 7651–7660. [Google Scholar] [CrossRef]
- Maurya, S.; Sarangi, P.; Jayandharan, G.R. Safety of Adeno-Associated Virus-Based Vector-Mediated Gene Therapy—Impact of Vector Dose. Cancer Gene Ther. 2022, 29, 1305–1306. [Google Scholar] [CrossRef]
- Vandenberghe, L.H.; Bell, P.; Maguire, A.M.; Cearley, C.N.; Xiao, R.; Calcedo, R.; Wang, L.; Castle, M.J.; Maguire, A.C.; Grant, R.; et al. Dosage Thresholds for AAV2 and AAV8 Photoreceptor Gene Therapy in Monkey. Sci. Transl. Med. 2011, 3, 88ra54. [Google Scholar] [CrossRef]
- Le Meur, G.; Lebranchu, P.; Billaud, F.; Adjali, O.; Schmitt, S.; Bézieau, S.; Péréon, Y.; Valabregue, R.; Ivan, C.; Darmon, C.; et al. Safety and Long-Term Efficacy of AAV4 Gene Therapy in Patients with RPE65 Leber Congenital Amaurosis. Mol. Ther. 2018, 26, 256–268. [Google Scholar] [CrossRef]
- Ye, G.; Komáromy, A.M.; Zeiss, C.; Calcedo, R.; Harman, C.D.; Koehl, K.L.; Stewart, G.A.; Iwabe, S.; Chiodo, V.A.; Hauswirth, W.W.; et al. Safety and Efficacy of AAV5 Vectors Expressing Human or Canine CNGB3 in CNGB3-Mutant Dogs. Hum. Gene Ther. Clin. Dev. 2017, 28, 197–207. [Google Scholar] [CrossRef]
- Koponen, S.; Kokki, E.; Tamminen, T.; Ylä-Herttuala, S. AAV2 and AAV9 Tropism and Transgene Expression in the Mouse Eye and Major Tissues after Intravitreal and Subretinal Delivery. Front. Drug Deliv. 2023, 3, 1148795. [Google Scholar] [CrossRef]
- Mallam, J.N.; Hurwitz, M.Y.; Mahoney, T.; Chévez-Barrios, P.; Hurwitz, R.L. Efficient Gene Transfer into Retinal Cells Using Adenoviral Vectors: Dependence on Receptor Expression. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1680–1687. [Google Scholar] [CrossRef]
- Ueyama, K.; Mori, K.; Shoji, T.; Omata, H.; Gehlbach, P.L.; Brough, D.E.; Wei, L.L.; Yoneya, S. Ocular Localization and Transduction by Adenoviral Vectors Are Serotype-Dependent and Can Be Modified by Inclusion of RGD Fiber Modifications. PLoS ONE 2014, 9, e108071. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Romanowski, E.; Araullo-Cruz, T. An Ocular Model of Adenovirus Type 5 Infection in the NZ Rabbit. Investig. Ophthalmol. Vis. Sci. 1992, 33, 574–580. [Google Scholar]
- Baudouin, C.; Labbé, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in Eyedrops: The Good, the Bad and the Ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef]
- Vadlapudi, A.D.; Mitra, A.K. Nanomicelles: An Emerging Platform for Drug Delivery to the Eye. Ther. Deliv. 2013, 4, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in Ocular Applications and Their Potential Toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef] [PubMed]
- Abud, M.B.; Louzada, R.N.; Isaac, D.L.C.; Souza, L.G.; dos Reis, R.G.; Lima, E.M.; de Ávila, M.P. In Vivo and in Vitro Toxicity Evaluation of Liposome-Encapsulated Sirolimus. Int. J. Retin. Vitr. 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.; Bozdağ Pehlivan, S.; Ünlü, N. Dendrimeric Systems and Their Applications in Ocular Drug Delivery. Sci. World J. 2013, 2013, 732340. [Google Scholar] [CrossRef] [PubMed]
- Krishna, T.R.; Jayaraman, N. Synthesis of Poly(Propyl Ether Imine) Dendrimers and Evaluation of Their Cytotoxic Properties. J. Org. Chem. 2003, 68, 9694–9704. [Google Scholar] [CrossRef]
- Rodrigues, G.A.; Shalaev, E.; Karami, T.K.; Cunningham, J.; Slater, N.K.H.; Rivers, H.M. Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye. Pharm. Res. 2019, 36, 29. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Tanabe, T.; Sun, D.; Zeng, Y.; Kjeldbye, H.; Gouras, P.; Maguire, A.M. Photoreceptor Cell Rescue in Retinal Degeneration (Rd) Mice by in Vivo Gene Therapy. Nat. Med. 1996, 2, 649–654. [Google Scholar] [CrossRef]
- Riley, D.J.; Nikitin, A.Y.; Lee, W.H. Adenovirus-Mediated Retinoblastoma Gene Therapy Suppresses Spontaneous Pituitary Melanotroph Tumors in Rb+/− Mice. Nat. Med. 1996, 2, 1316–1321. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Bazan-Peregrino, M.; Gil-Martín, M.; Álvarez, R.; Macarulla, T.; Riesco-Martinez, M.C.; Verdaguer, H.; Guillén-Ponce, C.; Farrera-Sal, M.; Moreno, R.; et al. Phase I, Multicenter, Open-Label Study of Intravenous VCN-01 Oncolytic Adenovirus with or without Nab-Paclitaxel plus Gemcitabine in Patients with Advanced Solid Tumors. J. Immunother. Cancer 2022, 10, e003255. [Google Scholar] [CrossRef]
- Yang, Y.; Jooss, K.U.; Su, Q.; Ertl, H.C.; Wilson, J.M. Immune Responses to Viral Antigens versus Transgene Product in the Elimination of Recombinant Adenovirus-Infected Hepatocytes in Vivo. Gene Ther. 1996, 3, 137–144. [Google Scholar]
- Gregory, S.M.; Nazir, S.A.; Metcalf, J.P. Implications of the Innate Immune Response to Adenovirus and Adenoviral Vectors. Future Virol. 2011, 6, 357–374. [Google Scholar] [CrossRef]
- Atchison, R.W.; Casto, B.C.; Hammon, W.M. Adenovirus-Associated Defective Virus Particles. Science 1965, 149, 754–756. [Google Scholar] [CrossRef]
- Frederick, A.; Sullivan, J.; Liu, L.; Adamowicz, M.; Lukason, M.; Raymer, J.; Luo, Z.; Jin, X.; Rao, K.N.; O’Riordan, C. Engineered Capsids for Efficient Gene Delivery to the Retina and Cornea. Hum. Gene Ther. 2020, 31, 756–774. [Google Scholar] [CrossRef]
- Lee, S.H.; Colosi, P.; Lee, H.; Ohn, Y.-H.; Kim, S.-W.; Kwak, H.W.; Park, T.K. Laser Photocoagulation Enhances Adeno-Associated Viral Vector Transduction of Mouse Retina. Hum. Gene Ther. Methods 2014, 25, 83–91. [Google Scholar] [CrossRef]
- Igarashi, T.; Miyake, K.; Asakawa, N.; Miyake, N.; Shimada, T.; Takahashi, H. Direct Comparison of Administration Routes for AAV8-Mediated Ocular Gene Therapy. Curr. Eye Res. 2013, 38, 569–577. [Google Scholar] [CrossRef]
- Song, H.; Bush, R.A.; Zeng, Y.; Qian, H.; Wu, Z.; Sieving, P.A. Trans-Ocular Electric Current In Vivo Enhances AAV-Mediated Retinal Gene Transduction after Intravitreal Vector Administration. Mol. Therapy. Methods Clin. Dev. 2019, 13, 77. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Laperrousaz, E.; Tribble, J.R.; Verhaagen, J.; Fawcett, J.W.; Martin, K.R.; Williams, P.A.; Osborne, A. Improving Adeno-Associated Viral (AAV) Vector-Mediated Transgene Expression in Retinal Ganglion Cells: Comparison of Five Promoters. Gene Ther. 2023, 30, 503–519. [Google Scholar] [CrossRef]
- Lotery, A.J.; Yang, G.S.; Mullins, R.F.; Russell, S.R.; Schmidt, M.; Stone, E.M.; Lindbloom, J.D.; Chiorini, J.A.; Kotin, R.M.; Davidson, B.L. Adeno-Associated Virus Type 5: Transduction Efficiency and Cell-Type Specificity in the Primate Retina. Hum. Gene Ther. 2003, 14, 1663–1671. [Google Scholar] [CrossRef]
- Pang, J.; Lauramore, A.; Deng, W.; Li, Q.; Doyle, T.J.; Chiodo, V.; Li, J.; Hauswirth, W.W. Comparative Analysis of in Vivo and in Vitro AAV Vector Transduction in the Neonatal Mouse Retina: Effects of Serotype and Site of Administration. Vision. Res. 2008, 48, 377–385. [Google Scholar] [CrossRef]
- Long, B.R.; Sandza, K.; Holcomb, J.; Crockett, L.; Hayes, G.M.; Arens, J.; Fonck, C.; Tsuruda, L.S.; Schweighardt, B.; O’Neill, C.A.; et al. The Impact of Pre-Existing Immunity on the Non-Clinical Pharmacodynamics of AAV5-Based Gene Therapy. Mol. Ther. -Methods Clin. Dev. 2019, 13, 440–452. [Google Scholar] [CrossRef]
- Acland, G.M.; Aguirre, G.D.; Ray, J.; Zhang, Q.; Aleman, T.S.; Cideciyan, A.V.; Pearce-Kelling, S.E.; Anand, V.; Zeng, Y.; Maguire, A.M.; et al. Gene Therapy Restores Vision in a Canine Model of Childhood Blindness. Nat. Genet. 2001, 28, 92–95. [Google Scholar] [CrossRef]
- Bukrinsky, M.I.; Haggerty, S.; Dempsey, M.P.; Sharova, N.; Adzhubel, A.; Spitz, L.; Lewis, P.; Goldfarb, D.; Emerman, M.; Stevenson, M. A Nuclear Localization Signal within HIV-1 Matrix Protein That Governs Infection of Non-Dividing Cells. Nature 1993, 365, 666–669. [Google Scholar] [CrossRef]
- Arsenijevic, Y.; Berger, A.; Udry, F.; Kostic, C. Lentiviral Vectors for Ocular Gene Therapy. Pharmaceutics 2022, 14, 1605. [Google Scholar] [CrossRef]
- Bemelmans, A.-P.; Kostic, C.; Crippa, S.V.; Hauswirth, W.W.; Lem, J.; Munier, F.L.; Seeliger, M.W.; Wenzel, A.; Arsenijevic, Y. Lentiviral Gene Transfer of Rpe65 Rescues Survival and Function of Cones in a Mouse Model of Leber Congenital Amaurosis. PLoS Med. 2006, 3, e347. [Google Scholar] [CrossRef]
- Kalidasan, V.; Ng, W.H.; Ishola, O.A.; Ravichantar, N.; Tan, J.J.; Das, K.T. A Guide in Lentiviral Vector Production for Hard-to-Transfect Cells, Using Cardiac-Derived c-Kit Expressing Cells as a Model System. Sci. Rep. 2021, 11, 19265. [Google Scholar] [CrossRef] [PubMed]
- Stocking, C.; Bergholz, U.; Friel, J.; Klingler, K.; Wagener, T.; Starke, C.; Kitamura, T.; Miyajima, A.; Ostertag, W. Distinct Classes of Factor-Independent Mutants Can Be Isolated after Retroviral Mutagenesis of a Human Myeloid Stem Cell Line. Growth Factors 1993, 8, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Schambach, A.; Zychlinski, D.; Ehrnstroem, B.; Baum, C. Biosafety Features of Lentiviral Vectors. Hum. Gene Ther. 2013, 24, 132–142. [Google Scholar] [CrossRef]
- Touchard, E.; Benard, R.; Bigot, K.; Laffitte, J.-D.; Buggage, R.; Bordet, T.; Behar-Cohen, F. Non-Viral Ocular Gene Therapy, pEYS606, for the Treatment of Non-Infectious Uveitis: Preclinical Evaluation of the Medicinal Product. J. Control. Release 2018, 285, 244–251. [Google Scholar] [CrossRef]
- Wolff, J.A.; Dowty, M.E.; Jiao, S.; Repetto, G.; Berg, R.K.; Ludtke, J.J.; Williams, P.; Slautterback, D.B. Expression of Naked Plasmids by Cultured Myotubes and Entry of Plasmids into T Tubules and Caveolae of Mammalian Skeletal Muscle. J. Cell Sci. 1992, 103 Pt 4, 1249–1259. [Google Scholar] [CrossRef]
- Wolff, J.A.; Budker, V. The Mechanism of Naked DNA Uptake and Expression. Adv. Genet. 2005, 54, 3–20. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and Limitations of Drug Delivery Systems Formulated as Eye Drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef]
- Patel, N.; Nakrani, H.; Raval, M.; Sheth, N. Development of Loteprednol Etabonate-Loaded Cationic Nanoemulsified in-Situ Ophthalmic Gel for Sustained Delivery and Enhanced Ocular Bioavailability. Drug Deliv. 2016, 23, 3712–3723. [Google Scholar] [CrossRef]
- Wu, C.; Qi, H.; Chen, W.; Huang, C.; Su, C.; Li, W.; Hou, S. Preparation and Evaluation of a Carbopol/HPMC-Based in Situ Gelling Ophthalmic System for Puerarin. Yakugaku Zasshi 2007, 127, 183–191. [Google Scholar] [CrossRef]
- Gupta, S.; Vyas, S.P. Carbopol/Chitosan Based pH Triggered in Situ Gelling System for Ocular Delivery of Timolol Maleate. Sci. Pharm. 2010, 78, 959–976. [Google Scholar] [CrossRef]
- Vittitow, J.; Kissling, R.; DeCory, H.; Borchman, D. In Vitro Inhibition of Evaporation with Perfluorohexyloctane, an Eye Drop for Dry Eye Disease. Curr. Ther. Res. Clin. Exp. 2023, 98, 100704. [Google Scholar] [CrossRef]
- de Paiva, C.S.; Pflugfelder, S.C.; Ng, S.M.; Akpek, E.K. Topical Cyclosporine A Therapy for Dry Eye Syndrome. Cochrane Database Syst. Rev. 2019, 9, CD010051. [Google Scholar] [CrossRef]
- Paton, D.M. Loteprednol Etabonate: A Formulation for Short-Term Use in Inflammatory Flares in Dry Eye Disease. Drugs Today 2022, 58, 77–84. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Evans, D.G.; Protzko, E.E. A Review of the First Anti-Evaporative Prescription Treatment for Dry Eye Disease: Perfluorohexyloctane Ophthalmic Solution. Am. J. Manag. Care 2023, 29, S251–S259. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Loftsson, T. Aqueous Prostaglandin Eye Drop Formulations. Pharmaceutics 2022, 14, 2142. [Google Scholar] [CrossRef] [PubMed]
- Batra, M.; Gupta, S.; Nair, A.B.; Dhanawat, M.; Sandal, S.; Morsy, M.A. Netarsudil: A New Ophthalmic Drug in the Treatment of Chronic Primary Open Angle Glaucoma and Ocular Hypertension. Eur. J. Ophthalmol. 2021, 31, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Skaat, A.; Rosman, M.S.; Chien, J.L.; Mogil, R.S.; Ren, R.; Liebmann, J.M.; Ritch, R.; Park, S.C. Effect of Pilocarpine Hydrochloride on the Schlemm Canal in Healthy Eyes and Eyes With Open-Angle Glaucoma. JAMA Ophthalmol. 2016, 134, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Hoyng, P.F.; Rasker, M.T. Four new drugs for glaucoma: Apraclonidine, brimonidine, dorzolamide and latanoprost. Ned. Tijdschr. Geneeskd. 1998, 142, 2138–2141. [Google Scholar] [PubMed]
- Shedden, A.; Laurence, J.; Tipping, R. Timoptic-XE 0.5% Study Group Efficacy and Tolerability of Timolol Maleate Ophthalmic Gel-Forming Solution versus Timolol Ophthalmic Solution in Adults with Open-Angle Glaucoma or Ocular Hypertension: A Six-Month, Double-Masked, Multicenter Study. Clin. Ther. 2001, 23, 440–450. [Google Scholar] [CrossRef]
- Januleviciene, I.; Siaudvytyte, L.; Barsauskaite, R. Ophthalmic Drug Delivery in Glaucoma—A Review. Pharmaceutics 2012, 4, 243–251. [Google Scholar] [CrossRef]
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent Perspectives in Ocular Drug Delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef] [PubMed]
- Hollyfield, J.G.; Varner, H.H.; Rayborn, M.E.; Liou, G.I.; Bridges, C.D. Endocytosis and Degradation of Interstitial Retinol-Binding Protein: Differential Capabilities of Cells That Border the Interphotoreceptor Matrix. J. Cell Biol. 1985, 100, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Heth, C.A.; Bernstein, M.H. Mannose-Sensitive HRP Endocytosis by the Retinal Pigment Epithelium. Exp. Eye Res. 1991, 52, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Finnemann, S.C.; Bonilha, V.L.; Marmorstein, A.D.; Rodriguez-Boulan, E. Phagocytosis of Rod Outer Segments by Retinal Pigment Epithelial Cells Requires Avβ5 Integrin for Binding but Not for Internalization. Proc. Natl. Acad. Sci. USA 1997, 94, 12932–12937. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Chug, I.; Khanna, H. The Ocular Gene Delivery Landscape. Biomolecules 2021, 11, 1135. [Google Scholar] [CrossRef]
- Tan, Y.F.; Lee, Y.S.; Seet, L.-F.; Ng, K.W.; Wong, T.T.; Venkatraman, S. Design and in Vitro Release Study of siRNA Loaded Layer by Layer Nanoparticles with Sustained Gene Silencing Effect. Expert. Opin. Drug Deliv. 2018, 15, 937–949. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, L.; Chi, H. Recent Development of Polymer Nanomicelles in the Treatment of Eye Diseases. Front. Bioeng. Biotechnol. 2023, 11, 1246974. [Google Scholar] [CrossRef]
- Liaw, J.; Chang, S.F.; Hsiao, F.C. In Vivo Gene Delivery into Ocular Tissues by Eye Drops of Poly(Ethylene Oxide)-Poly(Propylene Oxide)-Poly(Ethylene Oxide) (PEO-PPO-PEO) Polymeric Micelles. Gene Ther. 2001, 8, 999–1004. [Google Scholar] [CrossRef]
- De Campos, A.M.; Sánchez, A.; Gref, R.; Calvo, P.; Alonso, M.J. The Effect of a PEG versus a Chitosan Coating on the Interaction of Drug Colloidal Carriers with the Ocular Mucosa. Eur. J. Pharm. Sci. 2003, 20, 73–81. [Google Scholar] [CrossRef]
- Civiale, C.; Licciardi, M.; Cavallaro, G.; Giammona, G.; Mazzone, M.G. Polyhydroxyethylaspartamide-Based Micelles for Ocular Drug Delivery. Int. J. Pharm. 2009, 378, 177–186. [Google Scholar] [CrossRef]
- Terreni, E.; Chetoni, P.; Burgalassi, S.; Tampucci, S.; Zucchetti, E.; Chipala, E.; Alany, R.G.; Al-Kinani, A.A.; Monti, D. A Hybrid Ocular Delivery System of Cyclosporine-A Comprising Nanomicelle-Laden Polymeric Inserts with Improved Efficacy and Tolerability. Biomater. Sci. 2021, 9, 8235–8248. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, D.; Li, Y.; Yang, W.; Tu, J.; Shen, Y. Improving the Topical Ocular Pharmacokinetics of Lyophilized Cyclosporine A-Loaded Micelles: Formulation, in Vitro and in Vivo Studies. Drug Deliv. 2018, 25, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.-R.B.; Popa, M.; Rata, D.M.; Cadinoiu, A.N.; Parfait, F.; Delaite, C.; Atanase, L.I.; Solcan, C.; Daraba, O.M. Drug-Loaded Polymeric Micelles Based on Smart Biocompatible Graft Copolymers with Potential Applications for the Treatment of Glaucoma. Int. J. Mol. Sci. 2022, 23, 9382. [Google Scholar] [CrossRef]
- Elmowafy, E.; Gad, H.; Biondo, F.; Casettari, L.; Soliman, M.E. Exploring Optimized Methoxy Poly(Ethylene Glycol)-Block-Poly(ε-Caprolactone) Crystalline Cored Micelles in Anti-Glaucoma Pharmacotherapy. Int. J. Pharm. 2019, 566, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, J.; Xin, M.; Li, Q.; Wang, J.; Yang, H.; Wu, X. Rebaudioside A/TPGS Mixed Nanomicelles as Promising Nanocarriers for Nimodipine Ocular Delivery. Drug Deliv. Transl. Res. 2021, 11, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Seah, I.; Xue, K.; Wong, W.; Tan, Q.S.W.; Ma, X.; Lin, Q.; Lim, J.Y.C.; Liu, Z.; Parikh, B.H.; et al. Antiangiogenic Nanomicelles for the Topical Delivery of Aflibercept to Treat Retinal Neovascular Disease. Adv. Mater. 2022, 34, e2108360. [Google Scholar] [CrossRef] [PubMed]
- Tasharrofi, N.; Nourozi, M.; Marzban, A. How Liposomes Pave the Way for Ocular Drug Delivery after Topical Administration. J. Drug Deliv. Sci. Technol. 2022, 67, 103045. [Google Scholar] [CrossRef]
- Natarajan, J.V.; Chattopadhyay, S.; Ang, M.; Darwitan, A.; Foo, S.; Zhen, M.; Koo, M.; Wong, T.T.; Venkatraman, S.S. Sustained Release of an Anti-Glaucoma Drug: Demonstration of Efficacy of a Liposomal Formulation in the Rabbit Eye. PLoS ONE 2011, 6, e24513. [Google Scholar] [CrossRef]
- Bochot, A.; Fattal, E. Liposomes for Intravitreal Drug Delivery: A State of the Art. J. Control. Release 2012, 161, 628–634. [Google Scholar] [CrossRef]
- Vandamme, T.F.; Brobeck, L. Poly(Amidoamine) Dendrimers as Ophthalmic Vehicles for Ocular Delivery of Pilocarpine Nitrate and Tropicamide. J. Control. Release 2005, 102, 23–38. [Google Scholar] [CrossRef]
- Shaunak, S.; Thomas, S.; Gianasi, E.; Godwin, A.; Jones, E.; Teo, I.; Mireskandari, K.; Luthert, P.; Duncan, R.; Patterson, S.; et al. Polyvalent Dendrimer Glucosamine Conjugates Prevent Scar Tissue Formation. Nat. Biotechnol. 2004, 22, 977–984. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2022, 8, 787644. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.A.; MacKay, J.A. Protein and Polypeptide Mediated Delivery to the Eye. Adv. Drug Deliv. Rev. 2022, 188, 114441. [Google Scholar] [CrossRef] [PubMed]
- George, E.M.; Mahdi, F.; Logue, O.C.; Robinson, G.G.; Bidwell, G.L. Corneal Penetrating Elastin-Like Polypeptide Carriers. J. Ocul. Pharmacol. Ther. 2016, 32, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.H.; Wilson, C.G.; Seib, F.P. A Review of the Emerging Role of Silk for the Treatment of the Eye. Pharm. Res. 2018, 35, 248. [Google Scholar] [CrossRef] [PubMed]
- Zulliger, R.; Conley, S.M.; Naash, M.I. Non-Viral Therapeutic Approaches to Ocular Diseases: An Overview and Future Directions. J. Control. Release 2015, 219, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lee, C.; Pastuszka, M.; Laurie, G.W.; MacKay, J.A. Thermally-Responsive Loading and Release of Elastin-Like Polypeptides from Contact Lenses. Pharmaceutics 2019, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.L.; Wang, X.; Yucel, T.; York, L.; Keirstead, M.; Haggerty, L.; Kaplan, D.L. Silk Hydrogels for Sustained Ocular Delivery of Anti-Vascular Endothelial Growth Factor (Anti-VEGF) Therapeutics. Eur. J. Pharm. Biopharm. 2015, 95, 271–278. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Hao, J.-L.; Wang, S.; Zheng, Y.; Zhang, W.-S. Nanoparticles in the Ocular Drug Delivery. Int. J. Ophthalmol. 2013, 6, 390–396. [Google Scholar] [CrossRef]
- Ciolino, J.B.; Dohlman, C.H.; Kohane, D.S. Contact Lenses for Drug Delivery. Semin. Ophthalmol. 2009, 24, 156–160. [Google Scholar] [CrossRef]
- Kim, J.; Chauhan, A. Dexamethasone Transport and Ocular Delivery from Poly(Hydroxyethyl Methacrylate) Gels. Int. J. Pharm. 2008, 353, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, D.; Li, C.-C.; Chauhan, A. Dispersion of DMPC Liposomes in Contact Lenses for Ophthalmic Drug Delivery. Curr. Eye Res. 2005, 30, 1071–1080. [Google Scholar] [CrossRef]
- Gulsen, D.; Chauhan, A. Dispersion of Microemulsion Drops in HEMA Hydrogel: A Potential Ophthalmic Drug Delivery Vehicle. Int. J. Pharm. 2005, 292, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Tashakori-Sabzevar, F.; Mohajeri, S.A. Development of Ocular Drug Delivery Systems Using Molecularly Imprinted Soft Contact Lenses. Drug Dev. Ind. Pharm. 2015, 41, 703–713. [Google Scholar] [CrossRef]
- Ross, A.E.; Bengani, L.C.; Tulsan, R.; Maidana, D.E.; Salvador-Culla, B.; Kobashi, H.; Kolovou, P.E.; Zhai, H.; Taghizadeh, K.; Kuang, L.; et al. Topical Sustained Drug Delivery to the Retina with a Drug-Eluting Contact Lens. Biomaterials 2019, 217, 119285. [Google Scholar] [CrossRef] [PubMed]
- Schaffrath, K.; Lohmann, T.; Seifert, J.; Ingensiep, C.; Raffelberg, P.; Waschkowski, F.; Viga, R.; Kokozinski, R.; Mokwa, W.; Johnen, S.; et al. New Epiretinal Implant with Integrated Sensor Chips for Optical Capturing Shows a Good Biocompatibility Profile in Vitro and in Vivo. Biomed. Eng. Online 2021, 20, 102. [Google Scholar] [CrossRef] [PubMed]
- García-Estrada, P.; García-Bon, M.A.; López-Naranjo, E.J.; Basaldúa-Pérez, D.N.; Santos, A.; Navarro-Partida, J. Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics 2021, 13, 701. [Google Scholar] [CrossRef]
- Ranade, S.V.; Wieland, M.R.; Tam, T.; Rea, J.C.; Horvath, J.; Hieb, A.R.; Jia, W.; Grace, L.; Barteselli, G.; Stewart, J.M. The Port Delivery System with Ranibizumab: A New Paradigm for Long-Acting Retinal Drug Delivery. Drug Deliv. 2022, 29, 1326–1334. [Google Scholar] [CrossRef]
- Sharma, A.; Khanani, A.M.; Parachuri, N.; Kumar, N.; Bandello, F.; Kuppermann, B.D. Port Delivery System with Ranibizumab (Susvimo) Recall- What Does It Mean to the Retina Specialists. Int. J. Retin. Vitr. 2023, 9, 6. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Jaffe, G.J.; Johnson, C.A.; Farsiu, S.; Lad, E.M.; Guymer, R.; Rosenfeld, P.; Hubschman, J.-P.; Constable, I.; et al. Effect of Ciliary Neurotrophic Factor on Retinal Neurodegeneration in Patients with Macular Telangiectasia Type 2: A Randomized Clinical Trial. Ophthalmology 2019, 126, 540–549. [Google Scholar] [CrossRef]
- Lien, S.; Lowman, H.B. Therapeutic Anti-VEGF Antibodies. Handb. Exp. Pharmacol. 2008, 181, 131–150. [Google Scholar] [CrossRef]
- Tian, B.; Bilsbury, E.; Doherty, S.; Teebagy, S.; Wood, E.; Su, W.; Gao, G.; Lin, H. Ocular Drug Delivery: Advancements and Innovations. Pharmaceutics 2022, 14, 1931. [Google Scholar] [CrossRef] [PubMed]
- Pundlik, S.; Yi, H.; Liu, R.; Peli, E.; Luo, G. Magnifying Smartphone Screen Using Google Glass for Low-Vision Users. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.D.; Peli, E. An Augmented-Reality Edge Enhancement Application for Google Glass. Optom. Vis. Sci. 2014, 91, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Merabet, L.B. Building the Bionic Eye: An Emerging Reality and Opportunity. Prog. Brain Res. 2011, 192, 3–15. [Google Scholar] [CrossRef]
- Liu, X.; Chen, P.; Ding, X.; Liu, A.; Li, P.; Sun, C.; Guan, H. A Narrative Review of Cortical Visual Prosthesis Systems: The Latest Progress and Significance of Nanotechnology for the Future. Ann. Transl. Med. 2022, 10, 716. [Google Scholar] [CrossRef]
| Disease | Delivery System | Delivery Location | Clinical Trial Number | FDA-Approved Therapy |
|---|---|---|---|---|
| Achromatopsia | AAV | Subretinal | NCT03278873, NCT02935517, NCT02599922 | None |
| Dry AMD | Retinal prosthesis, Stem cells | Subretinal Intravitreal | NCT03392324, NCT01736059, NCT04339764, NCT04627428, NCT05187104 | None |
| Wet AMD | Lentivirus, Monoclonal antibody, AAV, Axitinib suspension, Axitinib implant, Durasert, Soluble protein decoy, Brachytherapy | Subretinal Intravitreal Suprachoroidal Episcleral | NCT01678872, NCT04777201, NCT04832724, NCT05891548, NCT04989699, NCT05381948, NCT04423718, NCT05112861, NCT02988895 | Aflibercept (Eylea), VEGF inhibitor, for intravitreal injection. Faricimab (Vabysmo), VEGF and Ang-2 inhibitor, for intravitreal injection. Ranibizumab (Lucentis, Byooviz), VEGF inhibitor, for intravitreal injection. Brolucizumab (Beovu), VEGF inhibitor, for intravitreal injection. |
| Geographic Atrophy Secondary to AMD | Pegcetacoplan (APL-2) C3 inhibitor, Antisense Inhibitor of Complement Factor B, AAV | Intravitreal Subcutaneous Subretinal | NCT04770545, NCT03815825, NCT04656561, NCT06018558 | Pegcetacoplan (Syfovre), C3 inhibitor, for intravitreal injection. Avacincaptad pegol (Izervay), C5 inhibitor, for intravitreal injection. |
| Choroideremia | AAV | Intravitreal | NCT04483440 | None |
| Diabetic Macular Edema | Triamcinolone acetonide, Ranibizumab, Anti-IL6 monoclonal antibody, Dexamethasone, Bevacizumab, Drug implant, aflibercept | Suprachoroidal Intravitreal Eye drops | NCT05512962, NCT05151744, NCT05066997, NCT05112861, NCT04469595, NCT04411693, NCT04108156, NCT04429503 | Ozurdex biodegradable implant for sustained dexamethasone release. Faricimab (Vabysmo), VEGF and Ang-2 inhibitor, for intravitreal injection. Ranibizumab (Lucentis, Byooviz), VEGF inhibitor, for intravitreal injection. Aflibercept (Eylea), VEGF inhibitor, for intravitreal injection. Brolucizumab (Beovu), VEGF inhibitor, for intravitreal injection. |
| Diabetic Retinopathy | Stem cells, Selective integrin inhibitor, Ranibizumab implant, Aflibercept, Brolucizumab | Intravitreal Eye drops | NCT01736059, NCT05409235, NCT04503551, NCT04708145, NCT04278417 | Aflibercept (Eylea), VEGF inhibitor, for intravitreal injection. Ranibizumab (Lucentis, Byooviz), VEGF inhibitor, for intravitreal injection. |
| Dry Eye Disease | Lipid conjugated chemerin peptide agonist, Small molecule, siRNA, rhNGF, TRPM8 agonist, synthetic peptide, Thermomechanical system | Eye drops Peri-orbital | NCT05759208, NCT05403827, NCT05310422, NCT05133180, NCT05493111, NCT05136170, NCT05467293, NCT05162261, NCT04795752 | Miebo, Restasis, a cyclosporin drug, and Eysuvis, an ophthalmic suspension with loteprednol etabonate as eye drops. |
| Glaucoma | Human retinal pigment epithelium cell therapy, carbonic anhydrase inhibitor, prostaglandin F2 alpha analog, EP2 receptor agonist | Intravitreal Eye drops Peri-orbital | NCT02862938, NCT02390284, NCT05397600, NCT04761705, NCT03850782, NCT03868124, NCT03519386 | Prostaglandins: Latanoprost, Ravoprost, Tafluprost, and Bimatoprost (implant/eye drops) as eye drops. Rho kinase inhibitors: Netarsudil as eye drops. Cholinergic agonists: Pilocarpine as eye drops. Alpha-adrenergic agonists: Apraclonidine and Brimonidine as eye drops. Beta blockers: Timolol as eye drops. Carbonic anhydrase inhibitors: Dorzolamide as eye drops. Omidenepag isopropyl (OMLONTI), EP2 receptor agonist, eye drops. |
| Leber Congenital Amaurosis | AAV, RNA antisense oligonucleotide, CRISPR/Cas9 | Subretinal Intravitreal | NCT01208389, NCT03920007, NCT00481546, NCT00999609, NCT03913143, NCT03872479 | AAV2-RPE65 (Luxturna) for subretinal injection gene replacement therapy. |
| Leber Hereditary Optic Neuropathy | AAV | Intravitreal | NCT02161380, NCT03293524 | None |
| RP | Small molecule, Stem cells, Human retinal progenitor cells, Electrical stimulation, AAV, RNA Antisense oligonucleotide, Retinal prosthesis | Intravitreal Subretinal Trans corneal | NCT05392751, NCT04925687, NCT02086890, NCT02464436, NCT02556736, NCT05285618, NCT04945772, NCT04123626, NCT05158296, NCT01736059, NCT05203939 | Argus II epiretinal Prosthesis System. AAV2-RPE65 (Luxturna) for subretinal injection gene replacement therapy. |
| X-linked RP | AAV | Intravitreal | NCT04517149 | AAV2-RPE65 (Luxturna) for subretinal injection gene replacement therapy. |
| X-linked Retinoschisis | AAV | Intravitreal | NCT02317887 | None |
| Usher Syndrome | Lentivirus, RNA Antisense oligonucleotide | Intravitreal Subretinal | NCT05158296, NCT02065011 | None |
| Stargardt Disease | Equine infectious anemia virus, AAV, complement factor C5 inhibitor | Subretinal Intravitreal | NCT01736592, NCT03364153, NCT05956626 | None |
| Name | Ocular Tissue Type | Duration | Efficacy | Toxicity |
|---|---|---|---|---|
| Adeno-associated virus | ||||
| AAV1 | Subretinal: RPE [125]. | Expression up to 6 months [125]. | High transduction efficiency in ONL [126]. | Inflammation above 5 × 1010 vg/eye [127]. |
| AAV2 | Intravitreal: Retinal ganglion cells, Müller cells, ciliary body, inner nuclear layer (INL). Subretinal: Mainly photoreceptors, also RPE. | Initial expression at 28 days post-injection [128]. Expression continues at 7–8 months post-injection [129]. | Moderate transduction efficiency of photoreceptors and RGCs, low transduction of RPE cells [128]. | Formation of AAV2 antibodies and possible retinal detachment above 8.0 × 1010 vg/eye [130,131]. |
| AAV4 | Subretinal: RPE, retina [125]. | Expression up to 6 months [125]. | High transduction efficiency of the ONL [128]. | Inflammation observed above 4.8 × 1010 vg/eye [132]. |
| AAV5 | Subretinal: RPE, photoreceptors [125]. | Initial expression starting at 14–21 days post-injection. Expression continues at 7–8 months post-injection [129]. | High transduction efficiency in the retina compared with other AAVs [129]. | Retinal thinning observed above 1.1 × 1012 vg/eye [133]. |
| AAV8 | Subretinal: photoreceptors. | Expression up to 6 months [125]. | Low transduction efficiency in the ONL [128]. | Inflammation and anti-AAV8 antibodies above a dose of 1 × 1010 vg/eye [130]. |
| AAV9 | Subretinal: Photoreceptors. | Expression up to 6 months [125]. | Moderate transduction efficiency in the ONL [128]. | Immunogenic responses observed at doses above 1 × 1010 vg/eye [134]. |
| Adenovirus | ||||
| Adenovirus serotype 5 (AdV5) | Subretinal: All retinal layers except ONL, some RPE cells [135]. | Expression begins ~2 weeks post-injection. Expression continues up to 6 months post-injection [136]. | Low due to high prevalence of Ad5 serotypes in humans. | Triggers immune responses starting at 4 × 105 IU/eye [137]. |
| Lentiviral vectors | ||||
| Lenti-VSVG (lentiviral vectors pseudotyped in a vesicular stomatitis virus glycoprotein envelope) | Intravitreal: INL. Subretinal: RPE, photoreceptors. | Initial expression by 7 days post-injection. Expression continues 4–5 months post-injection [128]. | High transduction efficiency. | Triggers immune response at 1.4 × 107 IU/eye [128]. Derived from virus that can integrate into host genome. |
| Lenti–Mokola (Mokola envelope) | Subretinal: RPE. | Initial expression by 7 days. Expression continues at 3 months post-injection [128]. | High transduction efficiency. | Triggers immune response at 6 × 105 IU/eye [128]. Derived from virus that can integrate into host genome. |
| Topical | ||||
| Eye drops | Cornea, trabecular meshwork. | A few hours. | Low to moderate due to leakage through nasolacrimal system. | Concentration of the preservative, benzalkonium chloride (BAK), should be below 0.0005% to avoid toxicity to eye [138]. |
| Nanoparticles | ||||
| Nanomicelles | Ocular anterior and poster segments. | Sustained release over several days [11]. | High for delivery of hydrophobic drugs to the cornea and trabecular meshwork. | Concentration should be less than 2 mg/mL [139,140]. |
| Liposomes | Cornea, retina (blood–retinal barrier). | Up to 3 months [11]. | High efficacy for corneal and blood–retinal barrier uptake. | Low toxicity up to concentrations of 50 µg/mL [141]. Dependent on addition of chemical groups. |
| Dendrimers | Cornea, retina. | A few weeks to a month [11]. | High corneal drug residence time. Can cross blood–retinal barrier depending on polymer chemistry [11]. | Low toxicity at concentrations up to 100 μg/mL [142,143]. Dependent on chemical modifications. |
| Devices | ||||
| Contact lens | Cornea, conjunctiva, trabecular meshwork. | Immersion in drug: temporary duration. Nanoparticle-loaded: several days. | Moderate drug uptake through the corneal layers [11]. | May cause corneal inflammation [11]. |
| Intraocular Implants | Lens, trabecular meshwork, retina. | Sustained release over several months. | High due to intraocular localization. | Low toxicity for many biodegradable implants [11]. |
| Retinal prosthesis | Retina. | Permanent electrical stimulation. | Moderate: limited by reduced specificity of cell targets and higher thresholds of inner retinal neurons compared with photoreceptors. | Generally considered biocompatible [90]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whalen, M.; Akula, M.; McNamee, S.M.; DeAngelis, M.M.; Haider, N.B. Seeing the Future: A Review of Ocular Therapy. Bioengineering 2024, 11, 179. https://doi.org/10.3390/bioengineering11020179
Whalen M, Akula M, McNamee SM, DeAngelis MM, Haider NB. Seeing the Future: A Review of Ocular Therapy. Bioengineering. 2024; 11(2):179. https://doi.org/10.3390/bioengineering11020179
Chicago/Turabian StyleWhalen, Maiya, Monica Akula, Shannon M. McNamee, Margaret M. DeAngelis, and Neena B. Haider. 2024. "Seeing the Future: A Review of Ocular Therapy" Bioengineering 11, no. 2: 179. https://doi.org/10.3390/bioengineering11020179
APA StyleWhalen, M., Akula, M., McNamee, S. M., DeAngelis, M. M., & Haider, N. B. (2024). Seeing the Future: A Review of Ocular Therapy. Bioengineering, 11(2), 179. https://doi.org/10.3390/bioengineering11020179







