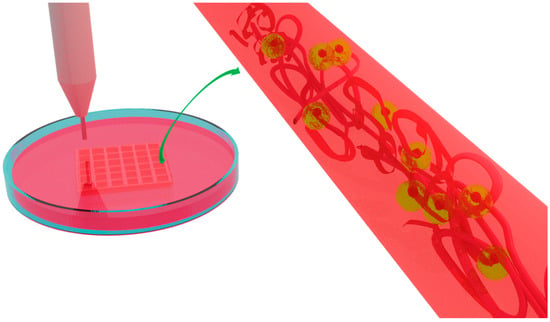
Nanofibrous Material-Reinforced Printable Ink for Enhanced Cell Proliferation and Tissue Regeneration
Abstract
:1. Introduction
2. Three-Dimensional Bioprinting
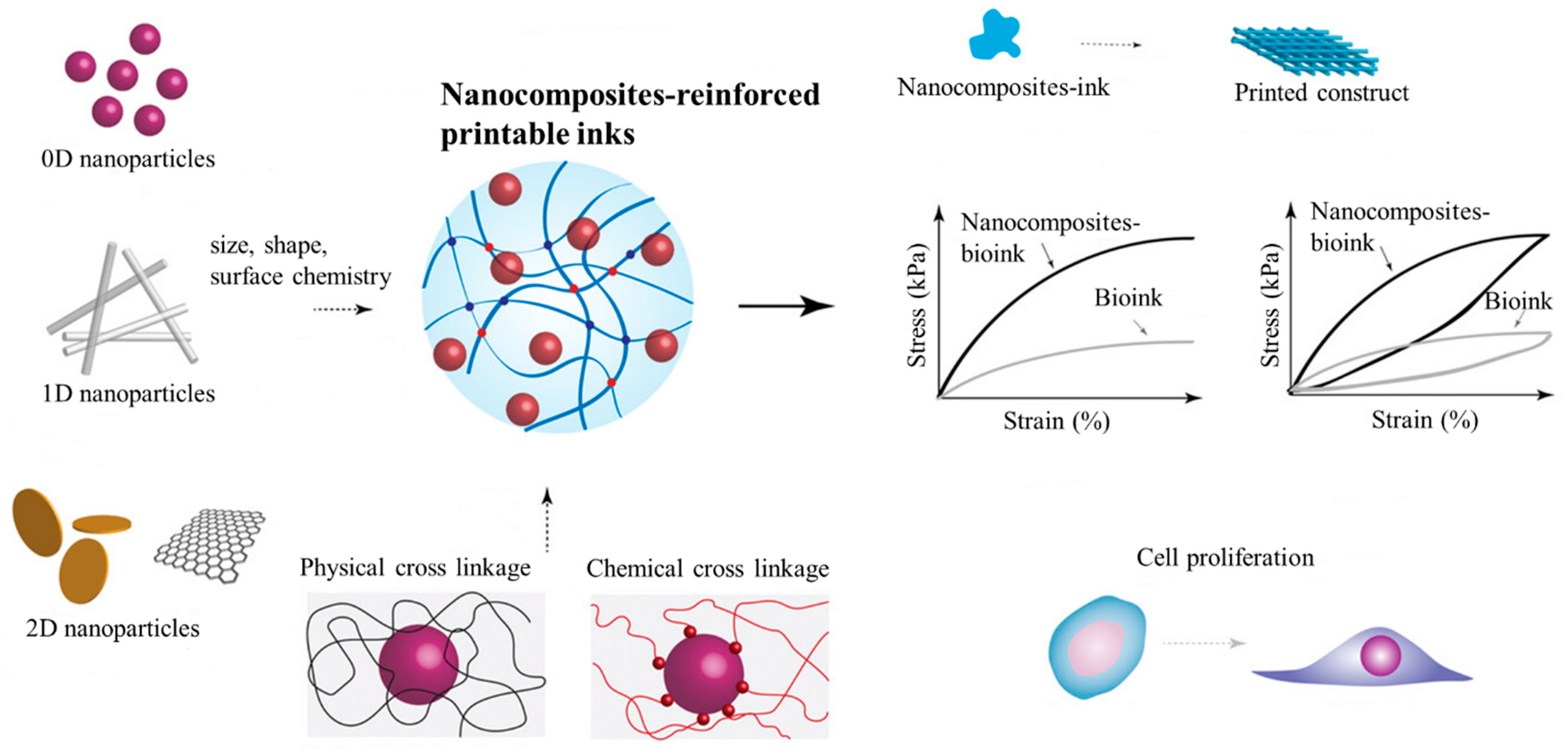
3. Nanocomposite-Reinforced Printable Ink
4. Nanofiber-Reinforced 3D-Printable Ink
4.1. Cellulose-Nanofiber-Reinforced Ink
4.2. Other Polymeric-Nanofiber-Reinforced Ink
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Lee, K.; Kawazoe, N.; Yang, Y.; Chen, G. ECM scaffolds mimicking extracellular matrices of endochondral ossification for the regulation of mesenchymal stem cell differentiation. Acta Biomater. 2020, 114, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk-Soczynska, B.; Zaszczyńska, A.; Zabielski, K.; Sajkiewicz, P. Hydrogel, Electrospun and composite materials for bone/cartilage and neural tissue engineering. Materials 2021, 14, 6899. [Google Scholar] [CrossRef] [PubMed]
- Buinov, A.S.; Gafarova, E.R.; Grebenik, E.A.; Bardakova, K.N.; Kholkhoev, B.C.; Veryasova, N.N.; Nikitin, P.V.; Kosheleva, N.V.; Shavkuta, B.S.; Kuryanova, A.S.; et al. Fabrication of conductive tissue engineering nanocomposite films based on chitosan and surfactant-stabilized graphene dispersions. Polymers 2022, 14, 3792. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, E.; Medina-Cruz, D.; Kalantari, K.; Taymoori, A.; Soltantabar, P.; Webster, T.J. Electroconductive nanobiomaterials for tissue engineering and regenerative medicine. Bioelectricity 2020, 2, 120–149. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Kang, M.S.; Kim, W.-H.; Jo, H.J.; Lee, S.-H.; Hahm, E.J.; Oh, J.H.; Hong, S.W.; Kim, B.; Han, D.-W. 3D printed membranes of polylactic acid and graphene oxide for guided bone regeneration. Nanoscale Adv. 2023, 5, 3619–3628. [Google Scholar] [CrossRef]
- Ko, Y.-G.; Kwon, O.H. Reinforced gelatin-methacrylate hydrogels containing poly(lactic-co-glycolic acid) nanofiber fragments for 3D bioprinting. J. Ind. Eng. Chem. 2020, 89, 147–155. [Google Scholar] [CrossRef]
- Carrow, J.K.; Cross, L.M.; Reese, R.W.; Jaiswal, M.K.; Gregory, C.A.; Kaunas, R.; Singh, I.; Gaharwar, A.K. Widespread changes in transcriptome profile of human mesenchymal stem cells induced by two-dimensional nanosilicates. Proc. Natl. Acad. Sci. USA 2018, 115, E3905–E3913. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv. Mater. 2013, 25, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelioğlu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel bioink reinforcement for additive manufacturing: A focused review of emerging strategies. Adv. Mater. 2020, 32, 1902026. [Google Scholar] [CrossRef]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced bioinks for 3D printing: A materials science perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, D.; Li, Y.; Zhou, X.; Hui, Z.; Lei, X.; Qiu, L.; Bai, Y.; Wang, C.; Xia, J.; et al. Collagen hydrogel with multiple antimicrobial mechanisms as anti-bacterial wound dressing. Int. J. Biol. Macromol. 2023, 232, 123413. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.J.; Kang, M.S.; Heo, H.J.; Jang, H.J.; Park, R.; Hong, S.W.; Kim, Y.H.; Han, D.-W. Skeletal muscle regeneration with 3D bioprinted hyaluronate/gelatin hydrogels incorporating MXene nanoparticles. Int. J. Biol. Macromol. 2024, 265, 130696. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.B.; Xu, C.P.; Li, W.Q.; Meng, Q.J.; Qu, H.Z. Halloysites modified polyethylene glycol diacrylate/thiolated chitosan double network hydrogel combined with BMP-2 for rat skull regeneration. Artif. Cells Nanomed. Biotechnol. 2021, 49, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Dawson, C.; Lamb, M.; Mueller, E.; Stefanek, E.; Akbari, M.; Hoare, T. Hydrogels for tissue engineering: Addressing key design needs toward clinical translation. Front. Bioeng. Biotechnol. 2022, 10, 849831. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tang, J.; Ji, F.; Lin, W.; Chen, S. Recent advances in zwitterionic hydrogels: Preparation, property, and biomedical application. Gels 2022, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Raja, I.S.; Kang, M.S.; Hong, S.W.; Bae, H.; Kim, B.; Hwang, Y.S.; Cha, J.M.; Han, D.W. State-of-the-art techniques for promoting tissue regeneration: Combination of three-dimensional bioprinting and carbon nanomaterials. Int. J. Bioprint. 2023, 9, 635. [Google Scholar] [CrossRef]
- Kaushik, S.N.; Kim, B.; Walma, A.M.; Choi, S.C.; Wu, H.; Mao, J.J.; Jun, H.W.; Cheon, K. Biomimetic microenvironments for regenerative endodontics. Biomater. Res. 2016, 20, 14. [Google Scholar] [CrossRef]
- Prabhakar, M.M.; Saravanan, A.K.; Lenin, A.H.; Leno, I.J.; Mayandi, K.; Ramalingam, P.S. A short review on 3D printing methods, process parameters and materials. Mater. Today Proc. 2021, 45, 6108–6114. [Google Scholar] [CrossRef]
- Luo, W.; Song, Z.; Wang, Z.; Wang, Z.; Li, Z.; Wang, C.; Liu, H.; Liu, Q.; Wang, J. Printability optimization of gelatin-alginate bioinks by cellulose nanofiber modification for potential meniscus bioprinting. J. Nanomater. 2020, 2020, 3863428. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. 3D bioprinting of photocrosslinkable hydrogel constructs. J. Appl. Polym. Sci. 2015, 132, 42458. [Google Scholar] [CrossRef]
- Lyu, Y.; Azevedo, H.S. Supramolecular hydrogels for protein delivery in tissue engineering. Molecules 2021, 26, 873. [Google Scholar] [CrossRef]
- Klein, A.; Whitten, P.G.; Resch, K.; Pinter, G. Nanocomposite hydrogels: Fracture toughness and energy dissipation mechanisms. J. Polym. Sci. B Polym. Phys. 2015, 53, 1763–1773. [Google Scholar] [CrossRef]
- Wu, D.; Yu, Y.; Tan, J.; Huang, L.; Luo, B.; Lu, L.; Zhou, C. 3D bioprinting of gellan gum and poly (ethylene glycol) diacrylate based hydrogels to produce human-scale constructs with high-fidelity. Mater. Design 2018, 160, 486–495. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zhu, L.; Qin, G.; Chen, Q. Double network hydrogels with controlled shape deformation: A mini review. J. Polym. Sci. B Polym. Phys. 2018, 56, 1351–1362. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Blokzijl, M.M.; Levato, R.; Peiffer, Q.C.; de Ruijter, M.; Hennink, W.E.; Vermonden, T.; Malda, J. Hydrogel-based reinforcement of 3D bioprinted constructs. Biofabrication 2016, 8, 035004. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Ruan, C.; Ma, Y.; Cheng, D.; Wu, M.; Liu, W.; Zhao, X.; Pan, H.; Lu, W.W. 3D-bioprinted osteoblast-laden nanocomposite hydrogel constructs with induced microenvironments promote cell viability, differentiation, and osteogenesis both in vitro and in vivo. Adv. Sci. 2018, 5, 1700550. [Google Scholar] [CrossRef]
- Kim, C.; Young, J.L.; Holle, A.W.; Jeong, K.; Major, L.G.; Jeong, J.H.; Aman, Z.M.; Han, D.W.; Hwang, Y.; Spatz, J.P.; et al. Stem cell mechanosensation on gelatin methacryloyl (GelMA) stiffness gradient hydrogels. Ann. Biomed. Eng. 2020, 48, 893–902. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking strategies for 3D bioprinting of polymeric hydrogels. Small 2020, 16, e2002931. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.E.; Anseth, K.S. Spatiotemporal hydrogel biomaterials for regenerative medicine. Chem. Soc. Rev. 2017, 46, 6532–6552. [Google Scholar] [CrossRef] [PubMed]
- Bertlein, S.; Brown, G.; Lim, K.S.; Jungst, T.; Boeck, T.; Blunk, T.; Tessmar, J.; Hooper, G.J.; Woodfield, T.B.F.; Groll, J. Thiol-ene clickable gelatin: A platform bioink for multiple 3d biofabrication technologies. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef] [PubMed]
- Parani, M.; Lokhande, G.; Singh, A.; Gaharwar, A.K. Engineered nanomaterials for infection control and healing acute and chronic wounds. ACS Appl. Mater. Interfaces 2016, 8, 10049–10069. [Google Scholar] [CrossRef]
- Wang, W.; Hou, Y.; Martinez, D.; Kurniawan, D.; Chiang, W.-H.; Bartolo, P. Carbon nanomaterials for electro-active structures: A review. Polymers 2020, 12, 2946. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Lokhande, G.; Singh, K.A.; Jaiswal, M.K.; Rajput, S.; Gaharwar, A.K. Light-triggered in situ gelation of hydrogels using 2D molybdenum disulfide (MoS2) nanoassemblies as crosslink epicenter. Adv. Mater. 2021, 33, 2101238. [Google Scholar] [CrossRef] [PubMed]
- Thakur, T.; Xavier, J.R.; Cross, L.; Jaiswal, M.K.; Mondragon, E.; Kaunas, R.; Gaharwar, A.K. Photocrosslinkable and elastomeric hydrogels for bone regeneration. J. Biomed. Mater. Res. A 2016, 104, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, C.; Chai, W.; Compaan, A.; Huang, Y. Self-supporting nanoclay as internal scaffold material for direct printing of soft hydrogel composite structures in air. ACS Appl. Mater. Interfaces 2017, 9, 17456–17465. [Google Scholar] [CrossRef]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef]
- Xin, H.; Brown, H.R.; Naficy, S.; Spinks, G.M. Mechanical recoverability and damage process of ionic-covalent PAAm-alginate hybrid hydrogels. J. Polym. Sci. B Polym. Phys. 2016, 54, 53–63. [Google Scholar] [CrossRef]
- Shin, J.Y.; Park, J.; Jang, H.K.; Lee, T.J.; La, W.G.; Bhang, S.H.; Kwon, I.K.; Kwon, O.H.; Kim, B.S. Efficient formation of cell spheroids using polymer nanofibers. Biotechnol. Lett. 2012, 34, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Matera, D.L.; Wang, W.Y.; Smith, M.R.; Shikanov, A.; Baker, B.M. Fiber density modulates cell spreading in 3D interstitial matrix mimetics. ACS Biomater. Sci. Eng. 2019, 5, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- Matera, D.L.; DiLillo, K.M.; Smith, M.R.; Davidson, C.D.; Parikh, R.; Said, M.; Wilke, C.A.; Lombaert, I.M.; Arnold, K.B.; Moore, B.B.; et al. Microengineered 3D pulmonary interstitial mimetics highlight a critical role for matrix degradation in myofibroblast differentiation. Sci. Adv. 2020, 6, eabb5069. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, H.L.; Matera, D.L.; Wang, W.Y.; Prabhu, E.S.; Zhang, Z.; Midekssa, F.; Argento, A.E.; Buschhaus, J.M.; Humphries, B.A.; Luker, G.D.; et al. Fiber density and matrix stiffness modulate distinct cell migration modes in a 3D stroma mimetic composite hydrogel. Acta Biomater. 2023, 163, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Polez, R.T.; Kimiaei, E.; Madani, Z.; Rojas, O.J.; Österberg, M.; Seppälä, J. 3D printing and properties of cellulose nanofibrils-reinforced quince seed mucilage bio-inks. Int. J. Biol. Macromol. 2021, 192, 1098–1107. [Google Scholar] [CrossRef]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-alginate hydrogel for cell encapsulation. Carbohydr. Polym. 2015, 116, 223–228. [Google Scholar] [CrossRef]
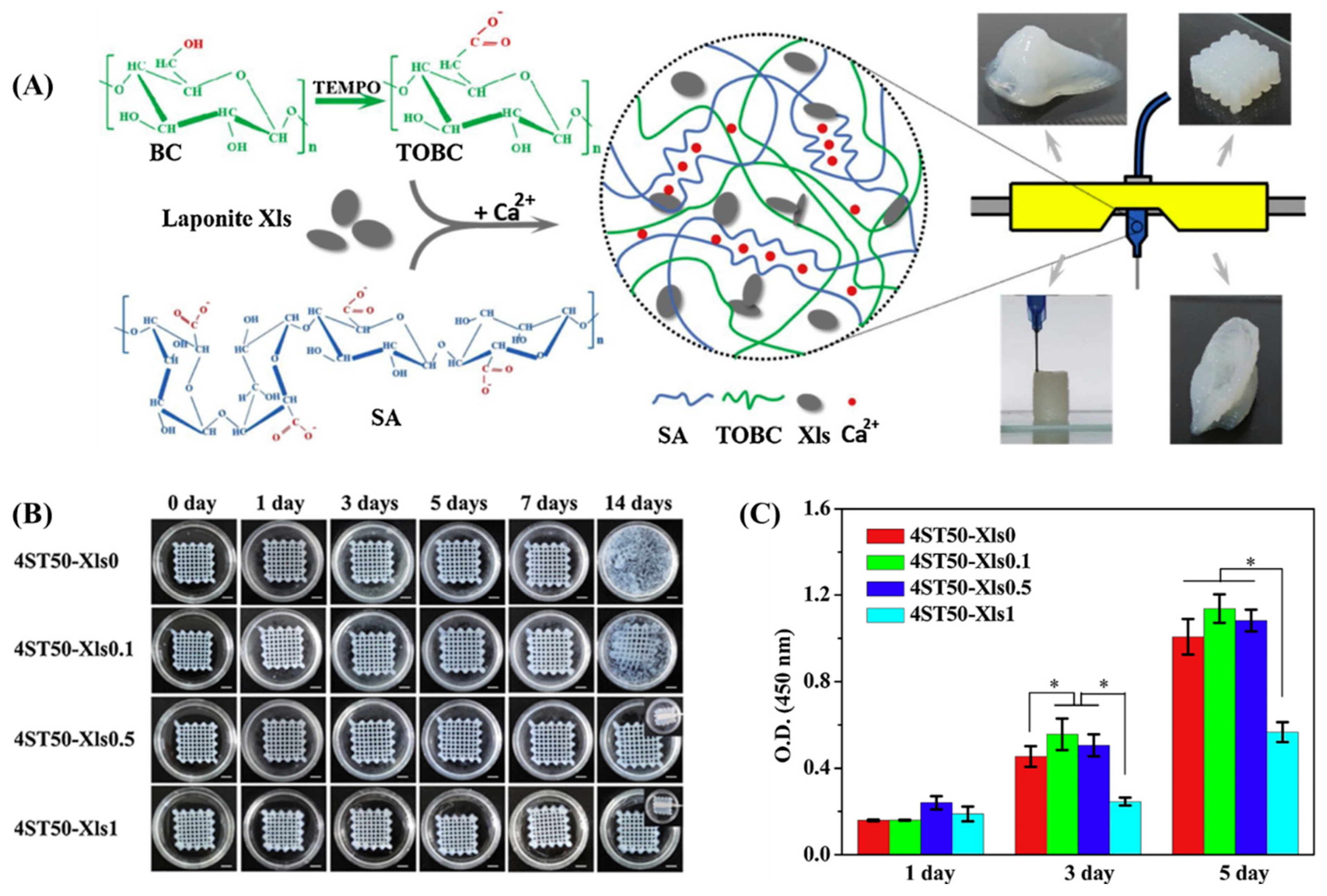
- Wei, J.; Wang, B.; Li, Z.; Wu, Z.; Zhang, M.; Sheng, N.; Liang, Q.; Wang, H.; Chen, S. A 3D-printable TEMPO-oxidized bacterial cellulose/alginate hydrogel with enhanced stability via nanoclay incorporation. Carbohydr. Polym. 2020, 238, 116207. [Google Scholar] [CrossRef]
- Pitton, M.; Fiorati, A.; Buscemi, S.; Melone, L.; Farè, S.; Contessi Negrini, N. 3D bioprinting of pectin-cellulose nanofibers multicomponent bioinks. Front. Bioeng. Biotechnol. 2021, 9, 732689. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Valente, B.F.A.; Luís, J.; Pires, L.; Oliveira, H.; Oliveira, M.; Silvestre, A.J.D.; Vilela, C.; et al. Alginate-lysozyme nanofibers hydrogels with improved rheological behavior, printability and biological properties for 3D bioprinting applications. Nanomaterials 2022, 12, 2190. [Google Scholar] [CrossRef]
- Chu, B.; He, J.-M.; Wang, Z.; Liu, L.-L.; Li, X.-L.; Wu, C.-X.; Chen, C.-S.; Tu, M. Proangiogenic peptide nanofiber hydrogel/3D printed scaffold for dermal regeneration. Chem. Eng. J. 2021, 424, 128146. [Google Scholar] [CrossRef]
- Narayanan, L.K.; Huebner, P.; Fisher, M.B.; Spang, J.T.; Starly, B.; Shirwaiker, R.A. 3D-bioprinting of polylactic acid (PLA) nanofiber-alginate hydrogel bioink containing human adipose-derived stem cells. ACS Biomater. Sci. Eng. 2016, 2, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, Z.; Kharaziha, M.; Zarrabi, A. 3D-printing of silk nanofibrils reinforced alginate for soft tissue engineering. Pharmaceutics 2023, 15, 763. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Ávila, H.M.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Han, C.; Wang, X.; Zhongjin, N.; Ni, Y.; Huan, W.; Lv, Y.; Bai, S.L. Effects of nanocellulose on alginate/gelatin bio-inks for extrusion-based 3D printing. Bioresources 2020, 15, 7357–7373. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Ávila, H.M.; Schwarz, S.; Feldmann, E.-M.; Mantas, A.; von Bomhard, A.; Gatenholm, P.; Rotter, N. Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl. Microbiol. Biotechnol. 2014, 98, 7423–7435. [Google Scholar] [CrossRef]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Zhang, Y.; Kuan, H.-C.; Lee, S.-H.; Ma, J. Polymer composite hydrogels containing carbon nanomaterials—Morphology and mechanical and functional performance. Prog. Polym. Sci. 2018, 77, 1–18. [Google Scholar] [CrossRef]
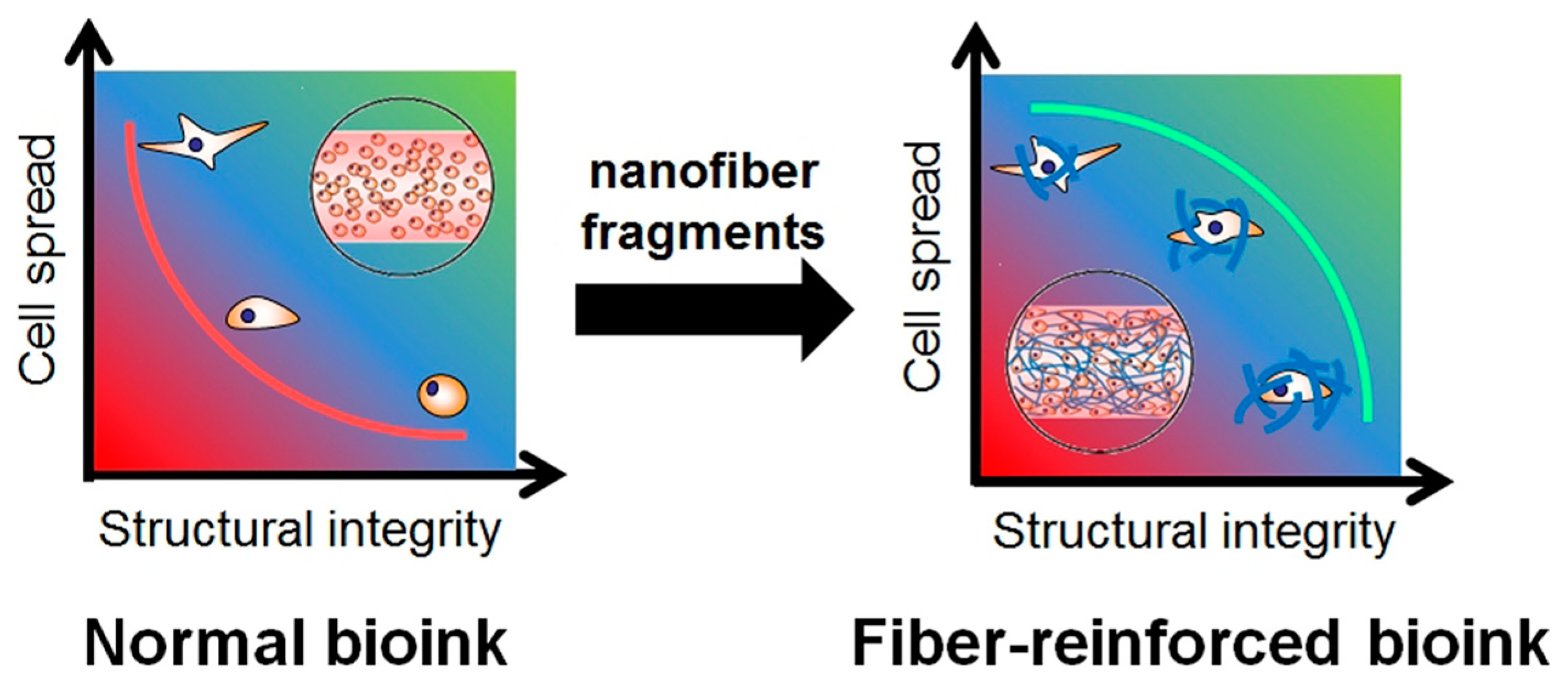
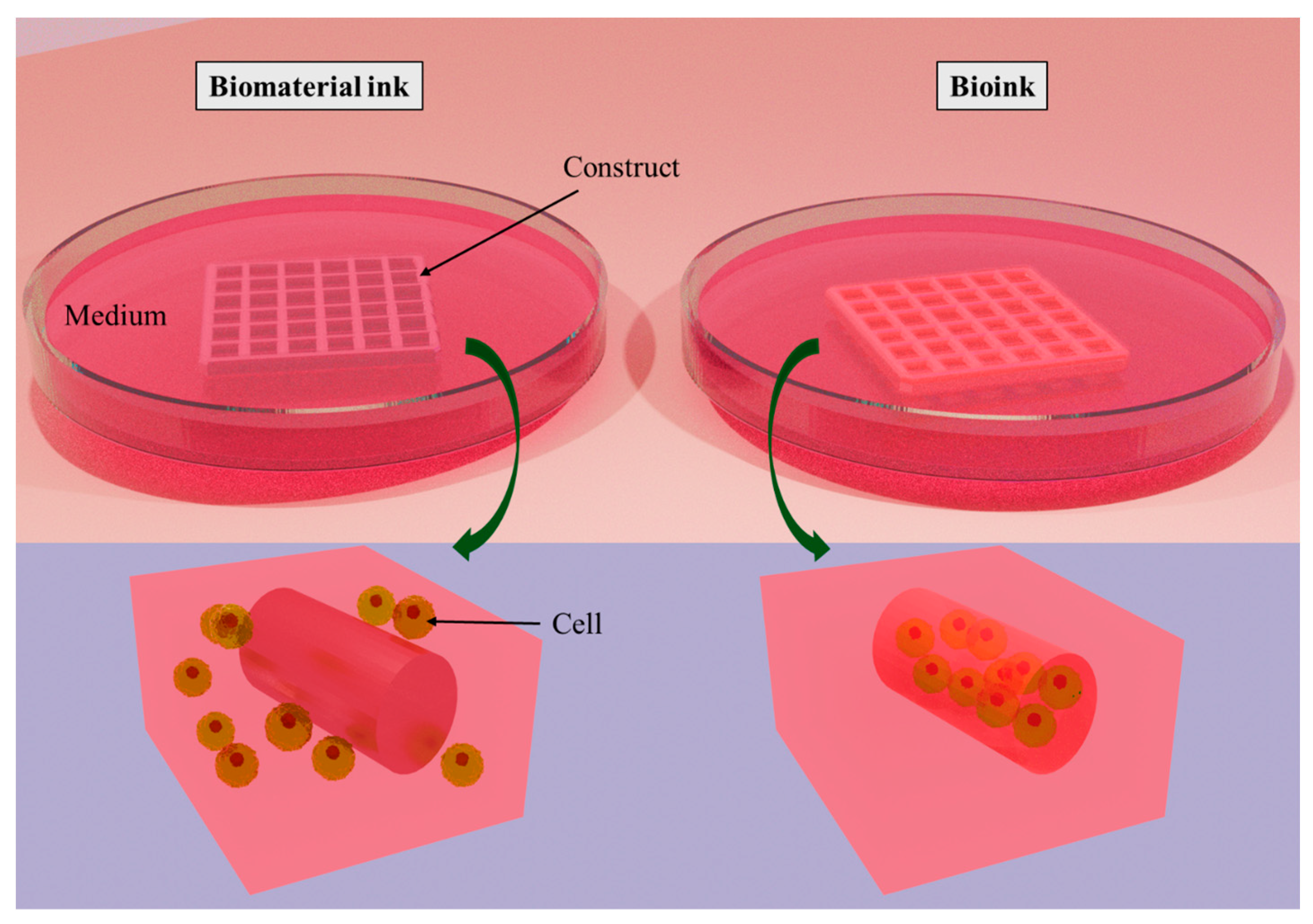
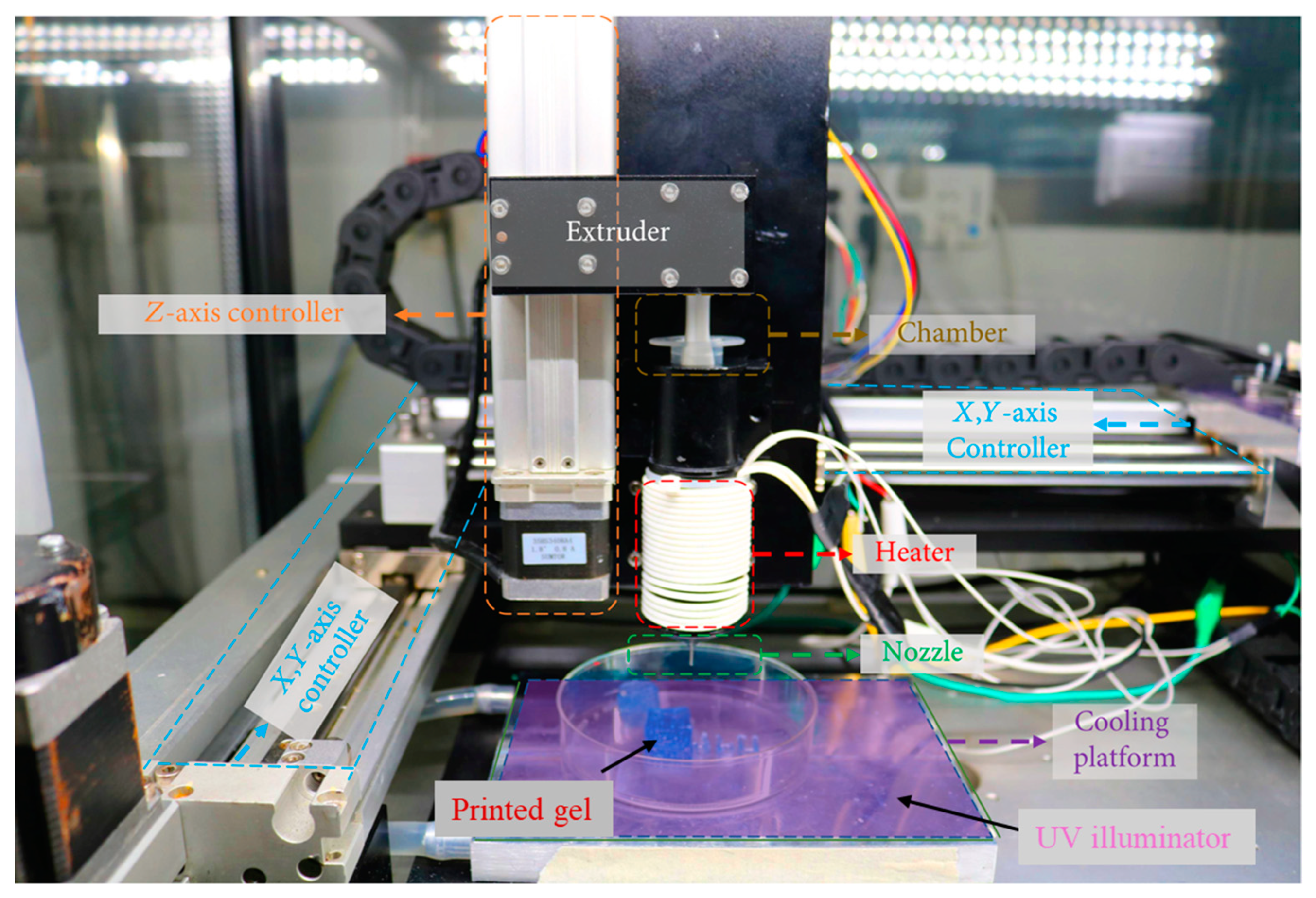


| Nanofiber-Reinforced Printable Inks | Specifications | Crosslinking Agent | Type of Printable Ink and Cell Quantity | Biological Outcomes | TE Application |
|---|---|---|---|---|---|
| Quince seed mucilage/CNF | Grid structure, 5 layers, 30 mm × 30 mm, 25% infill density (or) disc-shaped structure, 25 mm, 50% infill density | CaCl2 | Biomaterial ink HepG2, 50,000 cells/mL | ↑ cell viability (≥90%), cell attachment, and proliferation | Soft tissues [46] |
| Gelatin/alginate/CNF | Square blocks (15 mm × 15 mm × 2 mm) | CaCl2 | Bioink rFCs, 5 × 106 cells/mL | ↑ accumulation of collagen type I and type II | Meniscal reconstruction [21] |
| Sodium alginate/TOBC nanofiber | Beads | CaCl2 | Bioink NIH3T3, 2.3 × 106 cells/mL | ↑ aggregation and proliferation of cells | Skin tissue [47] |
| Sodium alginate/laponite nanoclay/TOBC nanofiber | Grid structure, 10 mm × 10 mm × 1 mm, line spacing 0.8–1.2 mm (or) 20 mm × 20 mm × 3 mm, line spacing 2 mm | CaCl2 | Biomaterial ink L929, 1 × 104 cells/well | ↑ cell-material interactions and cell spreading | Skin tissue [48] |
| Pectin/TEMPO-oxidized CNF | Printed rings (Ø internal = 2 cm, Ø external = 3.6 cm) | CaCl2 | Bioink L929, 10 × 106 cells/mL | ↑ cell viability (≥80%) and metabolic activity | Skin tissue [49] |
| Alginate/lysozyme nanofiber | 2 layers, 20 mm × 20 mm, line spacing 2.25 mm | CaCl2 | Biomaterial ink HaCaT, 2 × 106 cells/mL | ↑ cell viability (>80%) | Skin tissue [50] |
| GelMA/peptide nanofiber | 5 layers, fiber spacing 500 µm, layer height 150 µm | UV-curing | Biomaterial ink L929 and HUVECs, 1 × 105 cells/mL | ↑ formation of lumen structure and angiogenesis | Skin tissue [51] |
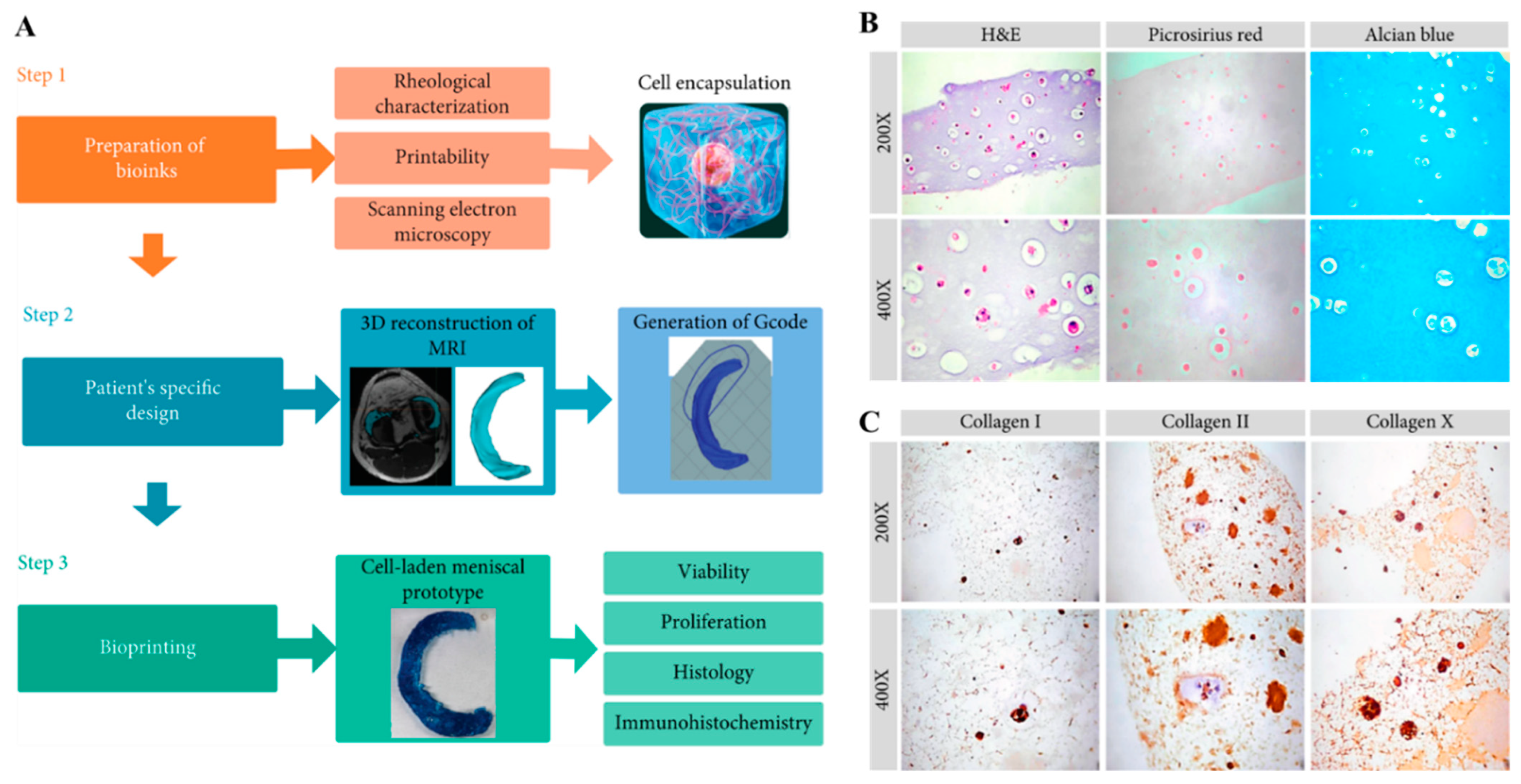
| Alginate/PLA nanofiber | Meniscus constructs, five strands, 25 mm × 0.5 mm × 0.5 mm, interstrand spacing 3 mm | CaCl2 | Bioink hASC, 1.375 × 106 cells/mL | ↑ metabolic activity and cell proliferation | Musculoskeletal soft tissue [52] |
| GelMA/PLGA nanofiber | Rectangle-shaped construct,10 mm × 10 mm, thickness 5 mm | UV-curing | Bioink NIH3T3, 5.0 × 106 cells/1.5 mL | ↑ cell spreading and proliferation | Soft tissues [6] |
| Alginate/silk nanofibrils | Five-layer grid pattern, 1 × 1 cm2 | CaCl2 | Biomaterial ink L929, 1 × 104 cells/well | ↑ cell viability and proliferation | Soft tissues [53] |
| Alginate/gelatin/carbon nanofiber | 2 layers, 2 mm per layer, 9:4 mm (w × h) | CaCl2 | Biomaterial ink NIH3T3, 0.04 × 106 cells/construct | ↑ cellular attachment and proliferation | Myocardial and neuronal tissues [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raja, I.S.; Kim, B.; Han, D.-W. Nanofibrous Material-Reinforced Printable Ink for Enhanced Cell Proliferation and Tissue Regeneration. Bioengineering 2024, 11, 363. https://doi.org/10.3390/bioengineering11040363
Raja IS, Kim B, Han D-W. Nanofibrous Material-Reinforced Printable Ink for Enhanced Cell Proliferation and Tissue Regeneration. Bioengineering. 2024; 11(4):363. https://doi.org/10.3390/bioengineering11040363
Chicago/Turabian StyleRaja, Iruthayapandi Selestin, Bongju Kim, and Dong-Wook Han. 2024. "Nanofibrous Material-Reinforced Printable Ink for Enhanced Cell Proliferation and Tissue Regeneration" Bioengineering 11, no. 4: 363. https://doi.org/10.3390/bioengineering11040363
APA StyleRaja, I. S., Kim, B., & Han, D.-W. (2024). Nanofibrous Material-Reinforced Printable Ink for Enhanced Cell Proliferation and Tissue Regeneration. Bioengineering, 11(4), 363. https://doi.org/10.3390/bioengineering11040363