Surface Modification of Polyetheretherketone (PEEK) Intervertebral Fusion Implant Using Polydopamine Coating for Improved Bioactivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PEEK
2.2. Preparation of the PEEK-Modified Surface
2.3. Characterizations
2.3.1. Physicochemical Characterization
2.3.2. In Vitro Cell Culture and Biocompatibility
- (1)
- WST-1 solution (30 µL) and the free DMEM (300 µL) were mixed and then added to each sample.
- (2)
- They underwent additional incubation at 37 °C for 30 min.
- (3)
- The solutions of each sample were carried to a 96-well plate and the absorbance at 450 nm was observed by using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
- (4)
- The percentage of cell viability was calculated as follows and the five specimens were subsequently analyzed repeatedly for each condition at three different times.
2.3.3. Mechanical Tests
2.4. Statistical Analysis
3. Results
3.1. Morphological Characterization
3.2. Surface Modification Analysis
3.3. Biocompatibility Study
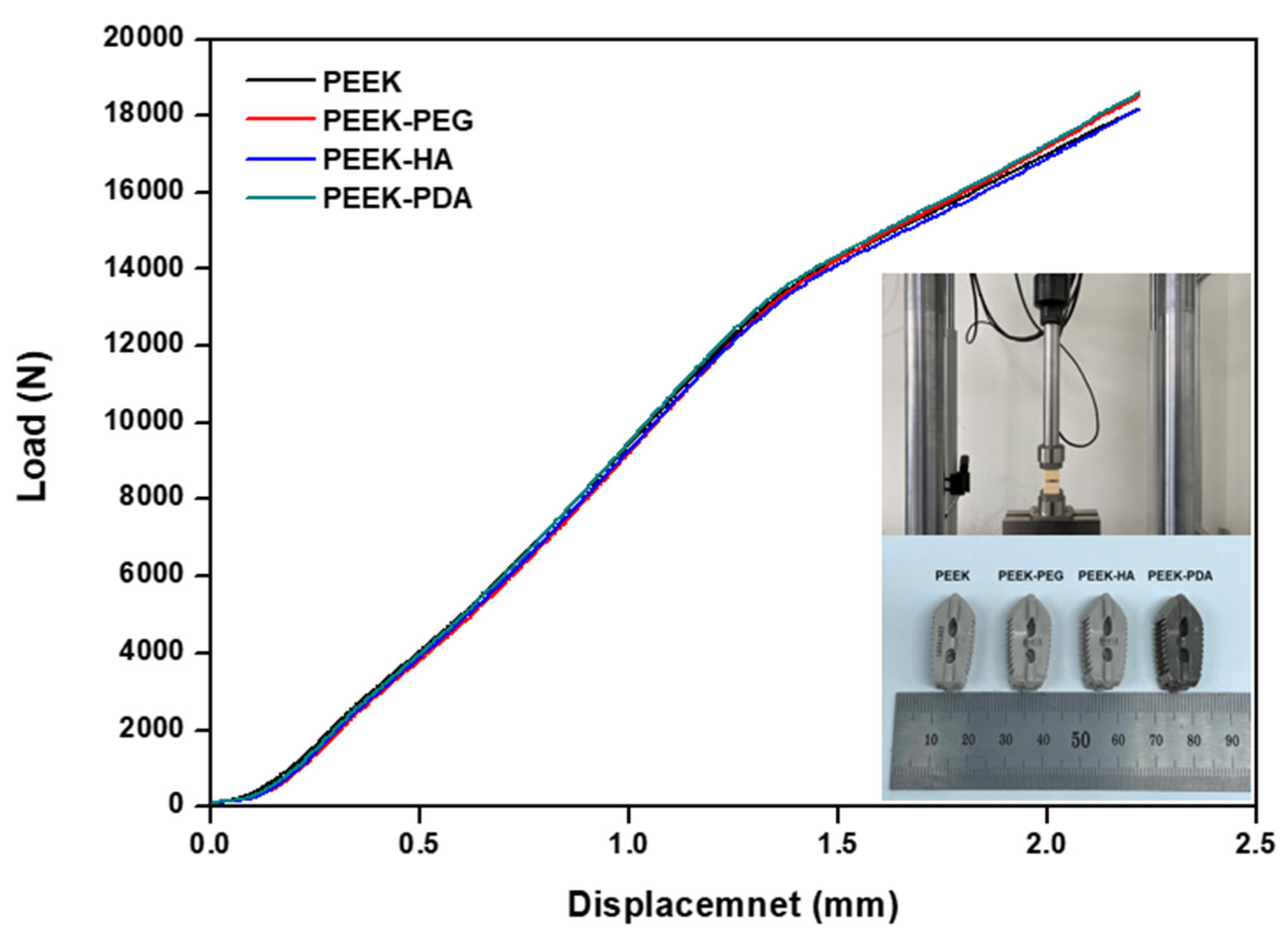
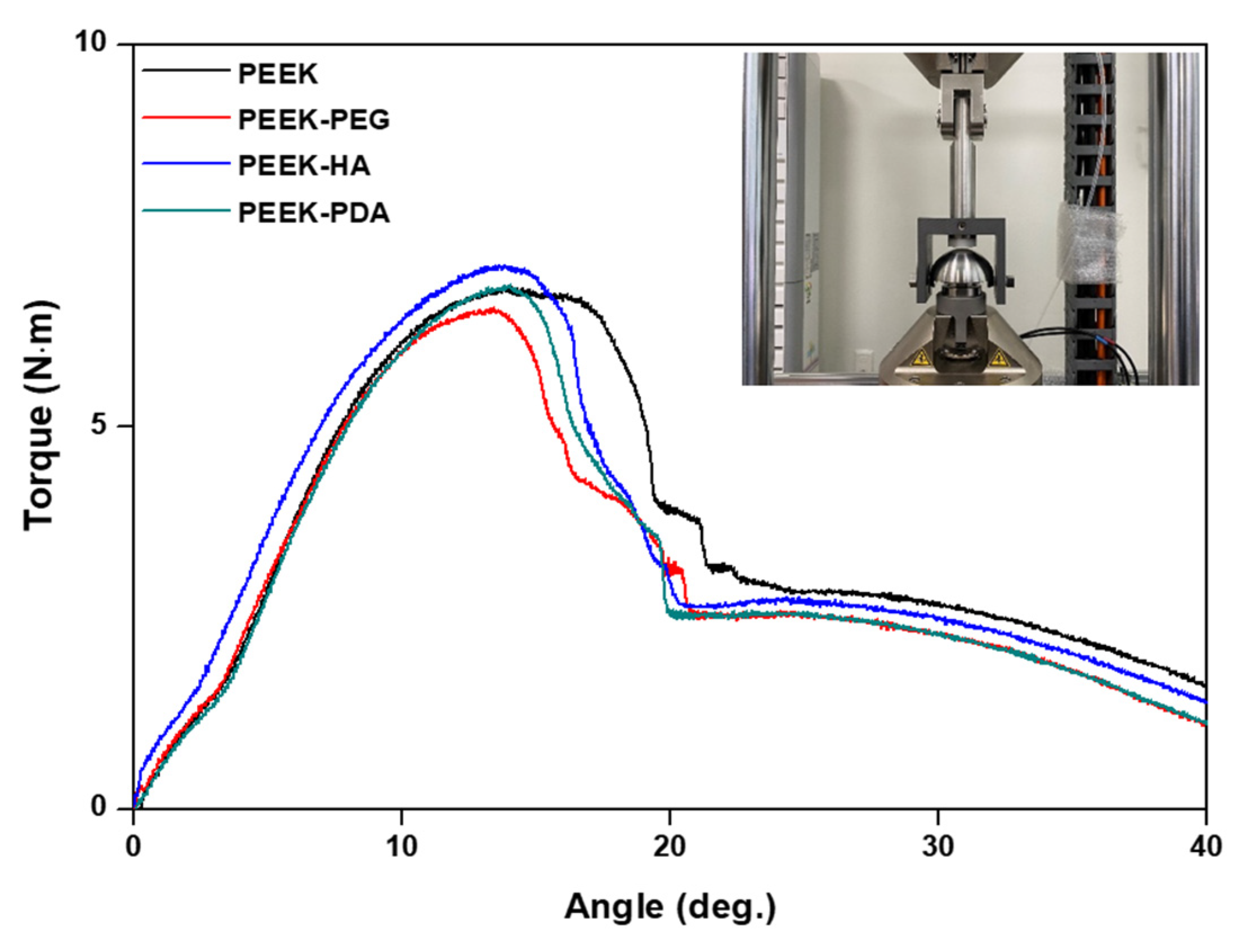
3.4. Mechanical Properties Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in spinal implants: A review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Phillips, F.M.; Tzermiadianos, M.N.; Voronov, L.I.; Havey, R.M.; Carandang, G.; Renner, S.M.; Rosler, D.M.; Ochoa, J.A.; Patwardhan, A.G. Effect of the Total Facet Arthroplasty System after complete laminectomy-facetectomy on the biomechanics of implanted and adjacent segments. Spine J. 2009, 9, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Zhang, Z.; Dai, Q.X.; Lin, D.Y.; Li, S.M. Microstructure and bond strength of HA(+ZrO2+Y2O3)/Ti6Al4V composite coatings fabricated by RF magnetron sputtering. Surf. Coatings Technol. 2006, 200, 5354–5363. [Google Scholar] [CrossRef]
- Lee, K.M.; Park, S.W.; Lim, H.P.; Koh, J.T.; Kang, S.S.; Kim, H.S.; Lee, D.J. A recent research and development tendency of dental titanium implant. Jaeryomadang 2009, 22, 33–40. [Google Scholar]
- Zheng, Y.; Xiong, C.; Zhang, S.; Li, X.; Zhang, L. Bone-like apatite coating on functionalized poly(etheretherketone) surface via tailored silanization layers technique. Sci. Eng. C 2015, 55, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed]
- Monich, P.R.; Henriques, B.; de Oliveira, A.P.N.; Souza, J.C.; Fredel, M.C. Mechanical and biological behavior of bio-medical PEEK matrix composites: A focused review. Mater. Lett. 2016, 185, 593–597. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Wei, J.; Ma, J.; Deng, F.; Wei, S. Nano-TiO2/PEEK bioactive composite as a bone substitute material: In vitro and in vivo studies. Int. J. Nanomed. 2012, 7, 1215–1225. [Google Scholar]
- Baştan, F.E.; Rehman, M.A.U.; Avcu, Y.Y.; Avcu, E.; Üstel, F.; Boccaccini, A.R. Electrophoretic co-deposition of PEEK-hydroxyapatite composite coatings for biomedical applications. Colloids Surf. B Biointerfaces 2018, 169, 176–182. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, T.; Jiang, C.; Zhong, T.; Su, Z.; Xu, Q.; Jiang, M.; Liu, P. Preparation of antifouling poly (ether ether ketone) hollow fiber membrane by ultraviolet grafting of polyethylene glycol. Mater. Today Commun. 2021, 27, 102326. [Google Scholar] [CrossRef]
- Wang, H.; Lu, X.; Lu, X.; Wang, Z.; Ma, J.; Wang, P. Improved surface hydrophilicity and antifouling property of polysulfone ultrafiltration membrane with poly(ethylene glycol) methyl ether methacrylate grafted graphene oxide nano-fillers. Appl. Surf. Sci. 2017, 425, 603–613. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Sadowitz, B.; Seymour, K.; Gahtan, V.; Maier, K.G. The role of hyaluronic acid in atherosclerosis and intimal hyperplasia. J. Surg. Res. 2012, 173, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Watanabe, C. Stimulation of osteoinduction in bone wound healing by high-molecular hyaluronic acid. Bone 1995, 16, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Wigg, M.E.; Amiel, D.; Vandeberg, J.; Kitabayashi, L.; Harwood, F.L.; Arfors, K.E. The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: An experimental study in rabbits. J. Orthop. Res. 1990, 8, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; You, I.; Cho, W.K.; Shon, H.K.; Lee, T.G.; Choi, I.S.; Lee, H. One-step modification of superhydrophobic surfaces by a mussel-inspired polymer coating. Angew. Chem. Int. Ed. 2010, 49, 9401–9404. [Google Scholar] [CrossRef] [PubMed]
- Lynge, M.E.; Ogaki, R.; Laursen, A.O.; Lovmand, J.; Sutherland, D.S.; Stadler, B. PolyPDAmine/liposome coatings and their interaction with myoblast cells. ACS Appl. Mater. Interfaces 2011, 3, 2142–2147. [Google Scholar] [CrossRef]
- Luo, R.; Tang, L.; Zhong, S.; Yang, Z.; Wang, J. In vitro investigation of enhanced hemocompatibility and endothelial cell proliferation associated with quinone-rich polyPDAmine coating. ACS Appl. Mater. Interfaces 2013, 5, 1704–1714. [Google Scholar] [CrossRef]
- Yang, K.; Lee, J.S.; Kim, J.; Lee, Y.B.; Shin, H.; Um, S.H.; Cho, S.W. PolyPDAmine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials 2012, 33, 6952–6964. [Google Scholar] [CrossRef]
- Kwon, G.; Kim, H.; Gupta, K.C.; Kang, I.K. Enhanced tissue compatibility of polyetheretherketone disks by dopamine-mediated protein immobilization. Macromol. Res. 2018, 26, 128–138. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Zhang, Y.; He, H.; Xiong, S.; Chen, P.; Li, C.; Wang, L.; Lu, G.; Xu, Y. A dual-functional PEEK implant coating for anti-bacterial and accelerated osseointegration. Colloids Surf. B Biointerfaces 2023, 224, 113196. [Google Scholar] [CrossRef] [PubMed]
- ASTM, F2077-18; Test Method For Intervertebral Body Fusion Devices. ASTM International: West Conshohocken, PA, USA, 2018.
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-inspired polyPDAmine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Carneiro, J.; Döll-Boscardin, P.M.; Fiorin, B.C.; Nadal, J.M.; Farago, P.V.; Paula, J.P. Development and characterization of hyaluronic acid-lysine nanoparticles with potential as innovative dermal filling. Braz. J. Pharm. Sci. 2016, 52, 645–651. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Sennerby, L.; Ericson, L.E.; Thomsen, P.; Lekholm, U.; Åstrand, P. Structure of the bone/titanium interface in retrieved clinical oral implants. Clin. Oral Implant. Res. 1992, 2, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Ektessabi, A.; Albrektsson, T.; Johansson, C.; Andersson, B. A 1-year follow-up of implants of different surface roughness placed in rabbit bone. Int. J. Oral Maxillofac. Implant. 1997, 12, 1–21. [Google Scholar]
- Huang, S.; Liang, N.; Hu, Y.; Zhou, X.; Abidi, N. Polydopamine-assisted surface modification for bone biosubstitutes. BioMed Res. Int. 2016, 2016, 2389895. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-B.; Chen, W.-T.; Chien, H.-W.; Kuo, W.-H.; Wang, M.-J. Poly(dopamine) coating of scaffolds for articular cartilage tissue engineering. Acta Biomater. 2011, 7, 4187–4194. [Google Scholar] [CrossRef]
- Lee, D.J.; Tseng, H.C.; Wong, S.W.; Wang, Z.; Deng, M.; Ko, C.-C. Dopaminergic effects on in vitro osteogenesis. Bone Res. 2015, 3, 15020. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, D.H.; Yoon, T.R.; Kim, H.K.; Jo, H.H.; Park, J.S.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater. 2016, 40, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, S.J.; Jung, T.G.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Surface modification of a three-dimensional polycaprolactone scaffold by polydopamine, biomineralization, and BMP-2 immobilization for potential bone tissue applications. Colloids Surf. B Biointerfaces 2021, 199, 111528. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef] [PubMed]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wu, Z.; Xu, K.; Wang, X.; Wang, S.; Qiu, H.; Li, X.; Chen, J. Multifunctional coatings of titanium implants toward promoting osseointegration and preventing infection: Recent developments. Front. Bioeng. Biotechnol. 2021, 9, 783816. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of an-tibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef]

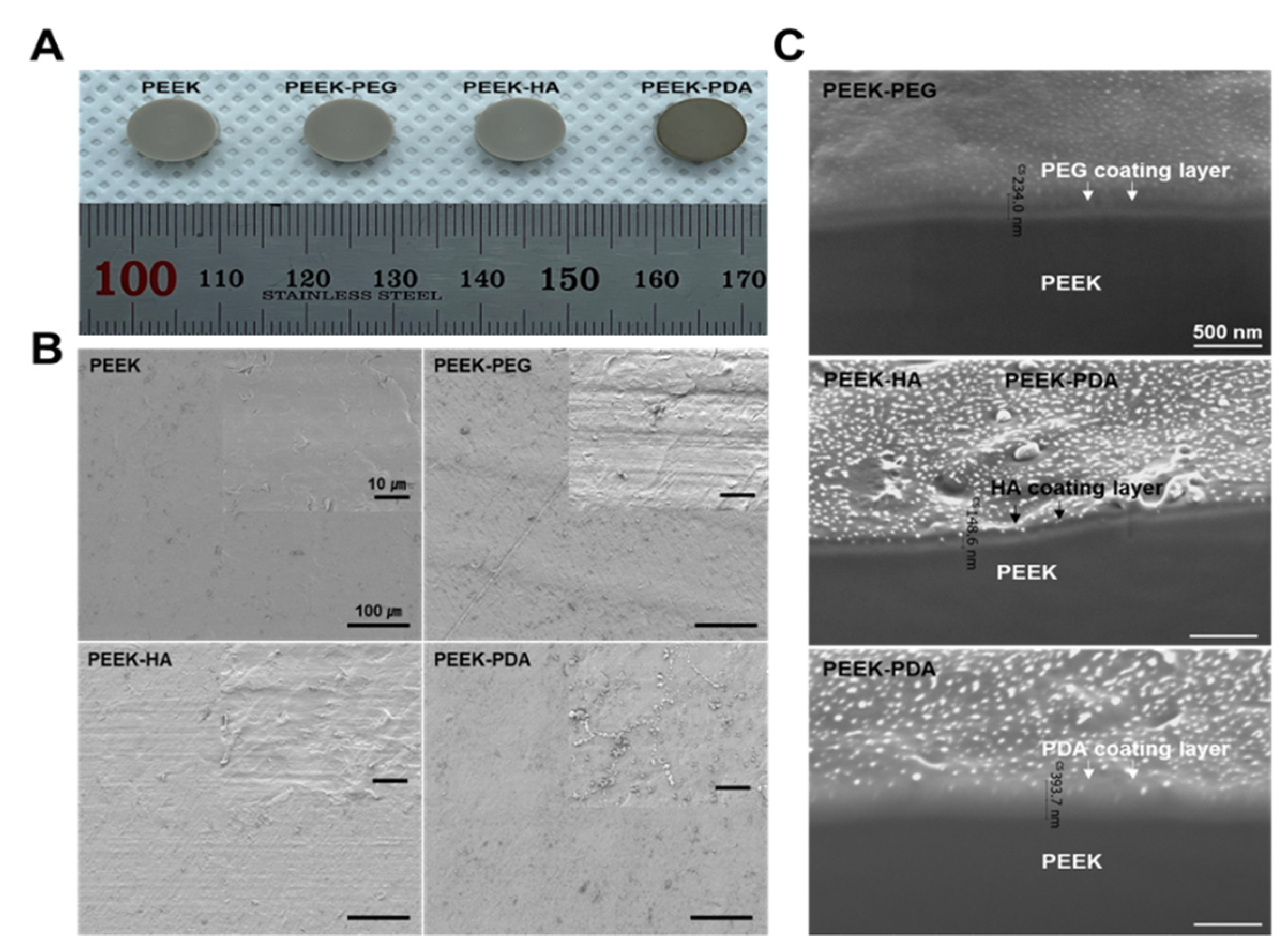


| Group | Ra/μm | Rq/μm | Rz/μm |
|---|---|---|---|
| PEEK | 0.635 ± 1.52 | 0.771 ± 1.65 | 4.261 ± 1.77 |
| PEEK-PEG | 0.756 ± 1.68 | 0.924 ± 1.76 | 4.416 ± 1.87 |
| PEEK-HA | 0.744 ± 1.71 | 0.871 ± 1.69 | 4.056 ± 1.74 |
| PEEK-PDA | 0.803 ± 1.83 | 0.963 ± 1.72 | 5.284 ± 1.82 |
| Group | Yield Load (N) | Stiffness (N/mm) | ||
|---|---|---|---|---|
| Average | Std. Dev. | Average | Std. Dev. | |
| PEEK | 10,072.430 | 179.276 | 8578.433 | 196.566 |
| PEEK-PEG | 10,074.479 | 70.874 | 8549.567 | 125.066 |
| PEEK-HA | 10,019.144 | 103.655 | 8561.833 | 199.118 |
| PEEK-PDA | 10,090.790 | 165.498 | 8487.683 | 207.593 |
| Group | Yield Torque (N·m) | Stiffness (N·m/deg.) | ||
|---|---|---|---|---|
| Average | Std. Dev. | Average | Std. Dev. | |
| PEEK | 6.525 | 0.181 | 0.845 | 0.045 |
| PEEK-PEG | 6.527 | 0.176 | 0.803 | 0.048 |
| PEEK-HA | 6.737 | 0.187 | 0.804 | 0.041 |
| PEEK-PDA | 6.685 | 0.184 | 0.839 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Jung, T.-G. Surface Modification of Polyetheretherketone (PEEK) Intervertebral Fusion Implant Using Polydopamine Coating for Improved Bioactivity. Bioengineering 2024, 11, 343. https://doi.org/10.3390/bioengineering11040343
Park S, Jung T-G. Surface Modification of Polyetheretherketone (PEEK) Intervertebral Fusion Implant Using Polydopamine Coating for Improved Bioactivity. Bioengineering. 2024; 11(4):343. https://doi.org/10.3390/bioengineering11040343
Chicago/Turabian StylePark, Suzy, and Tae-Gon Jung. 2024. "Surface Modification of Polyetheretherketone (PEEK) Intervertebral Fusion Implant Using Polydopamine Coating for Improved Bioactivity" Bioengineering 11, no. 4: 343. https://doi.org/10.3390/bioengineering11040343
APA StylePark, S., & Jung, T.-G. (2024). Surface Modification of Polyetheretherketone (PEEK) Intervertebral Fusion Implant Using Polydopamine Coating for Improved Bioactivity. Bioengineering, 11(4), 343. https://doi.org/10.3390/bioengineering11040343






