Release Profile and Antibacterial Activity of Thymus sibthorpii Essential Oil-Incorporated, Optimally Stabilized Type I Collagen Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication and Crosslinking of Collagen Type I Hydrogels
2.3. Screening of the Crosslinking Efficacy of starPEG Crosslinkers on Collagen Type I Hydrogels
2.3.1. Quantification of the Free Amine Groups
2.3.2. Enzymatic Degradation Analysis
2.4. Release Profile and Release Kinetics Analysis of Essential Oil
2.5. Biological Activity of Essential Oil-Loaded Hydrogels
2.5.1. Antimicrobial Activity
2.5.2. Cytocompatibility Analysis
2.6. Statistical Analysis
3. Results
3.1. Determination of the Optimal starPEG Type and Concentration on Hydrogel Stability
3.2. EO Release Profile and Release Kinetics
3.3. Antimicrobial Activity Analyses of the EO-Loaded Hydrogels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyriacou, H.; Kamaraj, A.; Khan, W.S. Developments in antibiotic-eluting scaffolds for the treatment of osteomyelitis. Appl. Sci. 2020, 10, 2244. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered drug delivery systems for enhancing antibiotic therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.A.; Roberts, I.; Gillespie, W.J. Antibiotics for preventing infection in open limb fractures. Cochrane Database Syst. Rev. 2004, 1, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against bone infection. Adv. Healthc. Mater. 2020, 9, 2000310. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development; European Union. Antimicrobial Resistance-Tackling the Burden in the European Union; OECD Publishing: Paris, France, 2019. [Google Scholar]
- U.S. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- Singh, A.; Amod, A.; Pandey, P.; Bose, P.; Pingali, M.S.; Shivalkar, S.; Varadwaj, P.K.; Sahoo, A.K.; Samanta, S.K. Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies. Biomed. Mater. 2022, 17, 022003. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. Apmis. 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Fragkou, I.A.; Skoufos, J.; Cripps, P.J.; Kyriazakis, I.; Papaioannou, N.; Boscos, C.M.; Tzora, A.; Fthenakis, G.C. Differences in susceptibility to Mannheimia haemolytica-associated mastitis between two breeds of dairy sheep. J. Dairy Res. 2007, 74, 349–355. [Google Scholar] [CrossRef]
- Kalelkar, P.P.; Riddick, M.; Garcia, A.J. Biomaterial-based antimicrobial therapies for the treatment of bacterial infections. Nat. Rev. Mater. 2021, 7, 39–54. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Rigas, D.; Grivas, N.; Nelli, A.; Gouva, E.; Skoufos, I.; Kormas, K.; Tzora, A.; Lagkouvardos, I. Persistent Dysbiosis, Parasite Rise and Growth Impairment in Aquacultured European Seabass after Oxytetracycline Treatment. Microorganisms 2023, 11, 2302. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Micol, V. Tackling antibiotic resistance with compounds of natural origin: A comprehensive review. Biomedicines 2020, 8, 405. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The progress of essential oils as potential therapeutic agents: A review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental chemistry of essential oils and volatile organic compounds, methods of analysis and authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef]
- Benkhoud, H.; M’Rabet, Y.; Gara Ali, M.; Mezni, M.; Hosni, K. Essential oils as flavoring and preservative agents: Impact on volatile profile, sensory attributes, and the oxidative stability of flavored extra virgin olive oil. J. Food Process. Pres. 2022, 46, e15379. [Google Scholar] [CrossRef]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Ersanli, C.; Tzora, A.; Skoufos, I.; Fotou, K.; Maloupa, E.; Gridoriadou, K.; Voidarou, C.; Zeugolis, D.I. The Assessment of Antimicrobial and Anti-Biofilm Activity of Essential Oils against Staphylococcus aureus Strains. Antibiotics 2023, 12, 384. [Google Scholar] [CrossRef]
- Kontogiorgis, C.; Ntella, M.; Mpompou, L.; Karallaki, F.; Athanasios, P.; Hadjipavlou-Litina, D.; Lazari, D. Study of the antioxidant activity of Thymus sibthorpii Bentham (Lamiaceae). J. Enzyme Inhib. Med. Chem. 2016, 31, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Unalan, I.; Endlein, S.J.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R. Evaluation of electrospun poly (ε-caprolactone)/gelatin nanofiber mats containing clove essential oil for antibacterial wound dressing. Pharmaceutics 2019, 11, 570. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. 2021, 109, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- David, G. Collagen-based 3D structures-versatile, efficient materials for biomedical applications. In Biopolymer-Based Formulations, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 881–906. [Google Scholar]
- Miyata, T.; Taira, T.; Noishiki, Y. Collagen engineering for biomaterial use. Clin. Mater. 1992, 9, 139–148. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef]
- Buehler, M.J. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar] [CrossRef]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.M.R.; Motta, A.; Fauzi, M.B. A comprehensive review on collagen type I development of biomaterials for tissue engineering: From biosynthesis to bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef]
- Kong, W.; Lyu, C.; Liao, H.; Du, Y. Collagen crosslinking: Effect on structure, mechanics and fibrosis progression. Biomed. Mater. 2021, 16, 062005. [Google Scholar] [CrossRef]
- Sallent, I.; Capella-Monsonís, H.; Zeugolis, D.I. Production and characterization of chemically cross-linked collagen scaffolds. In Collagen: Methods and Protocols; Springer: Berlin, Germany, 2019; pp. 23–38. [Google Scholar]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly (vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 61, 121–130. [Google Scholar] [CrossRef]
- Moshnikova, A.; Afanasyev, V.; Proussakova, O.; Chernyshov, S.; Gogvadze, V.; Beletsky, I. Cytotoxic activity of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide is underlain by DNA interchain cross-linking. Cell. Mol. Life Sci. 2006, 63, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Sanami, M.; Sweeney, I.; Shtein, Z.; Meirovich, S.; Sorushanova, A.; Mullen, A.M.; Miraftab, M.; Shoseyov, O.; O’Dowd, C.; Pandit, A. The influence of poly (ethylene glycol) ether tetrasuccinimidyl glutarate on the structural, physical, and biological properties of collagen fibers. J. Biomed. Mater. Res. 2016, 104, 914–922. [Google Scholar] [CrossRef]
- Delgado, L.M.; Fuller, K.; Zeugolis, D.I. Collagen cross-linking: Biophysical, biochemical, and biological response analysis. Tissue Eng. Part A 2017, 23, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, M.; Browne, S.; Schenke-Layland, K.; Pandit, A. A collagen-based scaffold delivering exogenous microrna-29B to modulate extracellular matrix remodeling. Mol. Ther. 2014, 22, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Collin, E.C.; Grad, S.; Zeugolis, D.I.; Vinatier, C.S.; Clouet, J.R.; Guicheux, J.J.; Weiss, P.; Alini, M.; Pandit, A.S. An injectable vehicle for nucleus pulposus cell-based therapy. Biomaterials 2011, 32, 2862–2870. [Google Scholar] [CrossRef] [PubMed]
- Hass, V.; Luque-Martinez, I.V.; Gutierrez, M.F.; Moreira, C.G.; Gotti, V.B.; Feitosa, V.P.; Koller, G.; Otuki, M.F.; Loguercio, A.D.; Reis, A. Collagen cross-linkers on dentin bonding: Stability of the adhesive interfaces, degree of conversion of the adhesive, cytotoxicity and in situ MMP inhibition. Dent. Mater. J. 2016, 32, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Capella-Monsonís, H.; Coentro, J.Q.; Graceffa, V.; Wu, Z.; Zeugolis, D.I. An experimental toolbox for characterization of mammalian collagen type I in biological specimens. Nat. Protoc. 2018, 13, 507–529. [Google Scholar] [CrossRef]
- Aker, S.D.; Tamburaci, S.; Tihminlioglu, F. Development of Cissus quadrangularis-Loaded POSS-Reinforced Chitosan-Based Bilayer Sponges for Wound Healing Applications: Drug Release and In Vitro Bioactivity. ACS Omega 2023, 8, 19674–19691. [Google Scholar] [CrossRef]
- Vyavahare, N.; Kulkarni, M.; Mashelkar, R. Zero order release from swollen hydrogels. J. Membrane Sci. 1990, 54, 221–228. [Google Scholar] [CrossRef]
- Ata, S.; Rasool, A.; Islam, A.; Bibi, I.; Rizwan, M.; Azeem, M.K.; Iqbal, M. Loading of Cefixime to pH sensitive chitosan based hydrogel and investigation of controlled release kinetics. Int. J. Biol. Macromol. 2020, 155, 1236–1244. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Rehman, Q.; Akash, M.S.H.; Rasool, M.F.; Rehman, K. Role of kinetic models in drug stability. In Drug Stability and Chemical Kinetics; Springer: Berlin, Germany, 2020; pp. 155–165. [Google Scholar]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Alexopoulos, A.; Giorgi, E.; Tzora, A.; Skoufos, I.; Varzakas, T.; Bezirtzoglou, E. An in vitro study of different types of Greek honey as potential natural antimicrobials against dental caries and other oral pathogenic microorganisms. Case study simulation of oral cavity conditions. Appl. Sci. 2021, 11, 6318. [Google Scholar] [CrossRef]
- Coentro, J.Q.; Di Nubila, A.; May, U.; Prince, S.; Zwaagstra, J.; Järvinen, T.A.; Zeugolis, D.I. Dual drug delivery collagen vehicles for modulation of skin fibrosis in vitro. Biomed. Mater. 2022, 17, 025017. [Google Scholar] [CrossRef]
- Calderon, L.; Collin, E.; Murphy, M.; O’Halloran, D.; Pandit, A. Type II collagen-hyaluronan hydrogel-a step towards a scaffold for intervertebral disc tissue engineering. Eur. Cells Mater. 2010, 20, 134–148. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Jadimurthy, R.; Jagadish, S.; Nayak, S.C.; Kumar, S.; Mohan, C.D.; Rangappa, K.S. Phytochemicals as invaluable sources of potent antimicrobial agents to combat antibiotic resistance. Life 2023, 13, 948. [Google Scholar] [CrossRef]
- Antoniadou, M.; Rozos, G.; Vaou, N.; Zaralis, K.; Ersanli, C.; Alexopoulos, A.; Dadamogia, A.; Varzakas, T.; Tzora, A.; Voidarou, C. Comprehensive Bio-Screening of Phytochemistry and Biological Capacity of Oregano (Origanum vulgare) and Salvia triloba Extracts against Oral Cariogenic and Food-Origin Pathogenic Bacteria. Biomolecules 2024, 14, 619. [Google Scholar] [CrossRef]
- Voidarou, C.; Alexopoulos, A.; Tsinas, A.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Bezirtzoglou, E. Effectiveness of bacteriocin-producing lactic acid bacteria and bifidobacterium isolated from honeycombs against spoilage microorganisms and pathogens isolated from fruits and vegetables. Appl. Sci. 2020, 10, 7309. [Google Scholar] [CrossRef]
- Sundar, G.; Joseph, J.; John, A.; Abraham, A. Natural collagen bioscaffolds for skin tissue engineering strategies in burns: A critical review. Int. J. Polym. Mater. 2021, 70, 593–604. [Google Scholar] [CrossRef]
- Ersanli, C.; Tzora, A.; Skoufos, I.; Voidarou, C.; Zeugolis, D.I. Recent advances in collagen antimicrobial biomaterials for tissue engineering applications: A review. Int. J. Mol. Sci. 2023, 24, 7808. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, E.; Helling, A.; Wall, J.; Bayon, Y.; Zeugolis, D. Battling bacterial infection with hexamethylene diisocyanate cross-linked and Cefaclor-loaded collagen scaffolds. Biomed. Mater. 2017, 12, 035013. [Google Scholar] [CrossRef]
- Li, Z.; Lu, F.; Liu, Y. A Review of the Mechanism, Properties, and Applications of Hydrogels Prepared by Enzymatic Cross-linking. J. Agric. Food Chem. 2023, 71, 10238–10249. [Google Scholar] [CrossRef]
- Xiang, C.; Wang, Z.; Zhang, Q.; Guo, Z.; Li, X.; Chen, W.; Wei, X.; Li, P. Tough physically crosslinked poly (vinyl alcohol)-based hydrogels loaded with collagen type I to promote bone regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2024, 261, 129847. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Alonso-González, M.; Jiménez-Rosado, M.; Benhnia, M.R.E.I.; Ruiz-Mateos, E.; Ostos, F.J.; Romero, A.; Perez-Puyana, V.M. Effect of different crosslinking agents on hybrid chitosan/collagen hydrogels for potential tissue engineering applications. Int. J. Biol. Macromol. 2024, 263, 129858. [Google Scholar] [CrossRef]
- Dattilo, M.; Patitucci, F.; Prete, S.; Parisi, O.I.; Puoci, F. Polysaccharide-based hydrogels and their application as drug delivery systems in cancer treatment: A review. J. Funct. Biomater. 2023, 14, 55. [Google Scholar] [CrossRef]
- Bindi, B.; Perioli, A.; Melo, P.; Mattu, C.; Ferreira, A.M. Bioinspired Collagen/Hyaluronic Acid/Fibrin-Based Hydrogels for Soft Tissue Engineering: Design, Synthesis, and In Vitro Characterization. J. Funct. Biomater. 2023, 14, 495. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef]
- Unalan, I.; Schruefer, S.; Schubert, D.W.; Boccaccini, A.R. 3D-printed multifunctional hydrogels with phytotherapeutic properties: Development of essential oil-incorporated ALG-XAN hydrogels for wound healing applications. ACS Biomater. Sci. Eng. 2023, 9, 4149–4167. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wu, Z.; McClements, D.J.; Zou, L.; Peng, S.; Zhou, W.; Liu, W. Improvement on stability, loading capacity and sustained release of rhamnolipids modified curcumin liposomes. Colloids Surf. B 2019, 183, 110460. [Google Scholar] [CrossRef] [PubMed]
- Poolman, J.T.; Anderson, A.S. Escherichia coli and Staphylococcus aureus: Leading bacterial pathogens of healthcare associated infections and bacteremia in older-age populations. Expert Rev. Vaccines 2018, 17, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Ersanli, C.; Tzora, A.; Voidarou, C.; Skoufos, S.; Zeugolis, D.I.; Skoufos, I. Biodiversity of Skin Microbiota as an Important Biomarker for Wound Healing. Biology 2023, 12, 1187. [Google Scholar] [CrossRef]
- Nelli, A.; Voidarou, C.; Venardou, B.; Fotou, K.; Tsinas, A.; Bonos, E.; Fthenakis, G.C.; Skoufos, I.; Tzora, A. Antimicrobial and Methicillin Resistance Pattern of Potential Mastitis-Inducing Staphylococcus aureus and Coagulase-Negative Staphylococci Isolates from the Mammary Secretion of Dairy Goats. Biology 2022, 11, 1591. [Google Scholar] [CrossRef]
- Bourgou, S.; Pichette, A.; Marzouk, B.; Legault, J. Bioactivities of black cumin essential oil and its main terpenes from Tunisia. S. Afr. J. Bot. 2010, 76, 210–216. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Aras, C.; Tümay Özer, E.; Göktalay, G.; Saat, G.; Karaca, E. Evaluation of Nigella sativa oil loaded electrospun polyurethane nanofibrous mat as wound dressing. J. Biomater. Sci. Polym. Ed. 2021, 32, 1718–1735. [Google Scholar] [CrossRef]
- Lo, S.; Fauzi, M.B. Current update of collagen nanomaterials—Fabrication, characterisation and its applications: A review. Pharmaceutics 2021, 13, 316. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Pérez, C.J.; Desimone, M.F. Collagen Hydrogels Loaded with Silver Nanoparticles and Cannabis Sativa Oil. Antibiotics 2021, 10, 1420. [Google Scholar] [CrossRef]
- Pugliese, E.; Sallent, I.; Ribeiro, S.; Trotier, A.; Korntner, S.H.; Bayon, Y.; Zeugolis, D.I. Development of three-layer collagen scaffolds to spatially direct tissue-specific cell differentiation for enthesis repair. Mater. Today Bio. 2023, 19, 100584. [Google Scholar] [CrossRef]
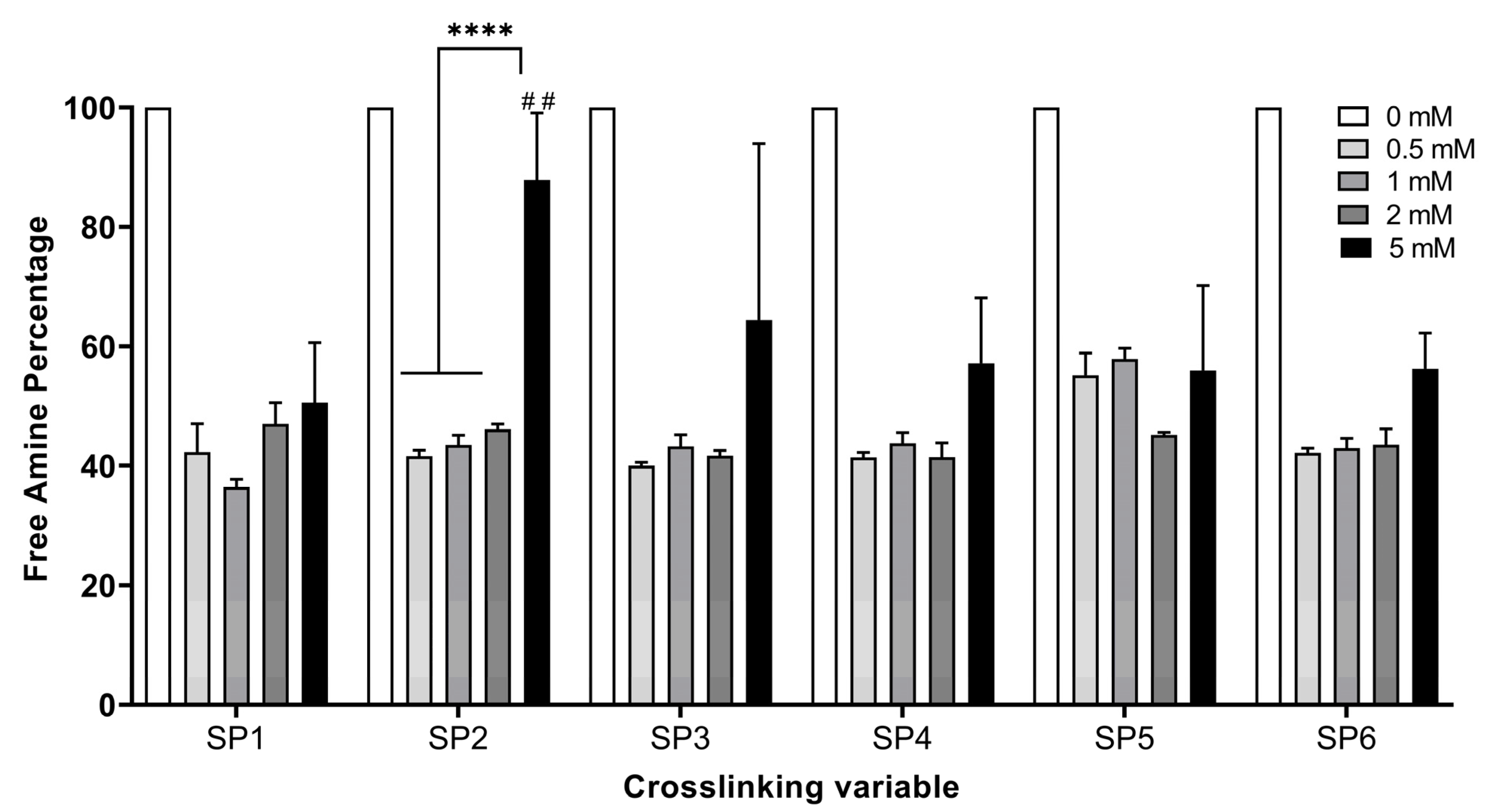
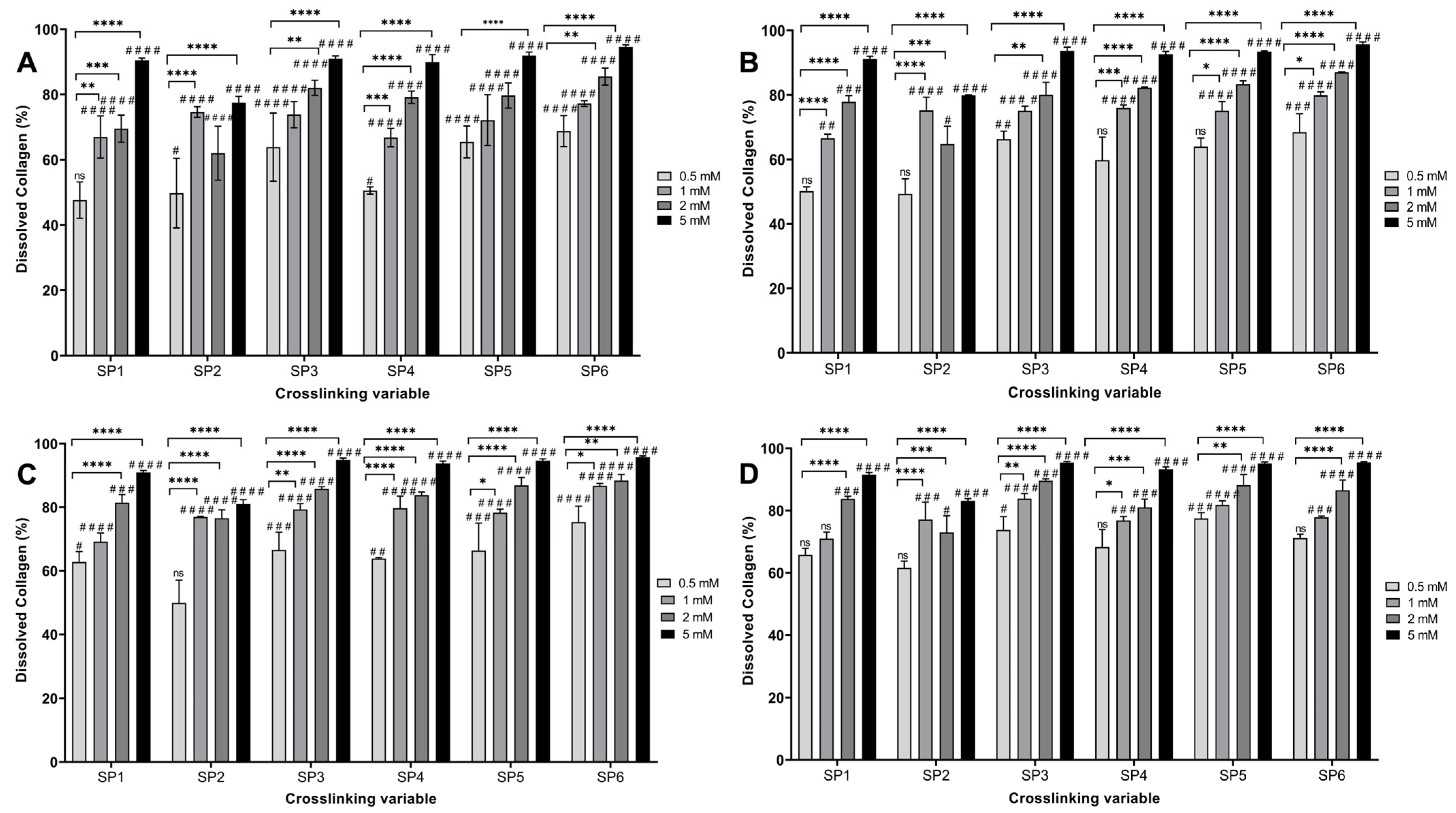
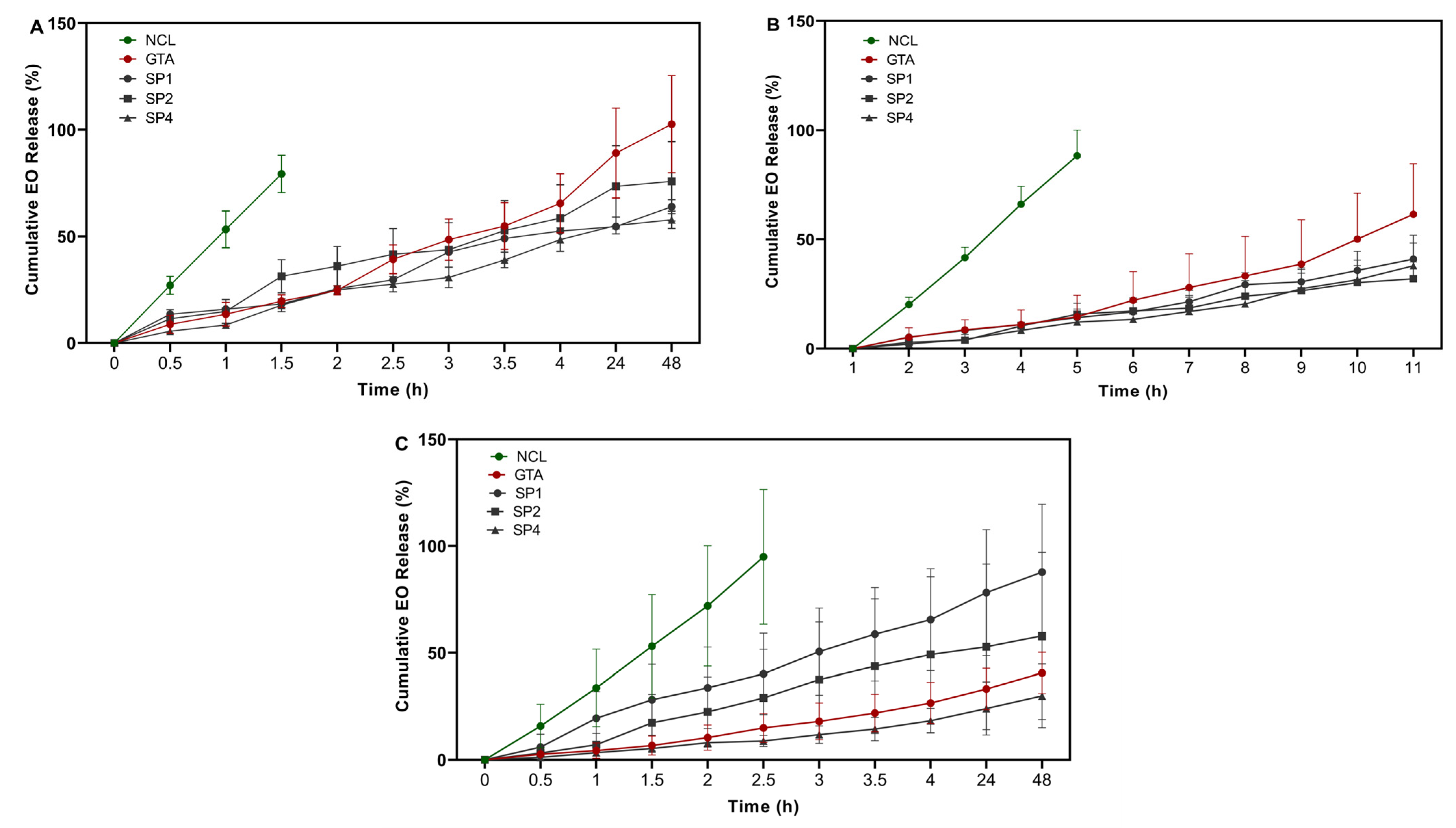
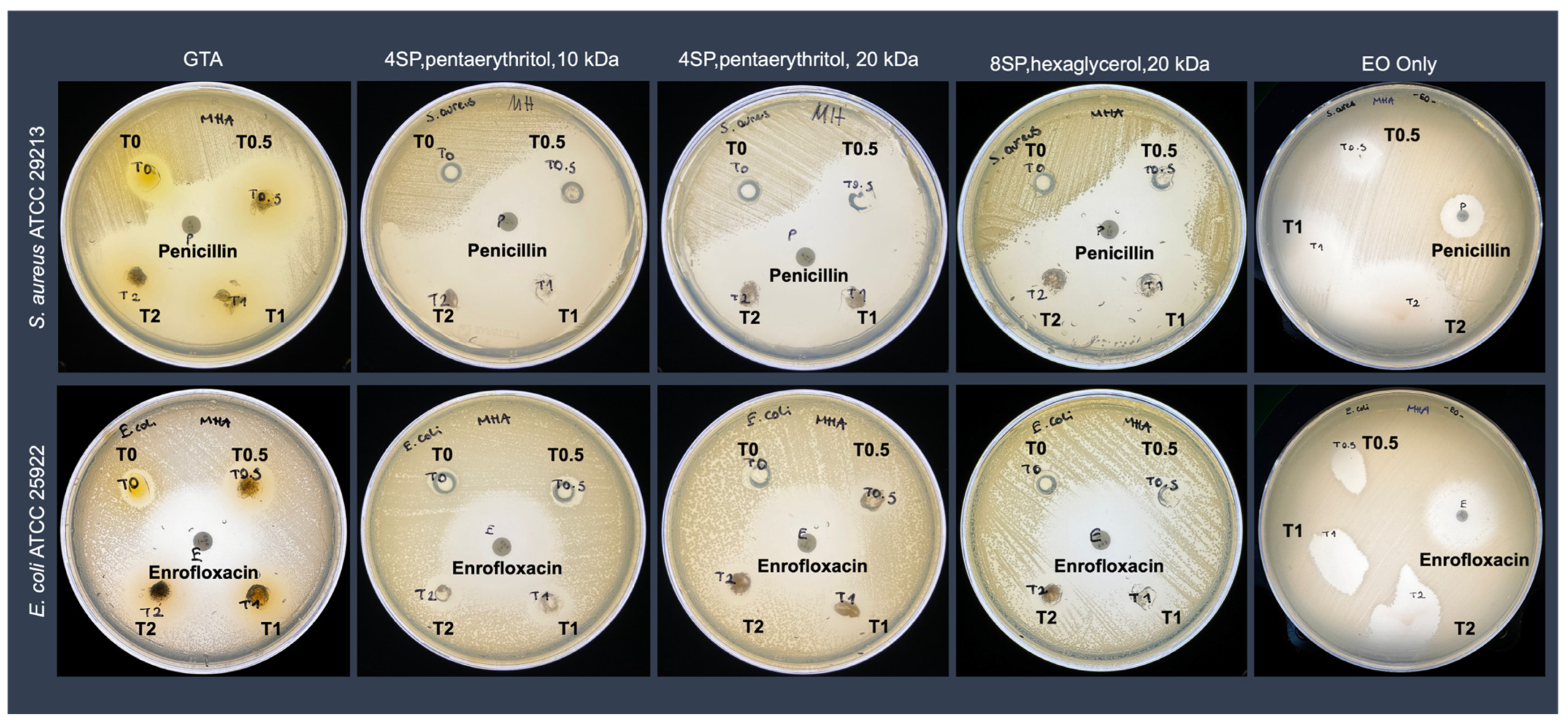


| Full Name | Abbreviation | Code | Arm Number | Functional Group | MW (kDa) | Concentration (mM) |
|---|---|---|---|---|---|---|
| 4-arm PEG Succinimidyl Glutarate, pentaerythritol, 10 kDa | 4SP, pentaerythritol, 10 kDa | SP1 | 4 | pentaerythritol | 10 | 0, 0.5, 1, 2, 5 |
| 4-arm PEG Succinimidyl Glutarate, pentaerythritol, 20 kDa | 4SP, pentaerythritol, 20 kDa | SP2 | 4 | pentaerythritol | 20 | 0, 0.5, 1, 2, 5 |
| 8-arm PEG Succinimidyl Glutarate, hexaglycerol, 10 kDa | 8SP, hexaglycerol, 10 kDa | SP3 | 8 | hexaglycerol | 10 | 0, 0.5, 1, 2, 5 |
| 8-arm PEG Succinimidyl Glutarate, hexaglycerol, 20 kDa | 8SP, hexaglycerol, 20 kDa | SP4 | 8 | hexaglycerol | 20 | 0, 0.5, 1, 2, 5 |
| 8-arm PEG Succinimidyl Glutarate, tripentaerythritol, 10 kDa | 8SP, tripentaerythritol, 10 kDa | SP5 | 8 | tripentaerythritol | 10 | 0, 0.5, 1, 2, 5 |
| 8-arm PEG Succinimidyl Glutarate, tripentaerythritol, 20 kDa | 8SP, tripentaerythritol, 20 kDa | SP6 | 8 | tripentaerythritol | 20 | 0, 0.5, 1, 2, 5 |
| Model | Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | Hixson–Crowell | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | R2 | |
| NCL-T0.5 | 0.9996 | 0.9538 | 0.9294 | 1.0000 | 0.9789 | 0.4040 |
| NCL-T1 | 0.9991 | 0.9050 | 0.8987 | 0.9996 | 1.0720 | 0.5536 |
| NCL-T2 | 0.9972 | 0.8101 | 0.8821 | 0.9997 | 1.1065 | 0.6183 |
| GTA-T0.5 | 0.9574 | 1.0000 | 0.9971 | 0.9985 | 0.1789 | 0.0235 |
| GTA-T1 | 0.9973 | 0.9998 | 0.9897 | 0.9666 | 0.1779 | 0.7033 |
| GTA-T2 | 0.9998 | 0.9997 | 0.9806 | 0.9494 | 0.1613 | 0.9434 |
| SP1-T0.5 | 0.9153 | 0.9039 | 0.8113 | 0.6980 | 0.0677 | 0.6412 |
| SP1-T1 | 0.9973 | 0.9991 | 0.9897 | 0.9573 | 0.1111 | 0.7033 |
| SP1-T2 | 0.9829 | 0.9997 | 0.9995 | 0.9830 | 0.1140 | 0.1856 |
| SP2-T0.5 | 0.8124 | 0.8400 | 0.9196 | 0.9747 | 0.1082 | 0.0953 |
| SP2-T1 | 0.9336 | 0.9382 | 0.9887 | 0.9996 | 0.0747 | 0.1965 |
| SP2-T2 | 0.9981 | 0.9952 | 0.9615 | 0.8993 | 0.0607 | 0.0152 |
| SP4-T0.5 | 0.9155 | 0.9277 | 0.9802 | 0.9999 | 0.0706 | 0.9991 |
| SP4-T1 | 0.9949 | 0.9916 | 0.9502 | 0.8958 | 0.1197 | 0.0280 |
| SP4-T2 | 0.9982 | 0.9996 | 0.9876 | 0.9645 | 0.1882 | 0.7830 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ersanli, C.; Skoufos, I.; Fotou, K.; Tzora, A.; Bayon, Y.; Mari, D.; Sarafi, E.; Nikolaou, K.; Zeugolis, D.I. Release Profile and Antibacterial Activity of Thymus sibthorpii Essential Oil-Incorporated, Optimally Stabilized Type I Collagen Hydrogels. Bioengineering 2025, 12, 89. https://doi.org/10.3390/bioengineering12010089
Ersanli C, Skoufos I, Fotou K, Tzora A, Bayon Y, Mari D, Sarafi E, Nikolaou K, Zeugolis DI. Release Profile and Antibacterial Activity of Thymus sibthorpii Essential Oil-Incorporated, Optimally Stabilized Type I Collagen Hydrogels. Bioengineering. 2025; 12(1):89. https://doi.org/10.3390/bioengineering12010089
Chicago/Turabian StyleErsanli, Caglar, Ioannis Skoufos, Konstantina Fotou, Athina Tzora, Yves Bayon, Despoina Mari, Eleftheria Sarafi, Konstantina Nikolaou, and Dimitrios I. Zeugolis. 2025. "Release Profile and Antibacterial Activity of Thymus sibthorpii Essential Oil-Incorporated, Optimally Stabilized Type I Collagen Hydrogels" Bioengineering 12, no. 1: 89. https://doi.org/10.3390/bioengineering12010089
APA StyleErsanli, C., Skoufos, I., Fotou, K., Tzora, A., Bayon, Y., Mari, D., Sarafi, E., Nikolaou, K., & Zeugolis, D. I. (2025). Release Profile and Antibacterial Activity of Thymus sibthorpii Essential Oil-Incorporated, Optimally Stabilized Type I Collagen Hydrogels. Bioengineering, 12(1), 89. https://doi.org/10.3390/bioengineering12010089










